Abstract
Key points
Sensory input from peripheral receptors are important for the regulation of walking patterns.
Cutaneous input mediates muscle responses to deal with immediate external perturbations.
In this study we focused on the role of cutaneous feedback in locomotor adaptation that takes place over minutes of training.
We show that interfering with cutaneous feedback reduced adaptation to ankle perturbations during walking.
These results help us understand the neural mechanisms underlying walking adaptation, and have clinical implications for treating walking impairments after neurological injuries.
Abstract
Locomotor patterns must be adapted to external forces encountered during daily activities. The contribution of different sensory inputs to detecting perturbations and adapting movements during walking is unclear. In the present study, we examined the role of cutaneous feedback in adapting walking patterns to force perturbations. Forces were applied to the ankle joint during the early swing phase using an electrohydraulic ankle–foot orthosis. Repetitive 80 Hz electrical stimulation was applied to disrupt cutaneous feedback from the superficial peroneal nerve (foot dorsum) and medial plantar nerve (foot sole) during walking (Choi et al. 2013). Sensory tests were performed to measure the cutaneous touch threshold and perceptual threshold of force perturbations. Ankle movement were measured when the subjects walked on the treadmill over three periods: baseline (1 min), adaptation (1 min) and post‐adaptation (3 min). Subjects (n = 10) showed increased touch thresholds measured with Von Frey monofilaments and increased force perception thresholds with stimulation. Stimulation reduced the magnitude of walking adaptation to force perturbation. In addition, we compared the effects of interrupting cutaneous feedback using anaesthesia (n = 5) instead of repetitive nerve stimulation. Foot anaesthesia reduced ankle adaptation to external force perturbations during walking. The results of the present study suggest that cutaneous input plays a role in force perception, and may contribute to the ‘error’ signal involved in driving walking adaptation when there is a mismatch between expected and actual force.
Key points
Sensory input from peripheral receptors are important for the regulation of walking patterns.
Cutaneous input mediates muscle responses to deal with immediate external perturbations.
In this study we focused on the role of cutaneous feedback in locomotor adaptation that takes place over minutes of training.
We show that interfering with cutaneous feedback reduced adaptation to ankle perturbations during walking.
These results help us understand the neural mechanisms underlying walking adaptation, and have clinical implications for treating walking impairments after neurological injuries.
Abbreviations
- EMG
electromyographic
- PEST
Parameter Estimation by Sequential Testing
Introduction
The perception of force plays an important role in many skilled motor tasks because motor commands are altered by changes in perceived force (Gandevia & McCloskey, 1977; Jones & Hunter, 1983; Carson et al. 2002). For example, a skilled driver controls speed by handling the accelerator pedal with only as much force as is required. A bicyclist must monitor the amount of force on the foot sole and foot dorsum when pushing and pulling on the pedal, respectively. Cutaneous afferents detect pressure, form, motion and vibration in the skin (Johnson, 2001), providing the nervous system with the information that is necessary for the control of movement. Recently, we have shown that interfering with cutaneous feedback from the foot dorsum with repetitive electrical nerve stimulation changes force perception and disrupts isometric force control at the ankle joint (Choi et al. 2013).
The nervous system must detect and correct unexpected foot contact to prevent tripping on obstacles during the swing phase of walking. Muscle responses mediated by cutaneous input during specific phases of walking have been demonstrated in both humans (Duysens et al. 1990; Yang & Stein, 1990; Schillings et al. 2000) and other animals (Rossignol et al. 2006). Cutaneous input is not only important for quick (i.e. within step) regulation of walking, but also for long‐term plastic changes in the locomotor circuitry (e.g. after central nervous system injury). In the absence of cutaneous inputs from the hindpaw of cats (Bouyer & Rossignol, 2003 a), abnormal electromyographic (EMG) patterns and walking deficits can be observed in more demanding tasks (e.g. incline and ladder walking). Moreover, spinalized cats that would normally recover walking function from treadmill training could not do so without cutaneous input (Bouyer & Rossignol, 2003 b).
In addition to making corrective feedback adjustments in response to unexpected perturbations, the nervous system must also make anticipatory feedforward adaptation to counteract predictable perturbations. In the present study, we focus on the role of cutaneous feedback in feedforward adaptation of human walking patterns that takes place over minutes of training. When a force field is applied to the ankle joint during walking, the resultant movement errors drive locomotor adaptation to counter the effects of external force on walking (Noel et al. 2009; Blanchette et al. 2011). Once the locomotor pattern is adapted to the force field, after‐effects can be observed when the force field is removed and the locomotor pattern gradually de‐adapts back to the normal conditions. The presence of after‐effects indicates the involvement of feedforward locomotor adaptation. We hypothesize that cutaneous input contributes to the error signals that drive feedforward locomotor adaptation, and predict that locomotor adaptation would be reduced when cutaneous input is unavailable. To test this, repetitive electrical stimulation was applied to disrupt cutaneous feedback from the superficial peroneal (foot dorsum) and medial plantar (foot sole) nerves during the swing phase of walking. In addition, separate experiments were performed using lidocaine instead of nerve stimulation to reduce cutaneous input. We measured the effects of altered cutaneous feedback on touch threshold, force perception and adaptation to force field perturbations during walking.
Methods
Subjects
Eleven healthy volunteers (two females, nine males; mean ± SD age: 30 ± 6 years) with no known neurological disorder participated in the present study. The study was approved by the local ethics committee (Protocol # H‐A‐2008‐029). All methods conformed to the Declaration of Helsinki. All subjects provided their written informed consent prior to participation.
