Abstract
Endothelial cells are covered with a polysaccharide rich layer more than 400 nm thick whose mechanical properties limit access of circulating plasma components to endothelial cell membranes. The barrier properties of this endothelial surface layer are deduced from the rate of tracer penetration into the layer and the mechanics of red and white cell movement through capillary microvessels. This review compares the mechanosensor and permeability properties of an inner layer (100–150 nm, close to the endothelial membrane) characterized as a quasi-periodic structure which accounts for key aspects of transvascular exchange and vascular permeability with those of the whole endothelial surface layers. We conclude that many of the barrier properties of the whole surface layer are not representative of the primary fiber matrix forming the molecular filter determining transvascular exchange. The differences between the properties of the whole layer and the inner glycocalyx structures likely reflect dynamic aspects of the endothelial surface layer including tracer binding to specific components, synthesis and degradation of key components, activation of signaling pathways in the endothelial cells when components of the surface layer are lost or degraded, and the spatial distribution of adhesion proteins in microdomains of the endothelial cell membrane.
Keywords: shear stress, fiber matrix, vascular, endothelium, fluid balance, inflammation, edema
INTRODUCTION
The primary focus of this review is the role of the endothelial glycocalyx surface layer as a barrier for blood constituents to access the endothelium. The subject falls within the scope of glycomechanics because the structure and function of the barrier is determined, at least in part, by the distinct disaccharide unit repeats of the glycosaminoglycans (GAGs) associated with the endothelial glycocalyx. Other components of the glycocalyx, including the protein backbones of the proteoglycans to which the GAGs are attached, glycoproteins, glycolipids, and plasma proteins are modified by glycosylation in ways that regulate binding of ligands, particularly to target immune cells.69 This review is constructed largely around three figures, each composed of images from recent reviews and publications. These figures illustrate aspects of the current state of our knowledge of the structure (Figure 1) and barrier and mechanosensor functions (Figures 2 and 3) of the endothelial glycocalyx surface layer. We have adopted the strategy of juxtaposing experimental results and conceptual diagrams that highlight distinctly different ways to describe the barrier. Using this approach we evaluate how well the properties of the localized glycocalyx structures that form at least part of the pathway for water and solutes (through inter-endothelial junctions and other specialized endothelial pathways such as fenestrations) are described by properties deduced from investigations of the extensive surface layer that covers most of the endothelium.62,70,86 Where this is not the case, new approaches are needed to understand glycocalyx function in separate microdomains of the cell surface.
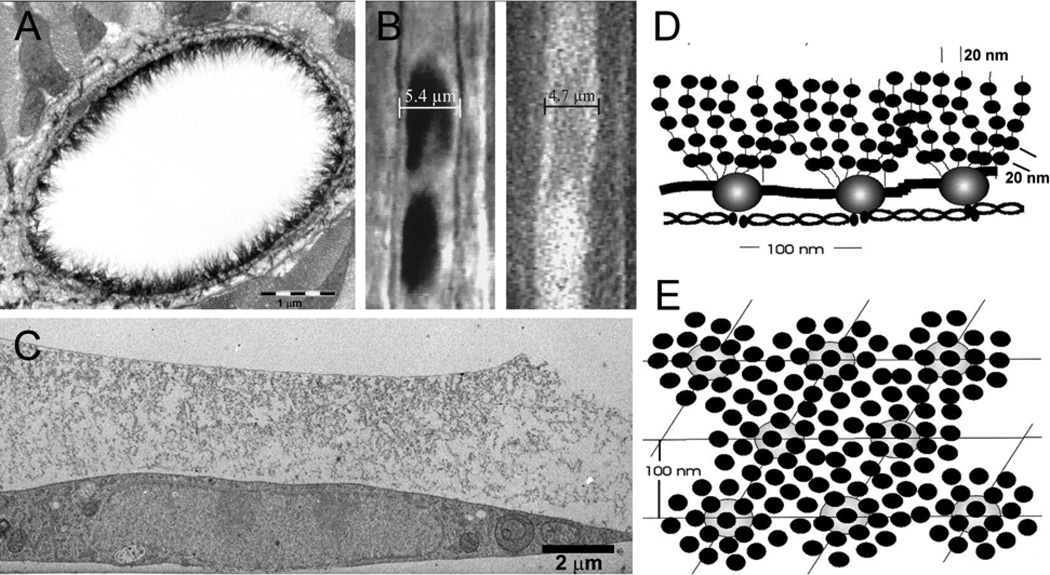
FIGURE 1.
Methods to estimate the endothelial glycocalyx thickness. (a) Alcian blue labeling and transmission electron microscopy of thin sectioned rat myocardial capillary reveals a layer up to 500 nm thick.79 (b) The width of the column of red blood cells (left) or the width of a column of fluorescently labeled dextran (right) is less than the anatomic width of the capillary in hamster cremaster (glycocalyx thickness estimated to be 400–500 nm).85 (c) Rapid freezing and freeze substitution of cultured endothelium reveals several micron depth of glycocalyx structures.16 Model of glycocalyx structure derived from autocorrelation functions and Fourier transforms of representative areas of electron micrograph images (from endothelial cells in frog mesentery and usually extending shorter distances (100–200 nm) from the endothelial cell membrane) showing quasi-periodic structure perpendicular (d) and parallel (e) to the endothelial surface.70 (a) and (b) Reprinted with permission of Circulation Research, (c) Reprinted with permission of Arteriosclerosis Thrombosis and Vascular Biology, and (d) & (e) Reprinted with permission from Elsevier.
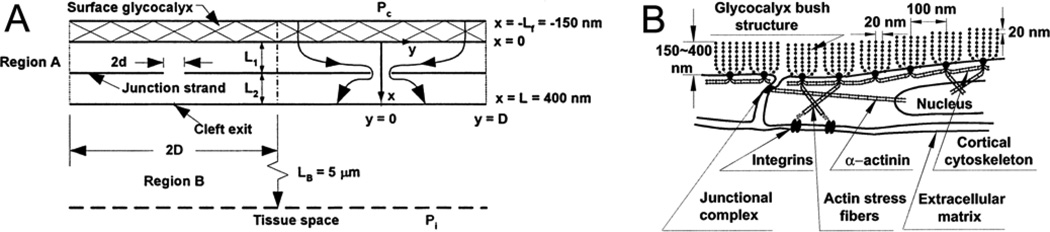
FIGURE 2.
