Abstract
Purpose
Macrophages are an important cellular reservoir in HIV, and exist in two phenotypically dissimilar subsets, the pro-inflammatory M1 phenotype, and the anti-inflammatory M2 phenotype. The role of these two subsets is uncertain. We hypothesized that differences in drug efflux transporters exist between the subsets, which would result in altered intracellular drug concentrations between these cells.
Methods
U937 monocytic cells were polarized to the M1 or M2 phenotype via treatment with interferon-gamma and LPS, or interleukins 4, 13, and LPS, respectively. PGP function was assessed with Hoechst 33342, and expression via western blotting. Intracellular lopinavir was assessed via LC-MS/MS. Data was confirmed with primary monocyte derived macrophages.
Results
We observed significant differences in intracellular concentrations of lopinavir, a PGP substrate, with higher concentrations in M1 cells. PGP function and expression was higher in the M2 macrophages. These results were confirmed with primary monocyte derived macrophages.
Conclusions
This data shows that there are previously unreported differences in P-glycoprotein expression between macrophage subsets, and suggests that there may be differences for other transporters. These differences can play a role in intracellular drug concentrations in these cells, and may allow for low-level HIV replication.
Keywords: Classically activated macrophage, alternatively activated macrophage, Hoechst 33342, P-glycoprotein
Introduction
Macrophages play an important role in a wide variety of disease states. Once activated, macrophages are polarized to one of two activation states. Macrophages are polarized to the classically activated (M1) phenotype via exposure to interferon-gamma (IFNγ), as well as exposure to a secondary inflammatory trigger, including bacterial lipopolysaccharide (LPS). These cells are pro-inflammatory and are primarily involved in the phagocytosis and destruction of foreign pathogens. The alternatively activated (M2) phenotype is anti-inflammatory and pro-fibrotic, and plays an important role in remodeling after an inflammatory response (1-3).
PGP is a transmembrane transporter responsible for the efflux of a number of drugs (4). It is expressed on many tissues, including the intestines, liver, and on the blood brain barrier (4). It is also expressed on monocytes and macrophages. Many drugs are substrates for PGP, including the vast majority of protease inhibitors utilized in the treatment of HIV-1 (5).
To date, no studies have shown differences in transporter expression between macrophage subsets. There has been one study that performed a microarray analysis comparing assessing differences in differentiated monocyte-derived macrophages that had been polarized to either the M1 or the M2 phenotype. While they found significant differences for many transcripts, including multiple solute carriers, they did not observe differences for any transporters that any pharmaceutical agents have been reported to be substrates of (6). It is important to note, however, that microarray data is not infallible, and differences may still be observed for transporters that microarray data does not show.
In individuals with HIV-1, macrophages also play an important role as an infected cell. While macrophages are not the primary infected cell in HIV, they are capable of being infected by HIV (7-11). HIV-infected macrophages function differently than infected T-cells, as an inhibitory intracellular concentration in a T-cell may be inadequate in a macrophage (12). Macrophages thus represent a long-lived sanctuary for HIV. Infected macrophages have a longer life than infected T-cells, and HIV-infection in macrophages is commonly both productive and non-cytopathic. Upon therapy discontinuation, it is thought that infected macrophages can provide a source for viral rebound to the rest of the body (13).
The role of polarized macrophage in HIV in uncertain. There is evidence that polarized macrophages of both phenotypes, are less susceptible to infection than resting monocytes and macrophages (14). Others have suggested that early in infection M1 macrophage are permissive to infection, while the cytokine shift that occurs in late stage HIV results in a shift towards the M2 phenotype, which is resistant to infection (15). Entirely uninvestigated, however, is whether there are any differences in transporter expression between the two subsets of macrophages. Differences in transporter expression between these two subsets of macrophages may result in differences in intracellular concentrations of many medications. These differences in transporter expression may then result in subtherapeutic intracellular concentrations, and may result in the inability for the elimination of virus from these cells. There are a number of transporters that medications are substrates for, including P-glycoprotein (PGP).
Materials and Methods
Cell Culture
The human monocytic cell lines U937 was utilized for initial experiments. U937 cells can be induced to differentiate into mature, polarized macrophages by several cytokines, including LPS, INF-γ, and various interleukins (16). U937 cells were stimulated with LPS (100ng/mL, E. Coli origin, Sigma Aldrich, St. Louis, MO, USA) + INF-γ (20ng/mL, Life Technologies, Carlsbad, CA, USA) or with LPS (100ng/mL) + IL4 (10ng/mL, CST, Danvers, MA, USA)+ IL13 (10ng/mL, CST, Danvers, MA, USA) for 48 hours in order to polarize them towards M1 or M2 phenotype, respectively. Unstimulated controls received no cytokines. In some experiments cells were treated with 1.25 nM lopinavir (LPV) a commonly utilized protease inhibitor, for 24 hours before harvesting.
For primary cell experiments monocytes were enriched via negative immunodensity separation with RosetteSep (StemCell Technologies, Vancouver, BC, Canada). In brief buffy coats were incubated with the RosetteSep enrichment cocktail for 20 minutes. After incubation the buffy coats were layered over lymphocyte separation medium (Corning Life Sciences, Tewksbury, MA), and centrifuged for 20 minutes at 1200g. The antibody enrichment cocktail binds to both residual red blood cells and non-monocytic cells, and the resulting complexes are separated from the mononuclear cells. Monocytes were treated with 50ng/ml m-CSF (Sigma Aldrich, St. Louis, MO) for 6 days to differentiate them into macrophages. After differentiation, cells were polarized to the M1 or M2 phenotype as previously described.
