Abstract
Background & Aims
Alcoholic liver disease (ALD) remains a major cause of morbidity and mortality, with no Food and Drug Administration–approved therapy. Chronic alcohol consumption causes a pro-oxidant environment and increases hepatic lipid peroxidation, with acrolein being the most reactive/toxic by-product. This study investigated the pathogenic role of acrolein in hepatic endoplasmic reticulum (ER) stress, steatosis, and injury in experimental ALD, and tested acrolein elimination/scavenging (using hydralazine) as a potential therapy in ALD.
Methods
In vitro (rat hepatoma H4IIEC cells) and in vivo (chronic+binge alcohol feeding in C57Bl/6 mice) models were used to examine alcohol-induced acrolein accumulation and consequent hepatic ER stress, apoptosis, and injury. In addition, the potential protective effects of the acrolein scavenger, hydralazine, were examined both in vitro and in vivo.
Results
Alcohol consumption/metabolism resulted in hepatic accumulation of acrolein-protein adducts, by up-regulation of cytochrome P4502E1 and alcohol dehydrogenase, and down-regulation of glutathione-s-transferase-P, which metabolizes/detoxifies acrolein. Alcohol-induced acrolein adduct accumulation led to hepatic ER stress, proapoptotic signaling, steatosis, apoptosis, and liver injury; however, ER-protective/adaptive responses were not induced. Notably, direct exposure to acrolein in vitro mimicked the in vivo effects of alcohol, indicating that acrolein mediates the adverse effects of alcohol. Importantly, hydralazine, a known acrolein scavenger, protected against alcohol-induced ER stress and liver injury, both in vitro and in mice.
Conclusions
Our study shows the following: (1) alcohol consumption triggers pathologic ER stress without ER adaptation/protection; (2) alcohol-induced acrolein is a potential therapeutic target and pathogenic mediator of hepatic ER stress, cell death, and injury; and (3) removal/clearance of acrolein by scavengers may have therapeutic potential in ALD.
Keywords: Lipid Peroxidation, Apoptosis, Therapeutic, CHOP
Abbreviations used in this paper: ADH, alcohol dehydrogenase; ALD, alcoholic liver disease; ALDH, aldehyde dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ATF, activating transcription factor; CHOP, CCAAT/enhancer-binding protein homologous protein; CYP2E1, cytochrome P4502E1; ER, endoplasmic reticulum; FDP-lysine, Nε-(3-formyl-3,4-dehydropiperidino)lysine; GRP, glucose regulated protein; GSTP, glutathione-s-transferase-Pi; IRE1, inositol-requiring enzyme 1; JNK, cJun N-terminal kinase; mRNA, messenger RNA; NIAAA, National Institute on Alcohol Abuse and Alcoholism; PERK, protein kinase RNA-like endoplasmic reticulum kinase; LPO, lipid peroxidation; PUFA, polyunsaturated fatty acids; siRNA, small interfering RNA; TRAF, TNF receptor-associated factor; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling; UPR, unfolded protein response; XBP1, X-box binding protein-1
Summary.
Acrolein is a pathogenic mediator of alcoholic liver disease; alcohol-induced acrolein accumulation triggers endoplasmic reticulum stress without endoplasmic reticulum adaptive/protective responses, leading to apoptosis and liver injury. Acrolein removal by scavengers (hydralazine) prevents alcohol-induced liver injury in mice, and shows therapeutic potential in alcoholic liver disease.
Alcohol consumption can lead to alcoholic liver disease (ALD), which remains a major cause of morbidity and mortality worldwide and in the United States. Despite the profound economic and health impact, there is no Food and Drug Administration–approved therapy for any stage of ALD,1 emphasizing the need for research into therapeutic interventions during the early initiating stages of the disease. Furthermore, only approximately 20% of heavy alcohol drinkers develop liver disease, and diet and environment are considered potential determining factors. The pathogenesis of ALD is multifactorial, and although oxidative stress, lipid peroxidation, and endoplasmic reticulum (ER) stress are known etiologic factors,2 the molecular mediators of hepatic injury remain poorly defined.
Hepatic ER stress and the unfolded protein response (UPR) are thought to play a critical role in the pathogenesis of ALD.3 ER stress is mediated primarily by the ER protein BiP/glucose-regulated protein (GRP)78 via 3 ER sensors: activating transcription factor (ATF)6, inositol-requiring enzyme 1 (IRE1), and protein kinase RNA-like endoplasmic reticulum kinase (PERK). ER stress comprises both pathologic and adaptive responses; UPR adaptive responses reduce the protein burden by decreased synthesis and increased ER-associated degradation of proteins, and by inducing ER chaperones to enhance folding capacity. If the adaptive/protective reactions are inadequate or if the ER stress is prolonged or extreme, apoptotic cell death ensues via pathways involving activation of cJun N-terminal kinase (JNK) and caspases, and up-regulation of proapoptotic proteins such as CCAAT/enhancer-binding protein homologous protein (CHOP), growth arrest DNA damage (GADD) 153 and GADD 34.4 Alcohol consumption is known to cause hepatic ER stress along with steatosis, inflammation, and apoptosis, and reducing ER stress decreases alcoholic liver injury.5, 6 Although oxidative stress is known to contribute to ER stress,7 the exact cause and pathogenic mediators of alcohol-induced ER stress in ALD remain unclear.
Alcohol consumption and metabolism via alcohol dehydrogenase (ADH), cytochrome P4502E1 (CYP2E1), and catalase pathways generates free radicals, leading to increased lipid peroxidation (LPO) of polyunsaturated fatty acids (PUFAs).6, 7, 8 The dietary consumption of PUFAs, particularly linoleic acid, has increased dramatically in the past 2 decades, thereby increasing the substrate availability for LPO. LPO gives rise to α, β-unsaturated aldehydes, such as 4-hydroxynonenal, malondialdehyde, and acrolein. Acrolein is the most reactive and toxic electrophile produced by LPO9, 10; moreover, it is found at approximately 40 times greater concentration and has a significantly longer half-life than other reactive oxidative species (days as compared with less than a second).11, 12 Acrolein is metabolized and cleared primarily by conjugation to glutathione catalyzed by glutathione-s-transferase-Pi (GSTP). If not removed, acrolein can form covalent protein adducts, generally leading to impaired protein structure and function. Accumulation of such adducted/misfolded proteins can trigger ER stress, and acrolein-induced ER stress is shown in vitro in various cell types.13 Increased acrolein adduct levels are linked pathologically with several diseases that are associated with oxidative stress, including cancer,14 Alzheimer’s,15 Sjogren’s syndrome autoimmune disorder,16 and cerebral stroke/infarction.17 However, the role of acrolein in ALD has not been investigated. In this study, we investigated the pathologic contribution of acrolein in alcohol-induced hepatic ER stress, steatosis, and liver injury, and we tested, both in vitro and in vivo, whether removal of acrolein by using scavengers is effective in preventing liver injury in ALD.
Materials and Methods
Reagents
General chemicals, hydralazine, carnosine, acrolein, and β-actin antibody were purchased from Sigma Aldrich (St. Louis, MO). Acrolein Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-lysine) antibodies were purchased from Abcam (Cambridge, MA). All other antibodies were purchased from Cell Signaling (Beverly, MA). Cell culture supplies were obtained from Invitrogen (Carlsbad, CA).
Cell Culture
H4IIEC, a rat hepatoma cell line, was obtained from American Type Culture Collection (Rockville, MD) and used according to the manufacturer's instructions. All treatments were performed on subconfluent monolayers of cells.18 Each experiment was replicated independently at least 3 times.
Animal Studies
We used an established model of ALD (National Institute on Alcohol Abuse and Alcoholism [NIAAA] model19). Male C57BL/6J mice (12 weeks of age) were obtained from Jackson Laboratories (Bar Harbor, ME), and maintained at 25°C with a 12:12-hour light/dark cycle. The mice were fed (ad libitum) a Lieber–DeCarli liquid diet (corn oil/LA enriched; Research Diet, New Brunswick, NJ.) containing 5% ethanol (wt/vol or 35% of calories) or an isocaloric maltose dextrin Lieber–DeCarli liquid diet as (pair-fed) control for 10 days. This was followed by a single oral gavage of ethanol (5 g/kg body weight, or maltose dextrin as control) on day 11. Blood and liver samples were collected 9 hours later. Hydralazine (5 mg/kg body weight) was administered by daily intraperitoneal injection along with alcohol feeding. We used 6 mice in each group based on our earlier observations and published literature in this mouse model. All experimental protocols were conducted under a protocol approved by the University of Louisville Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Office of Laboratory Animal Welfare Guidelines (available: http://grants.nih.gov/grants/olaw/olaw.htm).
Acrolein Adducts Assay
Cryostat mouse liver sections (5 μmol/L) and H4IIEC cells grown on coverslips were fixed, stained using polyclonal rabbit antibodies specific for acrolein FDP-lysine adducts, and examined by light microscopy. Quantification was performed using ImageJ Microscopy Image Analysis Software (National Institutes of Health, Bethesda, MD) by calculating the average intensity of the field-of-view from microscope fields (at least 4 images per sample from cells and 10 images/mouse from each group).
