Abstract
In learning and memory studies on honeybees (Apis mellifera), cold-induced narcosis has been widely used to temporarily immobilize honeybees. In this study, we investigated the effects of cold narcosis on the associative memories in honeybees by using the proboscis extension response (PER) paradigm. Severeimpairments in memory acquisitionwas found when cold narcosis was performed 30 min, instead of 1 h before training. Locomotor activities were reduced when honeybees were tested 15 min, instead of 30 min after cold narcosis. These results indicate that cold narcosis impairs locomotor activities, as well as memory acquisition in a time-dependent manner, but by comparison no such effects on memory retrieval have yet been observed.[0]
Keywords: Honeybee, Cold narcosis, Associative learning, Memory
Since the 1970s, honeybees (Apis mellifera) have become a useful model in the studies of learning and memory (Menzel, 1999, 2001, 2012). Bees respond reflexively to sucrose when a drop of solution is applied to the antennae, which is called proboscis extension reflex (PER) (Giurfa, 2003). PER conditioning is a well-established paradigm for investigating the mechanisms underlying associative memory processing in bees (Page & Erber, 2002). However, in this paradigm, the honeybee should be firstly harnessed to avoid flying away (Batson et al, 1992). Thus, the cold induced narcosis by putting honeybees on ice is frequently used to temporarily immobilize them for harnessing. It is shown that cold narcosis not only anaesthetizes bees, but also masks, blocks, or bypasses the putative effects of handling stress on bees (Pankiw & Page, 2003). However, the effects of cold narcosis on learning and memory of bees have received little attention.
The effects of anesthesia on learning and memory have been tested by many studies, e.g. short-term impairment of cognitive and psychomotor performance is common after general anesthesia (Moller et al, 1993); in isoflurane-treated rats, object recognition and reversal learning are significantly impaired (Zhu et al, 2010); sustained learning impairment is observed after anesthesia in aged rats (Culley et al, 2003), which is consistent with the cognitive impairment in elderly patients after anesthesia and surgery (Moller et al, 1998).
By comparison, studies on the effects of cold treatment in honeybees are very limited. Ebadi et al (1980) reported that short time chilling affects neither mortalities nor fecundity in honeybees. However, Robinson & Visscher (1984) found that the hoarding activities in bees decrease when they have being exposed to -20 ℃ for 3 min in laboratory setting. Moreover, Erber et al (1976, 1980) found that cooling leads to a retrograde amnesia if the treatments are applied between 30 s to 7 min after single trial learning in free flying bees, whereas, the whole body cooling impairs memory formation if it is performed within 5 min after conditioning. While all these studies were only focused on memory consolidation, the effects of cold treatment on memory acquisition and retrieval are to be elucidated. Memory processes include acquisition, consolidation and retrieval and during which many signaling molecules or pathways are involved or have selectively been recruited during certain memory stages (Abel & Lattal, 2001; Makkar et al, 2010).
In the present study, honeybees were trained to learn to respond to an odour associated with sucrose solution, meanwhile, to avoid responding to another odour associated with saturated NaCl solution in a two-odour discrimination PER paradigm (Fu et al, 2013; Maleszka & Helliwell, 2001; Si et al, 2004). Then, the retention tests were performed 1 h later to assess the mid-term olfactory memory. While, to evaluate the effects of cold narcosis on memory acquisition, consolidation and retrieval, honeybees were given cold treatment 30 min before and immediately after the training, as well as 30 min before the retention test, respectively. Meanwhile, honeybees were also given cold treatment 1 h prior to training to determine the time dependence of these effects. Moreover, due to the anesthesia induced by acute chilling, their locomotor activities 15 min and 30 min after cold treatment were evaluated.
MATERIALS AND METHODS
Animals
A colony of honeybees was obtained from the breeding center of Eastern Bee Research Institute of Yunnan Agricultural University, Kunming, Yunnan, China. Individual frames of brood combs containing pupae (sealed in cells) were removed from the experimental hives and placed in an incubator (32−33 ℃, 65% relative humidity, with good ventilation). Newly emerged honeybees were collected from the frame every day, ensuring that the experiment was performed only on honeybees of a known age. Honeybees of the same age were housed in an individual wooded cage covered with wire inside of the incubator (3.5 cm×20 cm×16.5 cm: width×length×height) and were fed ad libitum with 50% sugar solution. Freshly prepared solutions were supplied every the other day. All experiments were performed according to the National Care and Use of Animals Guideline approved by the National Animal Research Authority and were conformed within international guidelines for the ethical use of animals.