Models used for transport barrier and mechanosensor functions of the glycocalyx. (a) Flow through surface glycocalyx-junction break model of the endothelium.31 The resistance of the glycocalyx to water and solute flows is described using hydrodynamic models. Fiber diameters range from small (1–2 nm, representative of GAG side chains) to larger fibers (10 nm and adsorbed plasma proteins). Flows through the glycocalyx are funneled into infrequent breaks in the tight junction strands. Various combinations of fiber size and arrangement have been investigated which describe water and solute flows when the size and frequency of the breaks in the junction strand are measured (see text). (b) A specific form of the surface glycocalyx-junction break model based on the quasi periodic structures in Fig 1(d) and (e) is shown. The glycocalyx is modeled as branching clusters anchored to the peripheral actin band in the endothelial cell. The structures form the primary molecular filter on the luminal side of the intercellular junctions. When the fibers extend about 150 nm from the surface and breaks in the junction stand, up to 400 nm long and 20 nm wide, are present every 2–4 microns, the model describes the permeability properties of rat venular microvessels87. Shear stress on the edge of these clusters transmits displacements to their anchoring sites in the endothelial actin cytoskeleton of the order of 10 nm. (a) Reprinted with permission from Elsevier and (b) Copyright 2003 National Academy of Sciences, USA, used with permission.
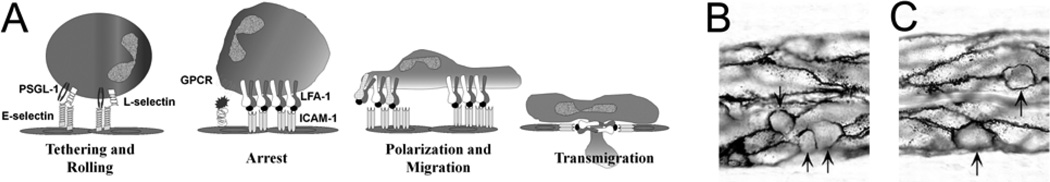
FIGURE 3.
(a) The multistep process of leucocyte recruitment requires interaction of membrane-bound macromolecules near the surface of the leucocyte and the endothelium.67 The adhesion molecules extend only tens of nm from the cell surface. The common cartoon of this cascade does not take into account the presence of a thick endothelial surface layer which limits access to the cell surface receptors. (b) Leucocytes (arrows) in frog mesentery vessels preferentially localize to endothelial cell borders as revealed by silver staining.27 (a) Courtesy of Professor Scott I. Simon and (b) used with permission of the American Physiological Society.
ENDOTHELIAL SURFACE LAYER STRUCTURE
Overview
In the first part of the review we focus on the properties of the surface layer. The endothelial surface layer is understood as a multilayer structure, which normally covers most of the surface of the endothelial cells, and reduces access of cellular and macromolecular components of the blood to the surface of the endothelium. This multilayer characteristic is the reason it is better to refer to the whole structure as an endothelial surface layer with the term “glycocalyx” restricted to the components close to the endothelial membrane. Taken together, recent major reviews highlight the remarkable progress in our understanding of the endothelial surface layer as a regulator of both microvascular and macrovascular functions. Highlights include new understanding of red cell mechanics,19,66,87 the regulation of microvascular hematocrit,15,56,85 the transmission of shear forces from the circulating blood to the endothelial cells,73,87 and its function as the primary molecular filter determining oncotic pressure difference across the vascular wall.2,39,45 There is also a growing understanding of the ways changes in the layer may be some of the earliest steps in the development of chronic disease including cardiovascular,5,52,81 infectious76 and metabolic.6,50,55
On the other hand there are fundamental gaps in our knowledge. Figure 1 illustrates different estimates of the functional thickness of the layer which may vary by more than an order of magnitude. Figure 2 highlights the fact that current models of the surface layer as the principal molecular filter in transvascular pathways use estimates of the layer thickness that fall at the lower range of measurements of the thickness in Figure 1. Further, the assumption that the same structures that determine the molecular filter also act to directly transmit shear force to the endothelial cytoskeleton (also illustrated in Figure 2) is challenged when the thickness of the whole layer significantly exceeds the height of the glycoproteins attached to the endothelial cell surface. A similar problem arises when the surface layer is ignored as a barrier to the access of key cell adhesion molecules on circulating inflammatory cells to adhesion proteins near the endothelial cell surface (Figure 3). Each of these issues is evaluated in the following sections.
General principles of glycocalyx organization
We start with the structure of the glycocalyx as understood from investigations that do not specifically focus on the area near the junctions between endothelial cells. Figure 1 illustrates results from several methods to image the glycocalyx in microvessels and cultured endothelial cells. Panels A-C show that an endothelial surface layer, which extends at least 200–400 nm from the endothelial membrane and covers most of the endothelial surface, has been demonstrated by several independent methods including (1) exclusion of high molecular weight fluorescent molecules from the endothelial cell membrane,20,28,56,82,85 2) exclusion of red cells from the vascular wall,15,59,85 and 3) alcian blue labeling and standard processing for transmission electron microscopy.79 Attempts to preserve the structure for TEM using rapid freezing and freeze substitution of endothelial monolayers avoiding use of conventional fixatives resulted in an apparent surface layer thickness of >5 µm.16 The source of this variability is not well understood. While some may be due to real differences in structure and composition, it is likely that different fixation methods preserve different components of the structure, and not necessarily in their undisturbed state. When applied in larger vessels these, and related techniques, demonstrated layers up to 3–4 µm thick.9,57,58,61 A detailed account of the composition of the glycocalyx is given in several of the recent reviews.58,62,74,86 The layer is formed from endothelial cell membrane associated proteoglycans, glycoproteins, and glycolipids with their oligosaccharide side chains (heparan sulfate and chondroitin sulfate). To these are attached constituents derived from the endothelial cells and plasma (including non membrane bound glycosaminoglycans such as hyaluronan, plasma proteins, and numerous regulator enzymes and peptides). The composition and porosity of these layers has been investigated by multiple methods including tracer molecules of different size, charge, and chemical composition, and differential digestion of the layer using enzymes to degrade heparan sulfate, chondroitin sulfate, and hyaluronan.
