Abstract
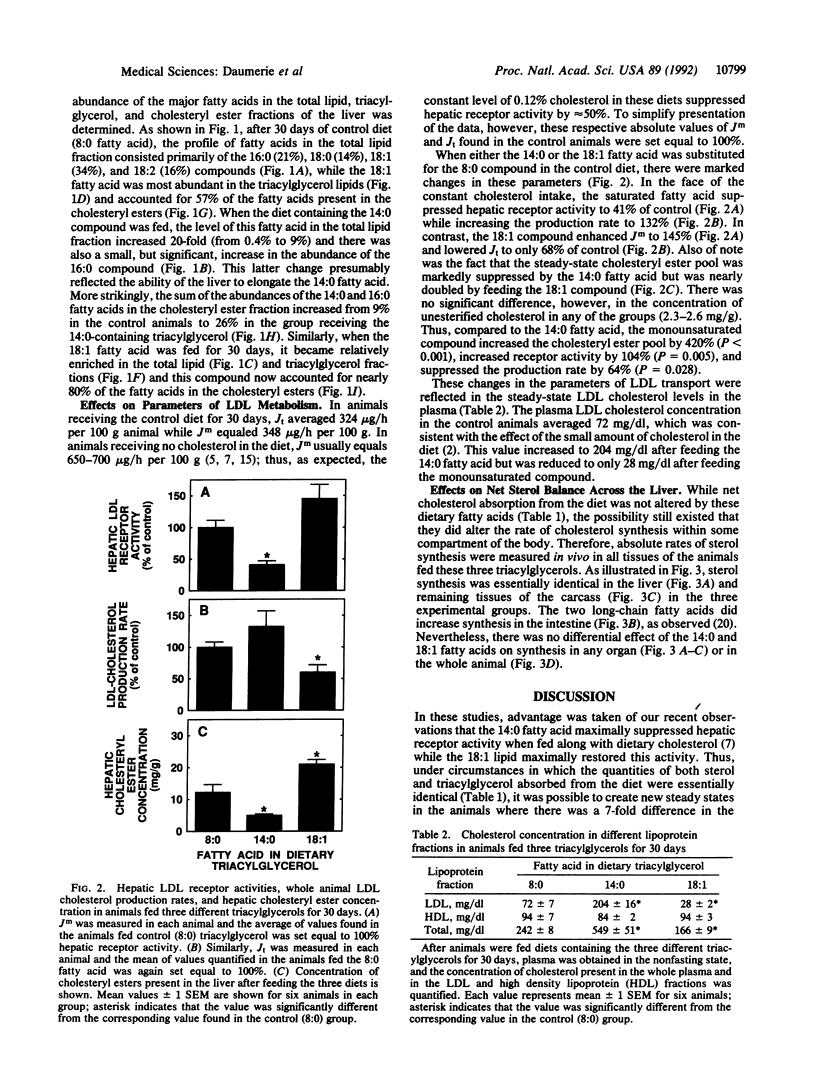
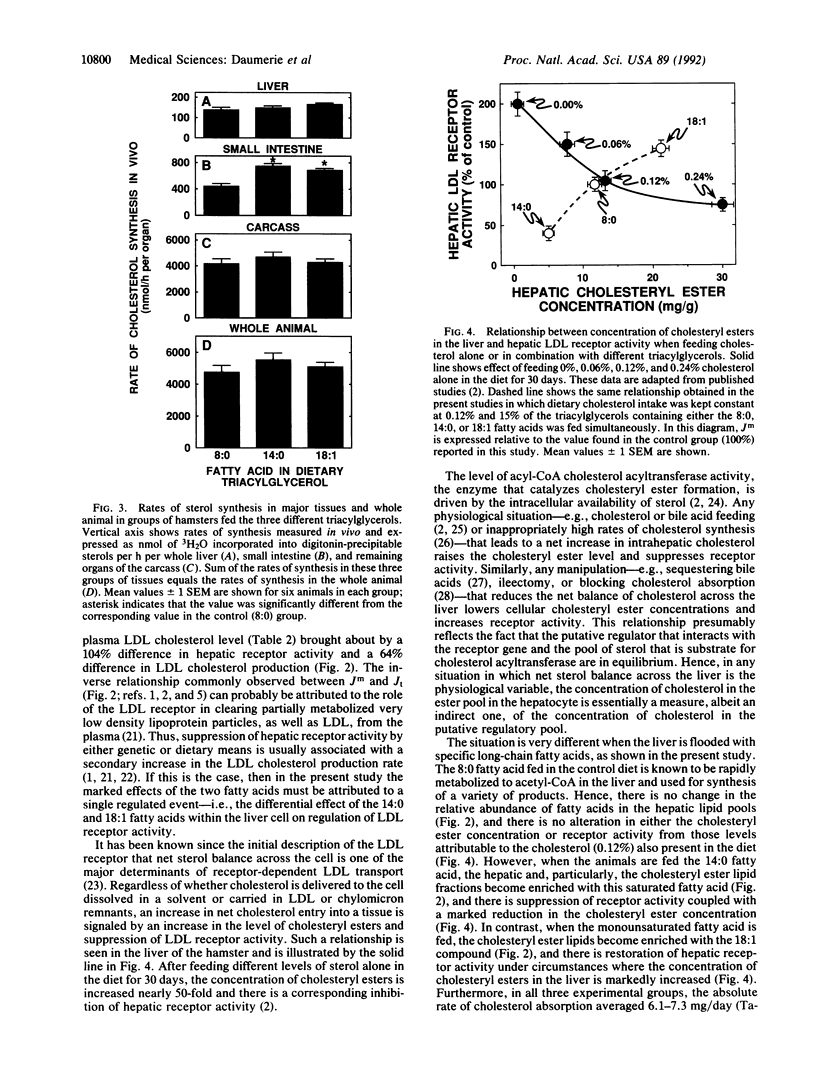
When the intake of dietary cholesterol in the hamster is constant, feeding the saturated 14:0 fatty acid (n-tetradecanoic acid) elevates the plasma low density lipoprotein (LDL) cholesterol concentration from 72 to 204 mg/dl, while the monounsaturated 18:1 fatty acid (cis-9-octadecenoic acid) lowers this level to 28 mg/dl. The 14:0 fatty acid lowers the hepatic cholesteryl ester concentration from 12 to 5 mg/g, while the abundance of this fatty acid in the ester fraction is increased 13-fold. Hepatic LDL receptor activity is depressed to 41% of control, while the LDL cholesterol production rate is increased to 132%. These changes account for the 3-fold increase in the plasma LDL cholesterol concentration. In contrast, feeding the 18:1 fatty acid increases hepatic cholesteryl ester concentration to 21 mg/g, and the abundance of this acid in the esters is increased 1.4-fold. Hepatic receptor activity is increased to 145%, while the production rate is suppressed to 68% of control. These changes account for the decrease in plasma LDL cholesterol level to 28 mg/dl. Despite these marked changes in LDL metabolism, however, the 14:0 and 18:1 fatty acids cause no change in net cholesterol balance across the liver. These results suggest that there are two fundamentally different mechanisms regulating hepatic LDL metabolism. One involves changes in net sterol balance across the liver brought about by alterations in the rate of cholesterol or bile acid absorption across the intestine, while the second is articulated through a redistribution of the putative sterol regulatory pool within the hepatocyte that is dictated by the type of long-chain fatty acid that reaches the liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Absolute rates of cholesterol synthesis in extrahepatic tissues measured with 3H-labeled water and 14C-labeled substrates. J Lipid Res. 1979 Aug;20(6):740–752. [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Bilhartz L. E., Spady D. K., Dietschy J. M. Inappropriate hepatic cholesterol synthesis expands the cellular pool of sterol available for recruitment by bile acids in the rat. J Clin Invest. 1989 Oct;84(4):1181–1187. doi: 10.1172/JCI114283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Hofmann S. L., van der Westhuyzen D. R., Südhof T. C., Brown M. S., Goldstein J. L. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. J Biol Chem. 1988 Mar 5;263(7):3372–3379. [PubMed] [Google Scholar]