PER conditioning
Nine days old (which is a proper age for learning and memory tests) honeybees were collected from the incubator cage, cooled on ice until immobile (within 90 s−2 min), and then were mounted in thin-walled plastic tubes (5 mm in diameter) by using a thin strip of rubberized fabric tape between head and thorax with antennae and proboscis moving freely. After revival, each honeybee was fed with 30% sucrose solution via a 1 mL syringe without needle. After feeding, honeybees were arranged in a wooden board holder and were then placed in an incubator overnight (29℃). The PER conditioning was performed on the following day.
The performance of honeybees on a two-odor discrimination learning task was evaluated by PER conditioning (Si et al, 2004; Maleszka & Helliwell, 2001). Bees were trained to associate the odour of limonene (Guangzhou Flower Flavour & Fragrances Co., Ltd, Guangdong, China) with sucrose (Shantou Xilong Chemical Factory, Guangdong, China) reward (CS+) and the odor of menthene (Guangzhou Flower Flavour & Fragrances Co., Ltd, Guangdong, China) with saturated NaCl (Tianjin Bodi Chemical Corporation, Tianjin, China) punishment (CS−). Limonene and menthene were mixed with 30% sucrose or saturated NaCl solution respectively into a proportion of 1:200, which is consistent with previous reports (Si et al, 2004; Hammer & Menzel, 1995).
The training session was consisted of two trials. Firstly, a drop of sucrose solution with limonene (CS+) was presented in front of the honeybee for 6 s, and then was applied to one antenna to lead a proboscis extension. Then the honeybee was fed with sucrose solution for 1 s (95% of the honeybees had extended their proboscis at the presence of the odor of limonene, and the 2% of honeybees did not respond with sucrose solution were excluded from the experiment). Secondly, after a short interval (1−2 min), a drop of salt solution with menthene (CS−) was applied to another antenna after being presented in front of the honeybee for 6 s. Then, if the proboscis extended, honeybees were fed with NaCl solution for 1 s. Approximately, 90% of the honeybees had extended their proboscis when the odor of menthene was presented, slightly less than the odor of lemonene. However, all honeybees shrunk back their antennae once touched with the salt solution.
The testing session was performed 1 h later after the conditioning. Honeybees were presented with the punishing stimulus (menthene) followed with the rewarding stimulus (limonene). Meanwhile, their proboscis activities were recorded. When a honeybee extended its proboscis at the presence of a stimulus, it was recorded as “right”, otherwise, it was recorded as “wrong”. Honeybees that extended their proboscis only at the presence of rewarding stimulus but not at the presence of non-rewarding stimulus were scored as having responded correctly, whereas, honeybees that did not respond to either of the stimuli or were unable to extend their proboscis when stimulated with sucrose were regarded as abnormal individuals and were not included in data analysis.
During the conditioning and testing, a mini exhaust fan (Deepcool, Beijing, China) was positioned behind honeybees to help air flow as well as to eliminate odours after stimulation.
Experimental procedures
The entire experimental procedure (three sets) was performed double-blinded with separated cohorts of honeybees.
Experiment 1 was to investigate the effects of cold narcosis on acquisition, consolidation and retrieval of olfactory memory. Honeybees received cold-induced narcosis 30 min before training (acquisition, n=34), immediately after training (consolidation, n=33) or 30 min before testing (retrieval, n=32), respectively. The control group (n=22) was not under any treatment (Figure 1).
Figure 1.
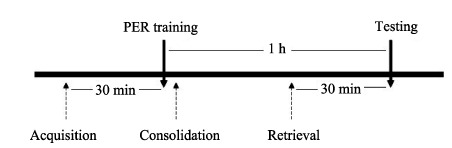
Flow diagram of experiment 1
In experiment 2, honeybees were given cold narcosis 1 h prior to the PER training (n=21), and the testing was performed 1 h later after training. Control honeybees (n=22) were not under any treatment.