Several general organizing principles emerge from such studies. The first is that there is a tendency toward a layered structure with different components modulating different levels of penetration. For example, treatment with hyaluronidase in cremaster muscle vessels caused increased penetration of dextrans D70 and D145 (approximately 70 kD and 145 kD, respectively), but no change in the exclusion of red cells and anionic proteins showing that significant components of the barrier remained in this tissue after hyaluronidase treatment. This barrier was restored by infusion of hyaluronic acid together with chondroitin sulfate.28 One the other hand, in the same vessels, treatment with heparinase has been shown to cause a significant increase in the penetration of red cells15 and white cells.12 While enzyme degradation studies have been used in several investigations in vivo to demonstrate change in barrier thickness and porosity from control state, systematic investigations using several enzymes in the same preparation are only beginning to be reported. This is necessary because there is significant variability in results using single enzymes in different microvascular and macrovascular preparations. Gao and Lipowsky used heparinase, chondroitinase, and hyaluronidase in rat mesentery microvessels.20 All treatments individually reduced the surface layer thickness to between 50% and 65% of untreated values. While there was no significant difference between different enzymes, there was a tendency for heparinase to be most effective. The combination of all three enzymes reduced the thickness to near 10% of control. From measurement of the rate of tracer penetration after chondroitin and hyaluronidase treatment, these investigators found evidence for a more dense layer near the endothelial surface. More investigations of this type are of particular interest to the focus of this review, because similar vessels are used for measurements of vascular permeability. In cultured endothelial cells a variety of techniques are becoming available to further extend analysis of structure and composition. These include fluorescence correlation spectroscopy to probe albumin dynamics inside lung endothelial glycocalyx71 and the development of new ways to preserve the layer such as rapid freezing and freeze substitution illustrated in Figure 1.16 The evidence to date suggests that the glycocalyx is not well developed in some cultured endothelial cells.57 Vink and colleagues have demonstrated differences in the structure and composition of the glycocalyx in cells exposed to long term shear when compared to cells grown under static conditions.24 It remains to be determined whether the structure of the surface layer of cultured endothelium can be used as a model of that seen in vivo.
The second organizational principle is that the high molecular weight GAG hyaluronan provides part of the scaffold for the endothelial surface layer. The properties of hyaluronan include its highly hydrated nature and the length of individual molecular strands that can attach to binding sites near the endothelial membrane surface.38 For example, one form of high molecular weight hyaluronan (1844 kD) has an extended length of 4.6 microns. Long chains of hyaluronan, attached to the endothelial membrane bound receptors such as CD44, are presumed to intertwine through the glycocalyx and contribute to the layered structure at distances above the endothelial surface much greater than the maximum extent of glycoproteins and other membrane attached molecules. Recent investigations of the endothelial surface after rapid freezing demonstrate strands at least as long as those suggested above, attached perpendicular to the surface,16 rather than intertwined within the structure (Figure 1C). The organization of the meshwork of proteoglycan side chains, their core proteins, and attached proteins within such a scaffold is far less well understood. However quantitative predictions of steric exclusion, restricted diffusion, and water flows within fiber matrices can be made using relatively well developed theory for both random and ordered matrices.13,45,53,54 For example, exclusion of macromolecules close to the size of albumin can be accounted for in a fibrous meshwork with fibers of typical radii less than 1 nm and which occupy 2–5% of the matrix volume. Such models can be extended to take into account the volume of adsorbed proteins and the effect of charge.44,45
A third organizational principle is the dynamic nature of the surface layer. There is evidence that the thickness of the glycocalyx is a balance between the rate of synthesis of glycocalyx components and the rate of degradation.24,46 Synthesis can be modulated by the supply of precursors of matrix components;6 degradation is modulated by enzymes such as heparinase, hyaluronidase, and metalloproteases.46 Synthesis and degradation are likely to modify the observed rates of tracer penetration. For example, it is not unreasonable to suggest that penetration of test tracers may be significantly retarded if tracers are preferentially removed near the outer layers after binding to components of the layer. The process may be further modified by synthesis of material to replace lost components of the matrix.
Further, the addition of new components to the matrix also modulates the organization of matrix components. For example, the binding of hyaluronan to its membrane linked binding proteins (CD44) causes clustering of the complex into endothelial caveolae and activation of the sphingosine-1-phosphate (S1P) receptor-1 (S1P1).68 Signaling via S1P1 stabilizes the cortical actin network in the endothelial cell periphery. It is also widely recognized that removal of plasma proteins can reduce the thickness and disrupt the organization of the endothelial layer structure.1,16,77 Removal of the plasma proteins results in calcium ion influx into endothelial cells.26 If this calcium influx is attenuated, the increase in permeability that characterizes frog microvessels after plasma protein removal is attenuated, even in the continued absence of plasma protein. A local increase in calcium ion concentration close to the peripheral actin band weakens adhesion mechanisms,34,88 just the opposite to the S1P1 action described above. These observations suggest that the function of the glycocalyx as a permeability barrier may depend as much on the organization of anchoring points to the cell cytoskeleton and the modulation of associated signaling pathways as on the organization within the matrix itself. Finally, we note that components of the glycocalyx act as co-receptors for key signaling agents such as endothelial cell growth factors.91 Heparan sulfate also modulates the uptake of agents such as precursors of nitric oxide synthesis.18
Evidence for quasi periodicities within the layer
Figure 1D and 1E highlight striking periodicities in the glycocalyx within 150 nm of the endothelial cell membrane. The model is based on analyses of glycocalyx images from frog mesenteric microvessels70, but is guided by images of the glycocalyx reported by Rostgaard and Qvortrup over the fenestrations in mammalian microvessels of gastrointestinal tissue, renal peri-tubular microvessels and the glomerulus.64,65 Use of autocorrelation functions and Fourier transforms of electron micrograph images demonstrated an ordered three dimensional meshwork within the glycocalyx. The figures depict a network with 10–12 nm diameter scattering foci that are spaced close to 20 nm apart in both the plane of the endothelial surface and perpendicular to the surface for a distance of up to 150 nm for normal vessels. Assuming these scattering foci represent matrix fibers, the investigators found that they occur in clusters with a common intercluster spacing of about 100 nm. These appear to be anchored at sites for the bush similar to structures described by Rostgaard and Qvortrup.64 A recent study which examined the glycocalyx of fenestrated and non-fenestrated endothelia of rats and rabbits using the same autocorrelation techniques yielded very similar results to the original investigation of frog microvessels suggesting a generality for the structural features of the glycocalyx.4 The thickness of the inner glycocalyx in these images is up to an order of magnitude smaller than the endothelial surface layers described by using methods depicted in Figure 1A–C. Some of the questions about this difference are addressed below.