Flow Cytometry staining for CD14
After isolation a representative buffy coat was stained for CD14 to confirm that we obtained a sufficiently pure cell population. Cells were stained for flow cytometry with a mouse monoclonal antibody to CD14 conjugated to APC (Tonbo Biosciences, San Diego, CA, USA). Cells were stained for flow cytometry using standard protocols, and analyzed on a BD LSR II Flow Cytometer.
Arginase Activity
Arginase activity was assessed using a colorimetric determination of urea byproduct from the reaction converting arginine to ornithine via the protocols first described by Corraliza et. al (17). Cells were lysed in 0.1% triton X-100. Briefly, 50μL of cell lysates was heated with 25μL of 10mM MnCl2 in 50mM Tris HCl at 55°C for 10 minutes. The mixtures were then incubated with 50μL of 0.5M arginine solution at 37°C for overnight to hydrolyze the L-arginine. The reaction was terminated by the addition of 400ul of an acid solution (H2SO4/H3PO4/water; 1/3/7). The mixture was then heated with 25μL of 9% isonitrosopropiophenone (ISPF) at 100°C for 45min. All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA). Absorbance was read on a μquant spectrophotometer (Bio-Tek, Winooski, VT, USA) at 540 nm. Readings were compared to a standard curve of known urea concentrations, and were then normalized to cell counts. The values for arginase activity shown in this research are expressed in terms of units of arginase activity. 1 unit of arginase activity is responsible for the catalysis of 1 μmole of L-arginine to ornithine and urea per minute at a pH of 9.7 at 37°C.
Quantification of intracellular lopinavir
Intracellular LPV concentrations were quantified utilizing a previously published validated method (18). Extracts were precipitated, and stable-isotope internal standard was spiked into the sample. Precipitants were centrifuged, and the supernatants were diluted with water and injected onto an API 6500 (AB SCIEX Corporation, Foster City, CA, USA). Separation was achieved with an ACE 3 C18 column. Detection was achieved via multiple reaction monitoring mass spectrometry in positive ion mode. This assay is linear from a range of 0.0500-25.0 fmol/μl.
Western blotting
Cellular proteins were analyzed by Western blot. Briefly, after 48hr treatment, cells were collected and rinsed with ice-cold HBSS. Cells were lysed in cold RIPA lysis buffer supplemented with protease/phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA). Total protein was measured using the BCA assay (Pierce, Rockford, IL, USA). For western blots, samples were prepared by adding 2× Laemmli Sample Buffer (with 5% β-mercaptoethanol) to cell lysates. Samples were heated at ∼70°C for 10 minutes, then equal amounts of protein were loaded (20∼50ug/well) on an 8% PAGE-SDS mini-gel. Immunoblotting was performed with PVDF membranes (Bio-Rad, Hercules, CA, USA), blocked using 5% non-fat milk in TBS buffer for at least 1hr then incubated at 4°C with the respective primary antibody overnight; anti-PGP primary antibodies (Abcam, Cambridge, UK, 1:250), or anti-iNOS antibody (Cayman, Ann Arbor, MI, USA, 1:1000). Whole cellular protein was normalized using GAPDH (Cell Signaling, Danvers, MA, USA, 1:10,00) or β-Actin (Cell Signaling Danvers, MA, USA, 1:2000). The secondary antibody (IRDye 800CW goat anti-rabbit) [1:5,000] was incubated in the dark at room temperature for 45 minutes. Dual-channel infrared scan and quantitation of immunoblots were conducted using the Odyssey Sa infrared imaging system with Image Studio (Ver. 3.1.4) (LI-COR, Lincoln, NE, USA).
PGP function
A hoechst 33342 cellular accumulation assay was utilized to assess PGP function, and was optimized from methods utilized in the literature (19, 20). U937-derived M1, M2, and unstimulated cells were collected after 48 hours of polarization with cytokines. Media was removed and cells were washed with HBSS and resuspended in serum free RPMI. 0.5 ×105 cells in 100uL RPMI were seeded in 96-Well Black Clear-Bottom Plates (Costar, Washington, DC, USA). Plates were incubated at 37°C for 10min with or without the PGP inhibitor elacridar. After incubation, Hoechst 33342 was added to the plates. Plates were immediately placed in FLx800 Fluorescence Reader (BioTek, Winooski, VT, USA) for 60mins and read at 5min intervals at 360/460 (ex/em).
Statistical Analysis
All data shown are expressed as mean ± SEM and obtained from at least three independent experiments. Samples were analyzed utilizing either paired T-tests, ANOVA with Tukey's multiple comparison test, or repeated measures ANOVA with Tukey's multiple comparison test, where appropriate. Statistical analysis was performed with Graphpad Prism (GraphPad Software, La Jolla, CA, USA).
Results
Confirmation of Macrophage Phenotype
Macrophage polarization was confirmed by both western blotting of inducible nitric oxide synthase (iNOS) and arginase activity. iNOS protein levels, a well-established marker of inflammatory macrophages (2, 21, 22), were significantly higher in the M1 group as compared to the M2 group with a mean of 2.55 times and 1.68 times of the untreated control group (U) (Fig. 1A, 1B). M2 macrophages have been shown to show upregulated levels of the enzyme arginase 1 (2, 21, 22). We observed significantly upregulated arginase activity in the macrophages polarized to the M2 phenotype, as compared to both the M1 and unstimulated macrophages (Fig. 1C). These data, coupled with our findings with iNOS expression, show that these cytokines are capable of strongly polarizing the U937 cells to both the M1 and the M2 phenotype.