To investigate the effects of cold narcosis on the locomotor activities in honeybee, in experiment 3, their locomotor activities were monitored 15 min (n=24) and 30 min (n=23) later after cold narcosis, respectively. Control bees (n=23) was not under any treatment.
Cold narcosis
Individual harnessed honeybee was cooled on ice for 90 s by being placed on a piece of tinfoil to avoid getting wet. They usually were immobilized within 1 min and got revived 3 min after being moved away from the ice.
Locomotor activity testing
Cold-treated honeybees were individually housed in a small glass vial (2 cm×4 cm : diameter×height) and then were put onto ice for 90 s, whereas, their controls were kept in the small vials without cooling. Cold-treated honeybees usually revived from the immobile state within 3 min. Locomotor activity test was carried out in 12 petri dishes (8.5 cm in diameter) in 3×4 arrays, with each dish accommodating one honeybee. The behaviors of honeybees were monitored for 5 min with a ceiling-mounted CCD camera (Tikal, Shenzhen, Guangdong, China). Video signals were then displayed and saved by video recording software (Smart Lab software). Video recordings were later analyzed and the ambulation paths and distances (cm) of honeybees were measured.
Data analysis
In PER conditioning, data were represented as the percentage of honeybees that responded correctly to limonene and menthene in testing session. Cold narcosis (30 min before, immediately after training, or 30 min before testing) was considered as a between-group factor. Data were analyzed by using SPSS statistical soft package (version 13.0). Between-group comparisons were completed with chi-square test (χ2-test). One-way ANOVA was used to analyze the locomotor activities of control and cold-treated honeybees.
RESULTS
Effects of cold narcosis on acquisition, consolidation and retrieval of mid-term olfactory memory in honeybees
Figure 2A showed that compared with the control group, cold narcosis administrated either 30 min prior to training (Acqu) (Ctrl: 81.8% (18/22); Acqu: 41.2% (14/34); χ2=9.01, P=0.005) or immediately after training (Cons) (Ctrl: 81.8% (18/22);Cons: 45.5% (15/33); χ2= 7.27, P=0.011) reduced the correct responding percentages of honeybees to conditioned stimuli, whereas, no significant differences were found when cold narcosis was administrated 30 min before testing (Retr: 75% (24/32); χ2=0.35, P=0.742). However, when cold narcosis was administrated 1 h before training, honeybees were showed with intact memories in discriminating the rewarding and punishing stimuli (Ctrl: 80.1% (17/22); Acqu 1 h: 90.5% (19/21); χ2=1.37, P=0.412) (Figure 2B).
Figure 2.
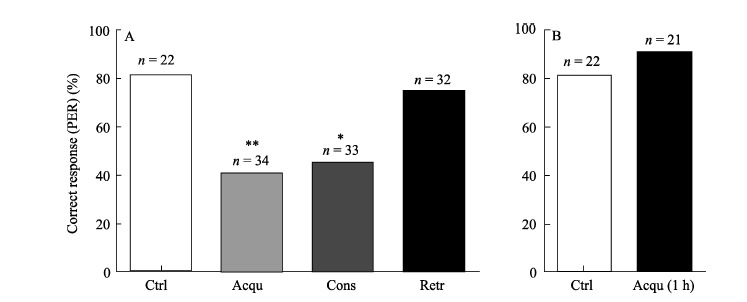
Effects of cold narcosis on the percentage of correct responses of honeybees in the associative olfactory tasks
Effects of acute cooling on the locomotor activities of individually isolated bees
Ambulation paths and distances of honeybees were monitored and measured by the video analysis system developed in our lab (Figure 3). One-way ANOVA showed that compared with the control group, 15 min after the cold narcosis, the 5 min ambulation distances of honeybees decreased significantly (Ctrl: 346.65±27.1 cm; 15 min: 212.46±36.4 cm; F (1, 45)=8.65, P=0.005). However, 30 min after the cold treatment, no difference was found (30 min: 358±39.9 cm) (Figure 4)
Figure 3.