Squire and colleagues70 suggested that a glycocalyx 100–200 nm was characteristic of normal endothelium and that the thick surface layers shown in Figure 1 were not characteristic of most endothelium. This does not appear to be the case given the consistent demonstration of thick layers in a variety of microvessels and larger vessels. The argument that the difference reflects species difference does not appear to be the case as the same labeled structures are described in mammalian microvessels41 and others.11,79 Another possibility is that the thinner layer is characteristic of a collapsed state of the matrix. It is difficult to reconcile such a collapsed state with the level of order and organization found with this analysis. Further, the same periodicity was found in microvessels prepared under a variety of fixation conditions. On the other hand, the possibility that the model describes an inner microdomain of the endothelial surface from which most of the outer layer has been lost cannot be excluded. This does not exclude the possibility that there may still be some ordering in regions of the glycocalyx more than 100–200 nm from the surface. In the surface coat of heat injured endothelium, Squire and colleagues still observed mean spacing of 20 nm in protuberances of the glycocalyx that extended up 300–400 nm, although larger periodicities were also seen and the glycocalyx was not necessarily continuous with the surface. For the purpose of this review, we suggest that the model in Figure 1D and 1E is characteristic of a microdomain within 100–150 nm of the endothelial surface. It may form the lower layer of a more extended matrix that can also contain some of the same periodicity at least perpendicular to the endothelial cell surface. We will also suggest that it may be characteristic of subdomains on the endothelial surface where the overall thickness of the layer differs significantly from that measured over most of the endothelial cell. We note that fibers 10–12 nm diameter, set 20 nm in a regular array have an interfiber spacing of 8 nm, close to the interfiber dimensions required to form a molecular filter for plasma proteins such as albumin. When arranged in a square or hexagonal array, these fibers therefore occupy 15% to 30% of the matrix volume, far more than occupied by the smaller diameter fibers associated with the proteoglycan side chains (close to 1 nm; fiber volume up to 5%) as described above, and also capable of forming a molecular filter to plasma protein. Squire and colleagues70 suggested that the fibers were the protein backbone of proteoglycans in the cell membrane, and that side chains might be responsible for the 20 nm periodicity if they were distributed along the protein backbone. Another possibility is that adsorbed plasma protein also contributes to the periodicity. However, the small size of individual proteoglycan core proteins such as the syndecans and glypicans makes this simple interpretation unlikely.
BARRIER FUNCTION OF THE ENDOTHELIAL SURFACE LAYER
Tracer penetration versus permeability of the vascular barrier
It is difficult to imagine a comprehensive model that would account for the diverse range of observations of tracer penetration within the endothelial surface layers. These include the fact that dextran D70, which was excluded from the matrix as in Figure 1B, penetrated when bound to albumin. Further, albumin and fibrinogen, both anionic, but with different molecular weights (65 kD vs 340 kD) penetrated at the same slow rate with an average equilibration time of 30 minutes. Also, dextran D40, which has a free diffusion coefficient similar to that of albumin, equilibrates within the layer within 1 minute. These observations indicate that preferential binding and chemical interaction are at least as important as size. Of particular note is the striking difference between the rates of penetration described above and the dependence of vascular permeability on size, charge and chemical composition. For example, in contrast to the 30-fold difference between the rate of equilibration of dextran D70 into cremaster surface layer and the rate of albumin equilibration, the rates of transvascular transport of albumin and dextran in the microvasculature of muscle and skin tissue in dog paw, measured as a permeability × surface area (PS) product, are close to that predicted from their diffusion coefficients (7.4 and 9.3 × 10−7 cm2/sec for D40 and albumin, respectively). PS for albumin is close to 3 × 10−4 cm3/sec/100g and PS for D40 is 1.6 × 10−4 cm3/sec/100g.63 In addition, in the same tissue, fibrinogen PS product is close to 30% of that for albumin, in accord with its higher molecular weight, whereas both proteins penetrate the endothelial surface layer at a similar rate as noted above. In the next section we evaluate the organizational principles above from the point of view of transport through the endothelial surface layer near the junctions between endothelial cells.
The glycocalyx and endothelial permeability
Figure 2 shows two forms of the endothelial surface–junction break model of permeability. The key feature of the model in Figure 2A is that the glycocalyx lies in series with the pathway for most water and solutes through the intercellular junctions of continuous capillaries.87 The idealized model based on the periodicities from Figures 1D and 1E describes water and solute flows from blood through the glycocalyx that are then funneled through infrequent breaks in the otherwise impermeable ring of tight junction proteins that link adjacent endothelial cells. The glycocalyx layer is modeled as a barrier with the same periodic structure described above (10–12 nm radius fibers spaced 20 nm apart in a hexagonal array). The interfiber spacing (close to 8 nm) significantly restricts entry of plasma proteins the size of albumin. Fibers extending 100–150 nm from the endothelial surface then form the primary diffusion barrier to molecules larger than 1 nm radius. This model is understood to provide a reasonable molecular level description of the classical pore theory of capillary permeability, with the fiber radius and interfiber spacing determining pore size, and the length and frequency of breaks in the junction strands determining pore density.14,86
The most rigorous test of the model has been to describe the coupled flows of water and albumin across the glycocalyx through the intercellular junctions and into adjacent tissue. The important result is that the magnitude of water and albumin fluxes directly measured in perfused rat venular microvessels can be accounted for only when most of the osmotic pressure of albumin is exerted across the glycocalyx, not across the whole endothelial barrier.2,39 In an extended form (with no modification to the proposed glycocalyx structure) the model also accounts for the observed readjustments, within 2 minutes or less, of these albumin gradients within the vascular wall in response to changes in microvessel pressure and tissue albumin concentrations.96 The model predictions are not consistent with equilibration times of macromolecules across the endothelial surface layer on the order of tens of minutes.80,84 These observations highlight one of the primary themes of this review. Specifically, resistances to tracer penetration of the endothelial surface layer, observed in situ over the bulk of endothelial surface layer in intact microvessels, are not representative of the resistance to the same molecules undergoing blood to tissue exchange. In other words the characteristics of tracer penetration deduced from experiments described in Figure 1 do not account for the permeability of the intact endothelial barrier whose permeability and selectivity is quite well described by the model in Figure 2A. This does not mean that the glycocalyx does not modulate exchange because modification of the glycocalyx does affect penetration of tracers into pathways across the endothelial cell.10,33 In the following section we review arguments that lead to a similar conclusion for water flow and then suggest the areas for further investigation that are necessary to understand this difference.