Figure 1. Cytokines polarize macrophages to the M1 and M2 phenotype.
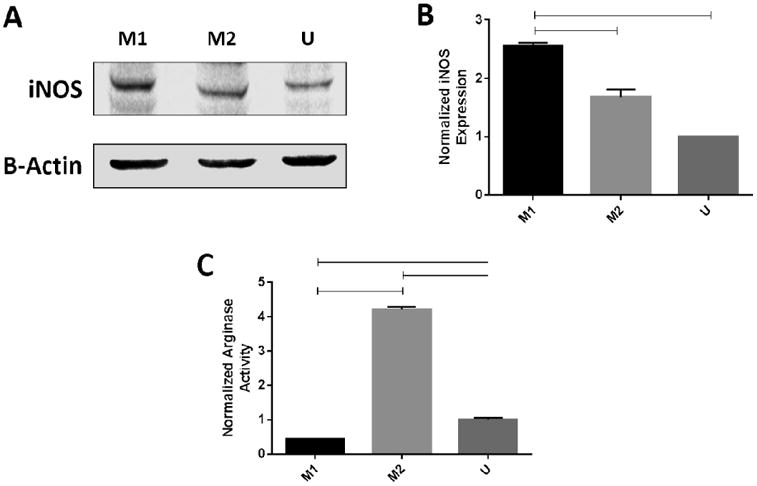
U937 cells were treated with either IFNγ + LPS (M1), IL4 + IL13 + LPS (M2), or no cytokines (U) for 48 hours. (A) Western blotting. Cell lysates were collected, and analyzed for iNOS with a rabbit polyclonal iNOS antibody, with an anti-Rb secondary antibody. (B) Densitometry: Blot density was analyzed using Image Studio, with sample density normalized to the loading control. Sample density was normalized to the unstimulated condition. Data represents averaged densitometry readings of 3 blots. (C) Arginase Assay: Lysates were collected and arginase activity was quantified as the conversion of L-arginine to urea. Samples were normalized to cell counts, then normalized to the unstimulated condition. Data represents a representative arginase assay, from 3 replicated. Samples were analyzed via ANOVA with Tukey's multiple comparison test. Lines between bars represent significant differences (p< 0.05).
Assessment of intracellular lopinavir in macrophage subsets
We next assessed the amount of intracellular LPV present in U937 cells that had been polarized to either the M1 or the M2 phenotype. The U937 cell line has previously been used for cellular pharmacology studies for a variety of antiretrovirals, most notably the protease inhibitors saquinavir, ritonavir, and indinavir (23, 24). Importantly, lopinavir is primarily a substrate of PGP, showing high affinity for the transporter (25). After initial polarization, cells were treated with 1.25 nM LPV for 4 hours (Fig. 2). This concentration was chosen as it is significantly lower than the reported plasma Cmax for the drug, in an attempt to more closely mimic the decreased concentrations that occurs in sanctuary sites. After sample collection, significant differences were observed between the M1 and M2 macrophages, with the M1 macrophages displaying almost two fold higher amounts of intracellular LPV than the M2 macrophages.
Figure 2. M1 macrophages have more intracellular lopinavir than M2 macrophages.
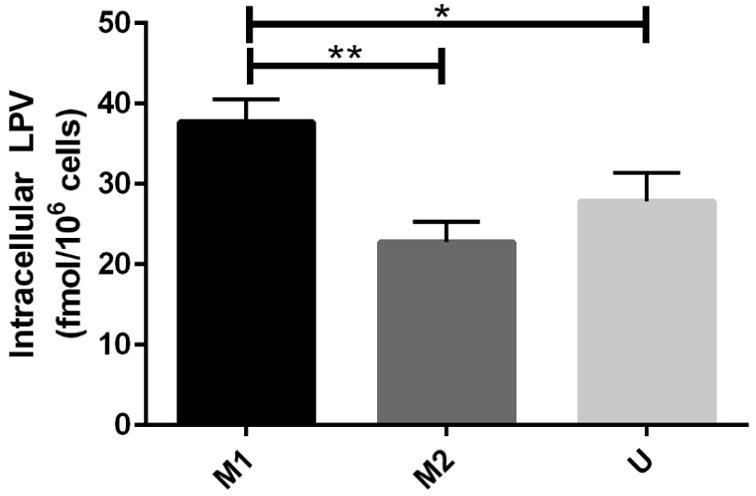
U937 cells were polarized to the M1 or the M2 phenotype for 48 hours via treatment with FNγ + LPS (M1), IL4 + IL13 + LPS (M2), then treated with LPV for 4 hours. After sample collection, cells were lysed in 70% methanol, and analyzed via LC-MS/MS. Intracellular LPV concentrations were normalized to cell counts in each condition. Data represents pooled results from n=3 experiments. Samples were compared via paired T-tests. Lines between bars represent significant results (p<0.05).