Small Interfering RNA Transfection
Before alcohol or acrolein treatment, small interfering RNAs (siRNAs) specific for rat GSTP or scrambled RNA (Thermo Fisher, Grand Island, NY) as a negative control were transfected into H4IIEC cells with Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. The final concentration of each transfected RNA was 25 nmol/L. Inhibition of GSTP was confirmed by examining GSTP messenger RNA (mRNA) and protein levels. Cells were treated 72 hours after transfection with alcohol or acrolein for 24 hours.
Cell Viability–3, (4, 5-Dimethylthiazol-2-Yl) 2, 5-Diphenyltetrazolium Bromide Assay
Cell survival/cell death was measured in treated cells by the 3, (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide assay as described.18
Western Blot Analysis
Equivalent proteins of total liver or cell extracts were analyzed by standard Western blot using specific commercial antibodies. Proteins were visualized using enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) and quantified by densitometry using Imagelab software (Hercules, CA). Blots were reprobed with antibody to β-actin to ensure equivalent loading. The density ratio of each band compared with its corresponding β-actin band was determined and normalized to the control value, which was set to 1. Numbers in the figures represent the density ratio or the group mean for each group of mice.
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction Analysis
Total RNA was isolated using TRIzol (Invitrogen) and subjected to real-time quantitative polymerase chain reaction using SYBR green I dye reagents and specific primers (Primer-BLAST; National Center for Biotechnology Information, National Institutes of Health) with an ABI prism 7500 system (Applied Biosystems, Foster City, CA). A dissociation curve analysis was performed to confirm primer specificity, and relative mRNA expression was calculated using the Delta-Delta-Ct method from duplicate samples after normalization to β-actin.
Liver Histology and Steatosis
Liver sections were stained with H&E or Oil Red O, and examined by light microscopy.20
Liver Cell Apoptosis
Liver cell apoptosis was assessed by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay using the ApopTag Peroxidase in Situ Apoptosis Detection kit (Chemicon, Temecula, CA). Quantification of apoptosis was performed by counting the positively stained cells in 10 fields from each mouse liver.
Liver Injury
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using Enzymatic Assay Kits (Thermo Scientific, Waltham, MA).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, Inc, La Jolla, CA). Data were analyzed, as appropriate, by the Student t test (for analysis of 2 groups) or by unpaired analysis of variance with Bonferroni post-test analysis (for >2 groups), with data from at least 3 experiments or 6 mice per group. Differences were considered statistically significant for a P value less than .05.
Results
In this study, we examined the contributory role of the lipid-derived aldehyde, acrolein, to alcohol-induced liver injury using cultured rodent hepatoma cells (H4IIEC), and the chronic+binge murine model of ALD (also called the NIAAA model). This is a well-accepted mouse model for hepatic steatosis and hepatocellular injury in ALD, and reflects a common drinking pattern in human beings, particularly in patients with ALD who often are both chronic and binge drinkers.19, 21
Alcohol Consumption Leads to Hepatic Acrolein Generation and Accumulation of Acrolein Adducts
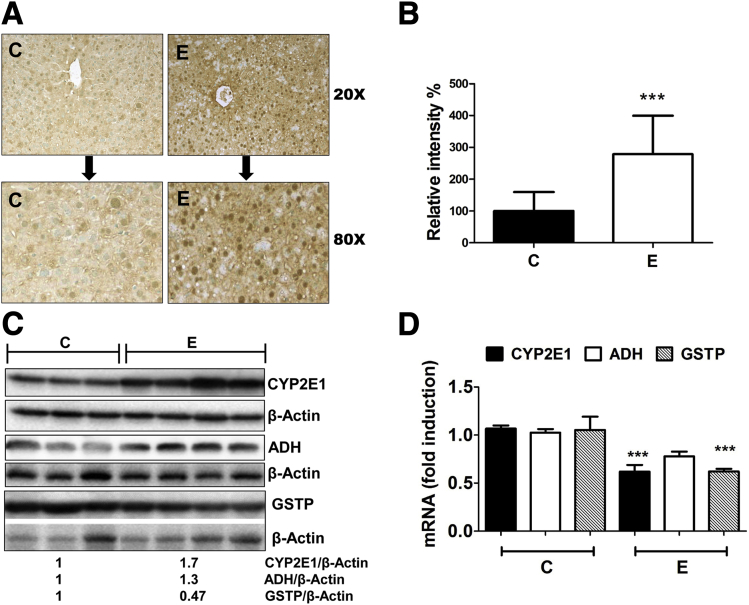
To investigate the theory that acrolein is a pathogenic mediator of alcoholic liver disease, we first examined whether alcohol consumption led to acrolein build-up in the liver. Although free acrolein is extremely labile and difficult to quantify, it readily reacts with cellular proteins to form covalent adducts that then can be assessed. Acrolein can form Michael addition-type adducts with cysteines, histidines, and lysines of proteins; the acrolein-lysine adduct, FDP-lysine, is readily detectable using specific antibodies. A marked increase was observed in the levels of acrolein–protein adducts (brown staining of acrolein–FDP-lysine adducts) in the livers of alcohol-fed mice (Figure 1A) compared with controls, showing that alcohol consumption led to the hepatic generation of acrolein. The alcohol-induced acrolein adduct accumulation was not largely zone-specific, but was increased slightly close to the portal vein in some animals; in addition, both cytoplasmic and nuclear accumulation was seen. Quantification of acrolein adducts showed a 3-fold, statistically significant difference between control and alcohol-fed livers (Figure 1B).
Figure 1.
Alcohol consumption leads to accumulation of acrolein-protein adducts, up-regulation of ADH and CYP2E1, and down-regulation of GSTP in mice livers. (A) Accumulation of acrolein adducts in mice livers by immunohistochemistry using specific FDP-lysine antibodies (magnification, 20× and 80×). (B) Quantification of acrolein adducts observed in panel A. Means ± SEM, n = 6 mice. ***P < .001 compared with control by analysis of variance–Bonferroni analysis. (C) ADH, CYP2E1, and GSTP protein levels in mice livers. Numbers represent mean densitometry ratios normalized to corresponding β-actin levels. (D) ADH, CYP2E1, and GSTP mRNA levels. Means ± SEM, n = 6 mice. ***P < .001 compared with control by analysis of variance–Bonferroni analysis. C, control; E, alcohol.
To determine the mechanisms underlying alcohol-induced acrolein accumulation, we examined enzymes that primarily metabolize alcohol, namely, ADH and CYP2E1. Alcohol is known to up-regulate CYP2E1 and increase its own metabolism, thereby leading to oxidative stress and enhanced LPO. Hence, it is likely to generate higher levels of the LPO-derived aldehydes, such as acrolein. Accordingly, we examined CYP2E1 and ADH expression in the livers of control vs alcohol-fed mice. As anticipated, alcohol feeding led to a robust increase in CYP2E1 protein levels in the alcohol-fed mice (Figure 1C). However, CYP2E1 mRNA was decreased by alcohol feeding (Figure 1D). Similar effects were seen in hepatic ADH, wherein ADH mRNA was decreased (Figure 1D) and ADH protein levels were increased slightly by alcohol (Figure 1C). An alcohol-induced increase in CYP2E1 protein in the absence of transcriptional up-regulation of the mRNA has been proposed to be caused by stabilization of the protein22; whether the same occurs with ADH remains to be investigated. The concentration of acrolein in the liver is modulated not only by its generation, but also by the rate of detoxification or removal. Because acrolein is metabolized and cleared primarily via conjugation with glutathione catalyzed by the enzyme GSTP,23 we also examined the effects of alcohol on hepatic GSTP levels. Alcohol consumption resulted in a decrease in the hepatic levels of both GSTP mRNA (Figure 1D) and GSTP protein (Figure 1C) as seen in alcohol-fed mice compared with control, suggesting that the decrease in GSTP may contribute to acrolein accumulation in the livers of alcohol-fed mice.
