Abstract
To dissect the genetic architecture of blood pressure and assess effects on target-organ damage, we analyzed 128,272 SNPs from targeted and genome-wide arrays in 201,529 individuals of European ancestry and genotypes from an additional 140,886 individuals were used for validation. We identified 66 blood pressure loci, of which 17 were novel and 15 harbored multiple distinct association signals. The 66 index SNPs were enriched for cis-regulatory elements, particularly in vascular endothelial cells, consistent with a primary role in blood pressure control through modulation of vascular tone across multiple tissues. The 66 index SNPs combined in a risk score showed comparable effects in 64,421 individuals of non-European descent. The 66-SNP blood pressure risk score was significantly associated with target-organ damage in multiple tissues, with minor effects in the kidney. Our findings expand current knowledge of blood pressure pathways and highlight tissues beyond the classic renal system in blood pressure regulation.
INTRODUCTION
There are considerable physiological, clinical and genetic data that point to the kidney as the major regulator of blood pressure (BP) and to renal damage as a consequence of long-term BP elevation. However, alternative hypotheses, such as increasing systemic vascular resistance, are also serious contenders to explain the rise of BP with increasing age, but with limited genetic support. The genetic basis of elevated blood pressure or hypertension (HTN) involves many loci that have been identified using large-scale analyses of candidate genes1,2, linkage studies, and genome-wide association studies (GWAS)3-12. The genes underlying BP regulation can help resolve many of the open questions regarding BP (patho-) physiology. While ~40-50% of BP variability is heritable13,14, the genetic variation identified to date explains only ~2%1-12.
The Cardio-MetaboChip is a custom genotyping microarray designed to facilitate cost-effective follow-up of nominal associations for metabolic and cardiovascular traits, including BP. This array comprises 196,725 variants, including ~5,000 SNPs with nominal (P <0.016) evidence of BP association in our previous GWAS meta-analysis5. Furthermore, the array includes several dense scaffolds for fine mapping of selected loci spanning, on average, genomic regions of 350 kilobases5,16, of which 24 include genome-wide significant BP association in the current study5,16.
RESULTS
Novel genetic loci associated with systolic and diastolic BP
We performed meta-analyses of association summary statistics from a total of 201,529 individuals of European (EUR) ancestry from 74 studies: (i) 109,096 individuals from 46 studies genotyped on Cardio-MetaboChip; and (ii) 92,433 individuals from 28 studies with imputed genotype data from genome-wide genotyping at variants included on the Cardio-MetaboChip. Twenty-four of the 28 studies with genome-wide genotyping data had contributed to previous analyses (Supplementary Tables 1-3)5,7.
BP was measured using standardized protocols in all studies5,17 (Supplementary Table 1, Online methods). Association statistics for systolic and diastolic BP (SBP and DBP) in models adjusting for age, age2, sex, and body mass index (BMI), were obtained for each study separately, with study-specific genomic control applied to correct for possible population structure. Fixed-effects meta-analysis proceeded in 4 stages, separately for the following SNP associations: Stage 1, using results based on 46 studies using Cardio-MetaboChip genotypes of 109,096 participants; Stage 2, using additional results based on imputed genotypes from genome-wide genotyping arrays in 4 previously unpublished studies; Stage 3 using imputed genotypes from genome-wide genotyping arrays in 24 previously published studies5; and Stage 4, the joint meta-analysis of Stages 1-3 including a total of 201,529 independent individuals (Supplementary Figure 1, Supplementary Tables 2-3, Supplementary Note). To account for population structure between studies in Stages 1-3 of our meta-analysis, genomic control correction was applied to meta-analysis results from each of these stages in an approach aggregating summary statistics from GWAS and Cardio-MetaboChip studies18,19.
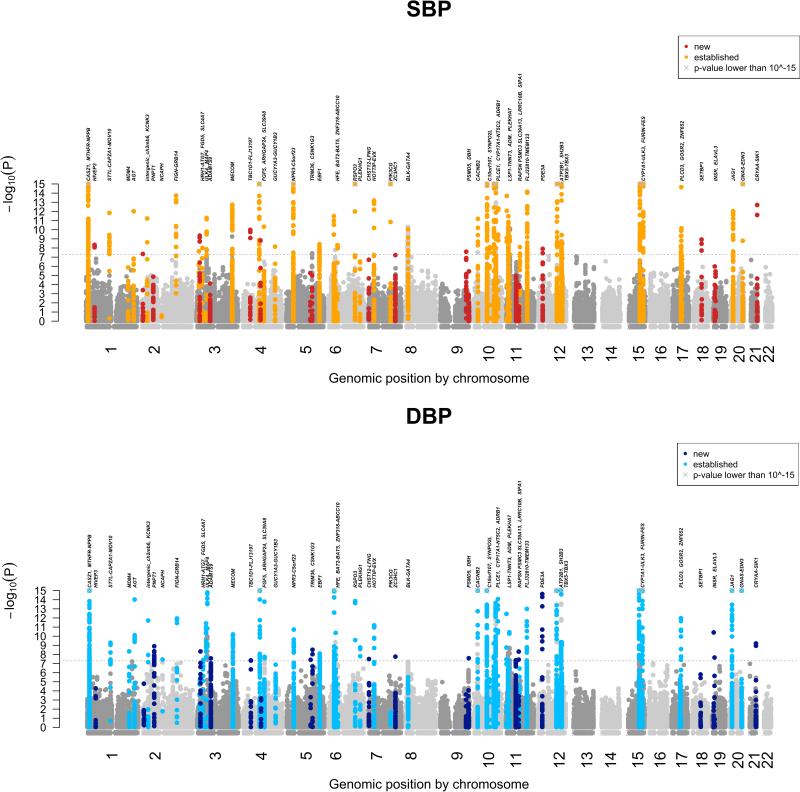
After stage 4, 67 loci attained genome-wide significance (P < 5 × 10−8), 18 of which were not previously reported in the literature (Supplementary Table 4). Quantile-quantile plots of the stage 4 meta-analysis showed an excess of small P values, with an elevated genomic control lambda estimate that was persistent, albeit attenuated, after excluding all 66 loci (Supplementary Figure 2). This observation is compatible with either residual uncorrected population stratification or the presence of a large number of variants that are truly associated with BP but fail to achieve genome-wide significance in the current meta-analysis. The Cardio-MetaboChip array's inclusion of SNPs from a prior BP GWAS5 does not appear to be the sole explanation, as we did not observe a significant decrease of the excess of small P values after exclusion of all SNPs that were included on the Cardio-MetaboChip based on nominal BP association (Supplementary Figures 3 and 4). Since the quantile-quantile plots continued to show deviation from the null expectation, we sought additional validation for 18 variants attaining genome-wide significance, but without prior support in the literature, in up to 140,886 individuals of European ancestry from UK Biobank20. For these SNPs, we performed a stage 5 meta-analysis combining the association summary statistics from stage 4 and UK Biobank, in a total of up to 342,415 individuals (Supplementary Table 5).
Upon stage 5 meta-analysis, 17 of 18 variants retained genome-wide significance for the primary trait (SBP or DBP result with the lower P value). The one variant that was not genome-wide significant had a borderline P value of 4.49 × 10−8 at stage 4. These findings are consistent with appropriate calibration of the association test statistics at stage 4 such that observing one failure among 18 validation tests is consistent with the use of a threshold (P < 5 × 10−8) designed to have a 1 in 20 chance of a result as or more extreme solely due to chance. In total, 66 loci attained genome-wide significance: 13 loci for SBP only, 12 loci for DBP only, and 41 loci for both traits. Of these, 17 BP loci were novel, while 49 were previously reported at genome-wide significance (Table 1 and Figure 1).
Table 1.
SBP and DBP association at 66 loci.
| Locus no. | Locus name | Lead SNP | Chr | Position (hg19) |
CA /NC |
Coded allele freq |
Traits | SBP |
DBP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | SE | P value | Total N | Effect | SE | P value | Total N# | ||||||||
| NEW 1 | HIVEP3 | rs7515635 | 1 | 42,408,070 | T/C | 0.468 | SBP | 0.307 | 0.0444 | 4.81E-12 | 340,969 | 0.1365 | 0.0263 | 2.05E-07 | 340,934 |
| NEW 2 | PNPT1 | rs1975487 | 2 | 55,809,054 | A/G | 0.464 | DBP | −0.2107 | 0.045 | 2.81E-06 | 337,522 | −0.1602 | 0.0266 | 1.75E-09 | 337,517 |
| NEW 3 | FGD5 | rs11128722 | 3 | 14,958,126 | A/G | 0.563 | SBP & DBP | −0.3103 | 0.0469 | 3.61E-11 | 310,430 | −0.1732 | 0.0279 | 5.16E-10 | 310,429 |
| NEW 4 | ADAMTS9 | rs918466 | 3 | 64,710,253 | A/G | 0.406 | DBP | −0.0865 | 0.0459 | 5.94E-02 | 336,671 | −0.1819 | 0.027 | 1.73E-11 | 336,653 |
| NEW 5 | TBC1D1-FLJ13197 | rs2291435 | 4 | 38,387,395 | T/C | 0.524 | SBP & DBP | −0.3441 | 0.0449 | 1.90E-14 | 331,382 | −0.156 | 0.0266 | 4.26E-09 | 331,389 |
| NEW 6 | TRIM36 | rs10077885 | 5 | 114,390,121 | A/C | 0.501 | SBP & DBP | −0.284 | 0.0444 | 1.64E-10 | 338,328 | −0.1735 | 0.0263 | 3.99E-11 | 338,323 |
| NEW 7 | CSNK1G3 | rs6891344 | 5 | 123,136,656 | A/G | 0.819 | DBP | 0.2811 | 0.058 | 1.24E-06 | 338,688 | 0.2311 | 0.0343 | 1.58E-11 | 338,678 |
| NEW 8 | CHST12-LFNG | rs2969070 | 7 | 2,512,545 | A/G | 0.639 | SBP & DBP | −0.2975 | 0.0464 | 1.44E-10 | 335,991 | −0.1821 | 0.0274 | 2.92E-11 | 335,972 |
| NEW 9 | ZC3HC1 | rs11556924 | 7 | 129,663,496 | T/C | 0.384 | SBP & DBP | −0.2705 | 0.0468 | 7.64E-09 | 325,929 | −0.2141 | 0.0276 | 8.15E-15 | 325,963 |
| NEW 10 | PSMD5 | rs10760117 | 9 | 123,586,737 | T/G | 0.415 | SBP | 0.283 | 0.0457 | 6.10E-10 | 333,377 | 0.0999 | 0.0269 | 2.08E-04 | 333,377 |
| NEW 11 | DBH | rs6271* | 9 | 136,522,274 | T/C | 0.072 | SBP & DBP | −0.5911 | 0.0899 | 4.89E-11 | 306,394 | −0.4646 | 0.0532 | 2.42E-18 | 306,463 |
| NEW 12 | RAPSN, PSMC3, SLC39A13 | rs7103648 | 11 | 47,461,783 | A/G | 0.614 | SBP & DBP | −0.3349 | 0.0462 | 4.43E-13 | 335,614 | −0.2409 | 0.0272 | 9.03E-19 | 335,592 |
