Abstract
Cerebellar alterations are a hallmark of Fetal Alcohol Spectrum Disorders and are thought to be responsible for deficits in fine motor control, motor learning, balance, and higher cognitive functions. These deficits are, in part, a consequence of dysfunction of cerebellar circuits. Although the effect of developmental ethanol exposure on Purkinje and granule cells has been previously characterized, its actions on other cerebellar neuronal populations are not fully understood. Here, we assessed the impact of repeated ethanol exposure on the number of inhibitory neurons in the cerebellar vermis. We exposed pregnant mice to ethanol in vapor inhalation chambers during gestational days 12–19 and offspring during postnatal days 2–9. We used transgenic mice expressing the fluorescent protein, Venus, in GABAergic/glycinergic neurons. Using unbiased stereology techniques, we detected a reduction in Venus positive neurons in the molecular and granule cell layers of lobule II in the ethanol exposed group at postnatal day 16. In contrast, ethanol produced a more widespread reduction in Purkinje cell numbers that involved lobules II, IV–V and IX. We also found a reduction in the volume of lobules II, IV–V, VI–VII, IX and X in ethanol-exposed pups. These findings indicate that second and third trimester ethanol exposure has a greater impact on Purkinje cells than interneurons in the developing cerebellar vermis. The decrease in the volume of most lobules could be a consequence of a reduction in cell numbers, dendritic arborizations, or axonal projections.
Keywords: Cerebellum, vermis, GABA, glycine, interneuron, ethanol, development
Introduction
It is well-established that exposure to ethanol during fetal development causes birth defects, with the developing brain being particularly sensitive to the teratogenic effects of ethanol [38]. Among the brain regions affected by developmental ethanol exposure is the cerebellum, which is involved, not only in motor coordination, but also in higher cognitive processes [22, 37]. Frequent problems in individuals with Fetal Alcohol Spectrum Disorder (FASD) include deficits in fine motor control, motor learning and balance, which have been linked to cerebellar damage [5, 14]. Moreover, studies suggest that alterations in timing, sensorimotor processing, and mathematical ability are also a consequence of cerebellar circuit dysfunction [10, 26]. Neuroimaging studies have demonstrated structural and functional alterations in cerebella of children with FASD [3, 9]. Although the precise mechanisms responsible for these effects are presently unknown, studies with rodent models suggest that ethanol disrupts neuronal migration and survival both in the developing cerebellar cortex and deep cerebellar nuclei [13, 15, 28, 30, 31]. Longer-term deficits in neuronal function, synaptic transmission, and plasticity have also been observed in Purkinje and granule cells of rodents exposed to ethanol during development [8, 19, 20, 29, 40, 43]. However, little is known regarding the impact of developmental ethanol exposure on other cerebellar neuronal populations.
The molecular layer of the cerebellar cortex contains two populations of specialized GABAergic interneurons that control the function of Purkinje cells [22, 42]. Stellate cells, located in the outer portion of the molecular layer, make synaptic connections with Purkinje cell dendrites. Basket cells are located in the inner portion of the molecular layer and provide inhibitory input to the soma and axonal initial segment. The granule cell layer also contains inhibitory interneurons: the Golgi cells, which release GABA onto granule cells, other Golgi cells and unipolar brush cells (they also release glycine onto the latter) and Lugaro cells which release GABA and glycine onto Golgi cells and molecular layer interneurons. Other less abundant subtypes of granule cell interneurons are globular and candelabrum cells [2, 18]. During the equivalent to the 2nd trimester of human pregnancy, cerebellar interneurons are born and gradually begin to migrate to their final destinations [27, 39]. This process continues during the first 2 weeks of postnatal development in rodents, which is, in part, equivalent to the 3rd trimester of human pregnancy. Specifically, between postnatal day (P) 4 and P7, undifferentiated molecular layer interneurons migrate toward the developing molecular layer and by P12 they begin to form synaptic connections with Purkinje cells [43]. This process is generally completed by P21. Golgi cells are present at birth and start to make synaptic connections with granule cells by P7 [43].In sum, cerebellar interneurons undergo profound developmental changes during the equivalent to 2nd and 3rd trimesters of human pregnancy, suggesting that they can be susceptible to damage during these critical developmental periods.
