Abstract
One hundred and fifty years ago Max Schultze first proposed the duplex theory of vision, that vertebrate eyes have two types of photoreceptor cells with differing sensitivity: rods for dim light and cones for bright light and colour detection. We now know that this division is fundamental not only to the photoreceptors themselves but to the whole of retinal and visual processing. But why are rods more sensitive, and how did the duplex retina first evolve? Cells resembling cones are very old, first appearing among cnidarians; the emergence of rods was a key step in the evolution of the vertebrate eye. Many transduction proteins have different isoforms in rods and cones, and others are expressed at different levels. Moreover rods and cones have a different anatomy, with only rods containing membranous discs enclosed by the plasma membrane. These differences must be responsible for the difference in absolute sensitivity, but which are essential? Recent research particularly expressing cone proteins in rods or changing the level of expression seem to show that many of the molecular differences in the activation and decay of the response may have each made a small contribution as evolution proceeded stepwise with incremental increases in sensitivity. Rod outer‐segment discs were not essential and developed after single‐photon detection. These experiments collectively provide a new understanding of the two kinds of photoreceptors and help to explain how gene duplication and the formation of rod‐specific proteins produced the duplex retina, which has remained remarkably constant in physiology from amphibians to man.
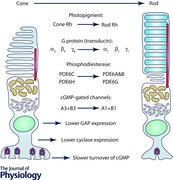
Keywords: cones, photoreceptor, rhodopsin, rods, vision
Abbreviations
- A
activation constant
- cGMP
cyclic guanosine monophosphate
- CNG
cyclic‐nucleotide gated
- GAP
GTPase‐accelerating protein
- GC
guanylyl cyclase
- GCAP
guanylyl cyclase‐activating protein
- GRK
G‐protein receptor kinase
- hν
light (photon)
- mG
mouse 508 nm cone rhodopsin
- mS
mouse 360 nm cone rhodopsin
- PDE6
phosphodiesterase 6
- PDE6*
light‐activated phosphodiesterase 6
- Rh*
light‐activated rhodopsin or metaII
- Tα
α subunit of photoreceptor G protein
- WT
wild‐type
Introduction
In 2016 we celebrate the 150th anniversary of the groundbreaking article of Max Schultze (1866), who first proposed that rod and cone photoreceptors have different functions. Schultze noticed that retinas of nocturnal animals tend to have a larger proportion of cells with rod‐shaped outer segments (Fig. 1 A), and that diurnal animals have greater numbers of cells with outer segments tapering like cones (Fig. 1 B). He then proposed the duplex theory of vision: that rods mediate perception in dim light and cones are specialized for bright light and colour vision. We now know that his division of visual detection into two systems is fundamental not only to the properties of photoreceptors but also to the connections these cells make with other neurons and to the whole of retinal and visual processing (Masland, 2012).
Figure 1. Rods and cones in nocturnal and diurnal animals .
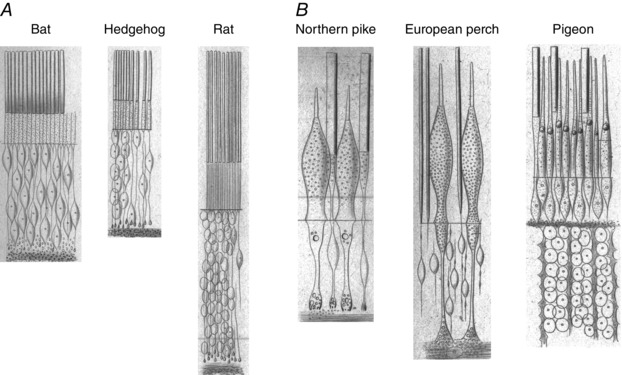
Drawings from Schultze's original paper (1866) of photoreceptors from nocturnal animals (A) and diurnal animals (B), magnification approximately 350–400 times. Schultze claimed that the bat retina lacked even a trace of cones, but in rat he noticed occasional gaps (Lücken) which he speculated could possibly correspond to cones, as we now know to be true. Fish and pigeon on the other hand have many easily observable cones in addition to rods. Schultze commented that these observations ‘would seem to indicate that rods are more advantageous than cones for quantitative light perception’, but that ‘cones would seem to be the nerve end‐organs for colour perception’.
Since the publication of Schultze's paper, we have wondered why rod vision is more sensitive. The first intracellular recordings showed that most of the sensitivity difference is inherent in the photoreceptors themselves: single rods are more sensitive than single cones (Fain & Dowling, 1973). Soon afterward, biochemists and molecular biologists discovered that the two photoreceptors have many of the same kinds of proteins and detect light in a similar way. Cones are much older than rods: from the sequences of a very large number of vertebrate photopigments, we can infer that gene duplication produced all of the different kinds of cone pigments before the evolution of rod pigments (Nickle & Robinson, 2007; Shichida & Matsuyama, 2009). Along with the pigment came the many other molecular and anatomical differences between the two kinds of cells, with the result that rods are able to integrate incoming light over a longer period and operate at the theoretical limit of single‐photon detection, whereas cones are less sensitive but exhibit adaptive properties that allow them to detect luminance changes and motion when the photon flux is less limiting. These differences in physiology must ultimately derive from differences in the mechanism of transduction in the two kinds of photoreceptors.