| NEW 13 | LRRC10B | rs751984 | 11 | 61,278,246 | T/C | 0.879 | SBP & DBP | 0.4074 | 0.0691 | 3.80E-09 | 334,583 | 0.3755 | 0.0409 | 4.20E-20 | 334,586 |
| NEW 14 | SETBP1 | rs12958173 | 18 | 42,141,977 | A/C | 0.306 | SBP & DBP | 0.3614 | 0.0489 | 1.43E-13 | 331,007 | 0.1789 | 0.0289 | 5.87E-10 | 331,010 |
| NEW 15 | INSR | rs4247374 | 19 | 7,252,756 | T/C | 0.143 | SBP & DBP | −0.5933 | 0.0673 | 1.23E-18 | 302,458 | −0.3852 | 0.0396 | 2.08E-22 | 302,459 |
| NEW 16 | ELAVL3 | rs17638167 | 19 | 11,584,818 | T/C | 0.047 | DBP | −0.4784 | 0.1066 | 7.13E-06 | 333,137 | −0.3479 | 0.0632 | 3.71E-08 | 333,107 |
| NEW 17 | CRYAA-SIK1 | rs12627651 | 21 | 44,760,603 | A/G | 0.288 | SBP & DBP | 0.3905 | 0.0513 | 2.69E-14 | 310,738 | 0.2037 | 0.0301 | 1.36E-11 | 310,722 |
| EST 1 | CASZ1 | rs880315 | 1 | 10,796,866 | T/C | 0.641 | SBP & DBP | −0.475 | 0.062 | 2.09E-14 | 184,226 | −0.257 | 0.038 | 1.34E-11 | 184,212 |
| EST 2 | MTHFR-NPPB | rs17037390 | 1 | 11,860,843 | A/G | 0.155 | SBP & DBP | −0.908 | 0.081 | 5.95E-29 | 195,493 | −0.499 | 0.05 | 1.20E-23 | 195,481 |
| EST 3 | ST7L-CAPZA1-MOV10 | rs1620668 | 1 | 113,023,980 | A/G | 0.822 | SBP & DBP | −0.535 | 0.076 | 1.45E-12 | 197,966 | −0.285 | 0.047 | 9.00E-10 | 197,948 |
| EST 4 | MDM4 | rs4245739 | 1 | 204,518,842 | A/C | 0.737 | DBP | 0.326 | 0.068 | 1.37E-06 | 191,594 | 0.243 | 0.041 | 4.63E-09 | 191,578 |
| EST 5 | AGT | rs2493134* | 1 | 230,849,359 | T/C | 0.579 | SBP & DBP | −0.413 | 0.058 | 9.65E-13 | 199,505 | −0.275 | 0.036 | 9.53E-15 | 199,502 |
| EST 6 | KCNK3 | rs2586886 | 2 | 26,932,031 | T/C | 0.599 | SBP & DBP | −0.404 | 0.059 | 5.94E-12 | 197,269 | −0.254 | 0.036 | 1.92E-12 | 197,272 |
| EST 7 | NCAPH | rs772178 | 2 | 96,963,684 | A/G | 0.64 | DBP | −0.072 | 0.061 | 2.39E-01 | 192,513 | −0.208 | 0.038 | 3.58E-08 | 192,501 |
| EST 8 | FIGN-GRB14 | rs1371182 | 2 | 165,099,215 | T/C | 0.443 | SBP & DBP | −0.444 | 0.058 | 1.89E-14 | 196,262 | −0.252 | 0.036 | 1.50E-12 | 196,240 |
| EST 9 | HRH1-ATG7 | rs2594992 | 3 | 11,360,997 | A/C | 0.607 | SBP | −0.334 | 0.06 | 2.31E-08 | 189,895 | −0.136 | 0.037 | 2.20E-04 | 189,854 |
| EST 10 | SLC4A7 | rs711737 | 3 | 27,543,655 | A/C | 0.604 | SBP | 0.334 | 0.058 | 9.93E-09 | 200,282 | 0.17 | 0.036 | 2.24E-06 | 200,260 |
| EST 11 | ULK4 | rs2272007* | 3 | 41,996,136 | T/C | 0.18 | DBP | −0.11 | 0.077 | 1.52E-01 | 193,915 | 0.328 | 0.047 | 3.94E-12 | 193,900 |
| EST 12 | MAP4 | rs6442101* | 3 | 48,130,893 | T/C | 0.692 | SBP & DBP | 0.396 | 0.062 | 1.62E-10 | 200,543 | 0.303 | 0.038 | 1.60E-15 | 200,534 |
| EST 13 | MECOM | rs6779380 | 3 | 169,111,915 | T/C | 0.539 | SBP & DBP | −0.439 | 0.06 | 1.85E-13 | 186,535 | −0.239 | 0.037 | 6.87E-11 | 186,521 |
| EST 14 | FGF5 | rs1458038 | 4 | 81,164,723 | T/C | 0.3 | SBP & DBP | 0.659 | 0.065 | 5.36E-24 | 188,136 | 0.392 | 0.04 | 7.36E-23 | 188,088 |
| EST 15 | ARHGAP24 | rs17010957 | 4 | 86,719,165 | T/C | 0.857 | SBP | −0.498 | 0.082 | 1.51E-09 | 196,325 | −0.173 | 0.051 | 6.63E-04 | 196,292 |
| EST 16 | SLC39A8 | rs13107325 | 4 | 103,188,709 | T/C | 0.07 | SBP & DBP | −0.837 | 0.127 | 4.69E-11 | 175,292 | −0.602 | 0.078 | 1.63E-14 | 175,372 |
| EST 17 | GUCY1A3-GUCY1B3 | rs4691707 | 4 | 156,441,314 | A/G | 0.652 | SBP | −0.349 | 0.06 | 7.10E-09 | 198,246 | −0.163 | 0.037 | 1.08E-05 | 198,226 |
| EST 18 | NPR3-C5orf23 | rs12656497 | 5 | 32,831,939 | T/C | 0.403 | SBP & DBP | −0.487 | 0.06 | 3.85E-16 | 194,831 | −0.228 | 0.037 | 4.73E-10 | 194,829 |
| EST 19 | EBF1 | rs11953630 | 5 | 157,845,402 | T/C | 0.366 | SBP & DBP | −0.38 | 0.065 | 3.91E-09 | 167,698 | −0.23 | 0.04 | 8.07E-09 | 167,708 |
| EST 20 | HFE | rs1799945* | 6 | 26,091,179 | C/G | 0.857 | SBP & DBP | −0.598 | 0.086 | 3.28E-12 | 185,306 | −0.43 | 0.053 | 3.10E-16 | 185,273 |
| EST 21 | BAT2-BAT5 | rs2187668 | 6 | 32,605,884 | T/C | 0.126 | DBP | −0.291 | 0.092 | 1.60E-03 | 189,806 | −0.372 | 0.057 | 4.31E-11 | 189,810 |
| EST 22 | ZNF318-ABCC10 | rs6919440 | 6 | 43,352,898 | A/G | 0.57 | SBP | −0.337 | 0.058 | 4.92E-09 | 200,733 | −0.125 | 0.035 | 4.25E-04 | 200,730 |
| EST 23 | RSPO3 | rs1361831 | 6 | 127,181,089 | T/C | 0.541 | SBP & DBP | −0.482 | 0.058 | 7.38E-17 | 197,027 | −0.271 | 0.036 | 2.34E-14 | 197,012 |
| EST 24 | PLEKHG1 | rs17080093 | 6 | 150,997,440 | T/C | 0.075 | DBP | −0.564 | 0.111 | 3.83E-07 | 194,728 | −0.411 | 0.068 | 1.71E-09 | 194,734 |
| EST 25 | HOTTIP-EVX | rs3735533 | 7 | 27,245,893 | T/C | 0.081 | SBP & DBP | −0.798 | 0.106 | 6.48E-14 | 197,881 | −0.445 | 0.065 | 1.09E-11 | 197,880 |
| EST 26 | PIK3CG | rs12705390 | 7 | 106,410,777 | A/G | 0.227 | SBP | 0.619 | 0.069 | 2.69E-19 | 198,297 | 0.059 | 0.042 | 1.63E-01 | 198,290 |
| EST 27 | BLK-GATA4 | rs2898290 | 8 | 11,433,909 | T/C | 0.491 | SBP | 0.377 | 0.058 | 8.85E-11 | 197,759 | 0.167 | 0.036 | 3.17E-06 | 197,726 |
| EST 28 | CACNB2 | rs12243859 | 10 | 18,740,632 | T/C | 0.326 | SBP & DBP | −0.402 | 0.061 | 6.13E-11 | 199,136 | −0.335 | 0.038 | 8.11E-19 | 199,124 |
| EST 29 | C10orf107 | rs7076398 | 10 | 63,533,663 | A/T | 0.188 | SBP & DBP | −0.563 | 0.076 | 1.72E-13 | 187,013 | −0.409 | 0.047 | 2.55E-18 | 187,024 |
| EST 30 | SYNPO2L | rs12247028 | 10 | 75,410,052 | A/G | 0.611 | SBP | −0.364 | 0.063 | 8.16E-09 | 180,194 | −0.159 | 0.039 | 3.89E-05 | 180,094 |
| EST 31 | PLCE1 | rs932764* | 10 | 95,895,940 | A/G | 0.554 | SBP & DBP | −0.495 | 0.059 | 6.88E-17 | 195,577 | −0.224 | 0.036 | 6.28E-10 | 195,547 |
| EST 32 | CYP17A1-NT5C2 | rs943037 | 10 | 104,835,919 | T/C | 0.087 | SBP & DBP | −1.133 | 0.105 | 2.35E-27 | 193,818 | −0.482 | 0.064 | 4.48E-14 | 193,799 |
| EST 33 | ADRB1 | rs740746 | 10 | 115,792,787 | A/G | 0.73 | SBP & DBP | 0.486 | 0.067 | 4.59E-13 | 184,835 | 0.32 | 0.041 | 8.63E-15 | 184,868 |
| EST 34 | LSP1-TNNT3 | rs592373 | 11 | 1,890,990 | A/G | 0.64 | SBP & DBP | 0.484 | 0.063 | 2.02E-14 | 177,149 | 0.282 | 0.039 | 3.61E-13 | 177,134 |
| EST 35 | ADM | rs1450271 | 11 | 10,356,115 | T/C | 0.468 | SBP & DBP | 0.413 | 0.059 | 3.40E-12 | 191,246 | 0.199 | 0.036 | 4.11E-08 | 191,221 |
| EST 36 | PLEKHA7 | rs1156725 | 11 | 16,307,700 | T/C | 0.804 | SBP & DBP | −0.447 | 0.072 | 5.65E-10 | 200,889 | −0.292 | 0.044 | 3.67E-11 | 200,899 |
| EST 37 | SIPA1 | rs3741378* | 11 | 65,408,937 | T/C | 0.137 | SBP | −0.486 | 0.084 | 8.04E-09 | 194,563 | −0.183 | 0.052 | 4.17E-04 | 194,551 |
| EST 38 | FLJ32810-TMEM133 | rs633185 | 11 | 100,593,538 | C/G | 0.715 | SBP & DBP | 0.522 | 0.067 | 6.97E-15 | 183,845 | 0.288 | 0.041 | 2.38E-12 | 183,825 |
| EST 39 | PDE3A | rs3752728 | 12 | 20,192,972 | A/G | 0.737 | DBP | 0.331 | 0.066 | 4.32E-07 | 200,440 | 0.319 | 0.04 | 2.35E-15 | 200,408 |
| EST 40 | ATP2B1 | rs11105354 | 12 | 90,026,523 | A/G | 0.84 | SBP & DBP | 0.909 | 0.081 | 3.88E-29 | 195,206 | 0.459 | 0.05 | 2.61E-20 | 195,195 |
| EST 41 | SH2B3 | rs3184504* | 12 | 111,884,608 | T/C | 0.475 | SBP & DBP | 0.498 | 0.062 | 9.97E-16 | 177,067 | 0.362 | 0.038 | 1.28E-21 | 177,122 |
| EST 42 | TBX5-TBX3 | rs2891546 | 12 | 115,552,499 | A/G | 0.11 | DBP | −0.529 | 0.1 | 1.36E-07 | 172,012 | −0.38 | 0.061 | 4.71E-10 | 171,980 |
| EST 43 | CYP1A1-ULK3 | rs936226 | 15 | 75,069,282 | T/C | 0.722 | SBP & DBP | −0.549 | 0.067 | 3.06E-16 | 187,238 | −0.363 | 0.041 | 1.03E-18 | 187,221 |
| EST 44 | FURIN-FES | rs2521501 | 15 | 91,437,388 | A/T | 0.684 | SBP & DBP | −0.639 | 0.069 | 3.35E-20 | 164,272 | −0.358 | 0.042 | 1.85E-17 | 164,255 |
| EST 45 | PLCD3 | rs7213273 | 17 | 43,155,914 | A/G | 0.658 | SBP | −0.413 | 0.066 | 4.71E-10 | 164,795 | −0.185 | 0.041 | 7.23E-06 | 164,788 |
| EST 46 | GOSR2 | rs17608766 | 17 | 45,013,271 | T/C | 0.854 | SBP | −0.658 | 0.083 | 2.27E-15 | 188,895 | −0.218 | 0.051 | 1.95E-05 | 188,928 |
| EST 47 | ZNF652 | rs12940887 | 17 | 47,402,807 | T/C | 0.38 | DBP | 0.321 | 0.06 | 7.06E-08 | 192,546 | 0.261 | 0.037 | 1.07E-12 | 192,524 |
| EST 48 | JAG1 | rs1327235 | 20 | 10,969,030 | A/G | 0.542 | SBP & DBP | −0.395 | 0.059 | 2.23E-11 | 192,680 | −0.308 | 0.036 | 1.78E-17 | 192,659 |
| EST 49 | GNAS-EDN3 | rs6026748 | 20 | 57,745,815 | A/G | 0.125 | SBP & DBP | 0.867 | 0.089 | 3.15E-22 | 192,338 | 0.552 | 0.055 | 4.86E-24 | 192,327 |
Meta-analysis results of up to 342,415 individuals of European ancestry for SBP and DBP: Established and new loci are grouped separately. Nearest genes are shown as locus labels but this should not be interpreted as support that the causal gene is the nearest gene. The lead SNP with the lowest P value for either BP trait is shown as the lead SNP and both SBP and DBP results are presented even if both are not genome-wide significant. The SNP effects are shown according to the effect in mm Hg per copy of the coded allele (that is the allele coded 0, 1, 2) under an additive genetic model.