The purpose of this study was to compare the impact of repeated ethanol exposure on the number of inhibitory neurons in the cortical region of the cerebellar vermis. We hypothesized that ethanol exposure during the mouse equivalent to the 2nd and 3rd trimesters results in a similar decrease in the number of molecular layer interneurons, granule cell layer interneurons, and Purkinje cells. We used transgenic mice expressing a fluorescent protein in these neurons and unbiased stereology techniques to test this hypothesis.
Methods
All animal procedures were approved by the University of New Mexico Health Sciences Center Institutional Care and Use Committee. For the studies described here, we used the vesicular GABA transporter (VGAT)-Venus mouse line expressing Venus in GABAergic and glycinergic neurons throughout the brain, including the cerebellum where virtually all inhibitory neurons expressed this fluorescent protein [44]. The Venus fluorescent protein (a variant of enhanced yellow fluorescent protein) was developed by Dr. Atsushi Miyawaki at RIKEN (Wako, Japan). Animals were housed in standard cages at a room temperature of 22 C. Lights were on at 6 am and off at 6 pm. We mated wild-type C57BL6 females with VGAT-Venus heterozygous males for 6 days, after which males were removed from the cage. The first day of mating was considered as the first day of gestation. At gestational day 12, females were transferred to custom built vapor inhalation chambers [34]. Pregnant dams were exposed for 4 hr/day (10 am to 2 pm) to ethanol between gestational days 12 and 19. Food was changed daily at the end of the 4 hr ethanol exposure. After pups were delivered on gestational days 20–21, the VGAT-Venus pups were identified during the first 2 postnatal days via transcranial illumination with fluorescence goggles containing a 480/40 nm excitation filter, and a long pass 520 nm emission filter (BLS ltd. Budapest, Hungary). Pups that did not express Venus were euthanized; the number of pups/litter was 9.2 ± 0.8 (n=5) and 8.5 ± 1.0, (n=4) for the air and ethanol groups, respectively (t(7)=0.54, p=0.6); the number of Venus positive pups was 4.8 ± 0.74 (n=6) and 4.5 ± 0.6, (n=6) for the air and ethanol groups, respectively (t(10)=0.33, p=0.7). Pup weight at the time of birth was not significantly different between the air (1.55 ± 0.2 g; n=7) and ethanol (1.39 ± 0.07 g; n=5) groups (t(10)=0.61, p=0.55). Ethanol vapor exposure for 4 hr/day was then resumed between postnatal days (P) 2–9. Pups remained with their mothers during exposure. Ethanol vapor chamber levels were gradually increased to allow the animals to adapt to the exposure paradigm and minimize toxicity. Ethanol chamber levels were 3–5 g/dl at gestational days 12–14, 6–7 g/dl at gestational days 15–17, 7–8 g/dl at gestational days 18–19, 3–4 g/dl at P2–3, 5–6 g/dl at P4–5 and 7–8 g/dl at P6–9. Blood alcohol levels were measured using an assay based on the activity of alcohol dehydrogenase [11]. In the dams, these were determined to be 0.1 ± 0.02 g/dl in tail blood obtained at gestational days 12–18 and 0.16 ± 0.06 g/dl in trunk blood obtained 7–8 days after giving birth (n=3 dams). The ethanol concentrations in trunk blood from P7–8 pups was 0.33 ± 0.02 g/dl (n=13 pups from 3 litters). For reference, the legal intoxication limit in the U.S. is 0.08 g/dl. At P9, average pup weights were 7.18 ± 0.2 (n=7) and 5.12 ± 0.23 (n=5) for the air and ethanol groups, respectively (t(10)=6.68, p<0.0001). At P16, average pup weights were 9.8 ± 0.3 (n=7) and 7.4 ± 0.35 (n=5) for the air and ethanol groups, respectively (t(10)=4.5, p<0.001).