Stimulation
A set of disposable surface electrodes (Ambu Blue Sensor, Ballerup, Denmark) was placed in the front of the right ankle joint to stimulate the medial cutaneous branch of the superficial peroneal nerve. Another set was placed behind the right medial malleolus to stimulate the medial plantar nerve, a branch of the tibial nerve. The electrode pairs were placed approximately 3 cm apart. Repetitive stimulation was administered with two constant current stimulators (DS7A; Digitimer, Welwyn Garden City, UK), one for each nerve, that delivered 1 ms monophasic pulses at 80 Hz. Subjects felt a tingling sensation on the foot dorsum during repetitive stimulation of the superficial peroneal nerve, as well as on the foot sole during stimulation of the tibial nerve. The sensation was intensified when both nerves were stimulated together.
Touch test
Von Frey monofilaments (20 piece Touch Test™ Sensory Evaluator; North Coast Medical, Gilroy, CA, USA) were used to measure touch thresholds on the foot dorsum and great toe with and without stimulation. The monofilament were pressed on the skin surface for <1 s and then removed when subjects rested on a chair, eyes closed. They were instructed to say ‘yes’ when the pressure was felt. For monofilaments in the size range 1.65–4.08, the touch was applied up to three times. For monofilaments in the size range 4.17–6.65, the touch was applied only once. The force from the smallest monofilament to elicit a positive response was defined as the touch threshold. To determine effects of electrical stimulation on touch sensation, trains of stimulation were applied every second for 500 ms, and monofilaments were applied when the stimulation was turned on.
Set‐up
Subjects wore an electrohydraulic ankle foot orthosis that applied torque to the right ankle during walking (Noel et al. 2008). The onset and magnitude of the force perturbation was adjusted according to each subject's swing phase. The onset of the force perturbation was set at the beginning of swing phase dorsiflexion for each subject (between 60% and 70% stride cycle). The magnitude of the force used depended on the task (see below). In both the perceptual and adaptation tasks, the applied force lasted for 150 ms, and terminated before heel strike in each step. Each perturbation consisted of a force field with gradual onset and offset (parabolic shape). Outside of the force field window, active torque cancellation was delivered, allowing subjects to walk normally with the 1.8 kg device. During baseline and post‐adaptation, subjects continued to wear the orthotics and active torque cancellation was provided over the entire gait cycle.
Data collection
Ankle angles were recorded using the optical encoder located on the orthosis. Footswitch sensors were placed under the right heel to record time of heel strike. EMG activity from the right tibialis anterior (TA) and soleus (SOL) muscles was measured using paired bipolar electrodes (Ag‐AgCl electrodes; 2 cm between poles; Ambu Blue Sensor), placed in accordance with the recommended locations of Zipp (1982). The EMG signals were amplified, bandpass filtered (10–500 Hz; Zerowire, Aurion, Italy), and sampled at 2000 Hz. All analogue signals were digitized online using custom data acquisition software at 1000 Hz.
Psychophysical procedure
Each subject's perception with respect to ankle movement perturbation was determined by measuring the threshold for 50% correct responses. During walking, perturbations of different magnitude (between 0.5 and 6.5 Nm) and direction (e.g. dorsiflexion or plantarflexion) were presented once every four to six strides. Using a forced‐choice paradigm, subjects were instructed to answer either ‘up’ (for dorsiflexion) or ‘down’ (for plantarflexion) when cued by the experimenter. Subjects were instructed to guess when they did not feel the movement. Instead of presenting a fixed set of stimuli of different magnitudes, an adaptive method was used to change the stimulus magnitude from one trial to the next (Taylor & Creelman, 1967; Treutwein, 1995). We chose the Parameter Estimation by Sequential Testing (PEST) method to reduce the number of measurements far from the threshold, which should be more efficient in principle (Taylor & Creelman, 1967). A schematic of the protocol is provided in the Appendix.
PEST was repeated during cutaneous stimulation. Nerve stimulation was turned on at 50% of right stride cycle (end of stance) and lasted for 500 ms in each stride (most of the swing phase).
Adaptation paradigm
Subjects walked on the treadmill at 3.6 km h–1, holding handrails in front of the treadmill during the experiment. Each block of testing consisted of a baseline (1 min), adaptation (1 min) and post‐adaptation (3 min) period of walking. In the baseline period, no force perturbation was applied (robotized orthosis under active force cancellation mode). In the adaptation period, force was applied to the right ankle joint using the orthosis. The magnitude of the perturbation was set to produce approximately 6° deviation towards plantarflexion in the ankle trajectory (Blanchette et al. 2011). The average force required was 4.3 ± 0.9 Nm across subjects. In the post‐adaptation period, the force was turned off to measure after‐effects from force exposure.
Experiment 1
Ten subjects completed two testing blocks, one with repetitive nerve stimulation (during all three periods) and one without stimulation. Nerve stimulation was turned on at 50% of right stride cycle (end of stance) and lasted for 500 ms in each stride (most of the swing phase). The testing was first performed with stimulation, and then repeated without stimulation. Both blocks of testing were conducted on the same day.
To determine potential order effects of repeated exposure to the force field, five of the subjects performed two additional blocks of testing, both without stimulation. These control experiments were conducted on a separate day.