Recent experiments are beginning to clarify these differences. Some of the most interesting observations have been made from the combined efforts of molecular biologists and physiologists inserting cone genes into mouse rods. These experiments along with more traditional observations by biochemists and single‐cell physiologists are gradually clarifying the roles of different proteins in rod sensitivity. Our initial expectation had been that one particular alteration might dramatically change the properties of the photoreceptor. Instead we have discovered what we should have suspected all along, that evolution proceeded by making small changes in many transduction proteins, incrementally increasing sensitivity to produce the rods and cones that emerged as long as 500 million years ago. Although the present state of research leaves many questions unanswered, we can now begin to see how rods became more sensitive.
Mechanism of transduction: rod/cone protein isoforms
Both rods and cones detect light according to the same basic scheme (Fig. 2). They use similar photopigments, which were once given distinct names but are now usually called rod or cone opsin or rhodopsin. The absorption of light produces a change in the pigment conformation to an intermediate called metaII or Rh*, which triggers a G‐protein cascade (for an overview, see for example Fain, 2014). The heterotrimeric G proteins of rods and cones (called transducins) are different: rods express α1, β1 and γ1, whereas cones express α2, β3 and γ8 (Sakmar & Khorana, 1988; Kubo et al. 1991; Ong et al. 1995; Deng et al. 2009). The G protein binds to Rh*, and exchange of GTP for GDP on the transducin α subunit (Tα) produces the active form Tα•GTP.
Figure 2. Phototransduction in vertebrate photoreceptors .
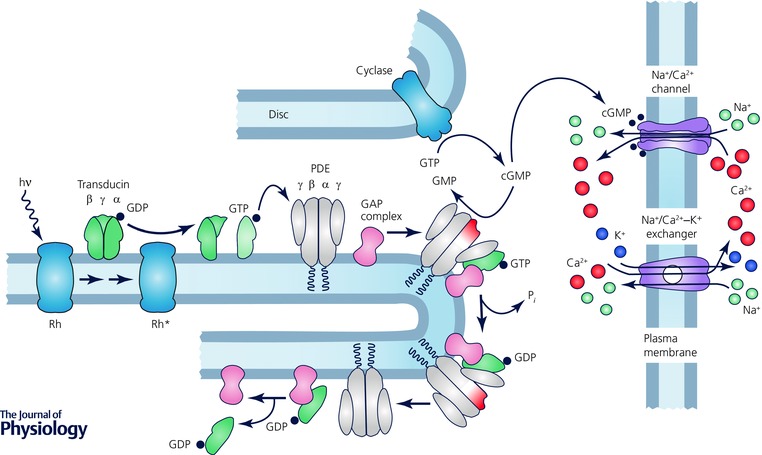
Redrawn and printed with permission from Fain et al. (2010).
Tα•GTP binds to the photoreceptor effector enzyme, which is phosphodiesterase 6 (PDE6). This protein has four subunits, two catalytic and two inhibitory. The catalytic subunits are slightly different from one another in rods and are called PDE6α and PDE6β (or PDE6A and PDE6B), whereas the two in cones are the same and called PDE6α′ (or PDE6C). Each PDE tetramer also has two inhibitory subunits, one for each catalytic subunit, which have somewhat different sequences in the two types of photoreceptors and are called rod or cone PDE6γ, or PDEG (in rods) and PDEH (in cones). Activated PDE6 hydrolyses cGMP, which acts as the second messenger of the cascade by binding to cGMP‐gated channels. The channels are tetramers again with different protein subunits called CNGA1 and CNGB1 in rods and CNGA3 and CNGB3 in cones (see Kaupp & Seifert, 2002; Zhong et al. 2002; Shuart et al. 2011).
Based on this general scheme, the activation of a single rhodopsin molecule is amplified across these stages to lead ultimately to the destruction of as many as one million cGMP molecules per Rh* in rods (Yee & Liebman, 1978). This reduction in cGMP concentration across vertebrate species is sufficient to reduce the cGMP‐gated current by more than its intrinsic noise in darkness (Baylor et al. 1979, 1984; Nakatani et al. 1991). The natural question to ask then is, can the lower sensitivity of cones be the result purely of reduced amplification within these steps? Let us suppose that rod and cone responses were to inactivate at the same rate. A reduced rate of activation would then cause cone responses to reach smaller peak amplitudes and might account entirely for the difference in sensitivity. But do rod and cone responses inactivate at the same rate? Not even close! In every vertebrate species from lamprey (Morshedian & Fain, 2015; Asteriti et al, 2015) to mouse (see Fig. 3), the rate is much faster in cones, and this difference must also contribute to the reduced cone sensitivity.
Figure 3. Responses of mouse rods and cones .