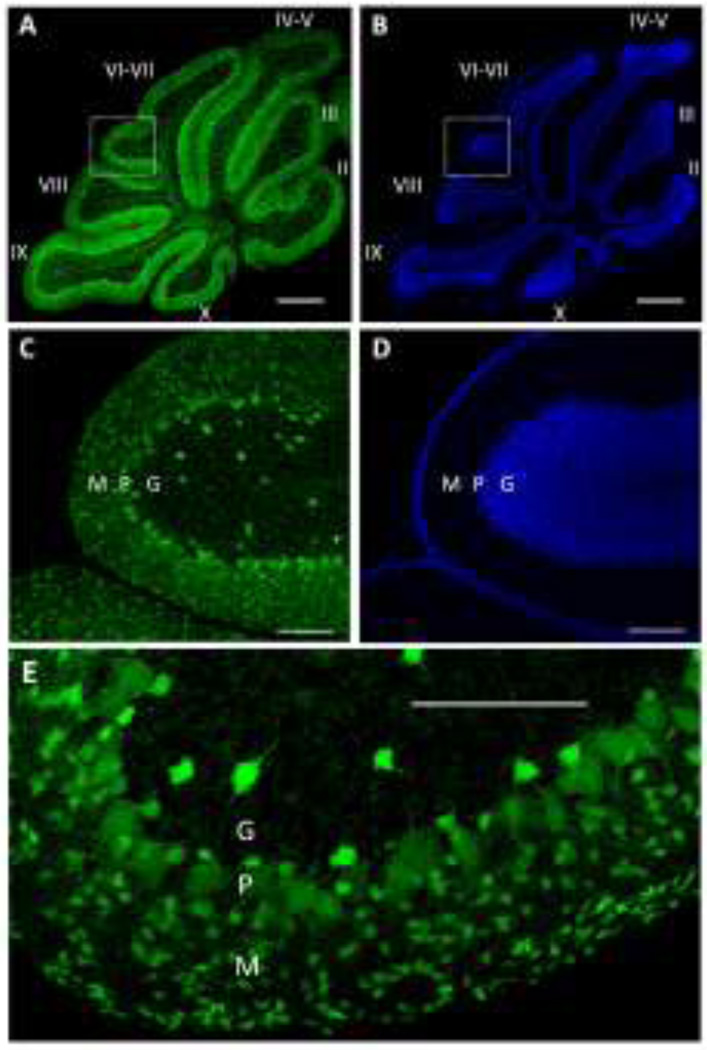
At P16, male pups were transcardially perfused with 4% paraformaldehyde under deep anesthesia with ketamine (500–700 mg/kg). Cerebella were cut from the forebrain and kept in 4% paraformaldehyde for 48 hours and then stored in phosphate buffered saline (PBS), all at 4 C. Each cerebellar vermis was blocked and sectioned at 50 µm thickness in the parasagittal plane using a vibrating tissue slicer (Vibratome 1000, The Vibratrome Company, St. Louis, MO). We chose to focus on the cerebellar vermis because studies indicate that this area is an important target of developmental ethanol exposure [7]. Fifteen consecutive sections of the vermis (see Fig 1 for representative images) were mounted sequentially on Superfrost plus slides (VWR Micro Slides, Radnor, PA), incubated for 20 min with 4',6-diamidino-2-phenylindole (DAPI; 1:4000 in PBS), washed with PBS 5 times, with each wash lasting 5 minutes, coverslipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA), and sealed with clear nail polish. The cell body numbers of molecular and granule cell layer interneurons, as well as Purkinje cells (Fig 1) was estimated using the optical fractionator method with the aid of an Olympus DSU spinning disk confocal microscope (Olympus, Melville, NY) and Stereo Investigator software (Version 9, MBF Bioscience, Williston, VT). Venus positive cells located in the outer and inner halves of the Purkinje cell layer were considered to be molecular and granule cell layer interneurons, respectively. The contours of each lobule (Fig 1A–B) were manually traced under 4× magnification. Starting with the third section from each vermis, Venus positive somata were counted in every 5th section using a 40× objective (i.e., a total of 3 sections per vermis were counted). If any of the sections were torn, the closest intact sections were counted. The guard zone was set at 5 µm and the dissector height was set at 15 µm. The section thickness was measured while counting at every sampling site. The counting frame size was set at 50×50 µm and 15% of the area of each lobule was sampled. The volume of each of the cerebellar vermis lobules was estimated according to the Cavalieri principle [32]. Every 5th section, starting with the 3rd section (total of 3 sections per vermis), was used to estimate the volume. The Gundersen coefficient of error was used for all volume and count estimates [16]. The images shown in Fig 1 were obtained with a Leica TCS-SP8 Confocal Microscope & Fluorescence Lifetime Imaging System (Leica Microsystems, Buffalo