Experiment 2
A lidocaine injection experiment was performed separately to determine the effects of peripheral blocking cutaneous afferents using anaesthesia, with no possibility of activating central mechanisms as with peripheral nerve stimulation. Lidocaine (25 mg ml–1) was injected around the ankle joint targeting the deep peroneal nerve, branches of the superficial peroneal nerve, the sural and tibial nerves. Up to 20 ml of lidocain was used in each subject (approximately 5 ml for each nerve). The extent of the nerve block was judged by the sensation of touch on the foot dorsum and foot sole.
Five subjects received lidocaine injection in the morning, and completed one block of testing. The anaesthesia lasted for ∼2 h. Subjects were retested in the afternoon to compare walking adaptation with and without foot anaesthesia.
Statistical analysis
Ankle angle data separated into individual strides, aligned on the time of right heel strike, with time normalized to 100% stride cycle. Stride‐by‐stride changes in kinematics were quantified by measuring ankle angle at the end of the force perturbation. Data were binned by three strides, and statistics were performed on the first 15 bins in each period: baseline, adaptation and post‐adaptation. Repeated measures ANOVA was used to test the effects of stimulation, time and stimulation × time. Post hoc analysis was performed using the Tukey's honestly significant test. The alpha level was set at 0.05 for all statistical comparisons.
EMG data were digitally filtered with a zero‐lag third order Butterworth bandpass filter (5–450 Hz). Rectified EMG data were separated into individual strides, aligned on the time of right heel strike. For each subject, EMG amplitude was normalized to the peak averaged EMG during baseline walking. Stride‐by‐stride changes in muscle activations were measured as the mean EMG over three time windows: from the beginning to the end of the force field, from the beginning to the peak of the force field and from the peak to the end of the force field. Note that EMG measured during 80 Hz stimulation (Experiment 1) was not included in the analysis as a result of stimulation artefacts in the EMG signal.
Results
Touch threshold
Repetitive stimulation of the peroneal and plantar nerves had a pronounced effect on the touch sensation in the foot across all subjects tested (Table 1). On average, touch threshold increased from 1.38 g to 58 g on the foot dorsum (P = 0.004), indicating a loss of superficial skin sensation during stimulation. In one subject, touch threshold increased to 300 g on the foot dorsum during stimulation, which means that he had deep pressure sensation only. There was also a significant loss of superficial skin sensation below the great toe, where touch threshold increased from 0.46 g to 14 g (P = 0.004).
Table 1.
Effects of repetitive nerve stimulation
| Touch thresholds (g) | Perturbation threshold (Nm) | |||||
|---|---|---|---|---|---|---|
| Foot dorsum | Toes | Ankle | ||||
| Subject | Stimulation OFF | Stimulation ON | Stimulation OFF | Stimulation ON | Stimulation OFF | Stimulation ON |
| S1 | 1 | 4 | 1 | 4 | 2.8 | 3.9 |
| S2 | 0.04 | 6 | 0.07 | 2 | 5.9 | 7.9 |
| S3 | 0.16 | 15 | 1 | 10 | 3.5 | 2.8 |
| S4 | 0.4 | 2 | 0.04 | 0.4 | 0.5 | 0.9 |
| S5 | 0.07 | 1.4 | 0.4 | 26 | 0.4 | 1.1 |
| S6 | 10 | 180 | 1 | 10 | 1.1 | 1.6 |
| S7 | 0.6 | 6 | 0.4 | 1.4 | 0.5 | 1.1 |
| S8 | 0.02 | 300 | 0.07 | 60 | 3.3 | 3.3 |
| S9 | 0.16 | 10 | 0.16 | 10 | 0.6 | 0.6 |
| S10 | 0.4 | 26 | 1 | 60 | 1.0 | – |
Force perception
Repetitive stimulation of the peroneal and plantar nerves also increased the perceptual threshold of detecting ankle perturbations during walking (Table 1). On average, the direction of ankle perturbation could be detected correctly for perturbations greater than 2.0 Nm without stimulation and increased to 2.6 Nm with stimulation (P = 0.07).
Adaptation
Figure 1 A shows the ankle angle from an example subject during the adaptation task. During the adaptation period, the ankle deviated towards plantarflexion when force perturbations were applied during the swing phase of walking. The subject gradually adapted to the force perturbations, and the ankle deviation was reduced at the end of the adaptation period. When the force was removed during the post‐adaptation period, the ankle deviated towards dorsiflexion compared to baseline (i.e. after‐effect). The presence of after‐effects indicates that a predictive feedforward mechanism was involved that anticipates the perturbation. This after‐effect was gradually washed out over the post‐adaptation period.
Figure 1. Ankle adaptation to force perturbations during walking .