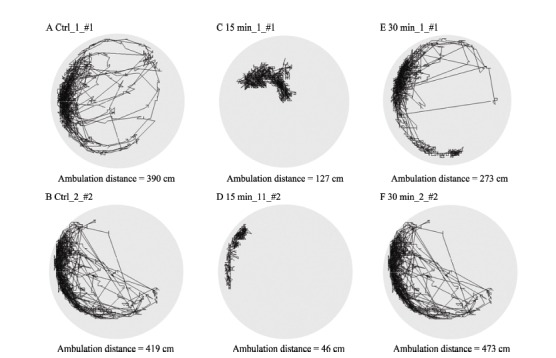
Effects of cold narcosis on the ambulation paths of honeybees
Figure 4.
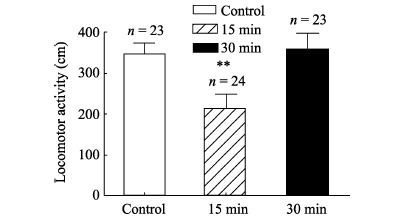
Effects of cold narcosis on ambulation distances of honeybees
DISCUSSION
Memory is a complex process involving three different stages: acquisition, consolidation, and retrieval. In the present study, we found that cold narcosis significantly impaired the memory acquisition and consolidation but not retrieval in honeybees.
Cold narcosis impairs memory acquisition in bees, which is consistent with previous studies showing that anesthesia destroys memory acquisition in mice and rats, e, g, ketamine treated rats need more time to reach the hidden platform in Morris Watermaze (Moosavi et al, 2012); Culley et al (2003) observed impaired acquisition of spatial memory in aged rats and mice, which were administrated with isoflurane anesthesia two weeks earlier.
In the present study, the impairing effects of cold narcosis on memory acquisition in honeybees were time-dependent and short-termed. Cold narcosis administrated 1 h before training did not affect the olfactory memory, which may be due to the fact that brain temperature could go back to normal after cold treatment, therefore the impairing effects of cold narcosis were reversed. These results also suggested that when using honeybees as an animal model, at least 1 h was necessary for them to revive from harnessing before any training trial.
Moreover, we also found the impairing effects of cold narcosis on memory consolidation in the present study. Fresh memories are sensitive to disruption. Consolidation starts after training and may need hours to stabilize. The effects of cold treatment on memory consolidation in honeybees have been reported in previous studies, e.g, the reversible block of neural activities induced by the localized cooling of mushroom bodies in honeybee brain impairs its memory formation within 3−10 min after training (Erber et al, 1980).
In the present study, memory retrieval was not significantly affected by cold narcosis, suggesting that it was more resistant to cold treatment than memory acquisition and consolidation were. Previous studies demonstrated that memory acquisition and consolidation shared with many molecular mechanisms while memory retrieval might recruit different ones (Abel & Lattal, 2001; Carlini et al, 2010). Our study supported this view and showed that cold narcosis impaired acquisition and consolidation, but not memory retrieval of mid-term olfactory memory in harnessed honeybees.
Besides of the anesthetic effects, brain activity is also temperature-dependent. In human beings, significant decrements have been observed in several types of cognitive measurements following cold stress, including vigilance, reaction time, reasoning skills, and short-term memory (Coleshaw et al, 1983; Patil et al, 1995). In rats that performed on a delayed matching-to-sample (DMTS) task at ambient temperatures of 23 ℃ and 2 ℃, matching accuracy was significantly decreased during exposure to 2 ℃ (Ahlers et al, 1991; Steele & Morris, 1999). Studies have found that temperature fluctuations in brain significantly affect the electrophysiological responses in many brain areas (Winter, 1973). The amplitude of action potentials in neurons could be reduced by cooling (Andersen & Moser, 1995). Stimulus-elicited neuron transmitter release was slower and less synchronized in cold conditions (Barrett et al, 1978). Furthermore, cold treatment as a stressor could affect learning and memory as reported in many researches (Zheng et al, 2008; Gunstad et al, 2009).
Since the time interval between training trial and testing trial was 1 h, the retrieval group received cold treatment 30 min after training. This finding was consistent with studies showing that signals were held in a long-term storage which was no longer sensitive to impairment 15 min after conditioning (Erber et al, 1980). These also indicated that post-acquisition cold stress could disrupt early consolidation process.