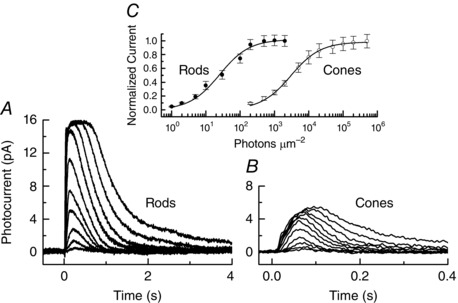
A, mean responses of 11 WT mouse rods to 20 ms flashes of 500 nm illumination from 0.5 to 2000 photons μm−2. B, mean responses of 18 mouse M (508 nm) cones to 20 ms flashes of 500 nm illumination from 200 to 500,000 photons μm−2. Responses in A and B were filtered with an 8‐pole Bessel filter with a low‐pass filter setting of 75 Hz. C, mean peak amplitudes (with SEM) of responses of mouse rods (●) and mouse cones (○) to 20 ms flashes of 500 nm illumination, normalized to maximum response and plotted as a function of flash intensity. Curves give best‐fitting Michaelis–Menten equation with flash intensities at half‐maximal amplitude of 25.3 (for rods) and 2960 (for cones) photons μm−2. All recordings were made from C57BL/6 mice from Jackson Laboratory (Bar Harbor, ME, USA), dark adapted for at least 4 h and usually overnight. All experiments were performed on mice of either sex in accordance with the rules and regulations of the NIH guidelines for research animals, as approved by the institutional animal care and use committee (IACUC) of the University of California, Los Angeles. Animals were kept in cyclic 12 h/12 h on/off lighting in approved cages and supplied with ample food and water. Animals in all experiments were killed before tissue extraction by approved procedures, usually CO2 inhalation or decerebration. Recordings were made at 37°C in Ames solution. Light intensities are given as photons effective at the lambda max of the rod or cone pigment calculated by convolving the spectrum of the stimulating beam with the rod or cone photopigment absorption curves.
The rate of inactivation is determined by the rates at which Rh*, transducin and PDE return to their basal conformations and the cGMP concentration goes back to its dark level. Rh* is silenced like other G‐protein receptors by phosphorylation and binding of arrestin. Rods and cones can have two different G‐protein receptor kinases, GRK1 in rods and GRK1 and/or GRK7 in cones, but rodents, including mice, have only GRK1 in both kinds of photoreceptors. Rods in mice have arrestin‐1 and cones both arrestin‐1 and arrestin‐4, though arrestin‐1 is by far the predominant species in both kinds of photoreceptors (Nikonov et al. 2008).
Activated transducin and phosphodiesterase are extinguished as in other G‐protein cascades by hydrolysis of Tα•GTP to Tα•GDP with the assistance of PDEγ and three GTPase‐accelerating proteins (GAPs): RGS9‐1, Gβ5 and the R9AP‐1 binding protein (see Arshavsky & Wensel, 2013). These proteins are required to speed PDE deactivation into the functional range of tens to hundreds of milliseconds, compared to the seconds or tens of seconds required in their absence (Hollinger & Hepler, 2002). Although these GAP complex proteins are the same in rods and cones, expression is significantly higher in cones (Cowan et al. 1998; Zhang et al. 2003), a point we return to later.
The cGMP concentration is restored by guanylyl cyclase (GC), which in photoreceptors is a member of the membrane guanylyl cyclase family (Potter, 2011). There are two cyclases in mammalian photoreceptors called retGC1 (or GC‐E) and retGC2 (or GC‐F); in mouse, rods have mostly retGC1 with some retGC2, whereas cones have only retGC1 (Wen et al. 2014). This difference is unlikely to be physiologically significant because when the gene for retGC2 is deleted there is little effect on rod sensitivity or response waveform (Baehr et al. 2007). The rate of cyclase activity is controlled by small molecular weight Ca2+‐binding proteins called guanylyl cyclase‐activating proteins or GCAPs. There are again two in mouse, GCAP1 and GCAP2, with somewhat different sensitivities for divalent ion binding (Dizhoor et al. 2010); rods express both GCAPs but cones mostly express GCAP1 (Dizhoor et al. 1995; Xu et al. 2013; Boye et al. 2015).
The differences in transduction proteins for rods and cones are summarized in Table 1. Rods and cones also display differences in anatomy: the photopigment in rods is contained almost entirely within the membrane of intracellular discs, whereas cone outer segments are formed from infoldings of the plasma membrane. We have long wondered whether this difference in anatomy might hold the key to the difference in sensitivity, but we now know the answer. Nature did the experiment for us: the rods and cones of lamprey have an identical morphology, which is like that of cones (see for example Dickson & Graves, 1979), but lamprey rods are nearly as sensitive as mouse rods and about 70 times more sensitive than lamprey cones (Morshedian & Fain, 2015; Asteriti et al. 2015). The discs of rods do not seem to be essential for high sensitivity vision (see also Ma et al. 2001) but may instead have evolved to allow more efficient renewal of outer segment membrane (Morshedian & Fain, 2015).
Table 1.