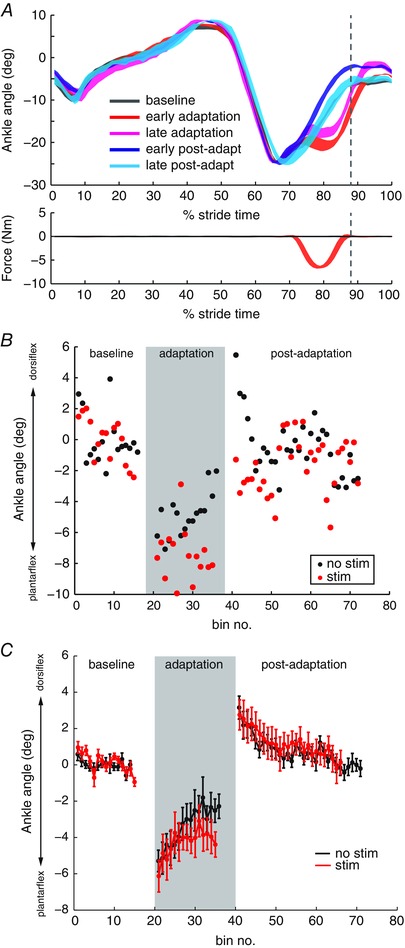
A, top: mean ankle angle from an example subject is plotted during baseline, the first and last three strides in adaptation, and the first and last three strides in post‐adaptation. Bottom: force was applied to the ankle joint during the early swing phase of walking. The dashed vertical line represents the end of force application. B, effects of stimulation on ankle adaptation. Changes in ankle kinematics were measured as the ankle angle at the end of force application. Stride‐by‐stride ankle adaptation with no stimulation (black) is compared to adaptation with stimulation (red) in an example subject (S7). Each point represents the average ankle angle over three strides. C, group averaged (n = 10) peak ankle dorsiflexion during baseline, adaptation and post‐adaptation periods with no stimulation (black) and with stimulation (red). Mean values are plotted for non‐overlapping bins over three strides. Error bars represent one SD. [Colour figure can be viewed at wileyonlinelibrary.com]
Ankle angle at the end of force application was calculated for each stride to measure adaptation. Figure 1 B shows the stride‐by‐stride ankle dorsiflexion in an example subject during the adaptation task. When no stimulation was applied, ankle deviation towards plantarflexion reduced from approximately –8° to –2° during the adaptation period. When stimulation was applied, the ankle deviation was maintained at approximately –8° throughout the adaptation period. This subject showed large after‐effects in post‐adaptation without stimulation, and the after‐effects were reduced with stimulation.
Group averaged data suggest that adaptation to ankle force perturbation was reduced when stimulation interfered with cutaneous input from the foot during walking (Fig. 1 C). The subject did not return to the same amount of ankle dorsiflexion in swing, even though there was no difference in the amount of ankle deviation in early adaptation between conditions. Group averaged data showed a significant effect of stimulation (P = 0.01) and time (P < 0.001). The interaction effect was not statistically significant (P = 0.7).
Group averaged data showed the same amount of after‐effects in early post‐adaptation in both conditions (Fig. 1 C). Group averaged data showed a significant effect of time (P < 0.001). The effect of stimulation (P = 0.1) and the interaction effect (P = 0.7) were not statistically significant. It must be noted, however, that, although the mean after‐effects is not statistically different between stimulation conditions, the variability in after‐effects across subjects is almost twice as large with stimulation (SD = 0.75) compared to no stimulation (SD = 0.45). The presence of after‐effects in post‐adaptation indicates that some subjects recalibrated ankle movement to the anticipated force perturbations in both conditions. To determine whether perceptual thresholds determined the size of after‐effects, Pearson's correlation coefficient was calculated between after‐effect size and the touch thresholds on the foot dorsum and great toe, as well as the ankle perturbation threshold. After‐effect size was not correlated with the touch thresholds (foot dorsum, r = –0.01, P = 0.9; toe, r = 0.3, P = 0.5), nor with ankle perturbation threshold (r = 0.02, P = 0.9).
We confirmed that there were no order effects of repeated exposure to the force field (twice on the same day). Five subjects performed two blocks of adaptation, both without stimulation. For the adaptation period, there was a significant effect of time (P = 0.01), although the effect of block (P = 0.9) and the interaction effect of block and time (P = 0.16) were not statistically significant. For the post‐adaptation period, the effect of block (P = 0.9) and the interaction effect of block and time (P = 0.18) were also not statistically significant (data not shown).
Foot anaesthesia
The foot dorsum was completely anaesthetized in five of five subjects (Table 2). The toes were fully anaesthetized in two of five subjects. One of five subjects had increased touch threshold with anaesthesia. Two of five subjects did not show an increased touch threshold with anaesthesia. The perceptual threshold for ankle perturbations was increased in three of five subjects, whereas two of five subjects did not show increased perceptual threshold with anaesthesia. On average, the direction of ankle perturbation could be detected correctly for perturbations greater than 1.3 Nm without anaesthesia, and increased to 2.3 Nm with anaesthesia.
Table 2.
Effects of foot anaesthesia
| Touch thresholds (g) | Perturbation threshold (Nm) | |||||
|---|---|---|---|---|---|---|
| Foot dorsum | Toes | Ankle | ||||
| Subject | No anaesthetic | Anaesthetic | No anaesthetic | Anaesthetic | No anaesthetic | Anaesthetic |
| S6 | 0.4 | >300 | 1 | 0.16 | 1.2 | 0.9 |
| S5 | 0.07 | >300 | 0.4 | 1.4 | 0.4 | 1.5 |
| S2 | 0.04 | >300 | 0.07 | >300 | 1.8 | 3.7 |
| S10 | 0.4 | >300 | 1 | 0.4 | 0.5 | 2.3 |
| S11 | 0.02 | >300 | 0.02 | >300 | 3.1 | 3.1 |
Figure 2 A shows the effects of foot anaesthesia in an example subject during the adaptation task. In the absence of anaesthesia, ankle deviation showed overcompensation from approximately –4° to + 4° during the adaptation period. When foot anaesthesia was applied, the ankle deviation was maintained in plantarflexion below –4° throughout the adaptation period. This subject showed large after‐effects in post‐adaptation without anaesthesia, and the after‐effects were reduced with foot anaesthesia.