Fifteen min after the cold narcosis, the locomotor activities of honeybees decreased severely compared with those of control honeybees. However, 30 min after the treatment, their locomotor activities revived to the normal level. Thus, the impairments of olfactory memory acquisition and consolidation were not due to the cold induced locomotor activity decreasing. The intact memory retrieval in this study also supports this view.
In conclusion, the PER paradigm applied in this study is widely used to test learning and memory in honeybees, through which, we found that the mid-term olfactory memory acquisition and consolidation in honeybees could be sensitively affected by cold-induced narcosis, whereas memory retrieval might be cold resistant.
Acknowledgments
We thank Chang LIU, Ping JIANG, Yu-Hua ZHANG, Jing-Kuan WEI and Tao ZENG for their assistance with the experiments.
Funding Statement
This study was supported by the National Science Foundation of China (91132307) and the K.C.Wong Education Foundation, Hong Kong.
References
- 1. Abel T, Lattal KM. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Current Opinion in Neurobiology, 11 (2): 180- 187. [DOI] [PubMed] [Google Scholar]
- 2. Ahlers ST, Thomas JR, Berkey DL. 1991. Hippocampal and body temperature changes in rats during delayed matching-to-sample performance in a cold environment. Physiology & Behavior, 50 (5): 1013- 1018. [DOI] [PubMed] [Google Scholar]
- 3. Andersen P, Moser EI. 1995. Brain temperature and hippocampal function. Hippocampus, 5 (6): 491- 498. [DOI] [PubMed] [Google Scholar]
- 4. Barrett EF, Barrett JN, Botz D, Chang DB, Mahaffey D. 1978. Temperature-sensitive aspects of evoked and spontaneous transmitter release at the frog neuromuscular junction. Journal Physiology, 279 253- 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batson JD, Hoban JS, Bitterman ME. 1992. Simultaneous conditioning in honeybees (Apis mellifera). Journal of Comparative Psychology, 106 (2): 114- 119. [DOI] [PubMed] [Google Scholar]
- 6. Carlini VP, Ghersi M, Schiöth HB, de Barioglio SR. 2010. Ghrelin and memory: differential effects on acquisition and retrieval. Peptides, 31 (6): 1190- 1193. [DOI] [PubMed] [Google Scholar]
- 7. Coleshaw SR, Van Someren RN, Wolff AH, Davis HM, Keatinge WR. 1983. Impaired memory registration and speed of reasoning caused by low body temperature. Journal of Applied Physiology:Respiratory, Environmental and Exercise Physiology, 55 (1 Pt 1): 27- 31. [DOI] [PubMed] [Google Scholar]
- 8. Culley DJ, Baxter M, Yukhananov R, Crosby G. 2003. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesthesia & Analgesia, 96 (4): 1004- 1009. [DOI] [PubMed] [Google Scholar]
- 9. Ebadi R, Gary NE, Lorenzen K. 1980. Effects of carbon dioxide and low temperature narcosis on honey bees, Apis mellifera. Environmental Entomology, 9 (1): 144- 148. [Google Scholar]
- 10. Erber J. 1976. Retrograde amnesia in honeybees (Apis mellifera carnica). Journal of Comparative Psychology and Physiological Psychology, 90 (1): 41- 46. [DOI] [PubMed] [Google Scholar]
- 11. Erber J, Masuhr TH, Menzel R. 1980. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiological Entomology, 5 (4): 343- 358. [Google Scholar]
- 12. Fu Y, Chen YM, Yao T, Li P, Ma YY, Wang JH. 2013. Effects of morphine on associative memory and locomotor activity in honeybee (Apis mellifera). Neuroscience Bulletin, 29 (3): 270- 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giurfa M. 2003. Cognitive neuroethology: dissecting non-elemental learning in a honeybee brain. Current Opinion in Neurobiology, 13 (6): 726- 735. [DOI] [PubMed] [Google Scholar]