Photoreceptor transduction protein isoforms in mouse rods and cones
| Rod | Cone | |
|---|---|---|
| Photopigment | Rod opsin (or rhodopsin) | Cone opsin (or rhodopsin) |
| G protein (transducin) | α1, β1 and γ1 | α2, β3 and γ8 |
| Phosphodiesterase 6 | PDE6A and PDE6B | PDE6C |
| Rod PDE6γ (PDE6G) | Cone PDE6γ (PDE6H) | |
| cGMP‐gated channels | CNGA1 and CNGB1 | CNGA3 and CNGB3 |
| Rhodopsin kinase | GRK1 | GRK1 |
| Arrestin | Arrestin‐1 | Arrestin‐1 and arrestin‐4 |
| GAPs | RGS9‐1, Gβ5 and R9AP‐1 | RGS9‐1, Gβ5 and R9AP‐1 |
| Guanylyl cyclase | retGC1 and retGC2 | retGC1 |
| GCAPs | GCAP1 and GCAP2 | GCAP1 |
| Na+/Ca2+–K+ exchanger | NCKX1 | NCKX2 and NCKX4 |
Activation of transduction
Although rods are universally more sensitive than cones, the value of the sensitivity difference varies among vertebrates, ranging from 25‐fold in mudpuppy (Fain & Dowling, 1973) to 1000‐fold between red‐sensitive cones and rods in carp (Tachibanaki et al. 2001). In our examination of the cause of this sensitivity difference, we will take as our example the mouse, because many of the most recent experiments have utilized transgenic mice. In Fig. 3 A and B, we show mean responses of mouse rods and cones recorded with suction electrodes. Rod responses decay much more slowly than cone responses (note ten‐fold difference in the scale of the abscissa) and are typically about twice as large; after normalizing response amplitudes to their maximum values, rods are a little more than 100 times more sensitive than cones (Fig. 3 C), as previously reported (see for example Nikonov et al. 2006). Part of this difference is the result of the larger volume of the rod outer segment, which increases the probability of absorption of a photon by pigment molecules. We can, however, correct for these differences by calculating the percentage decrease in photocurrent per photon absorbed. Calculations of this kind give about 0.2–0.25% per Rh* for cones (Nikonov et al. 2006; Sakurai et al. 2011; Cao et al. 2014) and 5% for rods (Sampath et al. 2005; see Reingruber et al. 2015). The resulting factor of between 20 and 30 is the difference in sensitivity produced by the transduction cascade.
One reason rods are more sensitive is that early events in the transduction cascade have greater gain and close channels more rapidly, as alluded to previously. As a consequence, rod responses rise more quickly per photon absorbed; with everything else being equal, rod responses would reach a commensurately larger peak amplitude for the same intensity of stimulus. Following the theoretical treatment of Pugh and Lamb (1993, 2000), we can use the rising phases to calculate values of an amplification constant A (see Fig. 4 A and legend), equal to the product of (1) the rate of formation of light‐activated PDE6* by the photopigment, (2) the rate of decline of cGMP concentration per PDE6* molecule, and (3) the Hill coefficient of binding of cGMP to the channels. The value of A is somewhat dependent on the frequency response of the recording (Chen et al. 2010 b) but is at least 2–3 times larger in rods than in cones (see Pugh & Lamb, 1993; Nikonov et al. 2006; Cao et al. 2014). This difference must be produced by the collective properties of the proteins responsible for activation. Since the Hill coefficient of rod and cone cGMP‐gated channels is nearly the same (Picones & Korenbrot, 1992; see Kaupp & Seifert, 2002), we can focus our attention on the photopigments, G proteins and PDE6s, which, as we have seen, all have different isoforms in rods and cones.
Figure 4. Differences in rate of activation and decay of WT and GNAT2C rods .
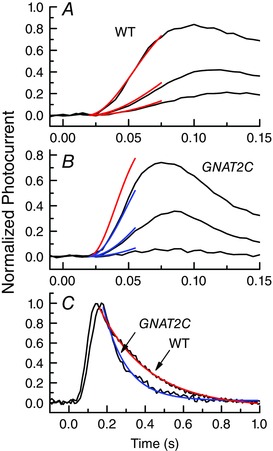
A, black traces are mean initial time courses of responses of 16 WT rods to 10 ms flashes at intensities of 8.6, 21 and 79 photons μm−2, after filtering with an 8‐pole Bessel filter with a low‐pass filter setting of 70 Hz. Responses have been normalized to the peak amplitude of the response. Red traces are fits to the data of the function
where r/r max is the normalized flash response, I is the flash intensity in photoisomerizations, A is the amplification constant, t is time, and t eff is the effective delay time of transduction (Pugh & Lamb, 1993), with the same mean values of A of 20.5 s−2 and t eff of 18 ms at all three intensities. B, black traces are mean initial time courses of responses recorded and normalized as in A but of 14 GNAT2C rods to 10 ms flashes at intensities of 21, 79 and 227 photons μm−2. Blue traces are fits to the data with an A of 10.2 s−2 and t eff of 19.3 ms. Single red curve gives prediction for brightest intensity with WT rod value of A (20.5 s−2). The value of A is about two times smaller in GNAT2C rods. C, mean small‐amplitude responses of 21 WT rods and 9 GNAT2C rods to flashes of intensities 17 photons μm−2 (WT) and 79 photons μm−2 (GNAT2C). Responses have been normalized rod by rod to the peak amplitude of the response to compare waveforms of response decay. Responses have been fitted with single exponentials of 258 ms (red trace, WT) and 122 ms (blue trace, GNAT2C). Responses of GNAT2C rods decay significantly more rapidly. (Panels A–C reprinted with permission from Chen et al. 2010 b).