Figure 2. Effects of foot anaesthesia on ankle adaptation .
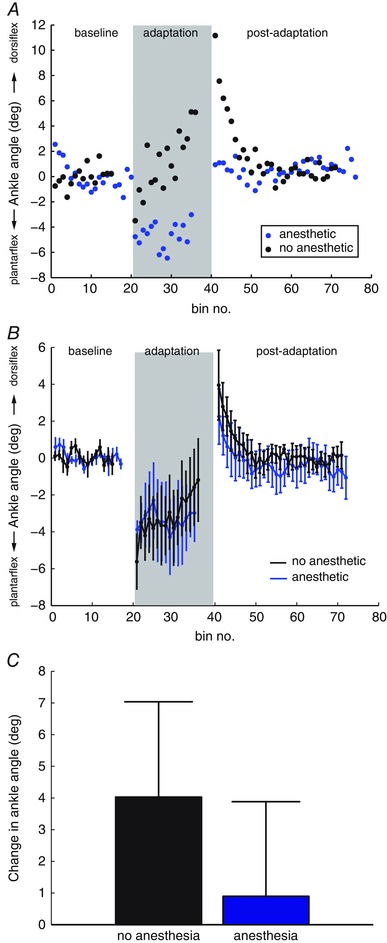
A, stride‐by‐stride ankle adaptation with no anaesthesia (black) is compared to adaptation with foot anaesthesia (blue) in an example subject (S10). Each point represents the mean ankle angle over three strides. B, group average (n = 5) peak ankle dorsiflexion during baseline, adaptation and post‐adaptation periods without anaesthetic (black) and with foot anaesthetic (blue). Error bars represent one SD. C, group average (n = 5) adaptation without anaesthetic (black) and with foot anaesthetic (blue), measured as the difference in ankle deviation in the first three trials in the adaptation period (A1) and the last three trials of the adaptation period (A2). Error bars represent one SD. [Colour figure can be viewed at wileyonlinelibrary.com]
The amount of adaptation was measured as the difference in ankle deviation from force perturbation in the first three trials in the adaptation period (A1) and the last three trials of the adaptation period (A2). Figure 2 B shows the group average adaptation (A2 – A1) with and without anaesthesia. We used the Wilcoxon signed‐rank test to compare adaptation between groups, and found no significant change with foot anaesthesia (P = 0.4) (i.e. anaesthesia prevented adaptation).
Modifications of EMG activity
Figure 3 shows the modifications in the TA muscle activations during adaptation and post‐adaptation relative to baseline period. During adaptation, TA activation was increased around the time of force application. The increased TA activity persisted during early post‐adaptation when the force field was removed. The after‐effect in TA activity was washed out by late post‐adaptation. By contrast, there was minimal change in TA muscle activations when foot anaesthesia was applied (Fig. 4). This analysis could not be performed during stimulation as a result of the presence of large artefacts.
Figure 3. EMG adaptation to force perturbations during walking .
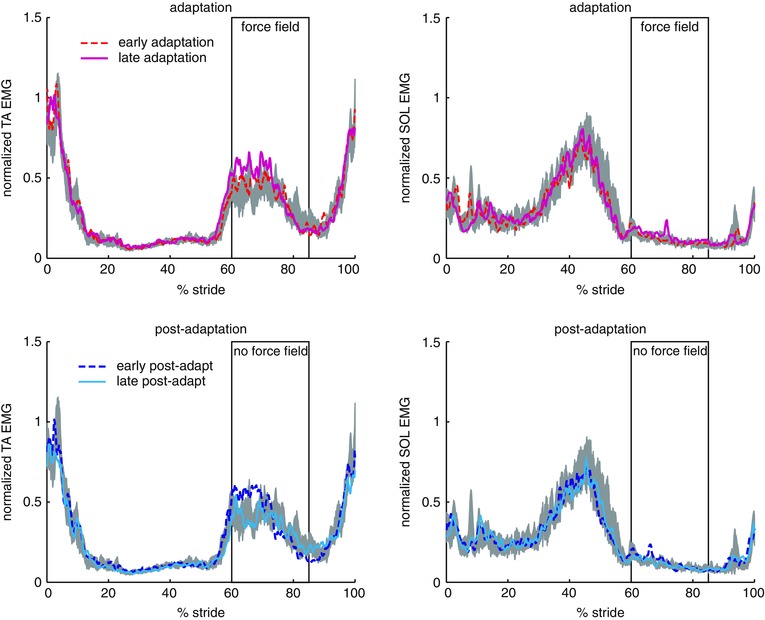
Mean (n = 10 subjects) EMG activity of TA and SOL muscles during adaptation (top) and post‐adaptation (bottom) compared to baseline (grey shaded area) under normal conditions (no stimulation). Rectangle indicates the average timing of force field application. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4. Effects of foot anaesthesia on EMG adaptation .