Alternative polarization induces P-glycoprotein expression in U937 cells
Based on our findings with LPV, we next assessed if there were differences in PGP function and expression between macrophage subsets. We tested whether the difference in LPV intracellular concentrations were dependent on PGP expression on polarized U937 cells. We measured PGP expression via Western blotting (Fig. 3A). We found that compared with untreated cells, M2 macrophages induced PGP expression level significantly. When PGP expression was normalized to unstimulated controls, M1 or M2 macrophages showed increased expression of PGP when compared to control. M2 macrophages also had significantly higher PGP than the M1 macrophages, consistent with the LPV data (Fig. 3B).
Figure 3. More PGP protein is present in M2 macrophages than M1 or unstimulated macrophages.
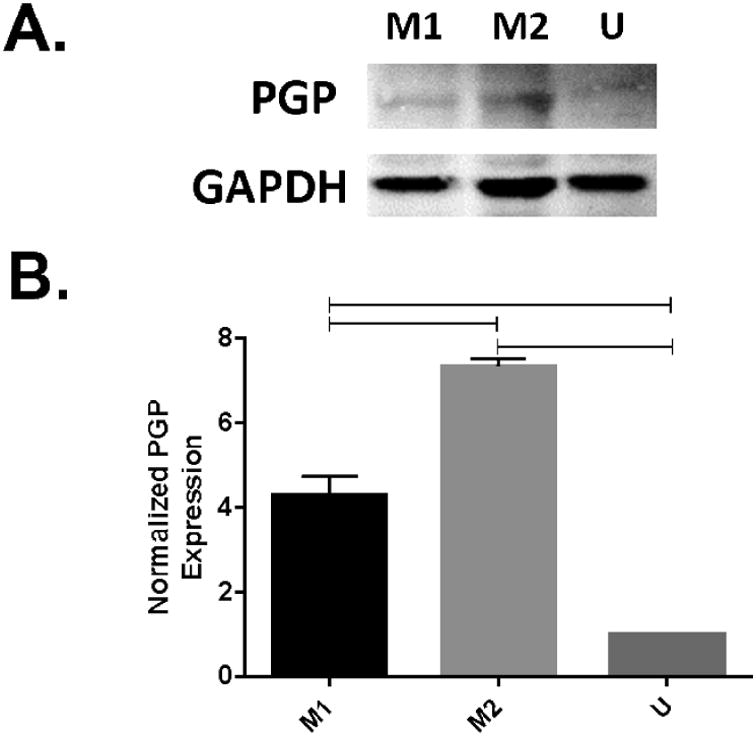
U937 cells were polarized to the M1 or the M2 phenotype for 48 hours via treatment with FNγ + LPS (M1), IL4 + IL13 + LPS (M2), or no cytokines (U). (A) cell lysates were collected, and probed for PGP via western blotting with a rabbit polyclonal to PGP antibody, with an anti-Rb secondary antibody. (B) Densitometry: Blot density was analyzed using Image Studio, with sample density normalized to the loading control. Sample density was normalized to the unstimulated condition. Data represents averaged densitometry readings of 4 blots.
We next tested the accumulation of the PGP substrate hoechst 33342 in cell groups (Fig. 4A). We utilized 10 μM hoechst dye and reached maximum fluorescence at approximately 30 minutes. The cells that had been polarized to the M1 phenotype showed significantly higher fluorescence than the cells that had been polarized to the M2 phenotype, or received no cytokines, showing that there is less PGP expression in the M1 cells. We then pre-treated the cells with elacridar, a small molecule inhibitor of PGP function (Fig. 4B). While we still observed significant differences between the M1 macrophages and the other two groups, the difference between the two groups was less than was observed in the cells that did not receive elacridar.
Figure 4. Macrophage subsets have different PGP function.
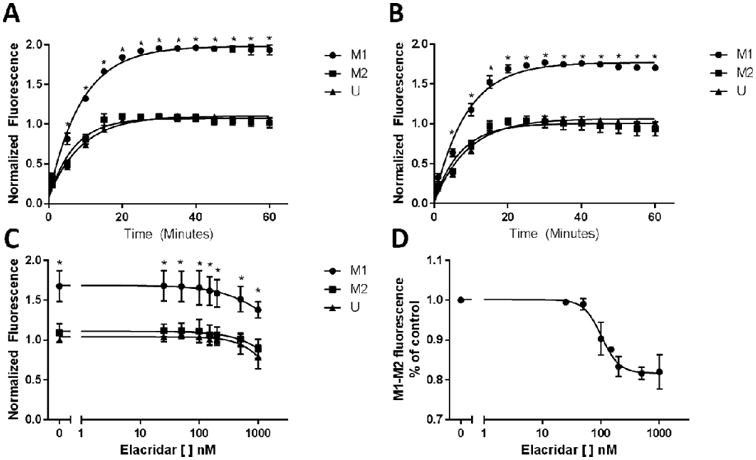
(A-D) U937 cells were polarized to the M1, M2, or unstimulated phenotype for 48 hours via treatment with FNγ + LPS (M1), IL4 + IL13 + LPS (M2), or no cytokines (U). Results were normalized to the unstimulated conditions, and lines were fit via either a one-phase association (figures A, B), or 4 parameter variable slope model (figures C, D). (A) Polarized cells were enumerated, and 5 × 105 cells were plated in 96 well plates. Hoechst 33342 was added at a final concentration of 10 μM, and fluorescence was analyzed every 5 minutes at 360/460 (ex/em). Fluorescence was analyzed for 60 minutes. (B) After being plated in 96 well plates, polarized macrophages were treated with elacridar for 10 minutes, then Hoechst dye was added, and fluorescence was analyzed for 60 minutes. (C) Polarized cells were treated with increasing concentrations of elacridar, then Hoechst dye was added for 30 minutes. (D) The difference in fluorescence between the M1 and M2 macrophages with increasing concentrations of elacridar was calculated and modeled. Comparisons between groups were made via repeated measures ANOVA with Tukey's multiple comparison test. * signifies a significant difference between the M1 and both the M2 and U group (p<0.05)
We then treated cells with increasing concentrations of elacridar, and assessed differences in fluorescence at 30 minutes (Fig. 4C). Increasing concentrations of elacridar resulted in declines in fluorescence. Interestingly, we observed a dose-dependent effect on the difference in fluorescence between the M1 and M2 macrophages, with a maximum effect observed at 500 nM (Fig. 4D). This clearly shows that differences in fluorescence between the two groups of macrophages are due at least in part to differences in function of PGP, and that blocking PGP function minimizes these differences.