One way to test the role of these proteins is by exogenous expression of cone proteins in rods or rod proteins in cones. Gene incorporation is easier for rods because there is only one rod photopigment in mouse with a reliable and widely used promoter, and rods are more convenient for physiology; so most experiments have put cone genes into rods. There is one complication: the value of A depends upon the rate of change of the cGMP concentration which is inversely proportional to cytoplasmic volume, because the larger the volume, the smaller the change in concentration per activated enzyme. Since mouse rods are about 2.5 times larger in volume than mouse cones, A would be 2.5 times smaller in rods even if the properties of all of the proteins were the same. To account for the greater value of A actually recorded from rods, activation would need to proceed at a rate at least 5–10 times faster (Nikonov et al. 2006). That is, if we could express the cone variants of all the activation proteins in a rod, activation should be at least something like 5–10 times slower. No one has yet expressed all of the proteins together, but many attempts have been made to express them one by one.
We begin with the photopigments. Sakurai and colleagues (2007) inserted the mouse 508 nm cone pigment gene (mG) in place of mouse rhodopsin. They found that mG/mG rods were about a factor of 3–4 less sensitive than wild‐type (WT) rods and gave smaller values for the activation constant A, but mG/mG rods expressed considerably less pigment and transducin, had smaller outer segments, and showed signs of degeneration. Clearer perhaps were experiments expressing the mG pigment on a background of mutant E112Q rod rhodopsin (Sakurai et al. 2007), whose peak absorbance is shifted into the blue so that rod and cone pigments in mG/RhEQ rods can be stimulated selectively. The cone mG pigment produced a response per Rh* only about a third as large as the rod E112Q rhodopsin.
In a similar study, Shi and colleagues (2007) expressed the mouse short wavelength‐sensitive (360 nm) pigment (mS) in mouse rods and recorded from homozygous mS/mS rods lacking rod rhodopsin as well as from heterozygous photoreceptors expressing both the mS pigment and rod rhodopsin. Although the single‐photon response of mS/mS rods was smaller than in WT rods, confirming the study of Sakurai et al. (2007), recordings from heterozygotes expressing both the mS cone and WT rod pigments and selectively stimulated with short‐ and long‐wavelength light showed no differences in sensitivity or response waveform. The two pigments seemed to produce nearly identical responses when expressed in the same rod.
Fu and colleagues (2008) then expressed the human long‐wavelength pigment in mouse rods. Responses to the rod and cone pigments were indistinguishable in sensitivity and waveform. The cone pigment produced greater dark noise as also in the experiments of Sakurai et al. (2007; but see Shi et al. 2007), perhaps as a result of the lower stability of cone pigments generally (Rieke & Baylor, 2000; Sampath & Baylor, 2002; Kefalov et al. 2003; Kefalov et al. 2005; but see Angueyra & Rieke, 2013). This increase in noise was, however, not large enough to affect photoreceptor sensitivity. In conclusion, cone pigments expressed in rods either have no effect on sensitivity or reduce it by as much as a factor of 2–3.
The first experiments expressing transducin used a viral vector approach to inject the rod or cone Tα gene into a mouse line that lacked both rod and cone transducins (Deng et al. 2009). Only a small fraction of the rods had any light response, probably reflecting the variability in expression level. From the few cells that could be recorded, there was no marked difference between cells expressing rod Tα and those expressing cone Tα. Since, however, the sensitivity of the rod is heavily dependent on transducin expression level (Sokolov et al. 2002), which was not (and could not) be measured for individual cells with this technique, the results were inconclusive.
Chen et al. (2010 b) used a more traditional transgenic approach to express cone transducin in Gnat1−/− mice lacking rod transducin. They were fortunate to isolate a GNAT2C line in which the level of cone transducin was nearly the same as the WT rod transducin level. Sensitivity in GNAT2C rods was reduced by a factor of about 3, and the amplification constant A was about a factor of 2 smaller. This effect on amplification can be seen in Fig. 4 A and B, which shows that the initial phase of the WT response rises more rapidly than that of GNAT2C rods.
Mao and colleagues (2013) then did a similar experiment also using a transgenic approach but with a different result. Rods in their mice expressed less cone Tα than GNAT2C rods and were less sensitive than WT rods, but the decrease in sensitivity seemed to depend only upon the expression level of the transducin and not upon the properties of cone Tα. They concluded that the species of transducin has no effect on the sensitivity difference between rods and cones. Thus incorporation of cone Tα in rods either has no effect on sensitivity or decreases it by as much as a factor of 3. No attempts have been made to express cone β3 or γ8 in place of rod β1 or γ1.
Two groups have attempted to express cone PDE6C in rods. Deng et al. (2013) injected viral vectors containing the PDE6C gene into the eyes of rd10 mice, a line that is deficient in rod PDE6 but does not lack it entirely. Rods with cone PDE6C were surprisingly about twice as sensitive as those with the rod PDE6 proteins and showed a slower time course of decay. This anomalous result may have been produced by an unphysiological level of expression of PDE6, which again could not be measured. A clearer result was obtained by Majumder and colleagues (2015), who used a transgenic approach and were able to compare rod and cone PDE6 at the same expression level. Rods with cone PDE6C had a higher PDE6 basal activity and a single‐photon response between 1.5 and 2 times smaller than WT rods, with a more rapid time course of decay (Fig. 5 A). No attempt has been made to substitute cone PDE6γ for rod PDE6γ. This experiment could be revealing in view of Muradov et al. (2007), who showed that lamprey rods and cones have the same catalytic PDE6 subunits but different γ subunits. In conclusion, substitution of cone PDE6 for rod PDE6 either has no effect or decreases sensitivity by about a factor of two.