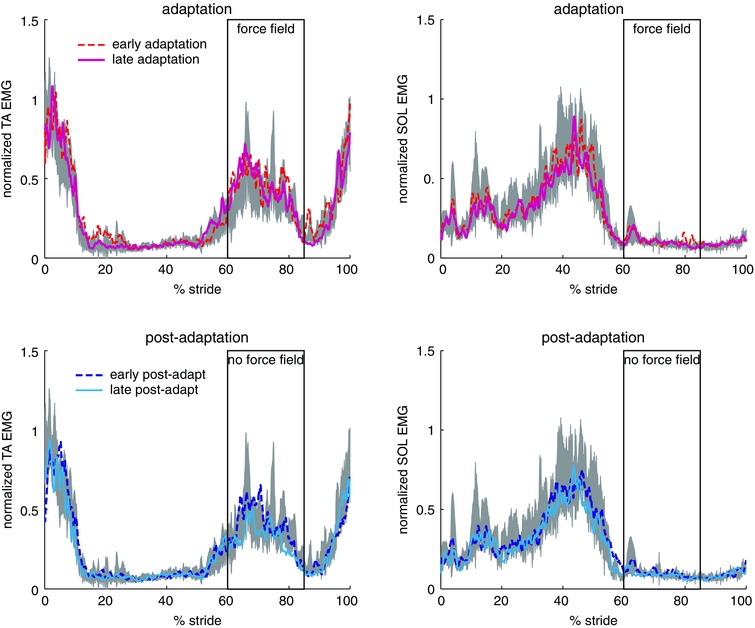
Mean (n = 5 subjects) EMG activity of TA and SOL muscles during adaptation (top) and post‐adaptation (bottom) compared to baseline (grey shaded area) when foot anaesthesia was applied. Rectangle indicates the average timing of force field application. [Colour figure can be viewed at wileyonlinelibrary.com]
Stride‐by‐stride changes in TA activity were measured as the mean EMG over a 150 ms time window from the beginning to the end of force perturbation (Fig. 5 A). Group average data showed that normal adaptation without anaesthesia was accompanied by a gradual increase in TA activity from early to late adaptation. When foot anaesthesia was applied, mean TA activation changed little throughout the adaptation period.
Figure 5. Group averaged (n = 5) mean TA activity during baseline, adaptation and post‐adaptation periods without anaesthetic (black) and with foot anaesthetic (blue) .
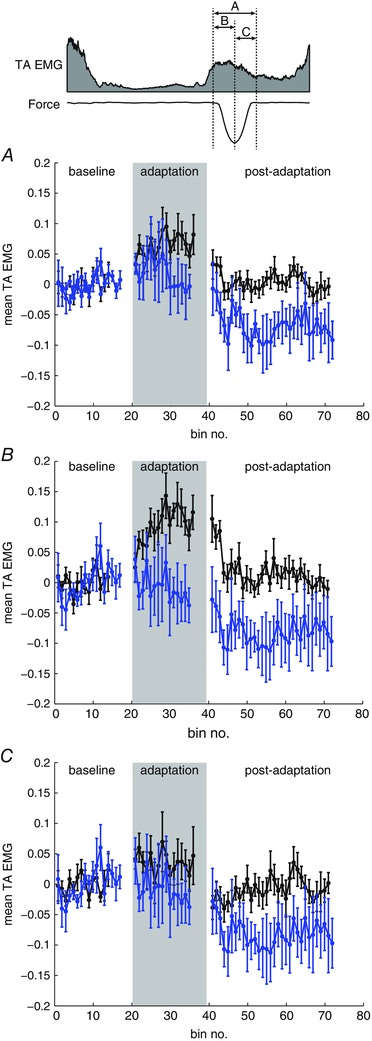
The schematic (top) shows the three time windows used for EMG averaging: from the beginning to the end of the applied force (A); from the beginning to the peak of the applied force (B); and from the peak to the end of the applied force (C). Mean values are plotted for non‐overlapping bins over three strides. Error bars represent one SD. [Colour figure can be viewed at wileyonlinelibrary.com]
In addition, TA EMG was averaged over two 75 ms time windows: from the beginning to the peak of the force command (Fig. 5 B) and from the peak to the end of the force command (Fig. 5 C). As a result of the gradual onset of the force field (Noel et al. 2009), short latency EMG responses are expected to be absent in the first window measuring the feedforward response to the force field. Group average data showed that the increase in TA activity during the force field was mainly driven by the increase in feedforward response (Fig. 5 B). The feedforward response to the force field was reduced with foot anaesthesia. Moreover, the feedforward response resulted in after‐effects in TA activation during early post‐adaptation. The after‐effect in TA activity was also reduced with foot anaesthesia. The second window measures both feedforward and feedback responses to the force field; the group average showed a lower increase in EMG response during the adaptation period and no after‐effect with respect to TA activity during post‐adaptation in this time window (Fig. 5 C). Taken together, these results show that removing cutaneous feedback reduced feedforward locomotor adaptation.
Discussion
Role of cutaneous inputs in walking adaptation
Cutaneous input has been shown to increase activity in the tibialis anterior muscle, an ankle dorsiflexor, during the swing phase of walking in humans (Duysens et al. 1990; Yang & Stein, 1990; Christensen et al. 1999). This cutaneous reflex is analogous to the ‘stumbling corrective reaction’ observed in the cat, and is considered to play a role in correcting the foot trajectory to overcome immediate obstacles in the walkway (Forssberg, 1979). More recently, it was shown that cutaneous input from the foot dorsum also plays a role in the control of foot trajectory during unperturbed level walking (Howe et al. 2015).