in the lead SNP column indicates a non-synonymous coding SNP (either the SNP itself or another SNP in r2 >0.8).
Established loci have smaller total sample sizes relative to novel loci (see Supplementary Note).
Figure 1. Manhattan plots for SBP and DBP from the stage 4 Cardio-MetaboChip-wide meta-analysis.
P values (expressed as -log10P) are plotted by physical genomic position labeled by chromosome. SNPs in new loci (3.5MB window around the index SNP), identified in this study, are labeled in dark red (SBP) or dark blue (DBP); SNPs in previously known loci are labeled in orange (SBP) or light blue (DBP). The locus names are indicated. The grey crosses indicate genomic positions at which the y-axis was truncated (SNPs with P < 10−15).
Compared with previously reported BP variants5,7,21, the average absolute effect size of the newly discovered variants is smaller, with comparable minor allele frequency (MAF), presumably owing to the increased power of a larger sample size (Table 2). As expected from the high correlation between SBP and DBP effects, the observed directions of effects for the two traits were generally concordant (Supplementary Figure 5), and the absolute effect sizes were inversely correlated with MAF (Table 1 and Supplementary Figure 6). The 66 BP SNPs explained 3.46% and 3.36% of SBP and DBP variance, respectively, a modest increase from 2.95% and 2.78% for SBP and DBP, respectively, for the 49 previously reported SNPs (Supplementary Note). The low percent variance explained is consistent with estimates that large numbers of common variants with weak effects at a large number of loci influence BP5.
Table 2.
Overview of novel and known BP variant properties.
| 17 new loci | 49 established loci | 66 loci | |
|---|---|---|---|
| Minor allele frequency (mean, range) | 32.1% [5%-50%] | 28.9% [7%-49%] | 29.8% [5%-50%] |
| Effect size SBP [mmHg] (range, mean) | 0.09-0.59, 0.34 | 0.07-1.13, 0.5 | 0.07-1.13, 0.46 |
| Effect size DBP [mmHg] (range, mean) | 0.1-0.46, 0.23 | 0.06-0.60, 0.3 | 0.06-0.6, 0.28 |
| Variance explained SBP | 0.52% | 2.95% | 3.46% |
| Variance explained DBP | 0.58% | 2.78% | 3.36% |
Key characteristics of the novel and established BP loci are shown. MAF and effect size estimates are derived from the Cardio-MetaboChip data. Variance explained estimates are estimated from one large study (Supplementary Note). Novel loci are classified as previously unknown to be linked to BP by a systematic PubMed review of all genes in a 200kb window (Supplementary Note).
Signal refinement at the 66 BP loci
To identify distinct signals of association at the 66 BP loci and the variants most likely to be causal for each, we started with an approximate conditional analysis using a model selection procedure implemented in the GCTA-COJO package22,23 as well as a detailed literature review of all published BP association studies. GCTA-COJO analysis was performed using the association summary statistics for SBP and DBP from the Stage 4 EUR ancestry meta-analyses, with the linkage disequilibrium (LD) between variants estimated on the basis of Cardio-MetaboChip genotype data from 7,006 individuals of EUR ancestry from the GoDARTS cohort24. More than one distinct BP association signal was identified at 13 loci at P < 5 × 10−8 (Supplementary Table 6, Supplementary Figures 7, and Supplementary Note). At six loci, the distinct signals were identified for both SBP and DBP analyzed separately; these trait-specific associations were represented by the same or highly correlated (r2 > 0.8) SNPs at 5 of the 6 loci (Supplementary Tables 7 and 8). We repeated GCTA-COJO analyses using the same summary association results, but with a different reference sample for LD estimates (WTCCC1-T2D/58BC, N = 2,947, Supplementary Note) and observed minimal differences arising from minor fluctuations in the association P value in the joint regression models (Supplementary Tables 7 and 8). LD-based comparisons of published association signals at established BP loci, and the current study's findings suggested that at 10 loci, the signals identified by the single-SNP and the GCTA-COJO analyses were distinct from those reported in the literature (Supplementary Table 9).
We then performed multivariable regression modeling in a single large cohort (Women's Genome Health Study, WGHS, N = 23,047) with simultaneous adjustment for both 1) all combinations of putative index SNPs for each distinct signal from the GCTA-COJO conditional analyses, and 2) all index SNPs for all potential distinct signals identified by our literature review (Supplementary Table 9, Supplementary Note). Although WGHS is very large as a single study, power is reduced in a single sample compared to that in the overall meta-analysis (23k vs. 342k individuals) and consequently the failure to reach significance does not represent non-replication for individual SNPs. The WGHS analysis supported two distinct association signals at eight of 13 loci identified in the GCTA-COJO analysis, but could not provide support for the remaining five (Supplementary Table 10). The joint SNP modeling in WGHS additionally supported two distinct signals of association at three other loci (GUCY1A3-GUCY1B3, SYNPO2L and TBX5-TBX3), at which the SNP identified in the current study is distinct from that previously reported in the literature5,11.
We sought to refine the localization of likely functional variants at loci with high-density coverage on the Cardio-MetaboChip. We followed a Bayesian approach to define, for each signal, credible sets of variants that have 99% probability of containing or tagging the causal variant (Supplementary Note). To improve the resolution of the method, the analyses were restricted to 24 regions selected to fine map (FM) genetic associations, and that included at least one SNP reaching genome-wide significance in the current meta-analyses (Supplementary Table 11). Twenty-one of the Cardio-MetaboChip FM regions were BP loci in the original design, with three of the newly discovered BP loci in FM regions that were originally selected for other non-BP traits. We observed that the 99% credible SNP sets at five BP loci spanned <20kb. The greatest refinement was observed at the SLC39A8 locus for SBP and DBP, and at the ZC3HC1 and PLCE1 loci for DBP, where the 99% credible sets included only the index variants (Supplementary Table 12). Although SNPs in credible sets were primarily non-coding, they included one synonymous and seven non-synonymous variants that attained high posterior probability of driving seven distinct association signals at six BP loci (Supplementary Table 12). Of these, three variants alone account for more than 95% of the posterior probability of driving the association signal observed at each of three loci (Supplementary Table 12 and 13). Despite reduced statistical power, the analyses restricted to the samples with Cardio-MetaboChip genotypes only (N = 109,096) identified the majority of SNPs identified in the GWAS+Cardio-MetaboChip data (Supplementary Table 12). The full list of SNPs in the 99% credible sets are listed in Supplementary Table 13.
What do the BP variants do?
Index SNPs or their proxies (r2 > 0.8) altered amino acid sequence at 11 of 66 BP loci (Table 1). Thus, the majority of BP-association signals are likely driven by non-coding variants hypothesized to regulate expression of some nearby gene in cis. To characterize their effects, we first sought SNPs associated with gene expression (eSNPs) from a range of available expression data which included hypertension target end organs and cells of the circulatory system (heart tissue, kidney tissue, brain tissue, aortic endothelial cells, blood vessels) and other tissue/cell types (CD4+ macrophages, monocytes lymphoblastoid cell lines, skin tissue, fat tissue, and liver tissue). Fourteen BP-associated SNPs at the MTHFR-NPPB, MDM4, ULK4, CYP1A1-ULK3, ADM, FURIN-FES, FIGN, and PSMD5 loci were eSNPs across different tissues (Supplementary Table 14). Of these 14 eSNPs, three were also predicted to alter the amino acid sequence at the MTHFR-NPPB, MAP4 and ULK4 loci, providing two potential mechanisms to explore in functional studies. Second, we used gene expression levels measured in whole blood in two different samples each including >5,000 individuals of EUR descent. We tested whether the lead BP SNP was associated with expression of any transcript in cis (<1Mb from the lead SNP at each locus) at a false discovery rate (FDR) of < 0.05, accounting for all possible cis-transcript association tests genome-wide. It is likely that we did not genotype the causal genetic variant underlying each BP association signal; a nearby SNP-transcript association, due to LD, may therefore reflect an independent genetic effect on expression that is unrelated to the BP effect. Consequently, we assumed that the lead BP SNP and the most significant eSNP for a given transcript should be highly correlated (r2 > 0.7). Furthermore, we assumed that the significance of the transcript association with the lead BP SNP should be substantially reduced in a conditional model adjusting for the best eSNP for a given transcript. Eighteen SNPs at 15 loci were associated with 22 different transcripts, with a total of 23 independent SNP-transcript associations (three SNPs were associated with two transcripts each, Supplementary Table 15, Supplementary Note). The genes expressed in a BP SNP allele-specific manner are clearly high-priority candidates to mediate the BP association. In whole blood, these genes included obvious biological candidates such as GUCY1A3, encoding the alpha subunit of the soluble guanylate cyclase protein, and ADM, encoding adrenomedullin, both of which are known to induce vasodilation25,26. There was some overlap of eSNPs between the whole blood and other tissue datasets at the MTHFR-NPPB, MDM4, PSMD5, ULK4 and CYP1A1-ULK3 loci, illustrating additional potentially causal genes for further study.