According to the model in Figure 2A, the removal of a 150 nm layer of glycocalyx is predicted to increase hydraulic conductivity (Lp) of microvessels 2-fold, close to the increase observed after the glycocalyx is removed by pronase treatment in frog vessels, and the increase in Lp with the removal of plasma proteins.1,32 A surface layer with the same structure, but greater thickness, offers greater resistance to water flow from blood to tissue. In particular, the resistance of a 400 nm layer with the same hydraulic resistivity as that in Figure 2A is too high to account for measured hydraulic conductivities, or the changes of Lp in frog vessels when the glycocalyx is removed. On the other hand, a thinner matrix or a matrix with lower resistance to water flow (see below) does not account for the measured microvessel Lp. Thus, as argued by Weinbaum and colleagues,86 measured Lp values constrain estimates of surface layer thickness to a relatively narrow band, close to 100–150 nm. It is hard to reconcile these conclusions with models of red cells moving through capillaries by effectively “skiing” across the endothelial surface layer when their velocity is greater than about 20 µm/sec.17 The latter is possible when the resistance to water flow through a surface layer 400–500 nm thick is sufficiently high.66,86 Specifically, for this to occur, the resistance to water flow throughout the layer must be similar to that in the matrix in Figure 2A.66,86 These differences also indicate that at least part of the structure of the surface layer in the vicinity of the junctions which are the main water pathways differs from that across most of the endothelial surface. One possibility is that the periodic structure in Figure 1 may be common to the inner 150 nm of endothelial layer. However surface layers (beyond 150 nm) in the region of the junctions may offer relative low resistance to water and albumin movement. We evaluate some of the feature of the endothelial surface cell near junctions and other pathways across the endothelial cells below.
Based on the measured length and maximum width of the inter-endothelial cleft, the entrance regions of the intercellular junctions occupy 0.2% of the whole endothelial cell surface. Furthermore, although a “cobblestone” appearance, where adjacent cells simply abut at junctions, is often used to describe endothelial monolayer geometry, the junctions are actually formed when two or more endothelial cells overlap in a plane more or less parallel to the endothelial surface. The extent of this region of overlap is actively regulated. Mechanisms include the peripheral actin band of endothelial cells and an array of junction protein regulatory complexes linked to intercellular adhesion proteins that regulate the ring of tight junction proteins (the solid lines in Fig 2) and the extent of overlap between adjacent cells. An important area for future research is the question whether mechanisms that regulate the ordering and composition of the endothelial surface in the region of the junction differ from those in other regions of the endothelial cell, and further from the junctions. There are several recent reports describing specific examples of localized expression of surface layer components. One is that the junction region is the site of up-regulation of additional adhesion proteins such as ICAM regulating immune cell attachment and migration at junctions (see Fig 3 and below). Another example is the observation mentioned above that binding of hyaluronan to CD44 causes redistribution of this membrane-associated protein to lipid rafts. The same argument may apply near other pathways for water and solutes across the endothelial barrier, particularly near fenestrations. The fact that the glycocalyx structures observed by Rostgaard and Qvortrup64,65 of fenestrated endothelium have provided a useful guide to understanding the ordered cluster model in Figure 2A suggests that more detailed evaluation of the glycocalyx in fenestrated microvessels where the fraction of membrane surface associated with transport pathways is high may provide new information about the glycocalyx in transvascular pathways.39 This is particularly the case since the glycocalyx in fenestrated vessels has been shown to be the primary osmotic barrier for plasma proteins.39
The model of the glycocalyx with dense fiber organization near the endothelial cell membrane in Figure 2A has played an important role in other recent investigations of glycocalyx functions. These include the attractive hypothesis that the same structures that determine vascular permeability also provide some of the mechanosensing functions of the glycocalyx.87 This hypothesis will be described in more detail below where we conclude that the link between mechnotransduction and permeability is less well established in intact vessels than is suggested by current investigations of cultured endothelial cell monolayers. It is therefore useful to briefly note alternate models of the glycocalyx structure such as that represented in Figure 2B. A fiber matrix extending 150 nm from the surface formed by thinner fibers (0.6 nm, characteristic of the side chains of glycosaminoglycans) organized in either a random assembly or as an ordered array (with regular fiber spacing determined, for example, by adsorbed albumin), has been used to describe most of the permeability properties of the venular microvessels.13,45 These fibers occupy only 2% to 5% of the network compared with 15% to 30% for the thicker fibers. Weinbaum et al87 have argued that the denser model described the resistance to water flow when restricted to 150 nm from the cell surface better than the fine fiber meshwork. Regular interfiber spacing also described the sharp cutoff of tracer entry into the layer better than a random fine fiber network.87 However a re-examination of some previous investigations suggest that the distinctions between the thin fiber model and the thick fiber model become less clear cut if the effects of plasma protein adsorbed to the thinner fibers is taken into account. The sharper cut-off in molecular size is achieved in a fine fiber network when it is ordered, for example by cross-linking with plasma proteins.44 Similarly, with respect to resistance to water flow, the increase in fiber volume taking into account adsorbed proteins will significantly increase hydraulic resistance. It may be necessary to revisit some of these ideas, as contributions of glycocalyx components in addition to the thicker fibers are re-evaluated.
Endothelial surface layer as mechanotransducer
The hypothesis is that deflections of the fibers in Figure 2A due to exposure to shear stress causes molecular displacement of signaling proteins in the endothelial cytoskeleton to modulate endothelial functions. These include cell-cell and cell-matrix adhesion, and the synthesis of nitric oxide.73 Weinbaum and colleagues87 used the time for the thick endothelial surface layer to recover its undisturbed thickness after being crushed by the passage of a white cell in a capillary to evaluate mechanical properties of mechanosensors in the endothelial surface layer which were assumed to be core proteins with the clusters shown in Figure 2A. These were modeled as cantilevers extending up to 400 nm from the surface. They concluded that, although the deflection of a single fiber, 400 nm long, was too small to cause significant molecular reorganization of regulatory pathways in the endothelial cell cytoskeleton, shear force on a whole cluster of fibers 400 nm long would cause significant molecular displacements of the order of 10 nm within the actin cytoskeleton. On the other hand, the cytoskeletal deformation estimated for shorter fibers (150 nm) are close to an order of magnitude smaller, and it is questionable whether such fibers, alone or in clusters would generate the displacement required for outside-in signaling. Furthermore, an important prediction from the models of water flows within the endothelial layer is that the applied shear force is restricted to the outer 10% of the surface layer. Structures below this surface region are not exposed to shear forces.86,87 Thus, in a matrix 400 nm thick all components of an inner layer (150 nm fiber clusters, as well as other possible fluid shear sensors, such as receptors or caveolae) will not be exposed to shear. They are likely to respond to applied shear only in the absence of the outer endothelial barrier.
