Abstract
Object
Variants of microRNA (miRNA)-binding sites in RAD51 gene’s 3′ untranslated region (3′UTR) are significantly associated with cancer risk, but the roles of these genetic variants in post-transcriptional regulation have not been elucidated.
Methods
The SNPs of RAD51 were identified both in the regulatory region and in the coding region by means of the online database. The bioinformatic tool SNP Function Prediction was used to predict the potential functional relevance of the miRNA-binding sites. We used additional data on RAD51 genotypes and mRNA levels available online for the genotype-phenotype association analysis.
Results
We found that rs12593359, rs7180135, rs11855560, and rs45507396 in the RAD51 3′UTR affect possible miRNA-binding sites according to bioinformatic analysis. Only rs12593359 was significantly associated with RAD51 mRNA expression in lymphoblastoid cell lines (P = 0.022).
Conclusion
This study demonstrated that rs12593359 may be a putative variant mediating the post-transcriptional regulation of the RAD51 gene. Deeper understanding of how 3′UTR variants influence RAD51 activity will pave the way to targeting of the RAD51 pathway as a cancer treatment.
Keywords: Genetic, MicroRNA, Polymorphism, RAD51, Variant
Background
Cancer is a common fatal disease and results from complex interactions between environmental and genetic factors (Pharoah et al. 2004). More and more studies have been focused on the role of gene polymorphisms in the etiology of cancers. Lately, there is growing evidence that single nucleotide polymorphisms (SNPs) play an important role in carcinogenesis (Zeljic et al. 2014; Vispé et al. 1998). DNA repair systems are believed to maintain genome integrity by countering threats posed by DNA lesions. Deficiencies in DNA repair pathways may leave such lesions unrepaired or repaired incorrectly, eventually leading to genomic instability or mutations that may contribute directly to cancer.
The RAD51 gene is located in chromosomal region 15q15.1 in humans (Shinohara et al. 1993). The RAD51 protein encoded by RAD51 is necessary for the repair of DNA damage. Growing evidence indicates that RAD51 performs an irreplaceable function in the maintenance of genomic stability and in the repair of DNA double-strand breaks (Baumann and West 1998). RAD51 genetic variations may contribute to the development of cancers (Thacker 2005). Overexpression of RAD51 was also found to be associated with a poor prognosis in squamous cell carcinoma of the head and neck, colorectal cancer, ovarian cancer, and breast cancer (Gresner et al. 2012; Krupa et al. 2011; Romanowicz-Makowska et al. 2012; Wieqmans et al. 2014). A variety of molecular epidemiological studies have been conducted to estimate the association between the RAD51 135G/C polymorphism and risk of cancers (Wang et al. 2013; Cheng et al. 2014). In addition, RAD51 is intimately involved in both the pathogenesis of malignant tumors and progression of various types of cancer.
It is well known that variants of microRNA (miRNA)-binding sites can alter gene functions. MiRNAs can regulate the activity of RAD51, and miRNA dysregulation has been implicated in neoplasms. For instance, Huang and colleagues used luciferase reporter constructs containing the RAD51 wild-type or mutant 3′UTRs, and they found that miR-103/107 directly targets the 3′UTR of RAD51 and that miR-103/107 overexpression in combination with cisplatin or other DNA-damaging agents may therefore have therapeutic utility in terms of chemosensitization of tumors (Huang et al. 2013). In addition, a number of studies have shown that RAD51 variants may perform crucial functions in carcinogenesis, but the role of variants of miRNA-binding sites of RAD51 is still unknown (Thacker 2005; Gresner et al. 2012). In the present study, we performed a bioinformatic analysis and genotype-phenotype association analysis by means of the HapMap database to test our hypothesis that RAD51 3′UTR variants are associated with miRNA regulation of this gene’s expression.