Grove, IL) equipped with 405 nm and white light (470–670 nm) diode lasers. Background was subtracted from the images using NIH ImageJ/Fiji software. Statistical analyses were performed with Prizm 6 (GraphPad, San Diego, CA). Data were analyzed using two-way ANOVA followed by Fisher’s Least Significant Difference (LSD) post hoc test; a p< 0.05 was considered to be statistically significant. For all statistical analyses, the experimental unit was an animal. A total of 4 mice per treatment group, each from a different litter, were used for these studies. The results are expressed as the mean ± SEM.
Figure 1. Sample images from the cerebellar vermis of a postnatal day 16 VGAT-Venus mouse.
A) Tile scan confocal image of Venus fluorescence illustrating the distribution of GABAergic/glycinergic neurons in the indicated lobules (labeled with roman numerals). Scale bar = 500 µm. B) Same as in A but for 4',6-diamidino-2-phenylindole (DAPI) nuclear stain. C) Enlarged image of the region outlined by the square in panel A, showing the distribution of Venus positive neurons in the molecular (M), Purkinje (P), and granule (G) cell layers. Scale bar = 100 µm. D) Same as in A but for DAPI nuclear stain. E) Higher magnification image showing, in more detail, the morphological characteristics of Venus positive neurons in the molecular (M), Purkinje (P), and granule (G) cell layers. Scale bar = 100 µm.
Results
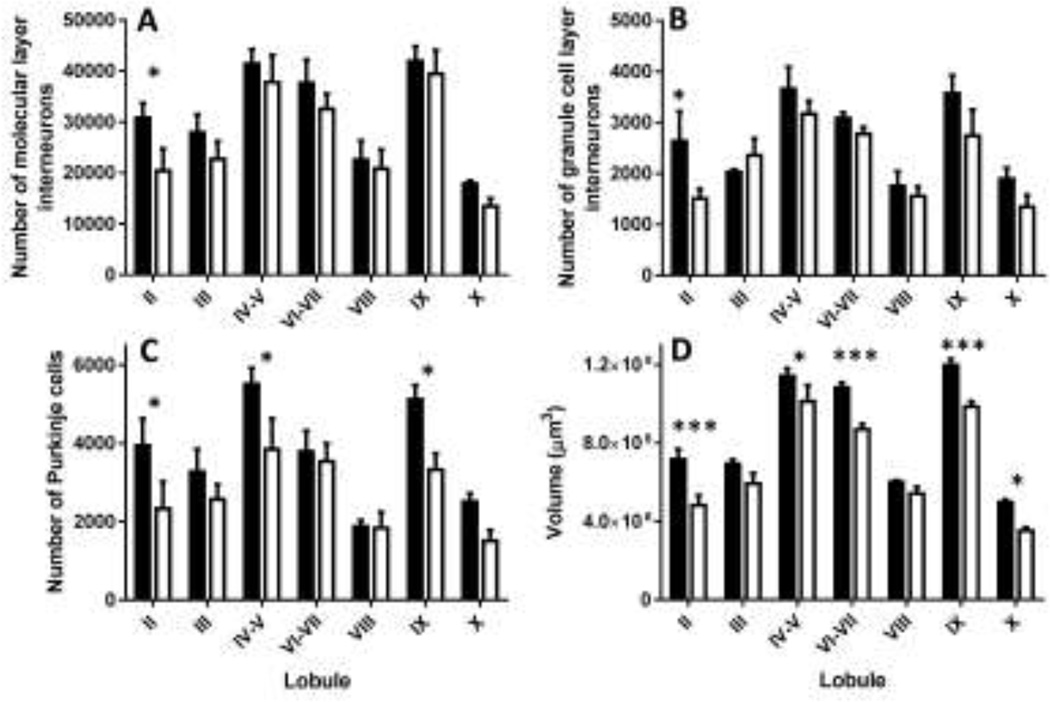
We found that developmental ethanol exposure significantly decreases the number of molecular layer interneurons in lobule II (Fig 2A; two-way ANOVA: interaction F(6, 42)=0.31, P=0.92; ethanol exposure F(1, 42)=6.123, P=0.017; lobule (F(6, 42)=14.07, P<0.0001; Fisher’s LSD post-hoc test for ethanol exposure, t(42)= 2.06; P=0.045 in lobule II). Interestingly, ethanol exposure also significantly reduced the number of granule cell layer interneurons in lobule II (Fig 2B; two-way ANOVA: interaction F(6, 42)=1.093; P=0.38; ethanol exposure F(1, 42)=7.249, P=0.01; lobule F(6, 42)=10.69, P<0.0001; LSD post-hoc test for ethanol exposure, t(42)= 2.52, P=0.015 in lobule II).In contrast, ethanol exposure had a more widespread effect on the number of Purkinje cells, which was significantly decreased in lobules II, IV–V, and IX (Fig 2C; two-way ANOVA: interaction F(6, 42)=1.047, P=0.4; ethanol exposure F(1, 42)=15.01, P=0.0004; lobule F(6, 42)=9.396, P<0.0001; LSD post-hoc test for ethanol exposure, t(42)= 2.33, P=0.02 in lobule II; t(42)= 2.40; P=0.02 in lobules IV–V, and t(42)= 2.59; P=0.01 in lobule IX). Moreover, ethanol exposure