Much remains to be done to evaluate these mechanical models of the endothelial surface layer. In particular, the role of the glycocalyx as a modulator of the endothelial cell responses to imposed shear remains an active area of investigation in cultured endothelial cell monolayers.73 It has been demonstrated that the removal of the glycocalyx from cultured endothelial cells attenuates shear dependent reorganization of the actin cytoskeleton that result when shear force is applied for several hours.75 The glycocalyx was removed using heparinase and by plasma protein depletion. Heparinase attenuated both nitric oxide production in response to shear and acute shear dependent increase in hydraulic conductivity of monolayers.74 Removal of the glycocalyx by heparinase prevents the reorientation of endothelial cells and the reduction in endothelial cell proliferation that normally results from sustained exposure over 24 hours.92 The modulation of endothelial permeability and junction structures after heparinase treatment is less clear. Some of the evidence that glycocalyx structures that regulate permeability are different from those responding to shear includes the observation that the same monolayers where heparinase attenuated shear dependent NO production, heparinase treatment did not increase baseline permeability (hydraulic conductivity) of the endothelial monolayer as expected if heparan sulfate was a principal component of both the permeability barrier and the mechanotransducer.18,40 Further, it has been recently reported that the application of shear to an endothelial monolayer causes the endothelial cell junctions to align with the direction of flow, with an angle close to 10 degrees to the direction of flow.43 Retrograde flow over the same monolayer results in a re-orientation of the angle of overlap in endothelial cells. The reorientation was not modified by treatment with heparinase. One conclusion from these observations is that parts of the endothelial surface near the region of cell overlap may be important for detecting the direction of flow and shear stress. An important question is the nature of forces on the endothelial cells transmitted via structures in the glycocalyx that are not directly involved in permeability regulation as reviewed in detail elsewhere.25,30,35,66,69,78,93
Mechanosensing in intact vessels
The action of imposed shear stress as a regulator of permeability in intact microvessels is less clear-cut. Increased flow rates were reported to increase albumin permeability in isolated cannulated coronary venules ranging from 30 to 70 µm in diameter,94 and increase Lp in frog mesenteric microvessels vessels.89 In both reports, the baseline permeability of the vessels investigated (>1 × 10−6 cm/s for albumin permeability, and >10 × 10−7 cm/s/cmH2O for Lp) was larger than expected for these vessels. Thus, the experimental condition (increased baseline permeability, loss of normal endothelial surface) may have biased the results. In individually perfused frog and rat mesentery vessels with normal permeability, no sustained increase in Lp with increased shear has been reported.3,36,48 However, a transient change in Lp (time course of tens of seconds to several minutes) following an abrupt change in shear has been described.37,89 Further, in rat mesenteric microvessels, where there was no shear dependent increase in Lp, an NO dependent increase in small solute permeably has been reported.36 The magnitude and solute size dependence of this shear dependent response indicates that this pathway represents a very small pore within the tight junction strands. One interpretation of these results is that, in microvessels with stable junctions, shear dependent signaling does not modify the common pathway for water and solutes larger than 500 kD. In our laboratory, we have confirmed that sustained high shear in the direction of normal blood flow in intact venules does not increase Lp. However, when the direction of flow is reversed (retrograde perfusion) there is a large increase in permeability apparently due to change in the organization of junction proteins.3 This result is similar to that described by Frangos and colleagues.43 The conclusion from these studies is that the application of the model of the glycocalyx in Figure 2A as both a molecular filter and as a mechanotransducer in intact microvessels requires further detailed evaluation.
Leukocyte access to endothelial receptors
Figure 3A illustrates the well known cascade of leukocyte capture, rolling, and firm attachment to the endothelial surface receptors, and highlights the problem that the sequence ignores the presence of the endothelial surface coat which, at a thickness of 400–500 nm, is up to ten times the maximum extension of the selectin and integrin receptors above the endothelial surface.46,86 The predictions of models of the penetration of long microvilli on leukocytes into the endothelial surface layer demonstrate that there will be no direct adhesion with these receptors on the endothelial surface unless the white cell is already tethered.86 Thus the intact surface coat serves as a barrier to leukocyte recruitment. Vascular injury and inflammatory stimuli (including ischemia reperfusion,7,60 TNF-alpha stimulation,8,29,42,49 free radical generation,83 and hyperglycemia50,51) all result in some modification of the surface layer to increase access of inflammatory cells to the endothelial surface. The physical and chemical nature of these changes,86 ways to protect the surface in situ (for example, inhibition of shedding46), and new strategies to establish a protective barrier using an artificial layer21,22 all remain important areas for further investigation.
With reference to the glycocalyx near the junctions, it is now well established that most firmly adherent leukocytes are preferentially localized near endothelial junctions in isolated human umbilical vein endothelial monolayers,23 in microvessels of frog and rat mesentery,27,95 and in mouse cremaster venular microvessels90 as illustrated in Figure 3B. In a recent series of papers, the Sarelius lab has demonstrated the fundamental role of differential ICAM expression patterns at intercellular junctions in the regulation of monocyte and neutrophil transmigration as well as the activation of endothelial pathways regulating leukocyte dependent increased permeability.72 These observations highlight the importance of investigations of the mechanisms whereby the products of activated inflammatory cells modify both the glycocalyx and the endothelial cell in a more extended form of the cascade depicted in Figure 3A.47
SUMMARY AND FUTURE INVESTIGATIONS
In summary, we have emphasized that the barrier properties of the whole endothelial surface layer are not representative of the molecular sieve and resistance to water and solute movement formed by a fiber matrix within the principal water and solute pathways for transvascular exchange. The barrier properties of thick endothelial surface layers (>400 nm) are deduced from the rate of tracer penetration into the layer that covers most of the endothelial cell surface and by the mechanics of red and white cell movement through capillary microvessels. In particular, an inner layer (100–150 nm) close to the endothelial membrane and characterized as a quasi-periodic structure with 20 nm spacing between fibers 10–12 nm in radius forms an effective size selective barrier. This structure describes key aspects of transvascular exchange and vascular permeability. However, in thicker endothelial surface layers (>400 nm) this same structure does not account for measured rates of penetration of tracers having different charge and chemical composition. These rates vary by as much as an order of magnitude even though the tracers have similar molecular size. We also emphasize that the proposed mechanosensor functions of the quasi-periodic structure and the ability of specific endothelial adhesion proteins such as ICAM1 to target inflammatory cells near endothelial junctions is compromised when the thick endothelial surface layer reduces access to these inner components of the layer. Thus while changes in the thickness and porosity of the endothelial surface layer may predict increased vascular permeability, mechanisms in addition to a simple reduction in resistance to transport are likely to be important. These include change in binding of tracers to the outer portion of the thick layer, and change in synthesis or degradation of the surface layer. They also likely reflect activation of signaling pathways in the endothelial cells that modify the endothelial cytoskeleton and cell–cell adhesion when key components of the surface layer (for example, plasma proteins and hyaluronic acid) are lost, and changes in the expression of adhesion proteins, possibly in specific microdomains such as those near the inter-endothelial cell junctions. These observations point to important areas for further research under both in vivo and in vitro conditions.