Figure 5. Single‐photon responses of mouse rods with altered transduction proteins .
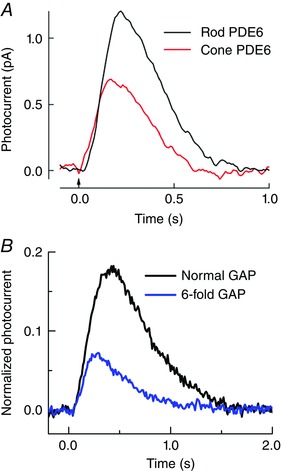
A, derived average single‐photon responses from control rods (black; rod PDE6) and cone‐PDE6C‐expressing rods (red; cone PDE6) (redrawn and reprinted with permission from Majumder et al. 2015). B, superimposed single‐photon responses of WT mouse rods and of R9AP95 rods with six times the normal expression of GAP proteins (Chen et al. 2010 a). Responses were plotted as a fraction of the peak current of the rod, effectively giving the fraction of channels closed per photon. Recordings were made from animals on a GCAPs−/− background to remove the effects of cyclase modulation on response amplitude and waveform (Gross et al. 2012). All experiments were performed on pigmented mice of either sex in accordance with the rules and regulations of the NIH guidelines for research animals, as approved by the institutional animal care and use committees (IACUCs) of the Virginia Commonwealth University and the University of California, Los Angeles. Animals were kept in cyclic 12 h/12 h on/off lighting in approved cages and supplied with ample food and water. Animals in all experiments were killed before tissue extraction by approved procedures, usually CO2 inhalation or decerebration. Rods were perfused at 37°C with Dulbecco's modified Eagle's medium (Sigma Chemical, St Louis, MO, USA), supplemented with 15 mm NaHCO3, 2 mm sodium succinate, 0.5 mm sodium glutamate, 2 mm sodium gluconate, and 5 mm NaCl, bubbled with 95% O2–5% CO2 (pH 7.4). Unless otherwise indicated, data were filtered at 35 Hz (8 pole, Bessel) and sampled at 100 Hz. (M. L. Woodruff, C. K. Chen & G. L. Fain, unpublished data).
In summary, activation in mouse cones is at least 2‐ to 3‐fold slower than activation in mouse rods. Taking outer segment volumes into account, we would predict that expression of cone pigment, cone transducin and cone PDE into a rod should together decrease the rate of activation by at least a factor of 5 with a commensurate decrease in sensitivity. Experiments expressing cone isoforms have, however, given conflicting results, with some showing a 2‐ to 3‐fold difference and some none at all. There are three possibilities: either papers showing significant differences are at least partially correct, or cone isoforms have to be expressed together (for example cone pigment with cone transducin), or other proteins (such as PDEγ or G‐protein β and γ) also have a role. One conclusion, however, seems clear: the contribution of any one isoform is individually small, such that no one protein by itself is responsible for the entire difference in activation or sensitivity between the two kinds of photoreceptors.
Inactivation
If the response per Rh* is 20–30 times smaller in mouse cones than in mouse rods and activation accounts for only part of this difference, the remainder must emerge from mechanisms of inactivation. The records in Fig. 3 show that rods decay much more slowly than cones and integrate incoming photons over a longer time period. This difference in decay could in theory be produced by any of the reactions terminating the response.
We begin with extinction of Rh*. Rods and cones in mouse both phosphorylate photopigment with the same GRK1 kinase with no marked difference in antibody labelling and presumably expression (Lyubarsky et al. 2000; Weiss et al. 2001). Moreover both rods and cones use arrestin‐1 with the small amount of arrestin‐4 in cones unlikely to affect the rate of Rh* decay (Nikonov et al. 2008). The mean lifetime of Rh* in rods is probably as short as 40–45 ms (see Burns & Pugh, 2011), which is already so short that it is difficult to understand how even two or three serines or threonines could be phosphorylated and arrestin bind in so little time (Gurevich et al. 2011). If phosphorylation is faster in cones as Tachibanaki and colleagues have argued (2005), it is probably not much faster at least in mouse, whose rods and cones both express GRK1 at a similar level. More likely suspects for the slower rate of rod inactivation may be differences in the rates of decay of light‐activated PDE6* and restoration of cGMP concentration by the cyclase.
Decay of PDE6* is produced by hydrolysis of Tα•GTP to Tα•GDP and rebinding of the PDE6γ inhibitory subunits to the PDE catalytic subunits. The rate of hydrolysis of Tα•GTP may be affected by the particular isoforms of transducin and PDE6: both cone transducin and cone PDE6C expressed in rods have been reported to produce responses that decay more rapidly than WT rod responses (see Figs 4 C and 5 A). The rate of hydrolysis may also be affected by the GAP proteins which, as we have said, are the same in rods and cones but are more abundantly expressed in cones at perhaps a 10‐fold higher concentration (Cowan et al. 1998; Zhang et al. 2003). This difference in expression could have a significant effect on sensitivity. In Fig. 5 B, we compare single‐photon responses from rods with the normal GAP level and mutant R9AP95 rods in which the GAP proteins are 6‐fold over‐expressed (Chen et al. 2010 a). This experiment was done on a GCAPs−/− background to obviate any effect of cyclase feedback on response waveform or amplitude (Gross et al. 2012). Rods with over‐expressed GAPs are about a factor of 2–3 less sensitive and decay more rapidly.