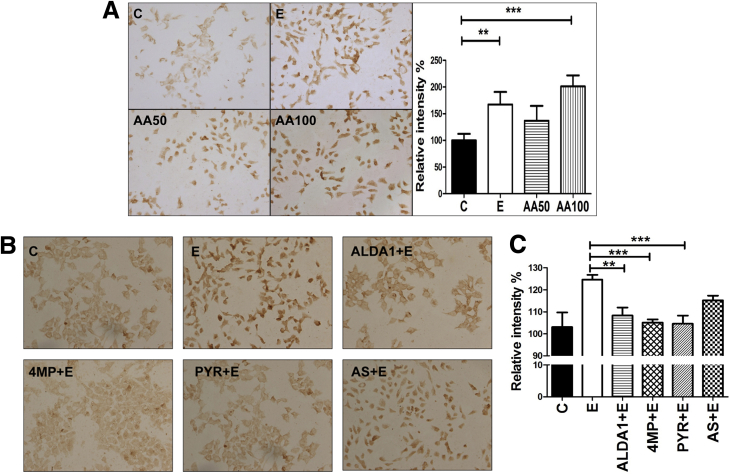
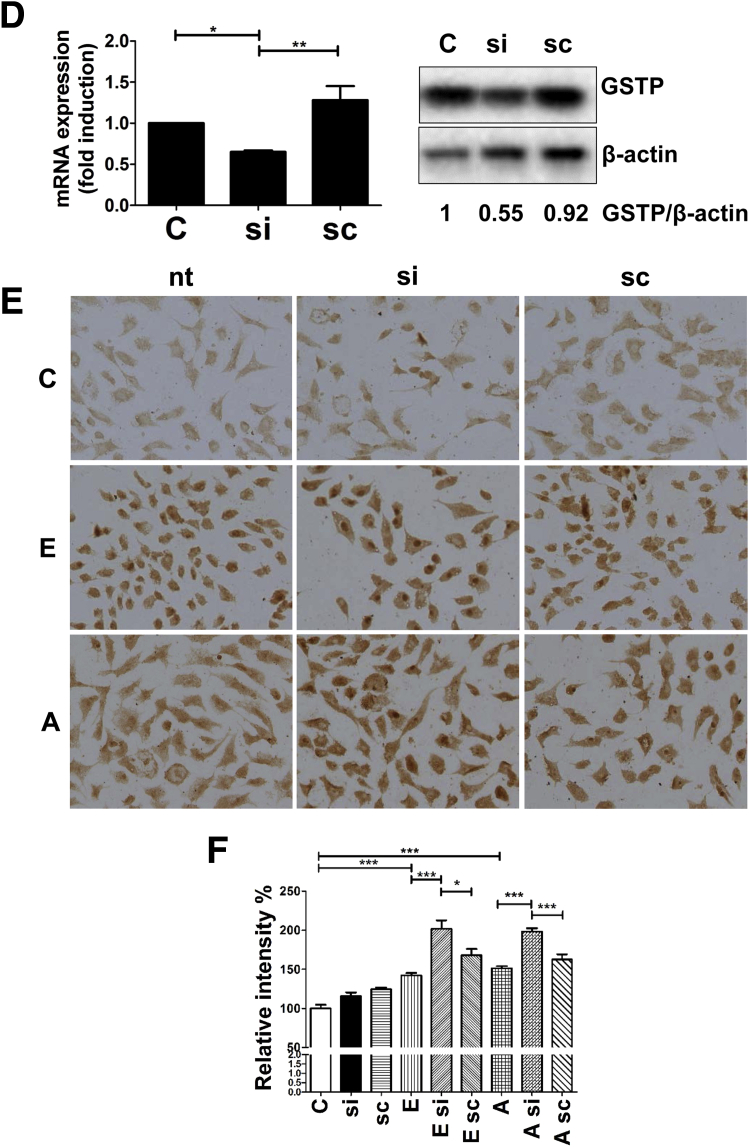
By using cultured rodent hepatic cells that are known to metabolize alcohol (H4IIEC cells), we assessed whether in vitro exposure to alcohol resulted in the accumulation of acrolein adducts, similar to that seen in livers of alcohol-fed mice. Considerable acrolein adduct accumulation was seen in H4IIEC cells that were treated for 24 hours with alcohol (200 mmol/L) compared with untreated cells (Figure 2A). Alcohol is metabolized in the liver by ADH or CYP2E1 into its primary metabolite, acetaldehyde, which causes increased reactive oxygen species production, leading to enhanced LPO. Based on the acrolein build-up seen in alcohol-exposed mice and cultured cells, we explored the direct role of the alcohol metabolite, acetaldehyde, in acrolein generation and adduct accumulation in hepatocytes. Cells were exposed in vitro to acetaldehyde (50 or 100 μmol/L) for 24 hours, and acrolein adduct levels were examined. Similar to alcohol, direct exposure of cells to acetaldehyde resulted in significant acrolein adduct accumulation (Figure 2A). Quantification showed that treatment with alcohol and 100 μmol/L acetaldehyde caused a statistically significant increase in acrolein adducts, and a similar trend (but not significant) was seen with the lower concentration of acetaldehyde (Figure 2A). Once formed, acetaldehyde is metabolized further by aldehyde dehydrogenase (ALDH) enzymes, particularly ALDH2, which also is known to metabolize and remove reactive unsaturated aldehydes such as acrolein. Hence, we tested whether (N-(1,3-Benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide), a known agonist of ALDH2, affected alcohol-induced acrolein build-up. Pretreatment of cells with (N-(1,3-Benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide) before alcohol exposure considerably decreased the accumulation of acrolein adducts (Figure 2B and C), showing that stimulating ALDH2 attenuated alcohol-induced acrolein accumulation. Furthermore, to determine whether acrolein build-up was dependent on alcohol metabolism, H4IIEC cells also were exposed to various known inhibitors of alcohol metabolism before alcohol exposure. Treatment of cells with either pyrazole or 4-methylpyrazole (which suppress both ADH and CYP2E1-dependent alcohol metabolism24), substantially attenuated the accumulation of alcohol-induced acrolein adducts (Figure 2B and C). In comparison, allyl sulfide, which is a more selective CYP2E1 inhibitor, was less effective in blocking alcohol-induced acrolein build-up, suggesting that both ADH and CYP2E1 pathways led to acrolein generation, and that inhibition of only one metabolic pathway was insufficient to eliminate acrolein adduct accumulation (Figure 2B and C). To further investigate the role of down-regulation of GSTP in the alcohol-induced build-up of acrolein adducts, we used siRNA in H4IIEC cells to effectively inhibit GSTP mRNA and protein (Figure 2D). Genetic inhibition of GSTP by siRNA led to an increase in the accumulation of acrolein adducts (Figure 2E and F) after treatment with alcohol or acrolein, which was not seen in untransfected cells or those transfected with scrambled RNA. These data confirmed that the down-regulation of GSTP by alcohol may be a novel mechanism that contributes to enhanced alcohol-induced hepatic acrolein generation and adduct accumulation. Thus, our data show that alcohol consumption and metabolism causes acrolein accumulation by simultaneously up-regulating enzymes that lead to acrolein generation and down-regulating the enzyme that metabolizes and removes acrolein.
Figure 2.
Effect of pharmacologic modulators of alcohol metabolism on alcohol-induced acrolein adduct accumulation in cultured hepatic cells. (A) Accumulation (magnification, 20×) and quantification of acrolein adducts by immunocytochemistry using specific FDP-lysine antibodies in H4IIEC cells treated for 24 hours as follows: untreated control cells (C) or cells treated with 200 mmol/L alcohol (E), 50 μmol/L acetaldehyde (AA50) or 100 μmol/L acetaldehyde (AA100). Means ± SEM (n = 4). **P < .01 and ***P < .001 compared with control by analysis of variance–Bonferroni analysis. (B) Accumulation of acrolein adducts (magnification, 20×) in H4IIEC cells treated for 24 hours as follows: untreated control cells (C), cells treated with 200 mmol/L alcohol alone (E), or 200 mmol/L alcohol in the presence of 10 μmol/L (N-(1,3-Benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide) ((N-(1,3-Benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide)+E), 10 μmol/L 4-methyl pyrazole (4MP+E), 10 μmol/L Pyrazole (PYR+E), or 10 μmol/L allyl sulfide (AS+E). (C) Quantification of acrolein adducts in panel B. Means ± SEM (n = 4). **P < .01 and ***P < .001 compared with control by analysis of variance–Bonferroni analysis. (D) Inhibition of GSTP mRNA and protein by siRNA transfection in H4IIEC cells. For polymerase chain reaction analysis: means ± SEM by analysis of variance–Bonferroni analysis. *P < .05 and **P < .01 compared with control. For Western blot: numbers represent mean densitometry ratios normalized to β-actin. (E) Accumulation of acrolein adducts (magnification, 20×) in transfected H4IIEC cells. (F) Quantification of acrolein adducts in panel E. Means ± SEM, n = 4. *P < .05 and ***P < .001 compared with control by analysis of variance–Bonferroni analysis. C, control; E, 200 mmol/L alcohol; A, 20 μmol/L acrolein; nt, not transfected; si, GSTP siRNA; and sc, scrambled RNA.