ACKNOWLEDGEMENTS
Supported by NIH HL28607 and HL44485. We thank Prof. Scott I. Simon for use of the illustration in Figure 3A.
REFERENCES
- 1.Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992;445:473–486. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557:889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson RH, Sarai RK, Weinbaum S, Curry FE. Retrograde shear stress modulates rat mesentery microvessel permeability and endothelial adhesion structures. FASEB J. 2010;23:950–955. [Google Scholar]
- 4.Arkill KP, Knupp C, Michel CC, Neal CR, Qvortrup K, Rostgaard J, Squire JM. Similar endothelial glycocalyx structures in microvessels from a range of Mammalian tissues: evidence for a common filtering mechanism? Biophys J. 2011;101:1046–1056. doi: 10.1016/j.bpj.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 6.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruegger D, Rehm M, Abicht J, Paul JO, Stoeckelhuber M, Pfirrmann M, Reichart B, Becker BF, Christ F. Shedding of the endothelial glycocalyx during cardiac surgery: on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2009;138:1445–1447. doi: 10.1016/j.jtcvs.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 8.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 9.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 10.Clough G, Michel CC. The role of vesicles in the transport of ferritin through frog endothelium. J Physiol. 1981;315:127–142. doi: 10.1113/jphysiol.1981.sp013737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clough G, Michel CC, Phillips ME. Inflammatory changes in permeability and ultrastructure of single vessels in the frog mesenteric microcirculation. J Physiol. 1988;395:99–114. doi: 10.1113/jphysiol.1988.sp016910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 13.Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res. 1980;20:96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- 14.Curry FR, Adamson RH. Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc Res. 2010;87:218–229. doi: 10.1093/cvr/cvq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol. 1990;258:H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- 16.Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Weinbaum S. Lubrication theory in highly compressible porous media: the mechanics of skiing, from red cells to humans. Journal of Fluid Mechanics. 2000;422:281–317. [Google Scholar]
- 18.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 19.Forsyth AM, Wan J, Owrutsky PD, Abkarian M, Stone HA. Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc Natl Acad Sci U S A. 2011;108:10986–10991. doi: 10.1073/pnas.1101315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giantsos-Adams K, Lopez-Quintero V, Kopeckova P, Kopecek J, Tarbell JM, Dull R. Study of the therapeutic benefit of cationic copolymer administration to vascular endothelium under mechanical stress. Biomaterials. 2011;32:288–294. doi: 10.1016/j.biomaterials.2010.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giantsos KM, Kopeckova P, Dull RO. The use of an endothelium-targeted cationic copolymer to enhance the barrier function of lung capillary endothelial monolayers. Biomaterials. 2009;30:5885–5891. doi: 10.1016/j.biomaterials.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 23.Gopalan PK, Burns AR, Simon SI, Sparks S, McIntire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68:47–57. [PubMed] [Google Scholar]
- 24.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–H452. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 25.Gudi S, Huvar I, White CR, McKnight NL, Dusserre N, Boss GR, Frangos JA. Rapid activation of Ras by fluid flow is mediated by Galpha(q) and Gbetagamma subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:994–1000. doi: 10.1161/01.ATV.0000073314.51987.84. [DOI] [PubMed] [Google Scholar]
- 26.He P, Curry FE. Albumin modulation of capillary permeability: role of endothelial cell [Ca2+]i. Am J Physiol. 1993;265:H74–H82. doi: 10.1152/ajpheart.1993.265.1.H74. [DOI] [PubMed] [Google Scholar]
- 27.He P, Wang J, Zeng M. Leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol. 2000;278:H1686–H1694. doi: 10.1152/ajpheart.2000.278.5.H1686. [DOI] [PubMed] [Google Scholar]
- 28.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 29.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 30.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Weinbaum S. A new view of Starling's hypothesis at the microstructural level. Microvasc Res. 1999;58:281–304. doi: 10.1006/mvre.1999.2177. [DOI] [PubMed] [Google Scholar]
- 32.Huxley VH, Curry FE. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985;248:H264–H273. doi: 10.1152/ajpheart.1985.248.2.H264. [DOI] [PubMed] [Google Scholar]
- 33.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000;278:H1177–H1185. doi: 10.1152/ajpheart.2000.278.4.H1177. [DOI] [PubMed] [Google Scholar]
- 34.Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kajimura M, Michel CC. Flow modulates the transport of K+ through the walls of single perfused mesenteric venules in anaesthetised rats. J Physiol . 1999;521(Pt 3):665–677. doi: 10.1111/j.1469-7793.1999.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MH, Harris NR, Tarbell JM. Regulation of capillary hydraulic conductivity in response to an acute change in shear. Am J Physiol Heart Circ Physiol. 2005;289:H2126–H2135. doi: 10.1152/ajpheart.01270.2004. [DOI] [PubMed] [Google Scholar]
- 38.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 39.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Quintero SV, Amaya R, Pahakis M, Tarbell JM. The endothelial glycocalyx mediates shear-induced changes in hydraulic conductivity. Am J Physiol Heart Circ Physiol. 2009;296:H1451–H1456. doi: 10.1152/ajpheart.00894.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25:1773–1783. [PubMed] [Google Scholar]
- 42.Marechal X, Favory R, Joulin O, Montaigne D, Hassoun S, Decoster B, Zerimech F, Neviere R. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008;29:572–576. doi: 10.1097/SHK.0b013e318157e926. [DOI] [PubMed] [Google Scholar]
- 43.Melchior B, Frangos JA. Shear-induced endothelial cell-cell junction inclination. Am J Physiol Cell Physiol. 2010;299:C621–C629. doi: 10.1152/ajpcell.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel CC. Capillary permeability and how it may change. J Physiol. 1988;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 46.Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16:657–666. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 47.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neal CR, Bates DO. Measurement of hydraulic conductivity of single perfused Rana mesenteric microvessels between periods of controlled shear stress. J Physiol. 2002;543:947–957. doi: 10.1113/jphysiol.2002.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MH, Hayden A, Levi M, Meijers JC, Ince C, Kastelein JJ, Vink H, Stroes ES. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 51.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 52.Noble MI, Drake-Holland AJ, Vink H. Hypothesis: arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM. 2008;101:513–518. doi: 10.1093/qjmed/hcn024. [DOI] [PubMed] [Google Scholar]