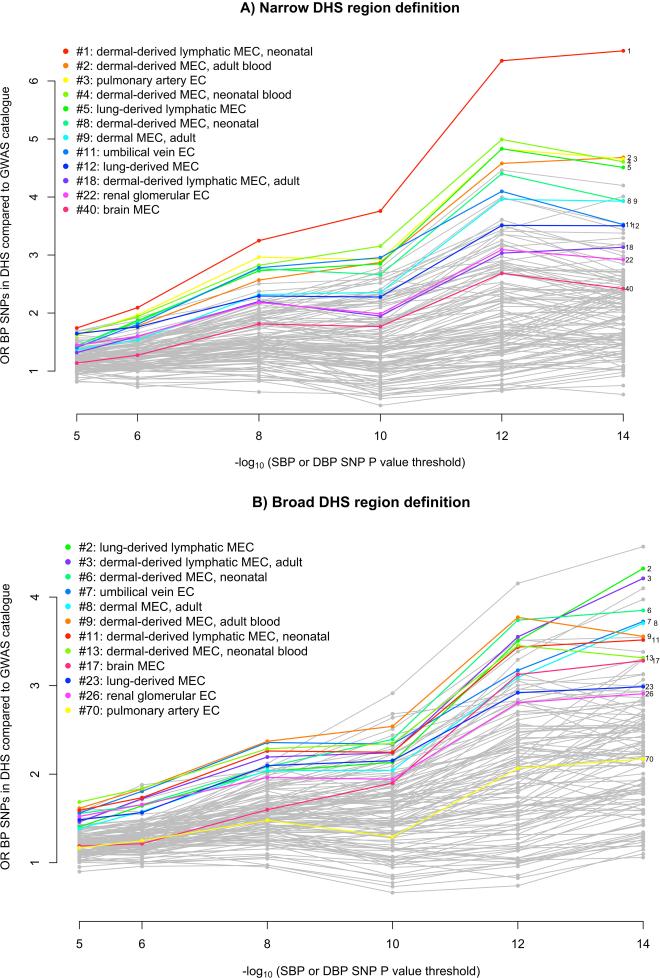
An alternative method for understanding the effect on BP of non-coding variants is to determine whether they fall within DNaseI hypersensitivity sites (DHSs). We performed two analyses to investigate whether BP SNPs or their LD proxies (r2 > 0.8) were enriched in DHSs in a cell-type-specific manner (Supplementary Note). First, we used Epigenomics Roadmap and ENCODE DHS data from 123 adult cell lines or tissues27-29 to estimate the fold increase in the proportion of BP SNPs mapping to DHSs compared to SNPs associated at genome-wide significance with non-BP phenotypes from the NHGRI GWAS catalog30. We observed that 7 out of the 10 cell types with the greatest relative enrichment of BP SNPs mapping to DHSs were from blood vessels (vascular or micro-vascular endothelial cell-lines or cells) and 11 of the 12 endothelial cells were among the top quarter most enriched among the 123 cell types (Figure 2 and Supplementary Table 16). In a second analysis of an expanded set of tissues and cell lines, in which cell types were grouped into tissues (Supplementary Table 17), BP-associated SNP enrichment in DHSs in blood vessels was again observed (P = 1.2 × 10−9), as well as in heart samples (P = 5.3 × 10−8; Supplementary Table 18).
Figure 2. Enrichment of DNAse hypersensitive sites among BP loci in different cell-types.
Enrichment analyses of SBP or DBP associated loci according to discovery P value using narrow peaks (panel A) or broad peaks (panel B). SNPs were selected according to different P value cutoffs (x-axis) and a fold enrichment of overlap with DNAse hypersensitive sites compared to unrelated GWAS SNPs was calculated (y-axis) (see Supplementary Note). The 12 endothelial cell-lines are indicated in color and for each endothelial cell-type the rank using the 10−14 P value cutoff is indicated. EC denotes endothelial cells.
We next tested whether there was enrichment of BP SNPs in H3K4me331 sites, a methylation mark associated with both promoter and enhancer DNA. We observed significant enrichment in a range of cell types including CD34 primary cells, adult kidney cells, and muscle satellite cultured cells(Supplementary Table 19). Enrichment of BP SNPs in predicted strong and weak enhancer states and in active promoters32 in a range of cell types was also observed (Supplementary Table 20, Supplementary Figure 8).
We used Meta-Analysis Gene-set Enrichment of variaNT Associations (MAGENTA)33 to attempt to identify pathways over-represented in the BP association results. No gene sets meeting experiment-wide significance for enrichment for BP association were identified by MAGENTA after correction for multiple testing, although some attained nominal significance (Supplementary Table 21, Supplementary Note). We also adapted the DEPICT34 pathway analysis tool (Data-driven Expression Prioritized Integration for Complex Traits) to identify assembled gene-sets that are enriched for genes near associated variants, and to assess whether genes from associated loci were highly expressed in particular tissues or cell types. Using the extended BP locus list based on genome-wide significant loci from this analysis and previously published SNPs that may not have reached genome-wide significance in the current analysis (Supplementary Table 9), we identified five significant (FDR ≤ 5%) gene sets: abnormal cardiovascular system physiology, G Alpha 1213 signaling events, embryonic growth retardation, prolonged QT interval, and abnormal vitelline vasculature morphology. We also found that suggestive SBP and DBP associations (P < 1 × 10−5) were enriched for reconstituted gene-sets at DBP loci (mainly related to developmental pathways), but not at SBP loci (Supplementary Table 22, Supplementary Note). In a final analysis, we assessed Cardio-MetaboChip SNPs at the fine-mapping loci using formaldehyde-assisted isolation of regulatory elements (FAIRE-gen) in lymphoblastoid cell lines35. Our results provided support for two SNPs, one of which SNP (rs7961796 at the TBX5-TBX3 locus) was located in a regulatory site. Although the other SNP (rs3184504 at the SH2B3 locus) is a non-synonymous variant, there was also a regulatory site indicated by DNaseI and H3K4me1 signatures at the locus, making the SNP a potential regulatory variant (Supplementary Table 23)36. Both SNPs were included in the list of 99% credible SNPs at each locus.
Asian- and African ancestry BP SNP association
We tested the 66 lead SNPs at the established and novel loci for association with BP in up to 20,875 individuals of South Asian (SAS) ancestry (PROMIS and RACE studies), 9,637 individuals of East Asian (EAS) ancestry (HEXA, HALST, CLHNS, DRAGON, and TUDR studies), and 33,909 individuals of African (AFR) ancestry (COGENT-BP consortium, Jupiter, SPT, Seychelles, GXE, and TANDEM studies). As expected, the effect allele frequencies are very similar across studies of the same ethnicity, but markedly different across different ancestry groups (Supplementary Figure 9). Many associations of individual SNPs failed to reach P < 0.05 for the BP trait with the lower P value (Supplementary Table 24), which could potentially be due to the much lower statistical power at the sample sizes available, different patterns of LD at each locus across ancestries, variability in allele frequency, or true lack of association in individuals of a given non-European ancestry. The low statistical power for the great majority of SNPs tested is visible considering SNP-by-SNP power calculations using European ancestry effect sizes (Supplementary Table 24). However, concordant directions of allelic effects for both SBP and DBP were observed for 45/66 SNPs in SAS, 36/60 SNPs in EAS, and 42/66 SNPs in AFR samples: the strongest concordance with SAS may not be surprising because South Asians are more closely related to Europeans than are East Asians or Africans. Moreover, strong correlation of effect sizes was observed between EUR samples with SAS, EAS, or AFR samples (r = 0.55, 0.60, and 0.48, respectively). A 66-SNP SBP or DBP risk score were significant predictors of SBP and DBP in all samples. A 1 mm Hg higher SBP or DBP risk score in EUR samples was associated with a 0.58/0.50 mm Hg higher SBP/DBP in SAS samples (SBP P = 1.5 × 10−19, DBP P = 3.2 × 10−15), 0.49/0.50 mm Hg higher SBP/DBP in EAS samples (SBP P = 1.9 × 10−10, DBP P = 1.3 × 10−7), and 0.51/0.47 mm Hg higher SBP/DBP in AFR samples (SBP P = 2.2 × 10−21, DBP P = 6.5 × 10−19). The attenuation of the genetic risk score estimates in non-European ancestries is presumably due to inclusion of a subset of variants that lack association in the non-European or admixed samples.
We subsequently performed a trans-ethnic meta-analysis of the 66 SNPs in all 64,421 samples across the three non-European ancestries. After correcting for 66 tests, 12/66 SNPs were significantly associated with either SBP or DBP (P < 7.6 × 10−4), with a correlation of EUR and non-EUR effect estimates of 0.77 for SBP and 0.67 for DBP; the European-ancestry SBP or DBP risk score was associated with 0.53/0.48 mm Hg higher BP per predicted mm Hg SBP/DBP respectively (SBP P < 6.6 × 10−48, DBP P < 1.3 × 10−38). For 7 of the 12 significant SNPs, no association has previously been reported in genome-wide studies of non-European ancestry. Some heterogeneity of effects was observed between European and non-European effect estimates (Supplementary Table 24). Taken together, these findings suggest that, in aggregate, BP loci identified using data from individuals of EUR ancestry are also predictive of BP in non-EUR samples, but larger non-European sample sizes will be needed to establish precisely which individual SNPs are associated in a given ethnic group.
Impact on hypertensive target organ damage
Long-term elevated BP causes target organ damage, especially in the heart, kidney, brain, large blood vessels, and the retinal vessels37. Consequently, the genetic effect of the 66 SBP and DBP SNPs on end-organ outcomes can be directly tested using the risk score, although some outcomes lacked results for a small number of SNPs. Interestingly, BP risk scores significantly predicted (Supplementary Note) coronary artery disease risk, left ventricular mass and wall thickness, stroke, urinary albumin/creatinine ratio, carotid intima-medial thickness and central retinal artery caliber, but not heart failure or other kidney phenotypes, after accounting for the number of outcomes examined (Table 3). Because outlier effects can affect risk scores, we repeated the risk score analysis removing iteratively SNPs that contributed to statistical heterogeneity (SNP-trait effects relative to SNP-BP effects). Heterogeneity was defined based on a multiple testing adjusted significance threshold for Cochran's Q test of homogeneity of effects (Supplementary Note). The risk score analyses restricted to the subset of SNPs showing no heterogeneity of effect revealed essentially identical results, with the exception that urinary albumin/creatinine ratio was no longer significant. The per-SNP results are provided in Supplementary Table 25 and Supplementary Figures 10. Because large-scale GWAS of non-BP cardiovascular risk factors are available, we examined the BP risk scores as predictors of other cardiovascular risk factors: LDL-cholesterol, HDL-cholesterol, triglycerides, type 2 diabetes, BMI, and height. We observed nominal (P <0.05) associations of the BP risk scores with risk factors, although mostly in the opposite direction to the risk factor-CVD association (Supplementary Table 26). The failure to demonstrate an effect of BP risk scores on heart failure may reflect limited power from a modest sample size, but the lack of significant effects on renal measures suggests that the epidemiologic relationship of higher BP and worse renal function may not reflect direct consequences of BP elevation.