significantly decreased the volume of lobules II, IV–V, VI–VII, IX and X (Fig 2D; two-way ANOVA: interaction F(6, 42)=1.298, P=0.27; ethanol exposure, F(1, 42)=52.73, P<0.0001; lobule (F(6, 42)=91.83, P<0.0001; LSD post-hoc test for ethanol exposure, t(42)= 4.08, P=0.0002 in lobule II; t(42)= 2.29; P=0.027 in lobules IV–V; t(42)= 3.74; P=0.0005 in lobules VI–VII; t(42)= 3.71; P=0.0006 in lobule IX; and t(42)= 2.55; P=0.014 in lobule X.
Figure 2. Effect of developmental ethanol exposure on the number of GABAergic/Glycinergic neurons and lobule volumes in the cerebellar vermis of VGAT-Venus mice.
Shown in the figure are the number of A) molecular layer interneurons, B) granule cell layer interneurons, and C) Purkinje cells in the indicated lobules of air (black bars) and ethanol (white bars) exposed mice at postnatal day 16. D) Same as above but for lobule volume. * p<0.05; ***p<0.001 (n=4 mice per treatment group, each from a different litter). For more details of statistical analyses, see text.
Discussion
We report here that exposure to ethanol during the equivalent to the 2nd and 3rd trimesters of human pregnancy significantly reduces the number of GABAergic/glycinergic interneurons in lobule II of the cerebellar vermis of mice. The number of both molecular layer interneurons (i.e., stellate and/or basket cells) and granule cell layer interneurons (i.e., Golgi, Lugaro, globular and/or candelabrum cells) was decreased. It is important to investigate in the future if ethanol exposure preferentially affects any of these subpopulations of cerebellar interneurons and their specialized functions [12, 22, 41]. The mechanisms responsible for the preferential ethanol sensitivity of developing interneurons in lobule II should be explored, including whether interneuronal development follows a differential time course in this lobule, as vulnerability of cerebellar neurons to ethanol is inversely correlated with expression of genes that control maturation [24]. Lobule II is part of the anterior cerebellar lobe and receives sensory input from the spinal cord [23]. It would be interesting to determine if a reduction in interneuronal numbers in this lobule alters processing of peripheral sensory information or if surviving interneurons are able to compensate, maintaining normal inhibitory tone to target neurons. It is important to perform additional studies with a larger sample size to determine whether the number of inhibitory interneurons is also reduced in other lobules, as there were trends towards a reduction in some of these (i.e., III and X; Figs 2A–B).