- 53.Ogston AG. The Spaces in a Uniform Random Suspension of Fibres. Transactions of the Faraday Society. 1958;54:1754–1757. [Google Scholar]
- 54.Ogston AG, Michel CC. General descriptions of passive transport of neutral solute and solvent through membranes. Prog Biophys Mol Biol. 1978;34:197–217. doi: 10.1016/0079-6107(79)90018-x. [DOI] [PubMed] [Google Scholar]
- 55.Perrin RM, Harper SJ, Bates DO. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys. 2007;49:65–72. doi: 10.1007/s12013-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platts SH, Duling BR. Adenosine A3 receptor activation modulates the capillary endothelial glycocalyx. Circ Res. 2004;94:77–82. doi: 10.1161/01.RES.0000108262.35847.60. [DOI] [PubMed] [Google Scholar]
- 57.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 58.Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol. 2006:1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- 59.Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P. Microvascular blood flow resistance: role of endothelial surface layer. Am J Physiol. 1997;273:H2272–H2279. doi: 10.1152/ajpheart.1997.273.5.H2272. [DOI] [PubMed] [Google Scholar]
- 60.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 61.Rehm M, Haller M, Orth V, Kreimeier U, Jacob M, Dressel H, Mayer S, Brechtelsbauer H, Finsterer U. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95:849–856. doi: 10.1097/00000542-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 62.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renkin EM, Curry FE. Transport of water and solutes across capillary endothelium. In: Giebisch G, Tosteson DC, Ussing HH, editors. Membrane Transport in Physiology. Heidelberg: Springer-Verlag; 1978. pp. 1–45. [Google Scholar]
- 64.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- 65.Rostgaard J, Qvortrup K. Sieve plugs in fenestrae of glomerular capillaries--site of the filtration barrier? Cells Tissues Organs. 2002;170:132–138. doi: 10.1159/000046186. [DOI] [PubMed] [Google Scholar]
- 66.Secomb TW, Hsu R, Pries AR. Motion of red blood cells in a capillary with an endothelial surface layer: effect of flow velocity. Am J Physiol Heart Circ Physiol. 2001;281:H629–H636. doi: 10.1152/ajpheart.2001.281.2.H629. [DOI] [PubMed] [Google Scholar]
- 67.Simon SI, Sarantos MR, Green CE, Schaff UY. Leucocyte recruitment under fluid shear: mechanical and molecular regulation within the inflammatory synapse. Clin Exp Pharmacol Physiol. 2009;36:217–224. doi: 10.1111/j.1440-1681.2008.05083.x. [DOI] [PubMed] [Google Scholar]
- 68.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 69.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136:239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 71.Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2007;293:L328–L335. doi: 10.1152/ajplung.00390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 75.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trung DT, Wills B. Systemic vascular leakage associated with dengue infections - the clinical perspective. Curr Top Microbiol Immunol. 2010;338:57–66. doi: 10.1007/978-3-642-02215-9_5. [DOI] [PubMed] [Google Scholar]
- 77.Turner MR, Clough G, Michel CC. The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries. Evidence that proteins in the endothelial cell coat influence permeability. Microvasc Res. 1983;25:205–222. doi: 10.1016/0026-2862(83)90016-x. [DOI] [PubMed] [Google Scholar]
- 78.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 79.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 80.van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol. 2003;285:H2848–H2856. doi: 10.1152/ajpheart.00117.2003. [DOI] [PubMed] [Google Scholar]
- 81.VanTeeffelen JW, Brands J, Vink H. Agonist-induced impairment of glycocalyx exclusion properties: contribution to coronary effects of adenosine. Cardiovasc Res. 2010;87:311–319. doi: 10.1093/cvr/cvq114. [DOI] [PubMed] [Google Scholar]
- 82.VanTeeffelen JW, Constantinescu AA, Brands J, Spaan JA, Vink H. Bradykinin- and sodium nitroprusside-induced increases in capillary tube haematocrit in mouse cremaster muscle are associated with impaired glycocalyx barrier properties. J Physiol. 2008;586:3207–3218. doi: 10.1113/jphysiol.2008.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer : implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 84.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278:H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 85.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 86.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 87.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Werthmann RC, Lohse MJ, Bunemann M. Temporally resolved cAMP monitoring in endothelial cells uncovers a thrombin-induced [cAMP] elevation mediated via the Ca(2)+-dependent production of prostacyclin. J Physiol. 2011;589:181–193. doi: 10.1113/jphysiol.2010.200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams DA. A shear stress component to the modulation of capillary hydraulic conductivity (Lp) Microcirculation. 1996;3:229–232. doi: 10.3109/10739689609148293. [DOI] [PubMed] [Google Scholar]
- 90.Wojciechowski JC, Sarelius IH. Preferential binding of leukocytes to the endothelial junction region in venules in situ. Microcirculation. 2005;12:349–359. doi: 10.1080/10739680590934763. [DOI] [PubMed] [Google Scholar]
- 91.Xu D, Wang TL, Sun LP, You QD. Recent progress of small molecular VEGFR inhibitors as anticancer agents. Mini Rev Med Chem. 2011;11:18–31. doi: 10.2174/138955711793564015. [DOI] [PubMed] [Google Scholar]
- 92.Yao Y, Rabodzey A, Dewey CF., Jr Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H1023–H1030. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- 93.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol. 1992;263:H641–H646. doi: 10.1152/ajpheart.1992.263.2.H641. [DOI] [PubMed] [Google Scholar]
- 95.Zeng M, Zhang H, Lowell C, He P. Tumor necrosis factor-alpha-induced leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol. 2002;283:H2420–H2430. doi: 10.1152/ajpheart.00787.2001. [DOI] [PubMed] [Google Scholar]
- 96.Zhang X, Adamson RH, Curry FE, Weinbaum S. Transient regulation of transport by pericytes in venular microvessels via trapped microdomains. Proc Natl Acad Sci U S A. 2008;105:1374–1379. doi: 10.1073/pnas.0710986105. [DOI] [PMC free article] [PubMed] [Google Scholar]