Methods
Bioinformatic analysis and selection of polymorphisms
The SNPs of RAD51 were identified both in the regulatory region and in the coding region by means of the online database (http://www.ncbi.nlm.nih.gov/SNP/). The bioinformatic tool SNP Function Prediction (FuncPred; http://www.snpinfo.niehs.nih.gov/cgi-bin/snpinfo/snpfunc.cgi) was used to predict the potential functional relevance of the miRNA-binding sites. Additionally, we limited our analysis to SNPs with minor allele frequency (MAF) >5 % in the HapMap populations “Utah residents with Northern and Western European ancestry (CEU)” and calculated pairwise linkage disequilibrium (LD) values of all the SNPs in the same gene. Then, we selected the SNPs that were not in LD (r2 < 0.8) and constructed LD maps of those SNPs of the RAD51 gene using a Web service (http://www.snpinfo.niehs.nih.gov/cgi-bin/snpinfo/snpfunc.cgi).
Genotype data and mRNA expression data on lymphoblastoid cell lines from the HapMap database
We used additional data on RAD51 genotypes and mRNA levels available online (http://www.app3.titan.uio.no/biotools/help.php?app=snpexp) for the genotype-phenotype association analysis (Holm et al. 2010). We used genome-wide expression arrays (47,294 transcripts) from Epstein–Barr virus-transformed lymphoblastoid cell lines from the same 270 HapMap individuals (142 males and 128 females) to analyze the gene expression variations (Stranger et al. 2007). The data on genotyping were retrieved from the HapMap phase II release 23 dataset consisting of 3.96 million SNP genotypes from 270 individuals from 4 populations (International HapMap Consortium 2003). A tool called SNPexp v1.2 was used for calculating and visualizing the correlations between HapMap genotypes and gene expression levels (Norwegian PSC Research Center, Clinic for Specialized Surgery and Medicine, Oslo University Hospital Rikshospitalet, Norway).
Ethics statement
The study was approved by the Ethics Committee of Union Hospital Tongji Medical College of Huazhong University of Science and Technology and conforms to the provisions of the Declaration of Helsinki.
Statistical methods
Genotype-phenotype correlations were analyzed by the χ2 test. All statistical tests were two-sided, and data with P < 0.05 were considered statistically significant.
Results
Selected variants of RAD51 3′UTR and putative miRNA-binding sites
In total, 849 SNPs were identified in the RAD51 gene region, and 38 in the mRNA-coding region (http://www.ncbi.nlm.nih.gov/SNP/). Among them, 24 SNPs are located in the 3′UTR, and only 4 SNPs of these (rs12593359, rs7180135, rs11855560, rs45507396) have a known MAF value >0.05. These four SNPs were predicted to affect the miRNA-binding site activity according to the bioinformatic analysis, as shown in Table 1. The most extensively studied SNPs of these affect putative binding sites of hsa-miR-129-3p, hsa-miR-1248, hsa-miR-1303, hsa-miR-197, hsa-miR-220b, hsa-miR-767-5p, hsa-miR-876-5p, hsa-miR-642, and hsa-miR-299-5p (http://www.snpinfo.niehs.nih.gov/cgi-bin/snpinfo/snpfunc.cgi). In combination with other SNPs in the 3′UTR or promoter region, variant rs7180135 is jointly involved in cancer susceptibility (Teo et al. 2012).
Table 1.