Table 3.
Prediction of hypertensive target organ damage by a multi-BP SNP score.
| Phenotype | Var. type (cont./ dic.) |
Eth. | Consort. | Total N or no. ca/co |
Total #SNPs |
SBP_score |
DBP_score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| effect (all) |
P value (all) |
het. P value (all) |
P value (p) |
# SNPs rem. |
effect (all) |
P value (all) |
het. P value (all) |
P value (p) |
# SNPs rem. |
||||||
|
HEART
| |||||||||||||||
| CAD | dich. | EUR SAS | CARDIoGRAMplusC4D | 63,746/130,681 | 61 | 1.042 | 1.72E-44 | 1.75E-25 | 4.08E-32 | 10 | 1.069 | 1.19E-42 | 6.63E-27 | 2.2E-38 | 10 |
| heart failure | dich. | EUR | CHARGE | 2,526/18,400 | 66 | 1.021 | 2.77E-02 | 1.63E-01 | 2.77E-02 | 0 | 1.035 | 2.31E-02 | 1.70E-01 | 2.31E-02 | 0 |
| LV mass | cont. | EUR | CHARGE | 11,273 | 66 | 0.480 | 6.43E-04 | 3.58E-01 | 6.43E-04 | 0 | 0.754 | 1.23E-03 | 3.21E-01 | 1.23E-03 | 0 |
| LV wall thickness | cont. | EUR | CHARGE | 11,311 | 66 | 0.004 | 4.45E-06 | 5.83E-02 | 4.45E-06 | 0 | 0.007 | 3.19E-06 | 6.40E-02 | 3.19E-06 | 0 |
|
KIDNEY | |||||||||||||||
| CKD | dich. | EUR | CHARGE | 6,271/68,083 | 65 | 1.010 | 1.37E-01 | 1.77E-03 | 2.65E-01 | 1 | 1.008 | 4.49E-01 | 1.25E-03 | 7.69E-01 | 1 |
| eGFR (based on cr) | cont. | EUR | CHARGE | 74,354 | 65 | 0.000 | 7.07E-01 | 3.12E-05 | 3.22E-01 | 2 | 0.000 | 9.41E-01 | 3.02E-05 | 9.65E-01 | 2 |
| eGFR (based on cystatin) | cont. | EUR | CHARGE | 74,354 | 65 | 0.001 | 9.05E-02 | 9.28E-06 | 4.11E-01 | 1 | 0.001 | 3.30E-01 | 5.64E-06 | 6.9E-01 | 1 |
| creatinine | cont. | EUR | KidneyGEN | 23,812 | 66 | 0.000 | 9.42E-01 | 6.31E-03 | 9.42E-01 | 0 | 0.000 | 4.11E-01 | 7.16E-03 | 4.11E-01 | 0 |
| microalbuminuria | dich. | EUR | CHARGE | 2,499/29,081 | 65 | 0.011 | 2.10E-01 | 4.79E-02 | 2.1E-01 | 0 | 0.023 | 1.02E-01 | 5.66E-02 | 1.02E-02 | 0 |
| urinary albumin/cr ratio | cont. | EUR | CHARGE | 31,580 | 65 | 0.009 | 2.52E-03 | 3.02E-04 | 0.53E-03 | 1 | 0.015 | 2.40E-03 | 3.08E-04 | 8.31E-03 | 1 |
|
STROKE | |||||||||||||||
| stroke, all subtypes | dich. | EUR | CHARGE | 1,544/18,058 | 66 | 0.056 | 6.11E-06 | 8.26E-02 | 6.11E-06 | 0 | 0.085 | 3.79E-05 | 4.98E-02 | 3.79E-05 | 0 |
| stroke, ischemic subtype | dich. | EUR | CHARGE | 1,164/18,438 | 66 | 0.067 | 3.33E-06 | 1.75E-01 | 3.33E-06 | 0 | 0.096 | 5.63E-05 | 8.82E-02 | 5.63E-05 | 0 |
| stroke, ischemic subtype | dich. | EUR | MetaStroke | 11,012/40,824 | 66 | 0.036 | 1.69E-10 | 4.72E-02 | 1.69E-10 | 0 | 0.056 | 1.29E-09 | 2.51E-02 | 1.29E-09 | 0 |
|
VASCULATURE | |||||||||||||||
| cIMT | cont. | EUR | CHARGE | 27,610 | 66 | 0.004 | 4.80E-15 | 5.06E-08 | 7.32E-10 | 4 | 0.005 | 4.15E-11 | 3.84E-10 | 6.2E-07 | 5 |
|
EYE | |||||||||||||||
| mild retinop. | dich. | EUR | CHARGE | 1,122/18,289 | 66 | 1.021 | 1.37E-01 | 6.01E-03 | 1.37E-01 | 0 | 1.046 | 5.78E-02 | 7.81E-03 | 5.78E-02 | 0 |
| central retinal artery caliber | cont. | EUR | CHARGE | 18,576 | 66 | 0.343 | 3.29E-14 | 2.56E-06 | 2.06E-13 | 2 | 0.570 | 3.61E-14 | 2.44E-06 | 7.05E-13 | 3 |
| mild retinop. | dich. | EAS | SEED | 289/5,419 | 66 | 1.033 | 2.55E-01 | 2.42E-01 | 2.55E-01 | 0 | 1.087 | 8.55E-02 | 2.87E-01 | 8.55E-02 | 0 |
| central retinal artery caliber | cont. | EAS | SEED | 6,976 | 63 | 0.320 | 1.39E-04 | 9.07E-01 | 1.39E-04 | 0 | 0.533 | 2.19E-04 | 8.91E-01 | 2.19E-04 | 0 |
Shown are the estimated effects of a BP risk score comprised of up to 66 SNPs (see column “Total #SNPs”) on risk of dichotomous outcome (as odds ratios) or increment in continuous measures per predicted mmHg of the SBP or DBP score. The effect sizes are expressed as incremental change in the phenotype for quantitative traits and natural logarithm of the odds ratio for binary traits, per 1 mmHg predicted increase in SBP or DBP. P values are bolded if they meet an analysis-wide significance threshold (< 0.05/18 = 0.0028). Results for all SNPs (“all”) and for pruned results (“p”) are shown. The pruned results were obtained by iterative removal of SNPs from the risk score starting with the SNP with lowest heterogeneity P value. Iterations to remove SNPs were continued until the heterogeneity P value was < 0.0028 (see Supplementary Note). The number of SNPs removed when calculating the pruned results is indicated by “# SNPs rem.”. The results per individual SNP can be found in Supplementary Table 15. CAD: coronary artery disease, LV: left ventricle, CKD: chronic kidney disease, eGFR: estimated glomerular filtration rate, cr: creatinine, cIMT: carotid intima: media thickness. Var. type denotes the variable type and cont. for continuous, or dic. for dichotomous. Eth. = Ethnicity, Consort. = Consortium, EUR = European ancestry, EAS = East Asian ancestry.
DISCUSSION
The study reported here is the largest to date to investigate the genomics of BP in multiple continental ancestries. Our results highlight four major features of inter-individual variation in BP: (1) we identified 66 (17 novel) genome-wide significant loci for SBP and DBP by targeted genotyping in up to 342,415 individuals of European ancestry that cumulatively explain ~3.5% of the trait; (2) the variants were enriched for cis-regulatory elements, particularly in vascular endothelial cells; (3) the variants had broadly comparable BP effects in South Asians, East Asian and Africans, albeit in smaller sample sizes; and, (4) a 66 SNP risk-score predicted target organ damage in the heart, cerebral vessels, carotid artery and the eye with little evidence for an effect in kidneys. Overall, there was no enrichment of a single genetic pathway in our data; rather, our results are consistent with the effects of BP arising from multiple tissues and organs.
Genetic and molecular analyses of Mendelian syndromes of hypertension and hypotension point largely to a renal origin, involving multiple rare deleterious mutations in proteins that regulate salt-water balance38. This is strong support for Guyton's hypothesis that the regulation of sodium excretion by the kidney and its effects on extracellular volume are a prime pathway determining intra-arterial pressure39. However, our genetic data from unselected individuals in the general community argues against a single dominant renal effect. The 66 SNPs we identified are not chance effects, but have a global distribution and impact on BP that are consistent as measured by their effects across the many studies meta-analyzed. That they are polymorphic across all continental ancestries argues for their origin and functional effects prior to human continental differentiation.
However several of the 17 novel loci contain strong positional biological candidates, these are described in greater detail in Supplementary Table 27 and the Supplementary Note. The single most common feature we identified was the enrichment of regulatory elements for gene expression in vascular endothelial cells. The broad distribution of these cells across both large and small vessels and across all tissues and organs suggest that functional variation in these cells affects endothelial permeability or vascular smooth muscle cell contractility via multiple pathways. These hypotheses will need to be rigorously tested in appropriate models, to assess the contribution of these pathways to BP control, and these pathways could also be targets for systemic anti-hypertensive therapy as they are for the pulmonary circulation42.
In summary, these genetic observations may contribute to an improved understanding of BP biology and a re-evaluation of the pathways considered relevant for therapeutic BP control.
ONLINE METHODS
Cohorts contributing to systolic (SBP) and diastolic blood pressure (DBP) analyses
Studies contributing to BP association discovery including community- and population-based collections as well as studies of non-BP traits, analyzed as case and control samples separately. Details on each of the studies including study design and BP measurement are provided in Supplementary Table 1, genotyping information in Supplementary Table 2, and participant characteristics in Supplementary Table 3. All participants provided written informed consent and the studies were approved by local Research Ethics Committees and/or Institutional Review Boards.
European ancestry meta-analysis
BP was measured using standardized protocols in all studies regardless of whether the primary focus was BP or another trait. We initially analyzed affected and unaffected individuals from samples selected as cases (e.g. type 2 diabetes) or controls, separately. However, because sensitivity analyses did not reveal any significant difference in BP effect size estimates between case and control samples (data not shown), we analyzed all samples combined. When available, the average of two BP measurements was used for association analyses (Supplementary Table 1). If an individual was taking a BP-lowering treatment, the underlying systolic BP (SBP) and diastolic BP (DBP) were estimated by adding 15 mmHg and 10 mmHg, respectively, to the measured values, as done in prior analyses.
A meta-analysis of 340,934 individuals of European descent was undertaken in four stages with subsequent validation in an independent cohort. Because stage 1 Cardio-MetaboChip samples included many SNPs selected on the basis of association with BP in earlier GWAS, we performed genomic control using a set of putative null SNPs based on P > 0.10 in earlier GWAS of SBP and DBP or both. Stage 2 samples with genome-wide genotyping used the entire genome-wide set of SNPs for genomic control given the lack of ascertainment. The study design is summarized in Supplementary Figure 1, and further details are provided in Supplementary Tables 2-5 and the Supplementary Note.
Systematic PubMed search +/− 100kb of each newly discovered index SNP
All genes with any overlap with a 200kb region centered around each of the 17 newly discovered lead SNPs were identified using the UCSC Genome Browser. A search term was constructed for each gene including the short and long gene name and the terms “blood pressure” and “hypertension” (e.g. for NPPA on chr 1: “NPPA OR natriuretic peptide A AND (blood pressure OR hypertension)”) and the search results of each search term from PubMed were individually reviewed.