There are two ways guanylyl cyclase could produce a difference in the rate of cGMP synthesis between rods and cones and alter sensitivity. Both rods and cones in mouse use the same retGC1 cyclase, but the expression level is likely to be higher in cones. Staining with a retGC1 antibody is brighter in cones than in rods (Dizhoor et al. 1994), and unpublished measurements on retinas lacking the neural retina leucine zipper (Nrl) transcription factor (Mears et al. 2001), where all photoreceptors are cone‐like, indicate that mouse cones may have something like 2–3 times more retGC1 than rods (A. Dizhoor, personal communication). Cone PDE6 expression may also be greater than rod but probably by no more than a factor of 1.5 (Zhang et al. 2003; Lobanova et al. 2010); however, cone PDE6 has a higher basal activity (Majumder et al. 2015). Together the cyclase and PDE6 would produce a higher rate of cGMP turnover in darkness, which in salamander has actually been measured and is about 3‐fold greater in cones than in rods (Cornwall & Fain, 1994; Cornwall et al. 1995). This increase in turnover rate would produce both an increase in the rate of response decay and a decrease in sensitivity (Rieke & Baylor, 1996; Nikonov et al. 2000; Fain et al. 2001). Measurements in salamander indicate that if turnover in a rod were increased by a factor of 3, sensitivity would be reduced by about a factor of about 2 (Cornwall & Fain, 1994; Nikonov et al. 2000).
The rate of cGMP synthesis is also controlled by GCAP proteins, which in turn are regulated by the outer‐segment Ca2+ concentration. Although the GCAPs themselves are similar in rods and cones, the change in Ca2+ concentration is considerably faster in cones, at least in salamander (Sampath et al. 1999). Rods and cones express different isoforms of the Na+/Ca2+–K+ exchanger (Lytton, 2007; Vinberg et al. 2015) and may have different concentrations or isoforms of Ca2+ buffers. This accelerated decline in Ca2+ would produce a more rapid modulation of the GCAPs and faster activation of the cyclase, which could in theory decrease cone sensitivity. This notion has, however, been tested by deleting the genes for the GCAP proteins, which increases sensitivity by about the same factor of 3 in both rods (M. L. Woodruff & G. L. Fain, unpublished observations; Gross et al. 2012) and cones (Sakurai et al. 2011). These observations indicate that GCAP‐mediated feedback makes little contribution to the sensitivity difference (however, see Wen et al. 2014). A similar conclusion emerges from comparison of salamander rod and cone responses under conditions that suppress changes in outer‐segment Ca2+ (Matthews et al. 1988, 1990; Nakatani & Yau, 1988, 1989).
In conclusion, the rate of inactivation of transduction is slower in rods than in cones, with the major effects apparently produced by the species of transducin and PDE6, the expression level of cyclase, PDE6 basal activity, and the expression level of the GAP proteins. Each of these differences seems, however, to make a relatively small contribution, and once again no single change predominates.
We have based our conclusions on results from mouse, but it is possible and even likely that additional adaptations are present in other species that contribute to the difference in rod and cone inactivation. Fish are of particular interest, because the difference in rod and cone sensitivity can be much larger than in mouse (Tachibanaki et al. 2001). Kawamura's laboratory has shown that fish cones have a very high rate of pigment phosphorylation by GRK7 (Tachibanaki et al. 2005), an enzyme highly expressed in fish but not present in mouse (Liu et al. 2005). Moreover carp also show a much higher rate of cGMP synthesis in cones than in rods, and therefore a higher cGMP turnover rate (Takemoto et al. 2009). These changes would collectively cause photoresponses from carp cones to be smaller and faster (Tachibanaki et al. 2005;Liu et al. 2005; Takemoto et al. 2009). In addition, Rebrik and Korenbrot have identified a Ca2+‐binding protein present in fish cones but not fish rods that reduces the affinity of cyclic nucleotide‐gated ion (CNG) channels for cGMP in high [Ca2+]i, a protein they first called CNG‐modulin (Rebrik et al. 2012), but later identified as echinoderm microtubule‐associated protein‐like 1 (EML‐1, Korenbrot et al. 2013). Knockdown of this protein in zebrafish produced a 5‐fold increase in cone sensitivity (Korenbrot et al. 2013), presumably by slowing the rate at which CNG channels open following illumination. This protein has not as yet been identified in mammalian cones.
Why are rods more sensitive?
The key step in the formation of the duplex retina of vertebrates was the evolution of more sensitive rods to accompany cones, so that the entire range of light intensities could be encoded by the photoreceptors. Molecular and biochemical studies tell us that rods and cones have many of the same transduction proteins but use different isoforms probably arising by gene duplication (see Table 1); in some cases they use the same isoform but at a different level of expression. No one change accounts for the difference in absolute sensitivity between rods and cones. Instead, each of the differences we have described seems to have produced a small increase in the rate of activation or prolongation of response decay, conferring an incremental advantage to the organism.