Finally, we assessed PGP expression in primary monocyte derived macrophages that had been collected from anonymized healthy subjects, to confirm that the observations that we made with the U937 cells were still true with primary cells. U937 cells are a commonly utilized model cell line for monocytes and macrophages which has been shown to correlate very well to primary cells, although some differences have been reported between the immortalized cell line and cells isolated from the blood (16, 26-28). To confirm the validity of these findings in primary cells, we finally assessed PGP expression in these cells. We first confirmed that the Rosettesep enrichment provided a sufficiently pure cell population (Fig. 5). We observed a purified population of CD14+ monocytic cells of 89.3% of cells, which is in line with the observations from others, which show that Rosettesep can yield a 95% pure population, with less than 5% contamination from B and T cells (29). Similar to the findings with the U937 cells, we observed significantly higher expression of PGP in the M2 polarized cells, with approximately a two-fold difference in expression between the M1 and the M2 cells (Fig. 6A, B). The similarities in PGP expression between the U937 and primary monocyte derived macrophages strongly suggest that PGP expression and function is unaltered between the cell line and primary cells.
Figure 5. Rosettesep purifies the monocyte population from human buffy coats.
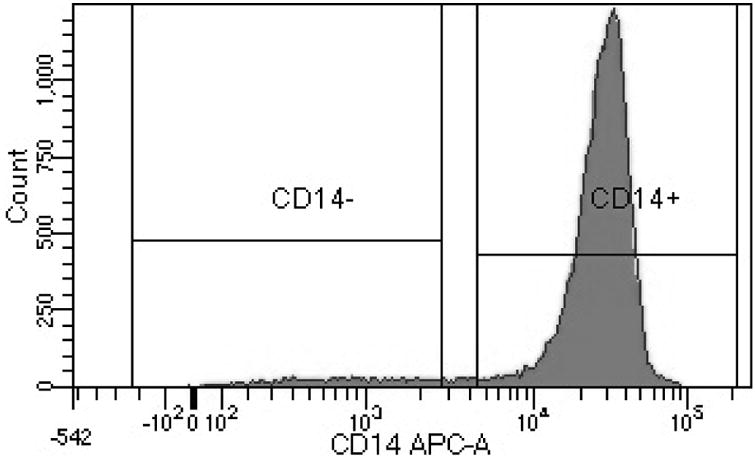
Buffy coats from healthy volunteers were obtained, and enriched via Rosettesep using standard protocols. After enrichment, cells were stained with fluorescently labeled monoclonal antibodies specific for CD14. Rosettesep isolation provided a cellular population of 89.3% CD14+ cells.
Figure 6. PGP expression is upregulated in primary monocyte derived macrophages that have been polarized to the M2 phenotype.
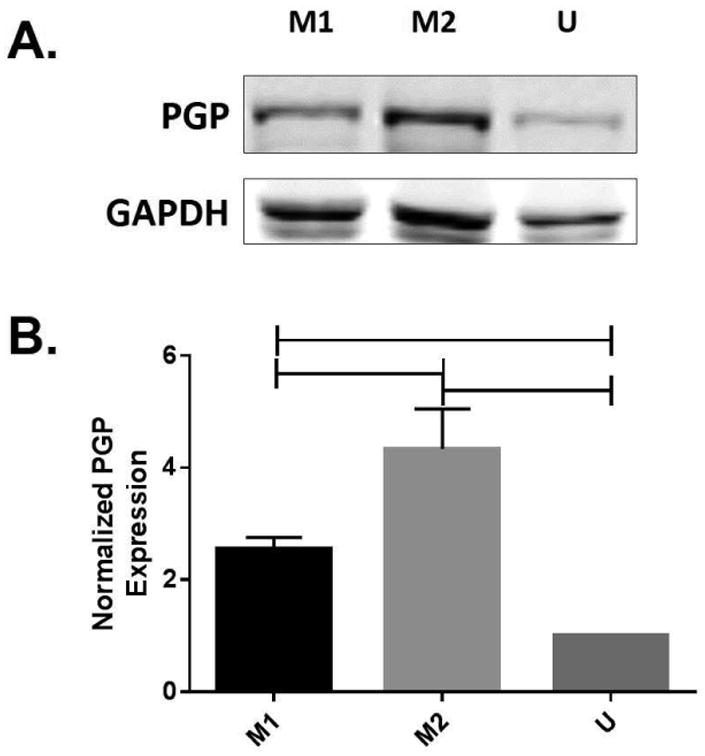
Peripheral blood mononuclear cells were obtained from healthy subjects, and enriched for monocytes via negative selection with RosetteSep. Monocytes were differentiated for 6 days with M-CSF, then treated with cytokines to polarize to the M1 or M2 phenotypes. (A) Cell lysates were collected, and probed for PGP via western blotting with a rabbit polyclonal to PGP antibody, with an anti-Rb secondary antibody. (B) Densitometry: Blot density was analyzed using Image Studio, with sample density normalized to the loading control. Sample density was normalized to the unstimulated condition. Data represents averaged densitometry readings of 3 blots. Comparisons between groups were made via repeated measures ANOVA with Tukey's multiple comparison test. Data represents averaged densitometry readings for 3 blots.