Trait variance explained
The trait variance explained by 66 lead SNPs at novel and known loci was evaluated in one study that contributed to the discovery effort: the Atherosclerosis Risk in Communities (ARIC) study. We constructed a linear regression model with all 66 or the subset of 49 known SNPs as a set of predictors of the BP residual after adjustment for covariates of the adjusted treatment-corrected BP phenotype (SBP or DBP). The r2 from the regression model was used as the estimate of trait variance explained.
European ancestry GCTA-COJO analysis
To identify multiple distinct association signals at any given BP locus, we undertook approximate conditional analyses using a model selection procedure implemented in the GCTA-COJO software package44,45. To evaluate the robustness of the GCTA-COJO results to the choice of reference data set, model selection was performed using the LD between variants in separate analyses from two datasets of European descent, both with individuals from the UK with Cardio-MetaboChip genotype data: GoDARTS with 7,006 individuals and WTCCC1-T2D/58BC with 2,947 individuals. Assuming that the LD between SNPs more than 10 Mb away or on different chromosomes is zero, we undertook the GCTA-COJO step wise model selection to select SNPs that were conditionally-independently associated with SBP and DBP, in turn, at a genome-wide significance, given by P < 5×10−8 (Supplementary Tables 6-8) using the stage 4 combined European GWAS+ Cardio-MetaboChip meta-analysis.
Conditional analyses in the Women's Genome Health Study (WGHS)
Multivariable regression modeling was performed for each possible combination of putative independent SNPs from a) model selection implemented in GCTA-COJO and b) a comprehensive manual review of the literature (Supplementary Table 9). Any SNP with P < 5×10−8 in a previous reported BP GWAS was considered. A total of 46 SNPs were examined (Supplementary Table 10). Genome-wide genotyping data imputed to 1000 Genomes in the WGHS (N = 23,047) were used. Regression modeling was performed in the R statistical language (Supplementary Table 10).
Fine mapping and determination of credible sets of causal SNPs
The GCTA-COJO and WGHS conditional analyses identified multiple distinct signals of association at multiple loci (Supplementary Tables 6 and 10). Of the 24 loci considered in fine-mapping analyses, 16 had no evidence for the existence of multiple distinct association signals, so it is reasonable to assume that there is a single causal SNP and therefore the credible sets of variants could be constructed using the association summary statistics from the unconditional meta-analyses. However, in the remaining eight loci, where evidence of secondary signals was observed from GCTA-COJO, we performed approximate conditional analyses across the region by conditioning on each index SNP (Supplementary Table 11). By adjusting for the other index SNPs at the locus, we can therefore assume a single variant is driving each “conditionally-independent” association signal, and we can construct the 99% credible set of variants on the basis of the approximate conditional analysis from GCTA-COJO (Supplementary Tables 12-13). At five of the eight loci with multiple distinct signals of association, one index SNP mapped outside of the fine-mapping region, so a credible set could not be constructed.
eQTL analysis: Whole Blood
NESDA/NTR: Whole blood eQTL analyses were performed in samples from the Netherlands Study of Depression and Anxiety (NESDA)46 and the Netherlands Twin Registry (NTR)47 studies. RNA expression analysis was performed in the statistical software R. The residuals resulting from the linear regression analysis of the probe set intensity values onto the covariates sex, age, body mass index (kg/m2), smoking status coded as a categorical covariate, several technical covariates, and three principal components were used. The eQTL effects were detected using a linear mixed model approach, including for each probe set the expression level (normalized, residualized and without the first 50 expression PCs) as dependent variable; the SNP genotype values as fixed effects; and family identifier and zygosity (in the case of twins) as random effects to account for family and twin relations48.
The eQTL effects were defined as cis when probe set–SNP pairs were at distance < 1M base pairs. At a FDR of 0.01 applied genome-wide, not just for candidate SNPs, the P value threshold was 1×10−4 for the cis-eQTL analysis. For each probe set that displayed a statistically significant association with at least one SNP located within its cis region, we identified the most significantly associated SNP and denoted this as the top cis-eQTL SNP. See Supplementary Note for details.
eQTL analysis: Selected published eQTL datasets
Lead BP SNP and proxies (r2 > 0.8) were searched against a collected database of expression SNP (eSNP) results. The reported eSNP results met criteria for statistical thresholds for association with gene transcript levels as described in the original papers. The non-blood cell tissue eQTLs searched included aortic endothelial cells49, left ventricle of the heart 50, cd14+ monocytes 51 and the brain 52. The results are presented in Supplementary Tables 14-15.
Enrichment analyses: Analysis of cell-specific DNase hypersensitivity sites (DHSs) using an OR method
The overlap of Cardio-MetaboChip SNPs with DHSs was examined using publicly available data from the Epigenomics Roadmap Project and ENCODE, choosing different cutoffs of Cardio-MetaboChip P values. The DHS mappings were available for 123 mostly adult cells and tissues 53 (downloaded from The DHS mappings were specified as both “narrow” and “broad” peaks, referring to reduction of the experimental data to peak calls at 0.1% and 1.0% FDR thresholds, respectively. Thus, the “narrow” peaks are largely nested within the “broad” peaks. Experimental replicates of the DHS mappings (typically duplicates) were also available for the majority of cells and tissues.
SNPs from the Cardio-MetaboChip genome-wide scan were first clumped in PLINK in windows of 100kb and maximum r2 = 0.1 among LD relationships from the 1000 Genomes European data. Then, the resulting index SNPs at each P value threshold were tagged with r2 = 0.8 in windows of 100kb, again using LD relationships in the 1000 Genomes, restricted to SNPs with MAF > 1% and also present in the HapMap2 CEU population. A reference set of SNPs was constructed using the same clumping and tagging procedures applied to GWAS catalog SNPs (available at http://www.genome.gov/gwastudies/, accessed 3/13/2013)54 with discovery P < 5×10−8 in European populations. A small number of reference SNPs or their proxies overlapping the BP SNPs or their proxies were excluded. After LD pruning and exclusions, there were a total of 1,196 reference SNPs. For each cell type and P value threshold, the enrichment of SBP or DBP SNPs (or their LD proxies) mapping to DHSs was expressed as an odds ratio (OR) relative to the GWAS catalog reference SNPs (or their LD proxies), using logistic mixed effect models treating the replicate peak determinations as random effects (glmer package in R). The significance of the enrichment ORs was derived from the significance of beta coefficients for the main effects in the mixed models (Figure 2, Supplementary Table 16).
Enrichment analyses: Analysis of tissue-specific enrichment of BP variants and H3K4me3 sites
An analysis to test for significant cell-specific enrichment in the overlap of BP SNPs (or their proxies) with H3K4me3 sites was performed as described in Trynka et al, 201355. The measure of overlap is a “score” that is constructed by dividing the height of an H3K4me3 ChIP signal in a particular cell by the distance between the nearest test SNP. The significance of the scores (i.e. P value) for all SNPs was determined by a permutation approach that compares the observed scores to scores of SNPs with similar properties to the test SNPs, essentially in terms of LD and proximity to genes (Supplementary Note). The number of permutations determined the number of significant digits in the P values and we conducted 10,000 iterations. Results are shown in Supplementary Table 19.
Enrichment analyses: Analysis of tissue-specific DHSs and chromatin states using GREGOR
The DNase-seq ENCODE data for all available cell types were downloaded in the processed “narrowPeak” format. The local maxima of the tag density in broad, variable-sized “hotspot” regions of chromatin accessibility were thresholded at FDR 1% with peaks set to a fixed width of 150bp. Individual cell types were further grouped into 41 broad tissue categories by taking the union of DHSs for all related cell types and replicates. For each GWAS locus, a set of matched control SNPs was selected based on three criteria: 1) number of variants in LD (r2 > 0.7; ± 8 variants), 2) MAF (± 1%), and 3) distance to nearest gene (± 11,655 bp). To calculate the distance to the nearest gene, the distance to the 5’ flanking gene (start and end position) and to the 3’ flanking gene was calculated and the minimum of these 4 values was used. If the SNP fell within the transcribed region of a gene, the distance was 0. The probability that a set of GWAS loci overlap with a regulatory feature more often than we expect by chance was estimated.
Enrichment analyses: FAIRE analysis of BP variants in fine-mapping regions in lymphoblastoid cell lines
FAIRE analysis was performed on a sample of 20 lymphoblastoid cell lines of European origin. All samples were genotyped using the Cardio-MetaboChip genotyping array, and BP SNPs and LD proxies (r2 > 0.8) at the fine mapping loci (N = 24, see Supplementary Table 23) were assessed to identify heterozygous imbalance between non-treated and FAIRE-treated chromatin. A paired t-test was used to compare the B allele frequency (BAF) arising from formaldehyde-fixed chromatin sheared by sonication and DNA purified to the BAF when the same chromatin sample underwent FAIRE to enrich for open chromatin. Three hundred and fifty-seven Cardio-MetaboChip BP SNPs were directly genotyped across the fine mapping regions. The Bonferroni-corrected threshold of significance is P < 0.0001 (0.05/357). The results for SNPs with P < 0.05 are reported in (Supplementary Table 23). FAIRE results were not available for some SNPs with missing data due to genotype failure or not having >3 heterozygous individuals for statistical analysis. Therefore there are no results for three lower frequency BP loci (SLC39A8, CYP17A1-NT5C2 and GNAS-EDN3) and for the second signal at the following loci: MTHFR-NPPB (rs2272803), MECOM (rs2242338) and HFE rs1800562).
Pathway analyses: MAGENTA
MAGENTA tests for enrichment of gene sets from a precompiled library derived from GO, KEGG, PATHTER, REACTOME, INGENUITY, and BIOCARTA was performed as described by Segré et al, 201056. Enrichment of significant gene-wide P values in gene sets is assessed by 1) using LD and distance criteria to define the span of each gene, 2) selecting the smallest P value among SNPs mapping to the gene span, and 3) adjusting this P value using a regression method that accounts for the number of SNPs, the LD, etc. In the second step, MAGENTA examines the distribution of these adjusted P values and defines thresholds for the 75%ile and the 95%ile. In the third step, MAGENTA calculates an enrichment for each gene set by comparing the number of genes in the gene set with P value less than either the 75th or 95th %ile to the number of genes in the gene set with P value greater than either the 75th or 95th %ile, and then comparing this quotient to the same quotient among genes not in the gene set. This gene-set quotient is assigned a P value based on reference to a hypergeometric distribution. The results based on our analyses are indicated in Supplementary Table 21.
Pathway analyses: DEPICT
We applied the DEPICT 57 analysis separately on genome-wide significant loci from the overall blood pressure (BP) Cardio-MetaboChip analysis including published blood pressure loci (see Supplementary Table 22). SNPs at the HFE and BAT2-BAT5 loci (rs1799945, rs1800562, rs2187668, rs805303, rs9268977) could not be mapped. As a secondary analysis, we additionally included associated loci (P < 1×10−5) from the Cardio-MetaboChip stage 4 combined meta-analyses of SBP and the DBP. DEPICT assigned genes to associated regions if they overlapped or resided within associated LD blocks with r2 > 0.5 to a given associated SNP.
Literature review for genes at the newly discovered loci
Recognizing that the most significantly associated SNP at a locus may not be located in the causal gene and that the functional consequences of a SNP often extends beyond 100kb, we conducted a literature review of genes in extended regions around newly discovered BP index SNPs. The genes for this extensive review were identified by DEPICT (Supplementary Table 22).