Alcohol-Induced Hepatic Acrolein Adduct Accumulation Is Associated With ER Stress, With Minimal Activation of ER Adaptive/Protective Responses
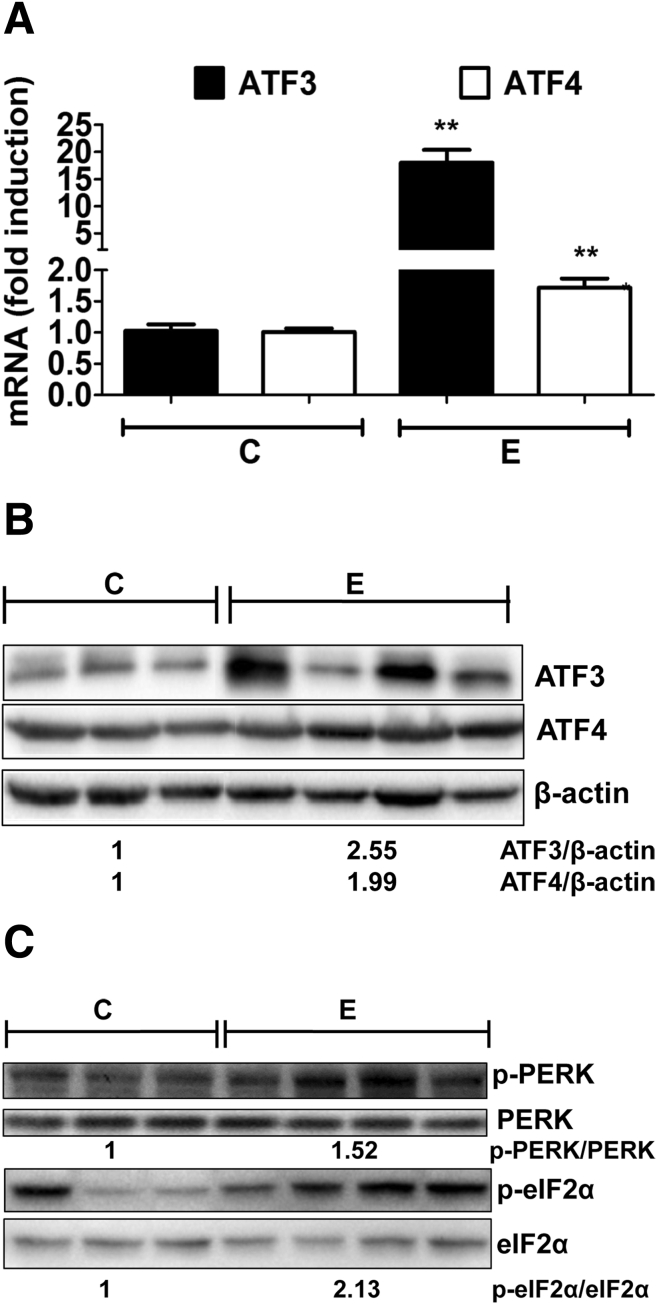
The alcohol-induced accumulation of acrolein-adducted proteins in the liver is likely to increase the burden on the cellular ER protein folding machinery, which if overwhelmed can trigger ER stress. Indeed, in our study, alcohol feeding and the resultant acrolein adduct accumulation was associated with up-regulation of the prototypical ER stress markers, activating transcription factors ATF3 and ATF4, at the mRNA (Figure 3A) and protein levels (Figure 3B). The increase in ATF3 and ATF4 was attributed to phospho-activation of the upstream signaling proteins, PERK and eukaryotic translation initiation factor 2α (Figure 3C).
Figure 3.
Alcohol-induced accumulation of acrolein adducts causes hepatic ER stress in mice livers. (A) ATF3 and ATF4 mRNA levels. Means ± SEM, n = 6 mice. **P < .01 compared with control by the Student t test. (B) ATF3 and ATF4 protein levels. (C) Phospho-PERK and phospho-eukaryotic translation initiation factor 2 α (eIF2α) protein levels. (B and C) Numbers represent mean densitometry ratios normalized to corresponding control proteins (β-actin, total PERK, or eIF2α). C, control; E, alcohol.
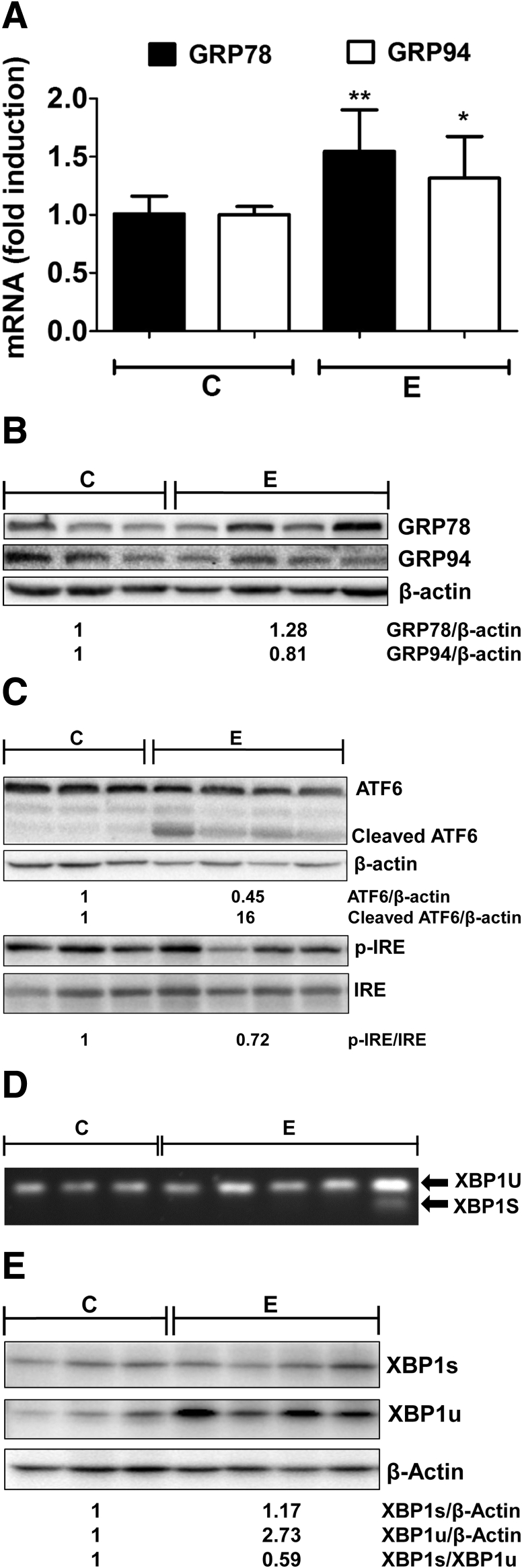
Furthermore, the effect of alcohol on the adaptation/protective responses associated with ER stress also was studied by examining the gene expression of hallmark ER chaperones, GRP78 and GRP94. These chaperones can promote protein folding, and thereby relieve ER stress and provide protection. A small (but statistically significant) increase was seen in the mRNAs of both GRP78 and GRP94 (Figure 4A), whereas at the protein level, only GRP78 was increased marginally (Figure 4B) in alcohol-fed mice, suggesting that cellular ER-adaptive responses were not activated robustly. Next, to determine the underlying cause, we examined the transcription factors that are thought to regulate chaperone gene expression, namely, ATF6 and X-box binding protein-1 (XBP1). Activation of ATF6 by proteolytic cleavage from the inactive approximately 90-kilodalton fragment into the active approximately 50-kilodalton cleaved form (Figure 4C) was seen in alcohol-fed mice, showing that the ATF6-mediated ER stress pathway also was triggered in this model of alcohol consumption in mice. XBP1 is activated by unconventional splicing of XBP1 mRNA owing to ER stress-induced activation of IRE1 endonuclease activity via oligomerization, and subsequent autophosphorylation. Accordingly, we examined IRE1 phosphorylation and XBP1 splicing in alcohol-fed mice and observed that alcohol consumption slightly decreased IRE1 phosphorylation (Figure 4C). In addition, we observed a lack of spliced XBP1 (Figure 4D), and, consequently, no induction of XBP1 protein in the majority of livers of alcohol-fed mice (Figure 4E). Interestingly, the unspliced XBP1u (∼26 kilodaltons) was increased slightly in alcohol-fed mice at the mRNA and protein level; the relevance of this finding remains to be clarified. These data show that alcohol feeding induced ER stress and activated ATF6, but failed to splice XBP1, and, consequently, did not up-regulate ER chaperones.
Figure 4.
Alcohol-induced accumulation of acrolein adducts does not induce UPR adaptive responses in mice livers. (A) GRP78 and GRP94 mRNA levels. Means ± SEM, n = 6 mice. *P < .05 and **P < .01 compared with control by the Student t test. (B) GRP78 and GRP94 protein levels. (C) Protein levels of ATF6 and cleaved/active ATF6, phospho-IRE1, and total IRE1. (D) XBP1 splicing by semiquantitative end point reverse-transcription polymerase chain reaction visualized by agarose gel electrophoresis. (E) Protein levels of XBP1s and XBP1u. (B, C, and E) Numbers represent mean densitometry ratios normalized to corresponding control proteins (β-actin or total IRE1). C, control; E, alcohol.