In contrast to its relatively limited impact on interneurons, our ethanol exposure paradigm caused a more widespread reduction in Purkinje cell numbers. Studies with rats have demonstrated that ethanol exposure during the first week of life causes Purkinje cell loss, whose magnitude varied in a lobule-dependent manner but could be detected in most cerebellar lobules [4, 6, 17, 21, 36]. Results of some of these studies suggest that lobules VI–VIII are among the least affected and this may be due to the fact that these mature later than other lobules [1, 4, 6, 17, 36]. In general agreement with these findings, we observed a significant decrease in Purkinje cell numbers in lobules II, IV–V, and IX. However, we did not observe a significant decrease in lobules that were shown to be affected in the above-mentioned studies (e.g., lobules III and X). Our findings suggest that the effect of developmental ethanol exposure on Purkinje cells depends on the experimental conditions, including the choice of exposure paradigm and animal model, including the possibility that transgenic expression of the Venus protein could affect sensitivity to ethanol exposure.
We also detected a significant reduction in lobule volume that involved additional lobules than those exhibiting a reduction in Purkinje cell numbers (i.e., all but III and VIII showed a reduction in volume). In lobules where Purkinje cell and/or interneuron numbers were reduced, loss of cell bodies, dendrites and/or axons (including climbing and parallel fiber inputs) is likely to be, in part, responsible for the decrease in volume [25, 33, 35]. However, it should be noted that our exposure paradigm produced a reduction in body weight, consistent with an ethanol-induced developmental delay that could explain the reduction in the volume and cell numbers in some cerebellar lobules.
In conclusion, we found that ethanol exposure during the 2nd and 3rd trimester-equivalent periods of development has limited effects on the number of GABAergic/glycinergic interneurons in the cerebellar vermis relative to its effects on Purkinje cell numbers and lobule volume. Future studies are needed to determine if these effects are also present in more lateral portions of the cerebellum and if these persist later in life. Importantly, the impact of these structural alterations on the function of cerebellar circuits should be characterized.
Highlights.
Used transgenic mice expressing fluorescent inhibitory neurons
Exposed to ethanol during 2nd and 3rd trimester equivalents
Inhibitory interneurons were reduced in lobule II at postnatal day 16
Purkinje cells were reduced in lobules II, IV–V and IX
Volume of lobules II, IV–V, VI–VII, IX and X was also decreased
Acknowledgments
This work was funded by NIH grant R01-AA014973, P50-AA022534, and internal funds from the Department of Obstetrics and Gynecology. The Fluorescence Microscopy Shared Resource of the UNM Cancer Center is funded as detailed on: http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml. We thank Brian Baculis, Jason Welch, and Jill Chavez for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Study conception and experimental design were contributed by C.F.V and P.N. Experiments were performed by P.N and D.H.T. Data analyses were carried out by P.N., D.H.T. and C.F.V. Venus-VGAT mice were generated by Y.Y. Writing and approval of manuscript was contributed by all authors.