Non-European meta-analysis
To assess the association of the 66 significant loci from the European ancestry meta-analysis in non-European ethnicities, we obtained lookup results for the 66 index SNPs for participants of South-Asian ancestry (8 datasets, total N = 20,875), East-Asian ancestry (5 datasets, total N = 9,637), and African- and African-American ancestry (6 datasets, total N = 33,909). The association analyses were all conducted with the same covariates (age, age2, sex, BMI) and treatment correction (+15/10 mm Hg in the presence of any hypertensive medication) as the association analyses for the discovery effort in Europeans. Tests for heterogeneity across effect estimates in European, South Asian, East Asian and African derived samples were performed using GWAMA58.
Genetic risk score and cardiovascular outcomes
The gtx package for the R statistical programming language was used to estimate the effect of the SNP-risk score on the response variable in a regression model59.
Supplementary Material
SUMMARY STATISTICS.
Full summary statistics (P values) are in the online version of the paper (file “ICBPCMfinalMeta.csv.zip”).
ACKNOWLEDGEMENTS
We thank all the study participants of this study for their contributions. Detailed acknowledgment of funding sources is provided in the Supplementary Note.
AUTHOR CONTRIBUTIONS
Analysis group
Design of secondary analyses: G.B.E., T.Ferreira, T.J., A.P.M., P.B.M., C.N.-C. Computation of secondary analyses: G.B.E., T.Ferreira, T.J., A.P.M., P.B.M., C.N.-C. Paper writing: A.C., G.B.E., T.Ferreira, T.J., A.P.M., P.B.M., C.N.-C. Study management: P.B.M., C.N.-C.
Cardio-MetaboChip or new GWAS
WGHS: Study phenotyping: P.M.R., D.I.C., L.M.R. Genotyping or analysis: P.M.R., D.I.C., L.M.R., F.Giulianini Study PI: P.M.R.
JUPITER: Study phenotyping: P.M.R., D.I.C., L.M.R. Genotyping or analysis: D.I.C., L.M.R., F.Giulianini Study PI: P.M.R., D.I.C.
deCODE: Study phenotyping: G.B. Genotyping or analysis: G.T. Study PI: K.S., U.T.
GoDARTS: Study phenotyping: C.N.A.P., L.A.D., A.D.M., A.S.F.D. Genotyping or analysis: C.N.A.P., L.A.D., A.D.M., M.I.M., C.G., N.W.W.R.R. Study PI: C.N.A.P., A.D.M.
KORA F3/F4: Study phenotyping: A.D., H.Schunkert, J.E. Genotyping or analysis: A.-K.P., M.M.-N., N.K., T.I. Study PI: H.-E.W., A.Peters
GLACIER: Study phenotyping: F.R., G.H. Genotyping or analysis: P.W.F., D.Shungin, I.B., S.Edkins, F.R. Study PI: P.W.F.
B58C: Genotyping or analysis: S.Kanoni, K.E.S., Wellcome Trust Case Control Consortium, E.M., T.Ferreira, T.J. Study PI: P.D.
MORGAM: Study phenotyping: K.Kuulasmaa, F.Gianfagna, A.Wagner, J.Dallongeville Genotyping or analysis: M.F.H., F.Gianfagna Study PI: J.V., J.F., A.E.
SardiNIA: Study phenotyping: E.G.L. Genotyping or analysis: E.G.L., O.Meirelles, S.Sanna, R.N., A.Mulas, K.V.T.
NFBC1986: Study phenotyping: M.R.J., S.Sebert, K.H.H., A.L.H. Genotyping or analysis: M.Kaakinen, A.L.H. Study PI: M.R.J.
DESIR: Genotyping or analysis: N.B.-N., L.Y., S.L. Study PI: P.F., N.B.-N., B.B.
DILGOM: Study phenotyping: S.M. Genotyping or analysis: K.Kristiansson, M.P., A.S.H. Study PI: V.S.
IMPROVE: Study phenotyping: D.B. Genotyping or analysis: R.J.S., K.G. Study PI: A.Hamsten, E.Tremoli
HyperGEN: Study phenotyping: S.C.H., D.C.R. Genotyping or analysis: A.C., V.P., G.B.E. Study PI: S.C.H.
FENLAND (MetaboChip): Study phenotyping: R.J.F.L., J.a.L., N.J.W., K.K.O. Genotyping or analysis: R.J.F.L., J.a.L., N.J.W., K.K.O. Study PI: N.J.W.
Whitehall II: Study phenotyping: M.Kumari Genotyping or analysis: M.Kumari, S.Shah, C.L. Study PI: A.Hingorani, M.Kivimaki
LURIC: Genotyping or analysis: M.E.K., G.Delgado Study PI: W.M.
MESA: Study phenotyping: W.P. Genotyping or analysis: W.P., X.G., J.Y., V.D., K.D.T., J.I.R., Y.-D.C. Study PI: W.P.
HUNT2: Study phenotyping: K.Kvaløy, J.H., O.L.H. Genotyping or analysis: A.U.J. Study PI: K.H.
FINCAVAS: Genotyping or analysis: T.L., L.-P.L., K.N., M.Kähönen Study PI: T.L., M.Kähönen
GenNet: Study phenotyping: R.S.C., A.B.W. Genotyping or analysis: A.C., V.P., M.X.S., D.E.A., G.B.E. Study PI: A.C., R.S.C., A.B.W.
SCARFSHEEP: Study phenotyping: B.G. Genotyping or analysis: R.J.S. Study PI: A.Hamsten, U.d.F.
DPS: Study phenotyping: J.L. Genotyping or analysis: A.U.J., P.S.C. Study PI: J.T., M.U.
DR's EXTRA: Study phenotyping: P.K. Genotyping or analysis: A.U.J., M.H. Study PI: R.Rauramaa, T.A.L.
FIN-D2D 2007: Genotyping or analysis: A.U.J., L.L.B. Study PI: J.Saltevo, L.M.
METSIM: Study phenotyping: H.M.S. Genotyping or analysis: A.U.J., A.Stančáková Study PI: M.L., J.K.
MDC-CVA: Study phenotyping: O.Melander Genotyping or analysis: O.Melander, C.F. Study PI: O.Melander
BRIGHT: Study phenotyping: A.F.D., M.J.B., N.J.S., J.M.C. Genotyping or analysis: T.J., P.B.M. Study PI: M.J.C., A.F.D., M.J.B., N.J.S., J.M.C., P.B.M.
NESDA: Study phenotyping: J.H.S. Genotyping or analysis: H.Snieder, I.M.N. Study PI: B.W.P.
EPIC (MetaboChip): Study phenotyping: R.J.F.L., J.a.L., N.J.W. Genotyping or analysis: J.a.L., N.J.W. Study PI: N.J.W., K.-T.K.
ELY: Study phenotyping: C.L., J.a.L., N.J.W. Genotyping or analysis: C.L., J.a.L., N.J.W. Study PI: N.J.W.
DIAGEN: Study phenotyping: J.G., G.M. Genotyping or analysis: A.U.J., G.M. Study PI: P.E.S., S.R.B.
GOSH: Study phenotyping: P.K.M., N.L.P. Genotyping or analysis: E.I., P.K.M., N.L.P., T.Fall Study PI: E.I.
Tromsø: Study phenotyping: T.W. Genotyping or analysis: A.U.J., A.J.S., N. Study PI: I.N.
ADVANCE: Study phenotyping: T.L.A., C.I. Genotyping or analysis: T.L.A., E.L.S., T.Q. Study PI: T.L.A., T.Q., C.I.
ULSAM: Study phenotyping: E.I., J.Sundstrom Genotyping or analysis: E.I., N.E., J.Sundstrom, A.-C.S. Study PI: J.Sundstrom
PIVUS: Study phenotyping: L.Lind, J.Sundstrom Genotyping or analysis: L.Lind, N.E., J.Sundstrom, T.A. Study PI: L.Lind, J.Sundstrom
MRC NSHD: Study phenotyping: D.K. Genotyping or analysis: A.Wong, J.a.L., D.K., K.K.O. Study PI: D.K.
ASCOT: Study phenotyping: A.Stanton, N.P. Genotyping or analysis: T.J., M.J.C., P.B.M. Study PI: P.S., M.J.C.
THISEAS: Genotyping or analysis: L.S.R., S.Kanoni, E.M., G.Kolovou Study PI: G.Dedoussis, P.D.
PARC: Study phenotyping: R.M.K. Genotyping or analysis: K.D.T., E.Theusch, J.I.R., X.L., M.O.G., Y.D.I.C. Study PI: R.M.K.
AMC-PAS: Genotyping or analysis: G.K.H., P.D. Study PI: G.K.H.
CARDIOGENICS: Genotyping or analysis: S.Kanoni, A.H.G. Study PI: P.D., A.H.G., J.E., N.J.S., H.Schunkert
Secondary analyses
Allele-specific FAIRE: Design of secondary analysis: A.J.P.S. Computation of secondary analysis: A.J.P.S., F.D., P.H.
ASAP eQTL: Design of secondary analysis: A.F.C. Computation of secondary analysis: L.Folkersen, P.Eriksson
CARDIOGENICS eQTL: Computation of secondary analysis: L.Lataniotis
CM design: P.B.M., C.N.-C., T.J., B.F.V.
Comprehensive literature review: Design of secondary analysis: P.B.M. Computation of secondary analysis: K.W., P.B.M.
DEPICT: Design of secondary analysis: L.Franke, T.H.P., J.N.H. Computation of secondary analysis: T.H.P.
DHS and methylation analysis by tissue:Design of secondary analysis: C.J.W. Computation of secondary analysis: E.M.S.
DHS and methylation by cell-line: Design of secondary analysis: D.I.C. Computation of secondary analysis: D.I.C., F.Giulianini
FHS eSNP: Design of secondary analysis: R.Joehanes Computation of secondary analysis: R.Joehanes
ICBP SC: C.N.-C., M.J.C., P.B.M., A.C., K.M.R., P.-O'R., W.P., D.L., M.D.T., B.M.P., A.D.J., P.Elliott, C.M.v.D., D.I.C., A.V.S., M.Bochud, L.V.W., H.Snieder, G.B.E.
Kidney eQTL: Computation of secondary analysis: H.J.G., S.K.K.
MAGENTA: Design of secondary analysis: D.I.C. Computation of secondary analysis: D.I.C.
Miscellaneous: Computation of secondary analysis: H.Warren
MuTHER eQTL: Design of secondary analysis: P.D. Computation of secondary analysis: L.Lataniotis, T.-P.Y.
NESDA eQTL: Design of secondary analysis: R.Jansen Computation of secondary analysis: R.Jansen, A.V.
NTR eQTL: Design of secondary analysis: R.Jansen Computation of secondary analysis: R.Jansen, J.-J.H. Study PI: D.I.B.
eQTL, EGCUT:Design of secondary analysis: A.Metspalu Computation of secondary analysis: T.E., A.Metspalu
eQTL, Groningen:Design of secondary analysis: L.Franke Computation of secondary analysis: H.J.W., L.Franke
Public eSNP and methylation: Design of secondary analysis: A.D.J., J.D.E. Computation of secondary analysis: A.D.J., J.D.E.
PubMed search: Design of secondary analysis: G.B.E. Computation of secondary analysis: G.B.E., L.Lin
WGHS conditional: Design of secondary analysis: D.I.C. Computation of secondary analysis: D.I.C., F.Giulianini, L.M.R.
Lookup of Cardio-MetaboChip variants
HEXA: Genotyping or analysis: Y.J.K., Y.K.K., Y.-A.S. Study PI: J.-Y.L.