Selected SNPs of RAD51 3′UTR and putative miRNA binding sites
| Name | Alleles | MAF | Putative miRNA binding sites |
|---|---|---|---|
| rs12593359 | G/T | 0.416 | hsa-miR-129-3p |
| rs7180135 | A/G | 0.273 | hsa-miR-1248,hsa-miR-1303,hsa-miR-197,hsa-miR-220b,hsa-miR-767-5p,hsa-miR-876-5p |
| rs11855560 | C/T | 0.416 | hsa-miR-642 |
| rs45507396 | A/G | 0.086 | hsa-miR-299-5p |
SNP single nucleotide polymorphism; 3′UTR 3′ untranslated region; MAF minor allele frequency; NA not available
Calculation of LD of all SNPs in the RAD51 gene
The bioinformatic tool FuncPred (http://www.snpinfo.niehs.nih.gov/snpfunc.htm) helped us to identify the potential functional relevance of the SNPs. We calculated pairwise LD values of all SNPs in the same gene to select the SNPs that were not in LD (r2 < 0.8), and plotted the LD maps of those RAD51 SNPs by means of FuncPred. The pairwise r2 correlations between the two relevant SNPs were represented by each square number. The color of each SNP spot reflects its D′ value, and when the D′ value decreases, the color changes from red to white. The haplotype blocks were estimated using the FuncPred software. The MAF of all of the above alleles was greater than 0.05. Polymorphisms rs12593359 and rs11855560 were the predicted tag SNPs in our study, whereas rs7180135 and rs45507396 in RAD51 were not included in the LD plot (Fig. 1).
Fig. 1.
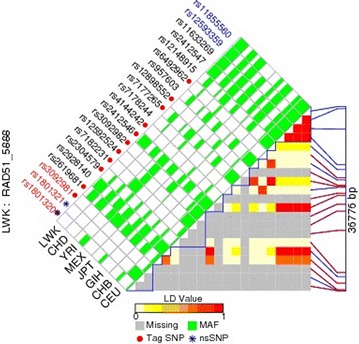
A linkage disequilibrium plot of the RAD51 region according to SNP Function Prediction software (FuncPred). Each square number represents the pairwise r2 correlations between the two relevant SNPs. The color of each SNP spot reflects its D′ value, which changes from red to white as the D′ value decreases. SNP single nucleotide polymorphism
RAD51 mRNA expression by genotypes in lymphoblastoid cell lines
For evaluation of mRNA expression of the prohibitin gene in the lymphoblastoid cell lines, we took advantage of the available HapMap-cDNA expression database to analyze the correlation of prohibitin genotype with mRNA expression in 270 HapMap lymphoblastoid cell lines. With the exception of the one cell line with unavailable values for rs12593359, 64 (24.24 %) cell lines with the TT genotype, 114 (43.18 %) cell lines with the TG genotype, and 86 (32.58 %) cell lines with the GG genotype were identified. For rs11855560, 64 (24.24 %) cell lines had the TT genotype, 114 (43.18 %) the TC genotype, and 86 (32.58 %) cell lines had the CC genotype. For rs7180135, 148 (55.43 %) cell lines had the AA genotype, 89 (33.33 %) cell lines the AG genotype, and 30 (11.24 %) cell lines had the GG genotype. Figure 2 shows the RAD51 mRNA expression levels of cell lines by RAD51 genotype. The rs12593359 GG genotype was found to yield significantly lower expression levels than the TT and TG genotypes did (P = 0.022; Fig. 2a), and there were significant differences in RAD51 mRNA expression levels among the cell lines carrying rs11855560 (P = 0.022) and rs7180135 (P = 0.016) genotypes (Fig. 2b, c).
Fig. 2.