Acrolein Adduct Accumulation and ER Stress Occur Concurrently With Proapoptotic Signaling
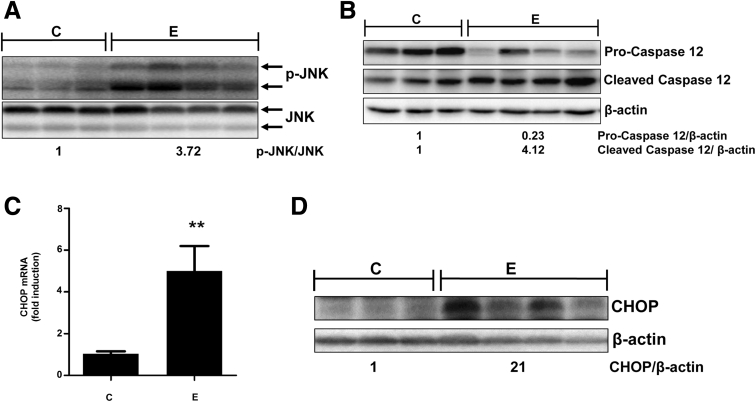
Uncontrolled ER stress in cells can result in apoptosis, which is thought to occur by various proapoptotic pathways.25 Accordingly, we examined the effects of alcohol consumption on relevant ER-associated apoptotic signaling in alcohol-fed mice. The activation of IRE1 leads to its interaction with TRAF2 and ASK1, and subsequent activation of mitogen/stress kinase, JNK, which is connected to ER stress-induced apoptosis. The sustained activation of JNK by phosphorylation is implicated in hepatocyte apoptosis and several forms of liver injury.26 Hepatocytes express both isozymes JNK1 (46 kilodaltons) and JNK2 (54 kilodaltons), which regulate inflammation, cell proliferation, and death in a cell type–dependent and contextual manner. Our data show that alcohol consumption caused significant phospho-activation of both JNK1 and JNK2, whereas total JNK was reduced very slightly compared with control mice (Figure 5A).
Figure 5.
Alcohol-induced hepatic acrolein build-up and consequent ER stress leads to proapoptotic signaling in mice livers. (A) Phospho-JNK and total JNK protein levels. (B) Pro- and cleaved/active caspase-12 protein levels. (C) CHOP mRNA levels. Means ± SEM, n = 6 mice. **P < .01 compared with control by the Student t test. (D) CHOP protein levels. (A, B, and D) Numbers represent mean densitometry ratios normalized to corresponding control proteins (total JNK or β-actin). C, control; E, alcohol.
Another relevant ER-driven apoptotic pathway involves the proteolytic activation of the ER-resident caspase-12, which is known to subsequently activate caspase-9 and caspase-3, resulting in apoptotic cell death.27 Correspondent to ER stress, alcohol consumption also caused proteolytic activation of caspase-12, as shown by a decrease in pro-caspase-12 (∼55 kilodaltons) and a concurrent increase in the cleaved form (∼38 kilodaltons) (Figure 5B). The third and possibly the most well-characterized pathway of ER stress-induced apoptosis is via up-regulation of CHOP, which primarily is responsible for ER stress-induced cell death.4, 28 Activated CHOP leads to alterations in Bcl2 and BAX, in turn, leading to activation of the mitochondrial death pathway, which is known to be involved in alcohol-induced liver injury. The CHOP gene promoter contains binding sites for transcription factors ATF4 and ATF6, which both are activated by alcohol (Figures 3 and 4, respectively). Correspondingly, alcohol consumption resulted in an approximately 5-fold up-regulation of CHOP mRNA (Figure 5C); and a concomitant increase in CHOP protein (Figure 5D). Thus, alcohol consumption and consequent acrolein build-up in mice activates ER-associated proapoptotic signaling pathways.
Hepatic Acrolein Accumulation and ER Stress Are Accompanied by Steatosis, Hepatocyte Apoptosis, and Liver Injury
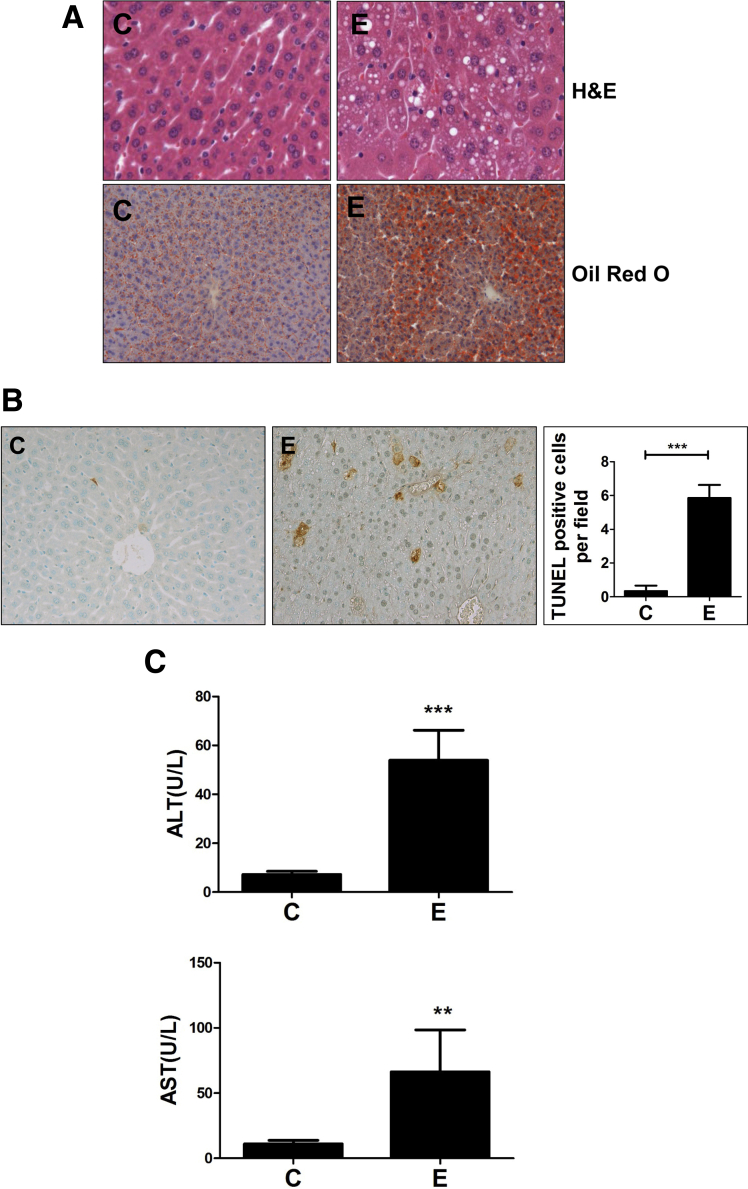
In keeping with literature showing that hepatic ER stress is linked causally to steatosis,29 we examined the effects on fat accumulation in the liver. Consistent with hepatic acrolein and ER stress, we observed substantial microvesicular and macrovesicular hepatic steatosis in alcohol-exposed mice compared with controls, as seen by lipid droplet accumulation by H&E and Oil Red O staining (Figure 6A). Furthermore, the activation of proapoptotic signaling culminated in apoptotic cell death in the livers of alcohol-fed mice, as seen by a significant increase in TUNEL-positive staining in the livers of alcohol-fed mice compared with control (Figure 6B), which led to greater liver injury as defined by serum ALT and AST levels (Figure 6C). Thus, concomitant with alcohol-induced accumulation of acrolein and ER stress, an increase was observed in hepatic steatosis and hepatocyte apoptosis, leading to liver injury in alcohol-fed mice.
Figure 6.
Alcohol-induced acrolein and ER stress leads to steatosis, hepatocyte apoptosis, and liver injury in mice livers. (A) Hepatic steatosis (by H&E; magnification, 40×) and Oil Red O staining; magnification, 20×). (B) Apoptosis by TUNEL staining (magnification, 20×), with quantification of apoptosis. Means ± SEM, n = 6 mice. ***P < .001 compared with control by Student t test. (C) Liver injury: serum ALT and AST. Means ± SEM, n = 6 mice. **P < .01 and ***P < .001 compared with control by the Student t test. C, control; E, alcohol.