- 14. Gunstad J, Smith J, Muller MD, Updegraff J, Spitznagel MB, Pierce K, Glickman E. 2009. Effects of acute cold exposure on cognitive function: evidence for sustained impairments. 11th International Conference on Environmental Ergonomics, Boston. Ref Type: Conference Proceeding, [Google Scholar]
- 15. Hammer M, Menzel R. 1995. Learning and memory in the honeybee. The Journal of Neuroscience, 15 1617- 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makkar SR, Zhang SQ, Cranney J. 2010. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology, 35 (8): 1625- 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maleszka R, Helliwell P. 2001. Effect of Juvenile Hormone on Short-Term Olfactory Memory in Young Honeybees (Apis mellifera). Hormones and behavior, 40 403- 408. [DOI] [PubMed] [Google Scholar]
- 18. Menzel R. 1999. Memory dynamics in the honeybee. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 185 (4): 323- 340. [DOI] [PubMed] [Google Scholar]
- 19. Menzel R. 2001. Searching for the memory trace in a mini-brain, the honeybee. Learning & Memory, 8 (2): 53- 62. [DOI] [PubMed] [Google Scholar]
- 20. Menzel R. 2012. The honeybee as a model for understanding the basis of cognition. Nature Reviews Neuroscience, 13 (11): 758- 768. [DOI] [PubMed] [Google Scholar]
- 21. Moller JT, Svennild I, Johannessen NW, Jensen PF, Espersen K, Gravenstein JS, Cooper JB, Djernes M, Johansen SH. 1993. Perioperative monitoring with pulse oximetry and late postoperative cognitive dysfunction. British Journal of Anaesthesia, 71 (3): 340- 347. [DOI] [PubMed] [Google Scholar]
- 22. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JEW, Gravenstein JS. 1998. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. The Lancet, 351 (9106): 857- 861. [DOI] [PubMed] [Google Scholar]
- 23. Moosavi M, Yadollahi Khales G, Rastegar K, Zarifkar A. 2012. The effect of sub-anesthetic and anesthetic ketamine on water maze memory acquisition, consolidation and retrieval. European Journal of Pharmacology, 677 (1/3): 107- 110. [DOI] [PubMed] [Google Scholar]
- 24. Page RE, Erber J. 2002. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften, 89 (3): 91- 106. [DOI] [PubMed] [Google Scholar]
- 25. Pankiw T, Page RE Jr. 2003. Effect of pheromones, hormones, and handling on sucrose response thresholds of honey bees (Apis mellifera L). Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 189 (9): 675- 684. [DOI] [PubMed] [Google Scholar]
- 26. Patil PG, Apfelbaum JL, Zacny JP. 1995. Effects of a cold-water stressor on psychomotor and cognitive functioning in humans. Physiology & Behavior, 58 (6): 1281- 1286. [DOI] [PubMed] [Google Scholar]
- 27. Robinson GE, Visscher PK. 1984. Effect of low temperature narcosis on honey bee (Hymenoptera: Apidae) foraging behavior. The Florida Entomologist, 67 (4): 568- 570. [Google Scholar]
- 28. Si A, Helliwell P, Maleszka R. 2004. Effects of NMDA receptor antagonists on olfactory learning and memory in the honeybee (Apis mellifera). Pharmacology Biochemistry and Behavior, 77 (2): 191- 197. [DOI] [PubMed] [Google Scholar]
- 29. Steele RJ, Morris RGM. 1999. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus, 9 (2): 118- 136. [DOI] [PubMed] [Google Scholar]
- 30. Winter C. 1973. The influence of temperature on membrane processes. In: Wieser W. Effects of Temperature on Ectothermic Organisms. New York: Springer, 45- 53. [Google Scholar]
- 31. Zheng G, Chen Y, Zhang X, Cai T, Liu M, Zhao F, Luo W, Chen J. 2008. Acute cold exposure and rewarding enhanced spatial memory and activated the MAPK cascades in the rat brain. Brain research, 1239 171- 180. [DOI] [PubMed] [Google Scholar]
- 32. Zhu CL, Gao JF, Karlsson N, Li Q, Zhang Y, Huang ZH, Li HF, Kuhn HG, Blomgren K. 2010. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. Journal of Cerebral Blood Flow & Metabolism, 30 (5): 1017- 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]