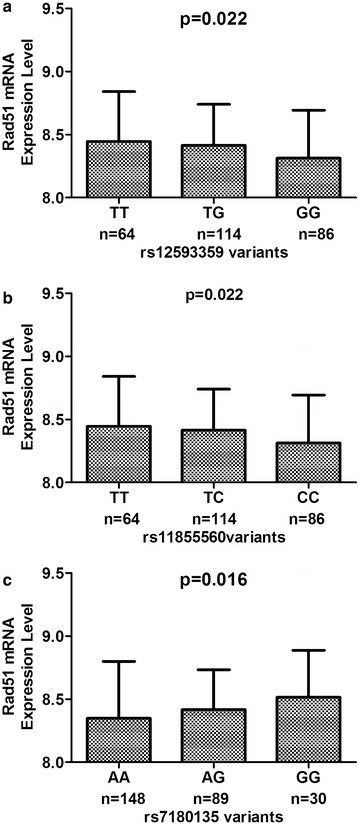
The mRNA expression levels of RAD51 uncovered by the genotype–phenotype association analysis of RAD51 variants, rs12593359 (a), rs11855560 (b), rs7180135 (c) and RAD51 mRNA expression in Epstein-Barr virus-transformed lymphoblastoid cell lines from the HapMap database
Discussion
The RAD51 gene, a homolog of recA from Escherichia coli, has been mapped to chromosomal region 15q15.1 in humans (Shinohara et al. 1993). It spans >39 kb, contains ten exons and encodes a 339-amino acid protein (GenBank accession No.: NM_133487). The RAD51 gene produces a protein also called RAD51, which is necessary for repair of damaged DNA (Lo et al. 2003). A number of studies, such as tissue expression experiments, animal models, and clinical trials suggest that RAD51 plays an important role in carcinogenesis, and numerous research groups have analyzed the link between RAD51 polymorphisms and cancer risk (Cheng et al. 2014; Maacke et al. 2000; Vispe et al. 1998). In the present study, the effect of SNPs in genes involved in DNA repair mechanisms on the response to treatment and survival in gastric cancer patients treated with platinum-based chemotherapy was investigated (Ding et al. 2015).
The present study supports the notion that mutations in miRNA-binding regions contribute to altered gene function. Although our findings indicate that rs12593359, rs7180135, rs11855560, and rs45507396 in the RAD51 3′UTR affect potential miRNA-binding sites according to bioinformatic analysis, we found that rs12593359 (P = 0.022), rs7180135 (P = 0.022), and rs11855560 (P = 0.016), but not rs45507396, were significantly associated with RAD51 mRNA expression levels in lymphoblastoid cell lines. Polymorphisms rs12593359 and rs11855560 were the predicted tag SNPs in our study; therefore, we believe that these variants may contribute the RAD51 post-transcriptional regulation to some extent. Our results point to the mechanism underlying the effects of rs12593359 and rs11855560 on the regulation of RAD51 expression and provide an explanation for the tumor susceptibility associated with these SNPs according to cancer research. It is possible that those genetic variants in RAD51 3′UTR may modulate its expression and the variants affecting RAD51 miRNA-binding sites are associated with carcinogenesis.
In conclusion, the results of the present study suggest that the RAD51 rs12593359 SNPs in DNA repair pathways may be utilized as predictive factors of the clinical outcome, and these SNPs may contribute to chemotherapy. In addition, our study may improve the understanding of the regulatory roles of miRNA variants of RAD51 3′UTR in its mRNA expression and the translation of pharmacogenetic predictors into clinical practice may lead to improved cancer treatment planning and outcome.
Conclusion
In summary, RAD51 variants have a strong influence on post-transcriptional regulation, and this finding highlights the importance of miRNA-mediated regulation of expression of cancer-associated genes, in particular, with respect to research on prognostic and diagnostic markers of malignancy. Besides, our findings should elucidate how the 3′UTR variants regulate RAD51 activity and thus will pave the way to targeting of the RAD51 pathway as a cancer treatment. Nonetheless, our data need to be validated by functional analysis of the mechanism underlying the association of RAD51 transcriptional activity with variants in the 3′UTR.
Authors’ contributions
FP designed the study, performed the data analysis and interpreted the manuscript. HZ has performed the experiments and analyzed the data. FC has wrote the manuscript. All authors contributed in the overall process of the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Fengxia Chen, Email: chenfengxiayangtze@163.com.
Haozhong Zhang, Email: zhanghaozhong112@163.com.
Feifei Pu, pufeifeiemail@163.com.