- GOODMAN D. S., DEYKIN D., SHIRATORI T. THE FORMATION OF CHOLESTEROL ESTERS WITH RAT LIVER ENZYMES. J Biol Chem. 1964 May;239:1335–1345. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hornick C. A., Kita T., Hamilton R. L., Kane J. P., Havel R. J. Secretion of lipoproteins from the liver of normal and Watanabe heritable hyperlipidemic rabbits. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6096–6100. doi: 10.1073/pnas.80.19.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- Kita T., Brown M. S., Bilheimer D. W., Goldstein J. L. Delayed clearance of very low density and intermediate density lipoproteins with enhanced conversion to low density lipoprotein in WHHL rabbits. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5693–5697. doi: 10.1073/pnas.79.18.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage G., Roy C. C. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986 Jan;27(1):114–120. [PubMed] [Google Scholar]
- Mattson F. H., Erickson B. A., Kligman A. M. Effect of dietary cholesterol on serum cholesterol in man. Am J Clin Nutr. 1972 Jun;25(6):589–594. doi: 10.1093/ajcn/25.6.589. [DOI] [PubMed] [Google Scholar]
- Spady D. K., Bilheimer D. W., Dietschy J. M. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Interaction of dietary cholesterol and triglycerides in the regulation of hepatic low density lipoprotein transport in the hamster. J Clin Invest. 1988 Feb;81(2):300–309. doi: 10.1172/JCI113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Meddings J. B., Dietschy J. M. Kinetic constants for receptor-dependent and receptor-independent low density lipoprotein transport in the tissues of the rat and hamster. J Clin Invest. 1986 May;77(5):1474–1481. doi: 10.1172/JCI112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Stange E. F., Bilhartz L. E., Dietschy J. M. Bile acids regulate hepatic low density lipoprotein receptor activity in the hamster by altering cholesterol flux across the liver. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1916–1920. doi: 10.1073/pnas.83.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange E. F., Dietschy J. M. The origin of cholesterol in the mesenteric lymph of the rat. J Lipid Res. 1985 Feb;26(2):175–184. [PubMed] [Google Scholar]