In the present study, we investigated the role of cutaneous inputs on walking adaptation, which is a feedforward learning process that occurs over minutes of training (Bastian, 2008). We show that interfering with cutaneous feedback, using either electrical stimulation or anaesthesia, reduced feedforward adaptation to ankle perturbations during walking. Under normal conditions, the ankle dorsiflexion would return to a level close to the baseline level by the end of the adaptation period. However, the overshoot in ankle movement as a result of applied perturbation was not corrected during the adaptation period with stimulation, which indicated a reduced adaptation with altered cutaneous input. Foot anaesthesia also reduced adaptation as measured by changes in ankle dorsiflexion over the adaptation period.
There was greater inter‐subject variability in the after‐effects observed during the post‐adaptation period with stimulation, with some subjects showing no after‐effects (Fig. 2) and others showing normal after‐effects. The absence of an after‐effect indicates that some subjects were unable to re‐calibrated ankle movement to the external perturbation by modifying their feedforward commands. The group average after‐effects size did not show a significant effect of stimulation, which suggests that some subjects were able to use other sensory input (e.g. muscle afferents) to re‐calibrate ankle movement.
Therefore, the results obtained in the present study suggest that cutaneous input is normally integrated with other sensory inputs to detect a change in ankle movement, and this information is normally used to adapt walking patterns to changes in the external environment. The ability to detect changes in ankle movement and to react adequately by scaling the dorsiflexion force output is reduced when cutaneous input is unavailable, and therefore adaptation to perturbations is reduced with stimulation or anaesthesia.
Role of cutaneous input in ankle force perception
The perception of force on the foot dorsum is disrupted when electrical nerve stimulation is applied to the superficial peroneal nerve (Choi et al. 2013). It is unclear how changes in force perception would affect walking adaptation. Motor adaptation does not depend on the ‘awareness’ of force perturbation because adaptation can occur when the perturbation is increased gradually (Mattar et al. 2013). Walking adaptation is highly automatic and is dependent in part on the cerebellum (Yanagihara & Kondo, 1996; Morton & Bastian, 2006), which receives afferent and efferent information via the dorsal and ventral spinocerebellar tracts, respectively (Bosco & Poppele, 2001). Although walking adaptation normally occurs with or without conscious effort, conscious attention can speed up the adaptation (Malone & Bastian, 2010).
We found that electrical nerve stimulation influenced the perceived aftereffects during early post‐adaptation period. Subjects commented that ‘they could not feel when the force field was removed’ when nerve stimulation was applied. This subjective report is consistent with changes in measured perturbation thresholds. On average, the ankle perturbation threshold is increased by 27% with electrical stimulation and by 73% with foot anaesthesia. We hypothesized that differences in conscious awareness of applied ankle force might explain variability in aftereffects across subjects. However, we were not able to show a statistically significant correlation between perturbation threshold and after‐effects magnitude with our sample size.
Contribution from other mechanisms
The data from the present study support the hypothesis that cutaneous input plays a role in force perception, and that it contributes to the ‘error’ signal involved in driving walking adaptation when there is a mismatch between expected and actual force. Our data do not exclude the contribution from other sensory mechanisms. Muscle spindle afferents (Gandevia & McCloskey, 1976) and joint receptors (Clark et al. 1979) are known to influence proprioception, and these sensory sources probbaly also play a role in detecting ankle deviation during walking. Indeed, some subjects showed normal walking after‐effects with cutaneous stimulation, which suggests that they may rely more on other sensory mechanisms (or were able to rapidly change to them; sensory re‐weighting) to adapt walking patterns to externally applied ankle forces.
It is possible that stimulation of the plantar nerve also influenced proprioceptive feedback. The plantar nerve consists partly of afferents from proprioceptors in foot muscles. However, proprioceptive feedback from the foot would probably not influence the proprioception of ankle joint position. Moreover, foot proprioceptors should have minimal affect on the adaptation to force perturbations that primarily affects ankle movement.
Conclusions
Cutaneous input contributes not only to the cutaneous reflex that mediates immediate responses to external perturbations, but also to force perception and is involved in the sensory signalling that drives the locomotor adaptation occurring over multiple steps. A better understanding of how the nervous system uses multiple sensory modalities to adapt movements would have clinical implications for treating walking impairments after neurological injuries.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
All authors have read and approved the final submission. All authors agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. All authors contributed to the conception and design of the study. J.T.C and L.J.B. collected data for the study. J.T.C. and L.J.B. contributed to the analysis and interpretation of the data. All authors contributed to the drafting of the manuscript.
Funding
This work is supported by grants from the Danish Medical Research Council, the Whitaker International Program, and the Natural Sciences and Engineering Research Council of Canada (NSERC).
PEST protocol
The stimulus size, A, was initially set at 6 Nm. The stimulation size on the next iteration changed depending on the subject's response (Fig. A1). If the subject's answer was correct, the magnitude was reduced; if the answer was incorrect, the magnitude was increased. The initial step size, x, was set at 1 Nm. Only responses to ‘down’ stimuli were recorded and used to determine the next stimulus magnitude. The ‘up’ stimuli were presented randomly to keep the subject's attention. The rules for changing x were: (1) x was halved on every reversal; (2) x remained unchanged on the second iteration in the same direction; (3) x was doubled on the third iteration if the last reversal resulted from doubling; x remained unchanged on the third iteration if the most recent reversal was not the result of a doubling; and (4) x was doubled on the fourth and subsequent iterations in the same direction. The test was terminated when x fell below 0.5 Nm (mechanical limit of the device) or after three reversals. The estimated threshold was the last magnitude tested.