Discussion
Here we show that PGP expression is altered between macrophage subsets, with higher expression in M2 macrophages as compared to the M1 macrophages. This was confirmed via western blotting, as well as by utilizing the fluorescent PGP substrate Hoechst 33342 with elacridar, an inhibitor of PGP function. We also showed that different subsets of alternatively activated macrophages also show differences in PGP expression that have not been previously reported. We also showed that M1 and M2 macrophages have different intracellular concentrations of the protease inhibitor LPV, consistent with our findings on PGP expression. PGP plays an important role in the efflux of a large number of drugs, both drugs utilized for the treatment of HIV, as well as drugs used to treat a wide variety of other conditions. Similarly, in HIV, as well as in other infectious states, intracellular concentrations, rather than plasma concentrations are of key importance. Differences in P-glycoprotein expression between macrophage subsets result in significant differences in intracellular concentrations with a commonly utilized antiretroviral. These alterations in intracellular concentrations may result in a decreased ability of these cells to combat infection with HIV-1.
Differences in intracellular LPV concentrations
We observed a significant difference in intracellular lopinavir concentrations at 4 hours, with two-fold more intracellular lopinavir being observed in the M1 macrophages than the M2 macrophages. This initially suggested that there may be differences in PGP expression between M1 and M2 macrophages, and suggests that these differences in PGP expression can play an important role in the amount of drug that stays inside of a cell, and the amount that is effluxed out of the cell. When these differences are considered in the context of sanctuary sites in HIV, where there is already low amount of drugs present to enter the cell, these differences may represent the final barrier resulting in subtherapeutic concentrations inside of these cells in these sites, for LPV or for other drugs (30). If antiretroviral concentration inside of cells that reside in sanctuary sites are in fact subtherapeutic, there is the potential for low level replication. This may contribute to viral latency in individuals with HIV-1.
PGP function and expression differences between M1/M2 macrophages
We observed significant differences in intracellular concentrations of the fluorescent dye Hoechst 33342 between M1 and M2 macrophages, with more dye present in the M1 macrophages. As PGP is an efflux transporter, this further strongly suggests that PGP expression is downregulated in M1 macrophages, as compared to M2 and unstimulated macrophages. When we next treated cells with a small molecule inhibitor of PGP, while there was still significant differences between M1 and M2 macrophages, the magnitude of the difference was decreased. When cells were treated with increasing concentrations of elacridar, a dose-dependent response was observed as expected. Interestingly, however, the differences in fluorescence between the two subsets of macrophages reaches a point where both the M1 and the M2 macrophages observe similar declines in fluorescence, suggesting that eventually full inhibition of PGP occurs. Remaining differences in fluorescence between the two subsets of macrophages are likely due to the expression of other transporters. These findings are consistent with the data on intracellular LPV concentrations, and explains the differences in LPV concentrations that we observed.
Conclusions
In conclusion, we have determined that there are phenotypic differences in PGP function and expression between M1 and M2 macrophages. These differences can result in altered intracellular drug concentrations between these two subsets of cells. This may result in subtherapeutic concentrations, and in the case of HIV, potentially low-level replication. Phenotypically, M1 and M2 macrophages are extremely dissimilar. They both express different surface proteins and receptors, enzymes, and effector molecules (3, 6). They also serve very different functions, with M1 macrophages being responsible for the phagocytosis and destruction of foreign pathogens, and M2 macrophages being responsible for repair and fibrotic deposition after an inflammatory event (31, 32). Differences in transporter expression can result in differences in intracellular drug concentrations, and may play a role in a variety of disease states. While in this paper we have shown significant differences in expression and function of PGP between macrophage subsets, it is likely that other previously unreported differences may exist for other transporters. Future work will assess differences in the expression and function for other common drug transporters, including BCRP and MRP1. The complex interplay between differences in intracellular drug concentrations, as well as differences in other immunological factors resulting in differences in the ability of these cells to be infected is unknown, and considerable research will be necessary in the future to determine how these factors interact. The main factors influencing differences in intracellular drug concentrations in polarized macrophages are drug influx/efflux, and metabolism of the drugs in the cell.
In HIV, the vast majority of antiretrovirals must enter cells before they can function. There is considerable evidence that intracellular concentrations differ between CD4+ T-cells and macrophages, with macrophages displaying lower intracellular concentrations for many antiretrovirals than T-cells (33). Similarly, there is considerable evidence that there are differences in effective concentrations between T-cells and macrophages; that is a concentration that may be sufficient to inhibit viral replication in T-cells may be insufficient to inhibit viral replication in a macrophage (12, 33, 34). To date, it is unknown if different intracellular concentrations are required for full viral inhibition between the two subsets of macrophages. If transporter expression on macrophage subsets results in lower intracellular concentrations in one subset of macrophages, it is possible that this may allowing low level ongoing replication, especially in sanctuary sites including lymph nodes and secondary lymphoid tissue (13, 30).