Acrolein Mimics the In Vivo Effects of Alcohol in Cultured Hepatic Cells
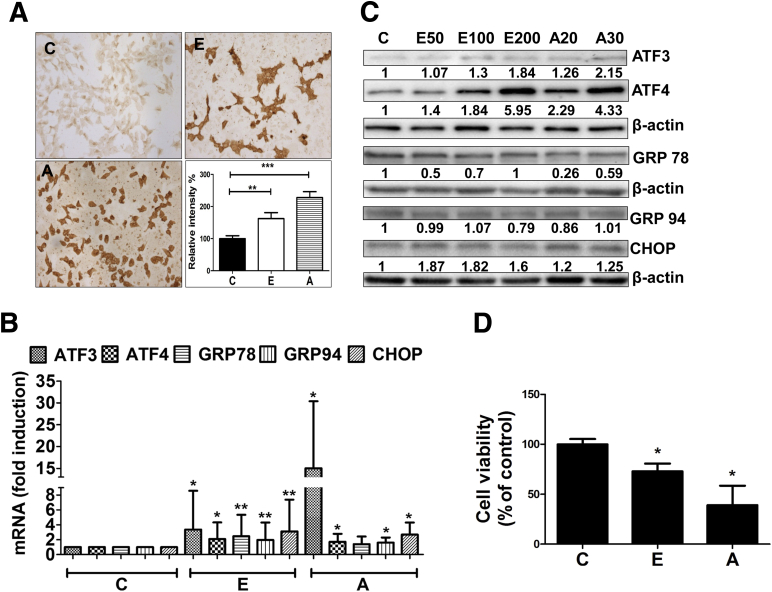
Along with acrolein, alcohol metabolism in the liver is capable of giving rise to many toxic metabolites including 4-Hydroxynonenal and malondialdehyde. Hence, to isolate and determine the sole contribution of acrolein in alcohol-induced hepatic injury, we used H4IIEC cells to examine the direct in vitro effects of acrolein in comparison with alcohol, particularly pertaining to induction of ER stress and hepatocyte cell death. Similar to alcohol exposure, direct acrolein exposure of H4IIEC cells resulted in considerable acrolein adduct accumulation (Figure 7A). Furthermore, analogous to alcohol exposure, direct in vitro acrolein exposure of hepatocytes resulted in ER stress and cell death. We exposed H4IIEC cells to different concentrations of either alcohol (50, 100, or 200 mmol/L) or acrolein (20 or 30 μmol/L) for 6 hours (for mRNA) or 24 hours (for protein). These concentrations of alcohol represent levels that may be encountered with moderate to high alcohol consumption. The exact levels of acrolein that may be generated in the liver after alcohol consumption are difficult to predict, and the acrolein concentrations used here are based on published studies and represent pathophysiological levels that caused hepatocyte apoptosis. Exposure of H4IIEC cells to either acrolein or alcohol yielded similar results: both triggered ER stress and increased ATF3 and ATF4, with minimal up-regulation of GRP78 and GRP94, but a robust increase in proapoptotic CHOP (Figure 7B [mRNA] and C [protein]). Furthermore, consistent with the induction of CHOP, we observed a corresponding loss of cell survival (Figure 7D). Thus, our data show that acrolein duplicates the effects of alcohol in cultured hepatic cells, indicating that the adverse effects of alcohol may indeed be attributed to increased acrolein in the liver occurring as a result of alcohol consumption.
Figure 7.
Acrolein mimics the in vivo effects of alcohol and causes ER stress and cell death in cultured hepatic cells. (A) Accumulation and quantification of acrolein adducts (immunocytochemistry using specific FDP-lysine antibodies; magnification, 20×) in H4IIEC cells treated for 24 hours. Means ± SEM, n = 4. **P < .01 and ***P < .001 compared with control by analysis of variance–Bonferroni analysis. (B) ATF3, ATF4, GRP78, GRP94, and CHOP mRNA levels at 6 hours. Means ± SEM, n = 3 experiments. *P < .05 and **P < .01 compared with control by analysis of variance–Bonferroni analysis. (C) ATF3, ATF4, GRP78, GRP94, and CHOP protein levels from untreated cells (C) or cells treated for 24 hours with alcohol at 50 mmol/L (E50), 100 mmol/L (E100), 200 mmol/L (E200), or acrolein at 20 μmol/L (A20) or 30 μmol/L (A30). Immunoblot analysis was repeated 3 times with similar results, and representative blots are shown. Numbers denote densitometry ratios normalized to corresponding β-actin levels. (D) Cell survival by 3, (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide assay (24 h). Means ± SEM, n = 3 experiments. *P < .05 compared with control by analysis of variance–Bonferroni analysis. C, control; E, 200 mmol/L alcohol; and A, 30 μmol/L acrolein.
Acrolein Scavengers Showed Protective Effects Both In Vitro and In Vivo
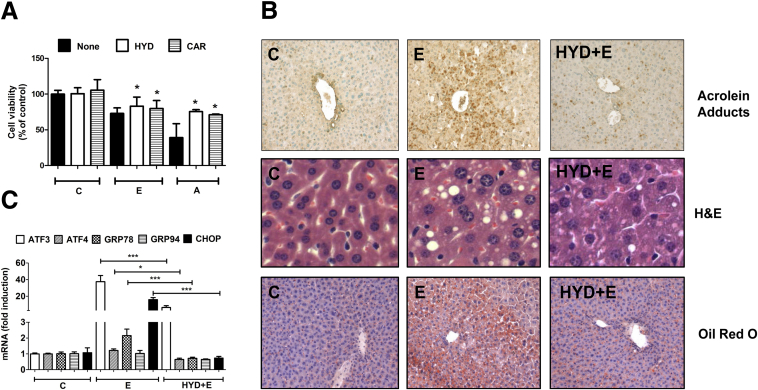
To confirm the contribution of acrolein and establish its pathogenic role in alcohol-induced ER stress and liver injury, we tested known acrolein scavengers in mitigating the injurious effects of acrolein build-up. In this study, we used a known acrolein scavenger, hydralazine, which is known to neutralize free acrolein30, 31 and acrolein-protein adducts,32, 33 both of which are cytotoxic. Also, hydralazine was shown effectively to prevent acrolein-mediated cell death and tissue damage in spinal cord injury.10, 34 In addition, we also used carnosine (β-alanyl histidine, an endogenous dipeptide) that is known to scavenge aldehydes such as acrolein, and have beneficial effects against acetaminophen-induced liver injury.35 H4IIEC cells were pretreated with 50 μmol/L hydralazine or 100 μmol/L carnosine for 1 hour before alcohol or acrolein exposure, and the effect on cell viability was monitored by the 3, (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide assay. Acrolein- or alcohol-induced cell death was attenuated significantly by both acrolein scavengers, and hydralazine was slightly more effective (Figure 8A).
Figure 8.
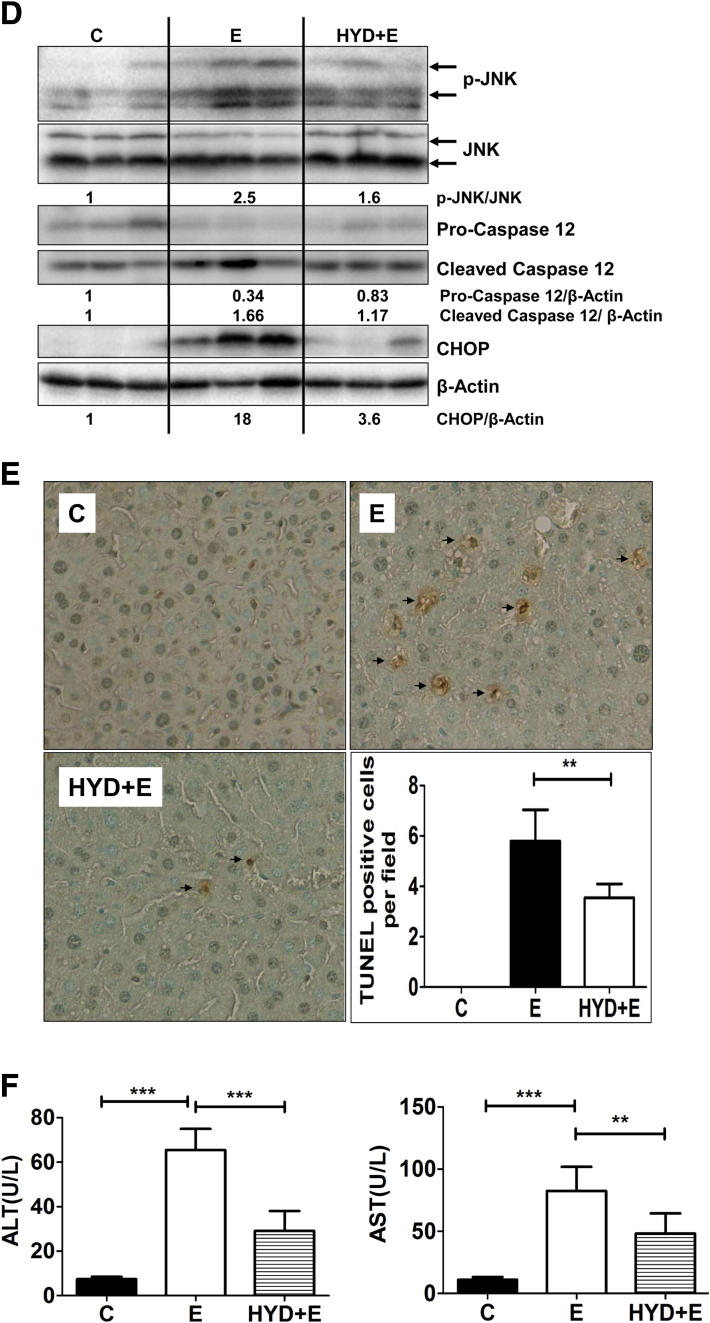
Acrolein scavengers show protective effects both in vitro and in vivo. (A) Cell survival in H4IIEC cells by 3, (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide assay (24 h). Means ± SEM, n = 3 experiments. *P < .05 compared with the corresponding treatments of alcohol (E) or acrolein (A) alone, by analysis of variance–Bonferroni analysis. C, control; E, 200 mmol/L alcohol; A, 30 μmol/L acrolein; HYD, 50 μmol/L hydralazine; CAR, 100 μmol/L carnosine. (B–F) Data are from livers of mice. E, alcohol; Hyd+E, hydralazine+alcohol. (B) Hepatic acrolein adduct accumulation in mice (magnification, 20×), hepatic steatosis by H&E (magnification, 80×), and Oil Red O staining (magnification, 20×). (C) ATF3, ATF4, GRP78, GRP94, and CHOP mRNA levels. Data are presented as means ± SEM (n = 6 mice). *P < .05 and ***P < .001 compared with alcohol (E) by analysis of variance–Bonferroni analysis. (D) Protein levels of phospho-JNK and total JNK, pro- and cleaved/active caspase 12, and CHOP. Numbers represent mean densitometry ratios normalized to corresponding control proteins (total JNK or β-actin). (E) Apoptosis by TUNEL staining (magnification, 80×), with quantification of apoptosis. **P < .01 compared with E by analysis of variance–Bonferroni analysis. (F) Liver injury: serum ALT and AST. Means ± SEM, n = 6 mice. **P < .01 and ***P < .001 by analysis of variance–Bonferroni analysis.