Figure A1.
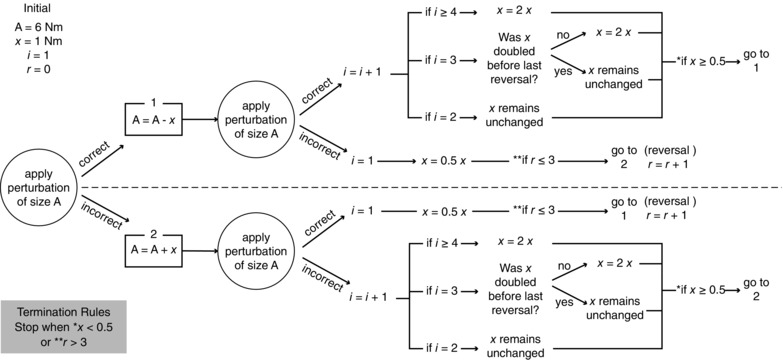
Flowchart showing the PEST protocol used to determine perturbation thresholds
References
- Bastian AJ (2008). Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21, 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette A, Lambert S, Richards CL & Bouyer LJ (2011). Walking while resisting a perturbation: effects on ankle dorsiflexor activation during swing and potential for rehabilitation. Gait Posture 34, 358–363. [DOI] [PubMed] [Google Scholar]
- Bosco G & Poppele RE (2001). Proprioception from a spinocerebellar perspective. Physiol Rev 81, 539–568. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ & Rossignol S (2003. a). Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol 90, 3625–3639. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ & Rossignol S (2003. b). Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol 90, 3640–3653. [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S & Shahbazpour N (2002). Central and peripheral mediation of human force sensation following eccentric or concentric contractions. J Physiol 539, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JT, Lundbye‐Jensen J, Leukel C & Nielsen JB (2013). Cutaneous mechanisms of isometric ankle force control. Exp Brain Res 228, 377–384. [DOI] [PubMed] [Google Scholar]
- Christensen LO, Morita H, Petersen N & Nielsen J (1999). Evidence suggesting that a transcortical reflex pathway contributes to cutaneous reflexes in the tibialis anterior muscle during walking in man. Exp Brain Res 124, 59–68. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Horch KW, Bach SM & Larson GF (1979). Contributions of cutaneous and joint receptors to static knee‐position sense in man. J Neurophysiol 42, 877–888. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA & Dietz V (1990). Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82, 351–358. [DOI] [PubMed] [Google Scholar]
- Forssberg H (1979). Stumbling corrective reaction: a phase‐dependent compensatory reaction during locomotion. J Neurophysiol 42, 936–953. [DOI] [PubMed] [Google Scholar]
- Gandevia SC & McCloskey DI (1976). Joint sense, muscle sense, and their combination as position sense, measured at distal interphalangeal joint of middle finger. J Physiol (Lond) 260, 387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC & McCloskey DI (1977). Changes in motor commands, as shown by changes in perceived heaviness, during partial curarization and peripheral anaesthesia in man. J Physiol 272, 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe EE, Toth AJ, Vallis LA & Bent LR (2015). Baseline skin information from the foot dorsum is used to control lower limb kinematics during level walking. Exp Brain Res 233, 2477–2487. [DOI] [PubMed] [Google Scholar]
- Johnson KO (2001). The roles and functions of cutaneous mechanoreceptors. CurrOpinNeurobiol 11, 455–461. [DOI] [PubMed] [Google Scholar]
- Jones LA & Hunter IW (1983). Effect of fatigue on force sensation. Exp Neurol 81, 640–650. [DOI] [PubMed] [Google Scholar]
- Malone LA & Bastian AJ (2010). Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103, 1954–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar AA, Darainy M & Ostry DJ (2013). Motor learning and its sensory effects: time course of perceptual change and its presence with gradual introduction of load. J Neurophysiol 109, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM & Bastian AJ (2006). Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26, 9107–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, Cantin B, Lambert S, Gosselin CM & Bouyer LJ (2008). An electrohydraulic actuated ankle foot orthosis to generate force fields and to test proprioceptive reflexes during human walking. IEEE Trans Neural Syst Rehabil Eng 16, 390–399. [DOI] [PubMed] [Google Scholar]
- Noel M, Fortin K & Bouyer LJ (2009). Using an electrohydraulic ankle foot orthosis to study modifications in feedforward control during locomotor adaptation to force fields applied in stance. J Neuroeng Rehabil 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R & Gossard JP (2006). Dynamic sensorimotor interactions in locomotion. Physiol Rev 86, 89–154. [DOI] [PubMed] [Google Scholar]
- Schillings AM, van Wezel BM, Mulder T & Duysens J (2000). Muscular responses and movement strategies during stumbling over obstacles. J Neurophysiol 83, 2093–2102. [DOI] [PubMed] [Google Scholar]
- Taylor MM & Creelman CD (1967). PEST: Efficient Estimates on Prabability Functions. J Acoust Soc Am 41. [Google Scholar]
- Treutwein B (1995). Adaptive psychophysical procedures. Vision Res 35, 2503–2522. [PubMed] [Google Scholar]
- Yanagihara D & Kondo I (1996). Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci USA 93, 13292–13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF & Stein RB (1990). Phase‐dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Zipp P (1982). Recommendations for the standardization of lead positions in surface electromyography. Eur J Appl Physiol 50, 41–54. [Google Scholar]