These reported differences in PGP expression may also be important in other disease states. Azithromycin, a macrolide antibiotic, is also a substrate for PGP (35). Intracellular concentrations in macrophages are extremely important for dealing with infections with other bacterial strains, including Listeria monocytogenes and Francisella tularensis (35, 36). These two bacteria that are capable of shifting macrophage polarization to the M1 or the M2 phenotype, respectively (37, 38). Polarization towards a certain macrophage phenotype with these or other bacteria may result in subtherapeutic anti-infective concentrations inside macrophages, preventing the clearance of the bacteria inside of these cells. A greater understanding of the differences in macrophage transporter expression between macrophage subsets, and the drug concentrations inside of these cells may result in improved outcomes in other disease states.
There are however, a few limitations associated with the experiments that we undertook here. First, macrophage phenotype exists on a spectrum. in vivo cells can be exposed to multiple stimuli, making categorization into distinct subsets considerably more difficult. While there may be difficulties fully translating the findings from our in vitro study to in vivo, the ability to fully manipulate this system utilizing in vitro and ex vivo stimulation of macrophages by cytokines provides a powerful tool to understand the interplay between polarized macrophages and transporters.
Second, the dye and small molecule inhibitor that we utilized display some cross-sensitivity for other transporters, including MRP1 and BCRP. All three of these transporters are ABC transporters, and many drugs are substrates for at least two, or all three of these transporters (39). Similarly, elacridar, while primarily a PGP inhibitor does also block BCRP, although IC50 concentrations for BCRP are 40-fold higher than IC50 concentrations for PGP (40). Elacridar does not have any substrate specificity for MRP1 (41). While we have chosen both fluorescent dyes and small molecule inhibitors to be primarily substrates of PGP, off-target effects cannot be eliminated. Third, LPV is not utilized as monotherapy as part of the current treatment of HIV, and it is possible that in the presence of other antiretrovirals these concentrations may be altered. Additionally, many antiretrovirals are substrates for a wide variety of efflux transporters, including MRP1 and BCRP. In these experiments we focused on LPV as it has high affinity for PGP, and significantly lower affinity for other transporters. LPV thus serves as a probe drug, albeit a therapeutically relevant one in this study. It is also important to first assess drugs as single agents, before later assessing the interplay between multiple drugs and efflux transporters, as if multiple agents are tested simultaneously it is difficult to discriminate which factors influences intracellular concentrations. Future work will assess differences in expression of other clinically relevant drug efflux transporters, as well as assess differences in intracellular concentrations of other antiretrovirals which are substrates for multiple transporters. The findings in this article describe a previously unreported difference between M1 and M2 macrophages, and suggests that this may result in differences in intracellular concentrations for LPV between these two subsets of cells. In vivo, there are a number of factors that likely influence differences in intracellular drug concentrations between macrophage subsets, including other transporters, and metabolic enzymes. This research describes the contribution of one efflux transporter. Future work will investigate other efflux transporters. A greater understanding of the various factors that influence intracellular drug concentrations may provide methods to increase drug concentrations in subsets of macrophages, including blocking transporters, modifying therapy to avoid drugs which are substrates of physiologically relevant transporters, and novel formulations of the drugs which may avoid efflux from the cell. Increasing intracellular concentrations of antiretrovirals in both subsets of macrophages may be an important tool in the elimination of HIV from the body.
Acknowledgments
TJC designed and implemented experiments, and organized the writing of the paper
HH ran experiments, and participated in the writing of the first draft of the paper
LCW ran the LC-MS/MS experiments
SK assisted with experimental design of the primary cell experiments
CVF assisted with the design of experiments, and provided guidance throughout the development of the project
Funds for this research was provided from an internal grant from the UTHSC College of Pharmacy (to TJC), and grants from the National Institute of Allergy and Infectious Diseases (P01 AI074340, R21 AI116208, and U01 AI105872, to CVF).