Finally, to firmly establish the role of acrolein in the development of ALD in vivo, we also tested the efficacy of hydralazine in scavenging acrolein and protecting against alcohol-induced liver injury in the NIAAA murine model of ALD. Hydralazine (5 mg/kg body weight, dose based on previous literature31, 34) was administered by daily intraperitoneal injection along with the 10-day alcohol feeding regimen. As noted previously, consumption of the alcohol diet resulted in significant hepatic acrolein adduct accumulation and steatosis in the liver, and the acrolein scavenger hydralazine effectively blocked alcohol-induced acrolein formation and adduct accumulation (Figure 8B) and dramatically reduced hepatic steatosis as seen by histologic examination and confirmed by Oil Red O staining (Figure 8B). Furthermore, alcohol-induced hepatic ER stress and transcriptional up-regulation of hallmark ER stress mRNAs (ATF3, ATF4, CHOP, and GRP78) also were attenuated substantially by hydralazine to control levels (Figure 8C). Importantly, hydralazine prevented alcohol-induced proapoptotic signaling and activation of JNK, pro-caspase-12, and CHOP (Figure 8D). A corresponding decrease was seen in alcohol-induced hepatic apoptosis as seen by decreased TUNEL-positive cells (Figure 8E). Finally, hydralazine showed dramatic protective effects against alcohol-induced liver injury with significantly reduced serum ALT and AST levels (Figure 8F). These data show that hydralazine administration, which targeted alcohol-induced hepatic acrolein via sequestration/neutralization, significantly attenuated ER stress, apoptosis, and liver injury, and protected against ALD in mice (Figure 9).
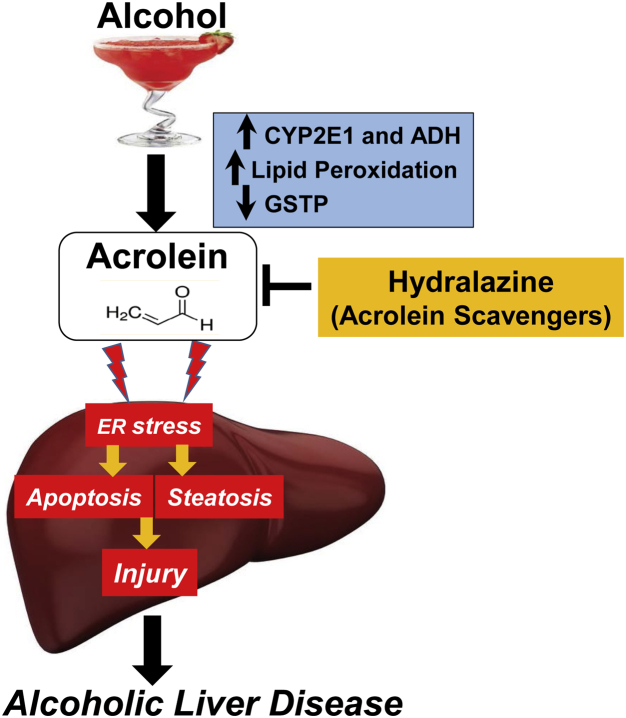
Figure 9.
Acrolein mediates alcohol-induced hepatic ER stress, apoptosis, and injury in ALD, and the scavenger, hydralazine, prevents these effects.
Discussion
Alcohol consumption is a major health problem worldwide and in the United States, with no Food and Drug Administration–approved therapy for any stage of ALD. Therefore, it is critical to investigate the mechanisms involved in ALD, and to identify novel therapeutic targets and strategies for the prevention and treatment of ALD. In this study, we pursued in vitro and in vivo studies to show the role of the lipid peroxidation by-product, acrolein, in contributing to alcohol-induced hepatic ER stress, steatosis, cell death and liver injury, and the pathogenesis of ALD. Our study shows the following: (1) alcohol consumption generates hepatic acrolein, triggers pathologic ER stress and hepatocyte apoptosis, with insufficient activation of ER-adaptive/protective responses; (2) alcohol-induced acrolein is a major mediator of hepatic ER stress, cell death and injury, and is a potential therapeutic target in ALD; and (3) removal/clearance of acrolein by scavengers has therapeutic potential in ALD (Figure 9).
Our results showed significant generation/accumulation of acrolein in the liver after alcohol exposure resulting in cytoplasmic and nuclear accumulation of acrolein-adducted proteins. The accumulation of acrolein–protein adducts triggered consequent hepatic ER stress, leading to hepatocyte cell death and liver injury. Notably, all 3 ER stress pathways involving the ER sensors PERK, IRE1, and ATF6 were activated; however, not all ER stress genes were affected in the same way. Alcohol consumption appeared to trigger ER stress and induced apoptotic signaling (JNK, caspase-12, and CHOP), while simultaneously suppressing UPR-protective responses (XBP1 splicing) and ER chaperones (GRP78 and 94). The differential regulation of ER stress genes may be via altered signal transduction, or by direct or indirect protein adduction. In addition, the nuclear accumulation of acrolein adducts may indicate adduction and alteration of function of transcription factors or chromatin-modifying proteins such as histone deacetylases and DNA/histone methylases, which likely have significant effects on downstream gene expression. The exact mechanisms by which acrolein adduct accumulation causes ER stress, and the identity and functionality of the proteins adducted by acrolein, currently are under investigation.
Oxidative stress is known to induce ER stress, however, the mediators responsible for such effects have not been identified clearly. Alcohol-induced oxidative stress and aldehyde generation is shown to occur in the absence of ER stress in a model of early ALD, suggesting that ER stress may be a downstream consequence of oxidant burden.36 Our data clearly show a direct mechanistic link and identify acrolein as a major pathogenic initiator of alcohol-induced hepatic injury. The causal role of acrolein in the pathogenesis of ALD is supported further by the observation that clearance of this toxic molecule by hydralazine (a known acrolein scavenger) largely prevented alcohol-induced acrolein build-up, and ER stress, cell death, and injury. With regard to acrolein generation during the development of ALD, our novel results show that alcohol-mediated down-regulation of GSTP may be a key mechanism that reduces the normal metabolism/clearance of acrolein, thereby contributing to hepatic accumulation of acrolein and consequent liver injury. Indeed, GSTP may be a potential therapeutic target, and pharmacologic activation of GSTP may be of benefit to attenuate ALD. In addition, our data indicate that alcohol metabolism is essential, and that both ADH- and CYP2E1-dependent pathways contribute to acrolein generation/accumulation. Importantly, our data show that acetaldehyde, the first metabolite of alcohol, plays a key role in alcohol-induced acrolein formation because direct exposure to acetaldehyde resulted in substantial acrolein accumulation in H4IIEC cells. Moreover, acetaldehyde may further support acrolein formation via up-regulation of spermine oxidase, which catalyzes the formation of free acrolein by oxidation of spermine as reported by Uemura et al.37 The vital role of ALDH2 in detoxification of aldehydes (both acetaldehyde and acrolein) was shown clearly by the observation that (N-(1,3-Benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide) (ALDH2 agonist) prevented alcohol-induced acrolein accumulation in cultured hepatocytes; these data are in keeping with the recent demonstration that (N-(1,3-Benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide) protects against alcohol-induced steatosis and cell death.38
Our data show that alcohol consumption causes pathologic ER stress without sufficient induction of the adaptive/protective responses involving XBP1s splicing and up-regulation of ER chaperones. Thus, insufficient adaptation to ER stress along with proapoptotic signaling may contribute significantly to hepatic injury in this model of alcohol consumption. Although XBP1 splicing occurred in a few alcohol-fed mice, GRP78 or GRP94 were not up-regulated significantly, suggesting that additional factors (such as other proteins or epigenetic modifications) also may be involved. A similar discordant lack of correlation between chaperone gene expression and upstream signal transduction (cleavage of ATF6 and splicing of XBP1)39 has been reported in dermal fibroblasts. Our data in the chronic binge murine model showing minimal to no increase in chaperones GRP78 and GRP94 differs from the murine intragastric alcohol feeding model, which showed substantial up-regulation of GRP78, along with CHOP and hepatic steatosis and injury.40 In addition, in guinea-pigs fed alcohol, liver steatosis and apoptosis were accompanied by increased mRNA and protein levels of CYP2E1 and GRP78, and activated caspase-12.41 These variations in ER stress-associated gene expression may reflect differences in model systems pertaining to animals, routes of alcohol feeding, or temporal patterns of gene expression. Our study emphasizes the important role of pathologic ER stress in alcohol-induced liver injury in this model of ALD, and indicates that impaired ER adaptive responses (eg, GRP78) may contribute significantly to injury in ALD, as has been described in other forms of liver injury.42 ER stress is known to result in JNK activation through the IRE1-TRAF pathway; however, several other stimuli also activate JNK, including TNF and TRAIL, both of which are known to be increased in alcohol-fed mice. The direct causative role of ER stress in JNK activation and alcohol-induced injury clearly is suggested, but was not established, by our work. Future studies to confirm this concept will involve investigating the effects of pharmacologic or genetic inhibition of ER stress on JNK activation, apoptosis, and liver injury.