RACe: Study phenotyping: D.Saleheen, W.Zhao, A.R., A.R. Genotyping or analysis: W.Zhao, A.R., A.R. Study PI: D.Saleheen
HALST: Study phenotyping: C.A.H. Genotyping or analysis: J.I.R., Y.-D.C., C.A.H., R.-H.C., I.-S.C. Study PI: C.A.H.
CLHNS: Study phenotyping: N.R.L., L.S.A. Genotyping or analysis: Y.W., N.R.L., L.S.A. Study PI: K.L.M., L.S.A.
GxE/Spanish Town: Study phenotyping: B.O.T., C.A.M., R.W. Genotyping or analysis: C.D.P. Study PI: R.S.C., C.A.M., R.W., T.Forrester, J.N.H.
DRAGON: Study phenotyping: W.-J.L., W.H.-H.S., K.-W.L., I-Te Lee Genotyping or analysis: J.I.R., Y.-D.C., E.K., D.A., K.D.T., X.G. Study PI: W.H.-H.S.
SEY: Study phenotyping: P.B. Genotyping or analysis: M.Bochud, G.B.E., F.M. Study PI: P.B., M.Bochud, M.Burnier, F.P.
TUDR: Study phenotyping: W.H.-H.S., I-Te Lee, W.-J.L. Genotyping or analysis: J.I.R., Y.-D.C., E.K., K.D.T., X.G. Study PI: W.H.-H.S.
TANDEM: Study phenotyping: P.B., M.Bochud Genotyping or analysis: G.B.E., F.M. Study PI: P.B., M.Bochud, M.Burnier, F.P.
Imputed genotypes
FHS: Study phenotyping: D.L. Genotyping or analysis: D.L. Study PI: D.L.
ARIC: Study phenotyping: E.B. Genotyping or analysis: G.B.E., E.B., A.C.M., A.C., S.K.G. Study PI: E.B., A.C.
RS: Genotyping or analysis: G.C.V., A.G.U. Study PI: A.Hofman, A.G.U., O.H.F.D.
CoLaus: Study phenotyping: P.V. Genotyping or analysis: Z.K. Study PI: P.V.
NFBC1966: Study phenotyping: M.R.J. Genotyping or analysis: P.O.R. Study PI: M.R.J.
SHIP: Study phenotyping: R.Rettig Genotyping or analysis: A.T.
CHS: Study phenotyping: B.M.P. Genotyping or analysis: K.M.R. Study PI: B.M.P.
EPIC (GWAS): Study phenotyping: N.J.W., R.J.F.L., J.a.L. Genotyping or analysis: N.J.W., J.H.Z., J.a.L. Study PI: N.J.W., K.-T.K.
SU.VI.MAX: Study phenotyping: S.H. Genotyping or analysis: S.H., P.M. Study PI: P.M.
Amish: Genotyping or analysis: M.E.M. Study PI: A.Parsa
FENLAND (GWAS): Study phenotyping: N.J.W., J.a.L., R.J.F.L., K.K.O. Genotyping or analysis: N.J.W., J.a.L., R.J.F.L., K.K.O. Study PI: N.J.W.
DGI: Study phenotyping: C.N.C. Genotyping or analysis: C.N.C., G.Kosova Study PI: C.N.C.
ERF (EUROSPAN): Genotyping or analysis: N.A. Study PI: C.M.v.D.
MIGEN: Study phenotyping: S.Kathiresan, R.E. Genotyping or analysis: S.Kathiresan, R.E. Design of secondary analysis: S.Kathiresan, R.E.
MICROS: Study phenotyping: P.P.P. Genotyping or analysis: A.A.H. Study PI: A.A.H., P.P.P.
FUSION: Genotyping or analysis: A.U.J. Study PI: M.Boehnke, F.S.C., K.L.M., J.Saramies
TwinsUK: Genotyping or analysis: C.M. Study PI: T.D.S.
PROCARDIS: Genotyping or analysis: M.Farrall, A.G. Study PI: M.Farrall
BLSA: Study phenotyping: L.Ferrucci Genotyping or analysis: T.T. Study PI: L.Ferrucci
ORCADES: Study phenotyping: J.F.W. Study PI: J.F.W.
Croatia-Vis: Genotyping or analysis: V.V., C.H. Study PI: V.V., C.H.
NSPHS: Genotyping or analysis: S.Enroth Study PI: U.G.
InCHIANTI: Genotyping or analysis: T.T. Study PI: S.Bandinelli
AGES Reykjavik: Study phenotyping: V.G. Genotyping or analysis: A.V.S. Study PI: V.G.
Lookup
CARDIoGRAMplusC4D: Genotyping or analysis: P.D. Study PI: J.Danesh, H.Schunkert, T.L.A., J.E., S.Kathiresan, R.Roberts, N.J.S., P.D.
CHARGE cIMT: Genotyping or analysis: C.O'D., J.C.B.
CHARGE EYE: Genotyping or analysis: T.Y.W., X.S., R.A.J. Study PI: T.Y.W.
CHARGE-HF consortium: Study phenotyping: R.S.V., J.F.F. Genotyping or analysis: H.L., J.F.F. Study PI: R.S.V.
CKDGen: Genotyping or analysis: M.G., V.M.
COGENT: Study phenotyping: N.F., J.R. Genotyping or analysis: N.F., X.Z., B.J.K., B.O.T., J.R.
EchoGen consortium: Study phenotyping: R.S.V., J.F.F. Genotyping or analysis: H.L., J.F.F. Study PI: R.S.V.
KidneyGen Consortium: Study phenotyping: J.C.C., J.S.K., P.Elliott Genotyping or analysis: W.Zhang, J.C.C., J.S.K. Study PI: J.C.C., J.S.K.
MetaStroke: Genotyping or analysis: S.Bevan, H.S.M.
NeuroCHARGE: Genotyping or analysis: M.Fornage, M.A.I. Study PI: M.A.I.
PROMIS: Study phenotyping: D.Saleheen, W.Zhao, J.Danesh Genotyping or analysis: W.Zhao Study PI: D.Saleheen
SEED: Study phenotyping: T.Y.W., C.-Y.C. Genotyping or analysis: E.-S.T, C.-Y.C., C.-Y.C. Study PI: C.-Y.C., T.Y.W.
UK Biobank: BP group leaders: Mark Caulfield, P.Elliott Genotyping or analysis: M.R.B., H.Warren, Claudia Cabrera, Evangelos Evangelou, He Gao.
Footnotes
SUPPLEMENTARY NOTE
Supplementary Note is available in the online version of the paper.
URLs
http://hgdownload.cse.ucsc.edu/goldenPath/hg19/encodeDCC/wgEncodeUwDnase for enrichment analyses. Accessed 3/13/2013.
http://www.genome.gov/gwastudies for enrichment analyses. Accessed 3/13/2013.
http://genome.ucsc.edu/ENCODE/cellTypes.html for enrichment analyses. Accessed 3/13/2013.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests (see corresponding section in the Supplementary Note).
REFERENCES
- 1.Johnson T, et al. Blood Pressure Loci Identified with a Gene-Centric Array. The American Journal of Human Genetics. 2011;89:1–13. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton-Cheh C, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nature genetics. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschini N, et al. Genome-wide association analysis of blood-pressure traits in African- ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet. 2013;93:545–54. doi: 10.1016/j.ajhg.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesh SK, et al. Effects of long-term averaging of quantitative blood pressure traits on the detection of genetic associations. Am J Hum Genet. 2014;95:49–65. doi: 10.1016/j.ajhg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehret GB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wain LV, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–11. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton-Cheh C, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–76. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simino J, et al. Gene-age interactions in blood pressure regulation: a large-scale investigation with the CHARGE, Global BPgen, and ICBP Consortia. Am J Hum Genet. 2014;95:24–38. doi: 10.1016/j.ajhg.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tragante V, et al. Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am J Hum Genet. 2014;94:349–60. doi: 10.1016/j.ajhg.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. From the Cover: Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci U S A. 2009;106:226–31. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato N, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nature genetics. 2011;43:531–8. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padmanabhan S, et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS genetics. 2010;6:e1001177. doi: 10.1371/journal.pgen.1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miall WE, Oldham PD. The hereditary factor in arterial blood-pressure. Br Med J. 1963;1:75–80. doi: 10.1136/bmj.1.5323.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy D, et al. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007;8(Suppl 1):S3. doi: 10.1186/1471-2350-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voight BF, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 18.Locke AE, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shungin D, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–96. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–75. S1–3. doi: 10.1038/ng.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimber CH, et al. TCF7L2 in the Go-DARTS study: evidence for a gene dose effect on both diabetes susceptibility and control of glucose levels. Diabetologia. 2007;50:1186–91. doi: 10.1007/s00125-007-0661-9. [DOI] [PubMed] [Google Scholar]
- 25.Erdmann J, et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504:432–6. doi: 10.1038/nature12722. [DOI] [PubMed] [Google Scholar]
- 26.Hirata Y, et al. Mechanisms of adrenomedullin-induced vasodilation in the rat kidney. Hypertension. 1995;25:790–5. doi: 10.1161/01.hyp.25.4.790. [DOI] [PubMed] [Google Scholar]
- 27.Epigenomics Roadmap et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–30. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurano MT, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welter D, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–6. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trynka G, et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat Genet. 2013;45:124–30. doi: 10.1038/ng.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernst J, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–9. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segre AV, et al. Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pers TH, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17:877–85. doi: 10.1101/gr.5533506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stergachis AB, et al. Conservation of trans-acting circuitry during mammalian regulatory evolution. Nature. 2014;515:365–70. doi: 10.1038/nature13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancia G, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 38.Lifton R, Somlo S, Giebisch G, Seldin D. Genetic Diseases of the Kidney. Academic Press; 2009. [Google Scholar]
- 39.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–6. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 40.Kim CH, et al. Mutations in the dopamine beta-hydroxylase gene are associated with human norepinephrine deficiency. American journal of medical genetics. 2002;108:140–7. [PubMed] [Google Scholar]
- 41.Deinum J, et al. DBH gene variants that cause low plasma dopamine beta hydroxylase with or without a severe orthostatic syndrome. Journal of medical genetics. 2004;41:e38. doi: 10.1136/jmg.2003.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghofrani HA, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–40. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–75. S1–3. doi: 10.1038/ng.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penninx BW, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–40. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boomsma DI, et al. Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet. 2006;9:849–57. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- 48.Visscher PM, Benyamin B, White I. The use of linear mixed models to estimate variance components from data on twin pairs by maximum likelihood. Twin Res. 2004;7:670–4. doi: 10.1375/1369052042663742. [DOI] [PubMed] [Google Scholar]
- 49.Romanoski CE, et al. Network for activation of human endothelial cells by oxidized phospholipids: a critical role of heme oxygenase 1. Circ Res. 2011;109:e27–41. doi: 10.1161/CIRCRESAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koopmann TT, et al. Genome-wide identification of expression quantitative trait loci (eQTLs) in human heart. PLoS One. 2014;9:e97380. doi: 10.1371/journal.pone.0097380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fairfax BP, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343:1246949. doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramasamy A, et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17:1418–28. doi: 10.1038/nn.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maurano MT, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welter D, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–6. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trynka G, et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat Genet. 2013;45:124–30. doi: 10.1038/ng.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segre AV, et al. Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pers TH, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magi R, Morris AP. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics. 2010;11:288. doi: 10.1186/1471-2105-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson T. Efficient Calculation for Multi-SNP Genetic Risk Scores. ASHG 2012 Annual Meeting. poster presentation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.