References
- 1.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. The Journal of comparative neurology. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosi G, Flace P, Lorusso L, Girolamo F, Rizzi A, Bosco L, Errede M, Virgintino D, Roncali L, Benagiano V. Non-traditional large neurons in the granular layer of the cerebellar cortex. Eur J Histochem. 2007;51(Suppl 1):59–64. [PubMed] [Google Scholar]
- 3.Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: a morphological study. Brain research. 1977;119:249–268. doi: 10.1016/0006-8993(77)90310-9. [DOI] [PubMed] [Google Scholar]
- 5.Bearer CF, Wellmann KA, Tang N, He M, Mooney SM. Choline Ameliorates Deficits in Balance Caused by Acute Neonatal Ethanol Exposure. Cerebellum. 2015;14:413–420. doi: 10.1007/s12311-015-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcoholism, clinical and experimental research. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng DT, Jacobson SW, Jacobson JL, Molteno CD, Stanton ME, Desmond JE. Eyeblink Classical Conditioning in Alcoholism and Fetal Alcohol Spectrum Disorders. Front Psychiatry. 2015;6:155. doi: 10.3389/fpsyt.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz MR, Vollmer CC, Zamudio-Bulcock PA, Vollmer W, Blomquist SL, Morton RA, Everett JC, Zurek AA, Yu J, Orser BA, Valenzuela CF. Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABA(A) receptor delta subunit in cerebellar granule neurons and delays motor development in rats. Neuropharmacology. 2014;79:262–274. doi: 10.1016/j.neuropharm.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.du Plessis L, Jacobson JL, Jacobson SW, Hess AT, van der Kouwe A, Avison MJ, Molteno CD, Stanton ME, Stanley JA, Peterson BS, Meintjes EM. An in vivo 1H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research. 2014;38:1330–1338. doi: 10.1111/acer.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Plessis L, Jacobson SW, Molteno CD, Robertson FC, Peterson BS, Jacobson JL, Meintjes EM. Neural correlates of cerebellar-mediated timing during finger tapping in children with fetal alcohol spectrum disorders. Neuroimage Clin. 2015;7:562–570. doi: 10.1016/j.nicl.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galindo R, Valenzuela CF. Immature hippocampal neuronal networks do not develop tolerance to the excitatory actions of ethanol. Alcohol. 2006;40:111–118. doi: 10.1016/j.alcohol.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geurts FJ, De Schutter E, Dieudonne S. Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum. 2003;2:290–299. doi: 10.1080/14734220310011948. [DOI] [PubMed] [Google Scholar]
- 13.Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- 14.Green CR, Lebel C, Rasmussen C, Beaulieu C, Reynolds JN. Diffusion tensor imaging correlates of saccadic reaction time in children with fetal alcohol spectrum disorder. Alcoholism, clinical and experimental research. 2013;37:1499–1507. doi: 10.1111/acer.12132. [DOI] [PubMed] [Google Scholar]
- 15.Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3:178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcoholism, clinical and experimental research. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirono M, Saitow F, Kudo M, Suzuki H, Yanagawa Y, Yamada M, Nagao S, Konishi S, Obata K. Cerebellar globular cells receive monoaminergic excitation and monosynaptic inhibition from Purkinje cells. PloS one. 2012;7:e29663. doi: 10.1371/journal.pone.0029663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao SH, Parrish AR, Nahm SS, Abbott LC, McCool BA, Frye GD. Effects of early postnatal ethanol intubation on GABAergic synaptic proteins. Brain Res Dev Brain Res. 2002;138:177–185. doi: 10.1016/s0165-3806(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 20.Idrus NM, McGough NN, Riley EP, Thomas JD. Administration of memantine during ethanol withdrawal in neonatal rats: effects on long-term ethanol-induced motor incoordination and cerebellar Purkinje cell loss. Alcoholism, clinical and experimental research. 2011;35:355–364. doi: 10.1111/j.1530-0277.2010.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idrus NM, Napper RM. Acute and long-term Purkinje cell loss following a single ethanol binge during the early third trimester equivalent in the rat. Alcoholism, clinical and experimental research. 2012;36:1365–1373. doi: 10.1111/j.1530-0277.2012.01743.x. [DOI] [PubMed] [Google Scholar]