Notably, in addition to showing the pathogenic contribution of acrolein in the development of experimental ALD, our study also provides novel and exciting evidence that acrolein removal or scavenging using hydralazine is an effective way to mitigate alcohol-induced hepatic ER stress, steatosis, and injury in experimental ALD, both in vitro and in vivo. Although the protective effects of hydralazine are reported primarily against acrolein,33 it is possible that hydralazine also may interact with and neutralize other toxic aldehydes such as 4-Hydroxynonenal, thereby providing added protection in ALD. In our study, hydralazine prevented liver injury when administered from the start of alcohol feeding; additional studies are needed to test its efficacy in a treatment paradigm. Although the data convincingly show that acrolein removal/clearance by hydralazine may be a novel and effective treatment modality, the use of hydralazine in the treatment of ALD patients may be somewhat limited because hydralazine has vasodilator properties. Alternative compounds, with the same hydrazine-based acrolein scavenging mechanism but without the vasodilator effects, such as dihydralazine and the antidepressant phenelzine,43 may need to be investigated for safety and efficacy in ALD. Further studies are needed to understand the exact mechanism by which alcohol-induced acrolein leads to ER stress, and to examine the contribution of acrolein in alcohol-induced hepatic inflammation. In addition to hepatic effects, alcohol-induced intestinal permeability and consequent systemic endotoxemia are known to be important in the pathogenesis of ALD; the possible involvement of acrolein in alcohol-induced intestinal dysfunction remains to be explored. These detailed mechanistic studies currently are under investigation in our laboratory.
Clinical Relevance
Only approximately 30% of heavy alcohol drinkers develop severe ALD, and dietary and environmental factors are thought to be critical determinants. Acrolein is a common environmental and dietary pollutant and our study shows that it is a major mediator of the adverse effects of alcohol and plays a causal role in experimental ALD pathogenesis. Our group has shown that diets enriched in linoleic acid (common dietary PUFA) exacerbate alcohol-induced liver injury,44 and consumption of linoleic acid has increased more than 3-fold over the past 2 decades, thereby increasing the substrate availability for LPO and subsequent acrolein generation. In addition, acrolein is a major aldehyde component of cigarette smoke45 and cigarette smoking is common in persons consuming alcohol and is known to impact alcoholic liver disease negatively. Thus, environmental and dietary acrolein exposures may add to endogenously generated acrolein with significant and clinically relevant pathogenic consequences. Our results indicate that acrolein is a major pathogenic contributor and may be an important therapeutic target in ALD. Notably, our study shows that acrolein neutralization/clearance may be an effective strategy for curtailing alcohol-induced, acrolein-mediated hepatic injury in the development of ALD. Hence, acrolein scavengers such as hydralazine may represent a novel therapeutic approach for the prevention/treatment of ALD.
Acknowledgments
The authors would like to thank Dr David Barker for designing and testing the quantitative polymerase chain reaction primers, and Mrs Marion McClain for critical revision of the manuscript.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by the National Institutes of Health grants K01ES017105 (S.J.B.), R01AA023681 (C.J.M.), R01AA018869 (C.J.M.), U01AA021901 (C.J.M.), and P50AA024337 (C.J.M.), and by the Department of Veterans Affairs (C.J.M.).
References
- 1.Rehm J., Samokhvalov A.V., Shield K.D. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Louvet A., Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 3.Dara L., Ji C., Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logue S.E. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 5.Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int J Hepatol. 2014;2014:513787. doi: 10.1155/2014/513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int. 2012;2012:216450. doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mari M., Colell A., Morales A. Redox control of liver function in health and disease. Antioxid Redox Signal. 2010;12:1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albano E. Free radical mechanisms in immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:110–114. doi: 10.1016/s0891-5849(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 9.Uchida K., Kanematsu M., Morimitsu Y. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 10.Park J., Muratori B., Shi R. Acrolein as a novel therapeutic target for motor and sensory deficits in spinal cord injury. Neural Regen Res. 2014;9:677–683. doi: 10.4103/1673-5374.131564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 12.Ghilarducci D.P., Tjeerdema R.S. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- 13.Moghe A., Ghare S., Lamoreau B. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarkovic K., Uchida K., Kolenc D. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic Res. 2006;40:543–552. doi: 10.1080/10715760500370048. [DOI] [PubMed] [Google Scholar]
- 15.Lovell M.A., Xie C., Markesbery W.R. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 16.Higashi K., Yoshida M., Igarashi A. Intense correlation between protein-conjugated acrolein and primary Sjogren's syndrome. Clin Chim Acta. 2010;411:359–363. doi: 10.1016/j.cca.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Saiki R., Park H., Ishii I. Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem Biophys Res Commun. 2011;404:1044–1049. doi: 10.1016/j.bbrc.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 18.Mohammad M.K., Avila D., Zhang J. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertola A., Mathews S., Ki S.H. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Liu Y., Sidhu A. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32–G41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews S., Xu M., Wang H. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G819–G823. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts B.J., Song B.J., Soh Y. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270:29632–29635. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- 23.Stevens J.F., Maier C.S. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feierman D.E., Cederbaum A.I. Inhibition of microsomal oxidation of ethanol by pyrazole and 4-methylpyrazole in vitro. Increased effectiveness after induction by pyrazole and 4-methylpyrazole. Biochem J. 1986;239:671–677. doi: 10.1042/bj2390671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sovolyova N. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem. 2014;395:1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 26.Seki E., Brenner D.A., Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao R.V., Castro-Obregon S., Frankowski H. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 28.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henkel A., Green R.M. The unfolded protein response in fatty liver disease. Semin Liver Dis. 2013;33:321–329. doi: 10.1055/s-0033-1358522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burcham P.C., Kerr P.G., Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- 31.Kaminskas L.M., Pyke S.M., Burcham P.C. Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J Pharmacol Exp Ther. 2004;310:1003–1010. doi: 10.1124/jpet.104.067330. [DOI] [PubMed] [Google Scholar]
- 32.Kaminskas L.M., Pyke S.M., Burcham P.C. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org Biomol Chem. 2004;2:2578–2584. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- 33.Burcham P.C., Pyke S.M. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- 34.Park J., Zheng L., Marquis A. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem. 2014;129:339–349. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan S.L., Wu S.T., Yin M.C. Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci. 2009;74:H259–H265. doi: 10.1111/j.1750-3841.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 36.Galligan J.J., Smathers R.L., Shearn C.T. Oxidative stress and the ER stress response in a murine model for early-stage alcoholic liver disease. J Toxicol. 2012;2012:207594. doi: 10.1155/2012/207594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uemura T., Tanaka Y., Higashi K. Acetaldehyde-induced cytotoxicity involves induction of spermine oxidase at the transcriptional level. Toxicology. 2013;310:1–7. doi: 10.1016/j.tox.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Zhong W., Zhang W., Li Q. Pharmacological activation of aldehyde dehydrogenase 2 by Alda-1 reverses alcohol-induced hepatic steatosis and cell death in mice. J Hepatol. 2015;62:1375–1381. doi: 10.1016/j.jhep.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang J., Lehrman M.A. Discordance of UPR signaling by ATF6 and Ire1p-XBP1 with levels of target transcripts. Biochem Biophys Res Commun. 2004;317:390–396. doi: 10.1016/j.bbrc.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 40.Ji C., Mehrian-Shai R., Chan C. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esfandiari F., Vaillnueva J.A., Wong D.H. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289:G54–G63. doi: 10.1152/ajpgi.00542.2004. [DOI] [PubMed] [Google Scholar]
- 42.Ji C., Kaplowitz N., Lau M.Y. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng L., Park J., Walls M. Determination of urine 3-HPMA, a stable acrolein metabolite in a rat model of spinal cord injury. J Neurotrauma. 2013;30:1334–1341. doi: 10.1089/neu.2013.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirpich I.A., Feng W., Wang Y. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith C.J., Fischer T.H. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]