Abbreviations
- M1
Classically activated macrophages
- M2
Alternatively activated macrophage
- IFNγ
Interferon gamma
- LPS
Lipopolysaccharide
- IL4
Interleukin 4
- IL13
Interleukin 13
- PGP
P-glycoprotein
- LPV
Lopinavir
- iNOS
Inducible nitric oxide synthase
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
References
- 1.Mantovani A, Locati M. Orchestration of macrophage polarization. Blood. 2009;114(15):3135–6. doi: 10.1182/blood-2009-07-231795. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Mosser DM. The many faces of macrophage activation. Journal of leukocyte biology. 2003;73(2):209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 4.Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 2007;7(3):154–79. doi: 10.1038/sj.tpj.6500413. [DOI] [PubMed] [Google Scholar]
- 5.Rathbun RC, Liedtke MD. Antiretroviral drug interactions: overview of interactions involving new and investigational agents and the role of therapeutic drug monitoring for management. Pharmaceutics. 2011;3(4):745–81. doi: 10.3390/pharmaceutics3040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 7.Aquaro S, Perno CF, Balestra E, Balzarini J, Cenci A, Francesconi M, et al. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocytes. Journal of leukocyte biology. 1997;62(1):138–43. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. Aids. 2014;28(15):2175–87. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Current Opinion in HIV and AIDS. 2013;8(3):190–5. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppensteiner H, Brack-Werner R, Schindler M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology. 2012;9:82. doi: 10.1186/1742-4690-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Douce V, Herbein G, Rohr O, Schwartz C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010;7:32. doi: 10.1186/1742-4690-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavegnano C, Kennedy EM, Kim B, Schinazi RF. The impact of macrophage nucleotide pools on HIV-1 reverse transcription, viral replication, and the development of novel antiviral agents. Molecular biology international. 2012:2012. doi: 10.1155/2012/625983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proceedings of the National Academy of Sciences of the United States of America. 2015 doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlaepfer E, Rochat MA, Duo L, Speck RF. Triggering TLR2, -3, -4, -5, and -8 reinforces the restrictive nature of M1- and M2-polarized macrophages to HIV. J Virol. 2014;88(17):9769–81. doi: 10.1128/JVI.01053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, Polentarutti N, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91(1):258–65. [PubMed] [Google Scholar]
- 17.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. Journal of immunological methods. 1994;174(1-2):231–5. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 18.Podany AT, Winchester LC, Robbins BL, Fletcher CV. Quantification of cell-associated atazanavir, darunavir, lopinavir, ritonavir, and efavirenz concentrations in human mononuclear cell extracts. Antimicrobial agents and chemotherapy. 2014;58(5):2866–70. doi: 10.1128/AAC.02551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller H, Klinkhammer W, Globisch C, Kassack MU, Pajeva IK, Wiese M. New functional assay of P-glycoprotein activity using Hoechst 33342. Bioorganic & medicinal chemistry. 2007;15(23):7470–9. doi: 10.1016/j.bmc.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunology and cell biology. 1999;77(6):499–508. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- 21.Murphy BS, Sundareshan V, Cory TJ, Hayes D, Jr, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. The Journal of antimicrobial chemotherapy. 2008;61(3):554–60. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 22.Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer research. 1998;58(4):717–23. [PubMed] [Google Scholar]
- 23.Jones K, Hoggard PG, Khoo S, Maher B, Back DJ. Effect of alpha1-acid glycoprotein on the intracellular accumulation of the HIV protease inhibitors saquinavir, ritonavir and indinavir in vitro. Br J Clin Pharmacol. 2001;51(1):99–102. doi: 10.1046/j.0306-5251.2001.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo SH, Hoggard PG, Williams I, Meaden ER, Newton P, Wilkins EG, et al. Intracellular accumulation of human immunodeficiency virus protease inhibitors. Antimicrob Agents Chemother. 2002;46(10):3228–35. doi: 10.1128/AAC.46.10.3228-3235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janneh O, Jones E, Chandler B, Owen A, Khoo SH. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. Journal of Antimicrobial Chemotherapy. 2007;60(5):987–93. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 26.Baek YS, Haas S, Hackstein H, Bein G, Hernandez-Santana M, Lehrach H, et al. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 2009;10:18. doi: 10.1186/1471-2172-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassol E, Alfano M, Biswas P, Poli G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. Journal of leukocyte biology. 2006;80(5):1018–30. doi: 10.1189/jlb.0306150. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Tikhonov I, Ruckwardt TJ, Djavani M, Zapata JC, Pauza CD, et al. Monocytes treated with human immunodeficiency virus Tat kill uninfected CD4(+) cells by a tumor necrosis factor-related apoptosis-induced ligand-mediated mechanism. J Virol. 2003;77(12):6700–8. doi: 10.1128/JVI.77.12.6700-6708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler JJ, Tuttle DL, Coberley CR, Sleasman JW, Goodenow MM. Human immunodeficiency virus type 1 (HIV-1) induces activation of multiple STATs in CD4+ cells of lymphocyte or monocyte/macrophage lineages. Journal of leukocyte biology. 2003;73(3):407–16. doi: 10.1189/jlb.0702358. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(6):2307–12. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S. Alternative activation of macrophages. Nature reviews. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antiviral chemistry & chemotherapy. 2009;20(2):63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavegnano C, Detorio MA, Bassit L, Hurwitz SJ, North TW, Schinazi RF. Cellular pharmacology and potency of HIV-1 nucleoside analogs in primary human macrophages. Antimicrob Agents Chemother. 2013;57(3):1262–9. doi: 10.1128/AAC.02012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seral C, Carryn S, Tulkens PM, Van Bambeke F. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J Antimicrob Chemother. 2003;51(5):1167–73. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad S, Hunter L, Qin A, Mann BJ, van Hoek ML. Azithromycin effectiveness against intracellular infections of Francisella. BMC Microbiol. 2010;10:123. doi: 10.1186/1471-2180-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181(6):3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 38.Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J Immunol. 2008;181(6):4159–67. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kis O, Robillard K, Chan GNY, Bendayan R. The complexities of antiretroviral drug–drug interactions: role of ABC and SLC transporters. Trends in pharmacological sciences. 2010;31(1):22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Boumendjel A, Baubichon-Cortay H, Trompier D, Perrotton T, Di Pietro A. Anticancer multidrug resistance mediated by MRP1: recent advances in the discovery of reversal agents. Med Res Rev. 2005;25(4):453–72. doi: 10.1002/med.20032. [DOI] [PubMed] [Google Scholar]
- 41.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146(2):117–26. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]