- 22.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Ji Z, Hawkes R. Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience. 1994;61:935–954. doi: 10.1016/0306-4522(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 24.Karacay B, Li S, Bonthius DJ. Maturation-dependent alcohol resistance in the developing mouse: cerebellar neuronal loss and gene expression during alcohol-vulnerable and -resistant periods. Alcoholism, clinical and experimental research. 2008;32:1439–1450. doi: 10.1111/j.1530-0277.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 25.Klintsova AY, Matthews JT, Goodlett CR, Napper RM, Greenough WT. Therapeutic motor training increases parallel fiber synapse number per Purkinje neuron in cerebellar cortex of rats given postnatal binge alcohol exposure: preliminary report. Alcoholism, clinical and experimental research. 1997;21:1257–1263. [PubMed] [Google Scholar]
- 26.Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcoholism, clinical and experimental research. 2010;34:354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- 27.Leto K, Rossi F. Specification and differentiation of cerebellar GABAergic neurons. Cerebellum. 2012;11:434–435. doi: 10.1007/s12311-011-0324-8. [DOI] [PubMed] [Google Scholar]
- 28.Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002;114:327–337. doi: 10.1016/s0306-4522(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 29.Light KE, Hayar AM, Pierce DR. Electrophysiological and Immunohistochemical Evidence for an Increase in GABAergic Inputs and HCN Channels in Purkinje Cells that Survive Developmental Ethanol Exposure. Cerebellum. 2015;14:398–412. doi: 10.1007/s12311-015-0651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J. Mechanisms of ethanol-induced death of cerebellar granule cells. Cerebellum. 2012;11:145–154. doi: 10.1007/s12311-010-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcussen BL, Goodlett CR, Mahoney JC, West JR. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol. 1994;11:147–156. doi: 10.1016/0741-8329(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 32.Miki T, Satriotomo I, Li HP, Matsumoto Y, Gu H, Yokoyama T, Lee KY, Bedi KS, Takeuchi Y. Application of the physical disector to the central nervous system: estimation of the total number of neurons in subdivisions of the rat hippocampus. Anat Sci Int. 2005;80:153–162. doi: 10.1111/j.1447-073x.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed SA, Nathaniel EJ, Nathaniel DR, Snell L. Altered Purkinje cell maturation in rats exposed prenatally to ethanol. II. Synaptology. Experimental neurology. 1987;97:53–69. doi: 10.1016/0014-4886(87)90281-0. [DOI] [PubMed] [Google Scholar]
- 34.Morton RA, Diaz MR, Topper LA, Valenzuela CF. Construction of vapor chambers used to expose mice to alcohol during the equivalent of all three trimesters of human development. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierce DR, Hayar A, Williams DK, Light KE. Olivary climbing fiber alterations in PN40 rat cerebellum following postnatal ethanol exposure. Brain research. 2011;1378:54–65. doi: 10.1016/j.brainres.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce DR, Serbus DC, Light KE. Intragastric intubation of alcohol during postnatal development of rats results in selective cell loss in the cerebellum. Alcoholism, clinical and experimental research. 1993;17:1275–1280. doi: 10.1111/j.1530-0277.1993.tb05241.x. [DOI] [PubMed] [Google Scholar]
- 37.Ramnani N. Frontal lobe and posterior parietal contributions to the cortico-cerebellar system. Cerebellum. 2012;11:366–383. doi: 10.1007/s12311-011-0272-3. [DOI] [PubMed] [Google Scholar]
- 38.Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychology review. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schilling K, Oberdick J, Rossi F, Baader SL. Besides Purkinje cells and granule neurons: an appraisal of the cell biology of the interneurons of the cerebellar cortex. Histochemistry and cell biology. 2008;130:601–615. doi: 10.1007/s00418-008-0483-y. [DOI] [PubMed] [Google Scholar]
- 40.Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci U S A. 2007;104:9858–9863. doi: 10.1073/pnas.0607037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sotelo C. Molecular layer interneurons of the cerebellum: developmental and morphological aspects. Cerebellum. 2015;14:534–556. doi: 10.1007/s12311-015-0648-x. [DOI] [PubMed] [Google Scholar]
- 42.Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int Rev Neurobiol. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]
- 43.Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. International review of neurobiology. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Kakizaki T, Sakagami H, Saito K, Ebihara S, Kato M, Hirabayashi M, Saito Y, Furuya N, Yanagawa Y. Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse. Neuroscience. 2009;164:1031–1043. doi: 10.1016/j.neuroscience.2009.09.010. [DOI] [PubMed] [Google Scholar]