References
- Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double-stranded break repair. Trends Biochem Sci. 1998;23(7):247–251. doi: 10.1016/S0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- Cheng D, Shi H, Zhang K, Yi L, Zhen G. RAD51 Gene 135G/C polymorphism and the risk of four types of common cancers: a meta-analysis. Diagn Pathol. 2014;9:18. doi: 10.1186/1746-1596-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Zhang H, Chen K, Zhao C, Gao J. Genetic variability of DNA repair mechanisms influences treatment outcome of gastric cancer. Oncol Lett. 2015;10(4):1997–2002. doi: 10.3892/ol.2015.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresner P, Gromadzinska J, Polanska K, Twardowska E, Jurewicz J, Wasowicz W. Genetic variability of Xrcc3 and Rad51 modulates the risk of head and neck cancer. Gene. 2012;504(2):166–174. doi: 10.1016/j.gene.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Holm K, Melum E, Franke A, Karlsen TH. SNPexp—a web tool for calculating and visualizing correlation between HapMap genotypes and gene expression levels. BMC Bioinform. 2010;11:600. doi: 10.1186/1471-2105-11-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JW, Wang Y, Dhillon KK, et al. Systematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivity. Mol Cancer Res. 2013;11(12):1564–1573. doi: 10.1158/1541-7786.MCR-13-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium The International HapMap Project. Nature. 2003;426(6968):789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Krupa R, Sliwinski T, Wisniewska-Jarosinska M, et al. Polymorphisms in RAD51, XRCC2 and XRCC3 genes of the homologous recombination repair in colorectal cancer-a case control study. Mol Biol Rep. 2011;38(4):2849–2854. doi: 10.1007/s11033-010-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T, Pellegrini L, Venkitaraman AR, Blundell TL. Sequence fingerprints in BRCA2 and RAD51: implications for DNA repair and cancer. DNA Repair (Amst) 2003;2(9):1015–1028. doi: 10.1016/S1568-7864(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Maacke H, Opitz S, Jost K, et al. Over-expression of wild-type Rad51 correlates with histological grading of invasive ductal breast cancer. Int J Cancer. 2000;88(6):907–913. doi: 10.1002/1097-0215(20001215)88:6<907::AID-IJC11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4(11):850–860. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- Romanowicz-Makowska H, Smolarz B, Samulak D, et al. A single nucleotide polymorphism in the 5′untranslated region of RAD51 and ovarian cancer risk in polish women. Eur J Gynaecol Oncol. 2012;33(4):406–410. [PubMed] [Google Scholar]
- Shinohara A, Ogawa H, Matsuda Y, Ushio N, Ikeo K, Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993;4(3):239–243. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- Stranger BE, Forrest MS, Dunning M, et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007;315(5813):848–853. doi: 10.1126/science.1136678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo MT, Landi D, Taylor CF, et al. The role of microRNA-binding site polymorphisms in DNA repair genes as risk factors for bladder cancer and breast cancer and their impact on radiotherapy outcomes. Carcinogenesis. 2012;33(3):581–586. doi: 10.1093/carcin/bgr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker J. The RAD51 gene family, genetic instability and cancer. Cancer Lett. 2005;219(2):125–135. doi: 10.1016/j.canlet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Vispé S, Cazaux C, Lesca C, Defais M. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 1998;26(12):2859–2864. doi: 10.1093/nar/26.12.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li JL, He XF, et al. Association between the RAD51 135 G > C polymorphism and risk of cancer: a meta-analysis of 19,068 cases and 22,630 controls. PLoS One. 2013;8(9):e75153. doi: 10.1371/journal.pone.0075153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieqmans AP, Al-Ejeh F, Chee N, et al. Rad51 supports triple negative breast cancer metastasis. Oncotarget. 2014;5(10):3261–3272. doi: 10.18632/oncotarget.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeljic K, Supic G, Jovic N, et al. Association of TLR2, TLR3, TLR4 and CD14 genes polymorphism with oral cancer risk and survival. Oral Dis. 2014;20(4):416–424. doi: 10.1111/odi.12144. [DOI] [PubMed] [Google Scholar]