- Suckling K. E., Stange E. F., Dietschy J. M. In vivo modulation of rat liver acyl-coenzyme A:cholesterol acyltransferase by phosphorylation and substrate supply. FEBS Lett. 1983 Aug 8;159(1-2):29–32. doi: 10.1016/0014-5793(83)80410-4. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Brown M. S., Goldstein J. L. 42 bp element from LDL receptor gene confers end-product repression by sterols when inserted into viral TK promoter. Cell. 1987 Mar 27;48(6):1061–1069. doi: 10.1016/0092-8674(87)90713-6. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Andersen J. M., Dietschy J. M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981 May;22(4):551–569. [PubMed] [Google Scholar]
- Turley S. D., Daggy B. P., Dietschy J. M. Cholesterol-lowering action of psyllium mucilloid in the hamster: sites and possible mechanisms of action. Metabolism. 1991 Oct;40(10):1063–1073. doi: 10.1016/0026-0495(91)90131-f. [DOI] [PubMed] [Google Scholar]
- Woollett L. A., Spady D. K., Dietschy J. M. Mechanisms by which saturated triacylglycerols elevate the plasma low density lipoprotein-cholesterol concentration in hamsters. Differential effects of fatty acid chain length. J Clin Invest. 1989 Jul;84(1):119–128. doi: 10.1172/JCI114131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett L. A., Spady D. K., Dietschy J. M. Regulatory effects of the saturated fatty acids 6:0 through 18:0 on hepatic low density lipoprotein receptor activity in the hamster. J Clin Invest. 1992 Apr;89(4):1133–1141. doi: 10.1172/JCI115694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett L. A., Spady D. K., Dietschy J. M. Saturated and unsaturated fatty acids independently regulate low density lipoprotein receptor activity and production rate. J Lipid Res. 1992 Jan;33(1):77–88. [PubMed] [Google Scholar]
- Zilversmit D. B., Hughes L. B. Validation of a dual-isotope plasma ratio method for measurement of cholesterol absorption in rats. J Lipid Res. 1974 Sep;15(5):465–473. [PubMed] [Google Scholar]