Accumulated changes in a large number of proteins eventually produced a sensitivity great enough in the rod to allow it to operate in dim light, with cones remaining for enhanced temporal resolution when photon flux is no longer limiting. These changes also have implications for the dynamic properties of rods and cones, namely their ability to adapt to increasing light intensity. While we have not discussed these mechanisms in this review, the fundamental tradeoff between sensitivity and dynamic range between rods and cones will also depend upon differences in their transduction mechanisms. The properties of the two receptor types form the basis of our duplex visual system, whose fundamental nature was first proposed by Schultze 150 years ago.
Additional information
Competing interests
None declared.
Funding
This work was supported by individual grants from the National Eye Institute of the NIH to G.L.F. (EY001844) and to A.P.S. (EY017606), as well as a core grant to the Jules Stein Eye Institute (EY000331).
Acknowledgements
We are grateful to past and present members of our laboratories for many useful discussions and for participating in some of the experiments we have described, and to Margery J. Fain for help with the figures.
Biography
Gordon L. Fain is Distinguished Professor of Integrative Biology/Physiology and of Ophthalmology at the University of California Los Angeles (UCLA) and a member of the Jules Stein Eye Institute. His laboratory works on the physiology of vertebrate photoreceptors. Norianne Ingram is a graduate student in the Molecular, Cellular, and Integrative Physiology Program at UCLA and is presently doing her thesis on cone photoreceptors and retinal signal processing. Alapakkam P. Sampath is Professor of Ophthalmology at UCLA and a member of the Jules Stein Eye Institute. His laboratory works on vertebrate photoreceptor physiology and signal integration in vertebrate retina.

References
- Angueyra JM & Rieke F (2013). Origin and effect of phototransduction noise in primate cone photoreceptors. Nat Neurosci 16, 1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY & Wensel TG (2013). Timing is everything: GTPase regulation in phototransduction. Invest Ophthalmol Vis Sci 54, 7725–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteriti S, Grillner S & Cangiano L (2015). A Cambrian origin for vertebrate rods. Elife 4, e07166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM & Palczewski K (2007). The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem 282, 8837–8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD & Yau KW (1979). Responses of retinal rods to single photons. J Physiol 288, 613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ & Schnapf JL (1984). The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis . J Physiol 357, 575–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SL, Peterson JJ, Choudhury S, Min SH, Ruan Q, McCullough KT, Zhang Z, Olshevskaya EV, Peshenko IV, Hauswirth WW, Ding XQ, Dizhoor AM & Boye SE (2015). Gene therapy fully restores vision to the all‐cone Nrl −/− Gucy2e −/− mouse model of Leber congenital amaurosis‐1. Hum Gene Ther 26, 575–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns ME & Pugh EN Jr (2011). Lessons from photoreceptors: turning off G‐protein signaling in living cells. Physiology (Bethesda) 25, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LH, Luo DG & Yau KW (2014). Light responses of primate and other mammalian cones. Proc Natl Acad Sci USA 111, 2752–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Woodruff ML, Chen FS, Chen D & Fain GL (2010. a). Background light produces a recoverin‐dependent modulation of activated‐rhodopsin lifetime in mouse rods. J Neurosci 30, 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Woodruff ML, Chen FS, Shim H, Cilluffo MC & Fain G (2010. b). Replacing the rod with the cone transducin α subunit decreases sensitivity and accelerates response decay. J Physiol 588, 3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC & Fain GL (1994). Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol 480, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Matthews HR, Crouch RK & Fain GL (1995). Bleached pigment activates transduction in salamander cones. J Gen Physiol 106, 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CW, Fariss RN, Sokal I, Palczewski K & Wensel TG (1998). High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc Natl Acad Sci USA 95, 5351–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WT, Sakurai K, Kolandaivelu S, Kolesnikov AV, Dinculescu A, Li J, Zhu P, Liu X, Pang J, Chiodo VA, Boye SL, Chang B, Ramamurthy V, Kefalov VJ & Hauswirth WW (2013). Cone phosphodiesterase‐6α′ restores rod function and confers distinct physiological properties in the rod phosphodiesterase‐6β‐deficient rd10 mouse. J Neurosci 33, 11745–11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WT, Sakurai K, Liu J, Dinculescu A, Li J, Pang J, Min SH, Chiodo VA, Boye SL, Chang B, Kefalov VJ & Hauswirth WW (2009). Functional interchangeability of rod and cone transducin α‐subunits. Proc Natl Acad Sci USA 106, 17681–17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DH & Graves DA (1979). Fine structure of the lamprey photoreceptors and retinal pigment epithelium (Petromyzon marinus L.). Exp Eye Res 29, 45–60. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP & Hurley JB (1994). The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron 12, 1345–1352. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I & Hurley JB (1995). Cloning, sequencing, and expression of a 24‐kDa Ca2+‐binding protein activating photoreceptor guanylyl cyclase. J Biol Chem 270, 25200–25206. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Olshevskaya EV & Peshenko IV (2010). Mg2+/Ca2+ cation binding cycle of guanylyl cyclase activating proteins (GCAPs): role in regulation of photoreceptor guanylyl cyclase. Mol Cell Biochem 334, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL (2014). Molecular and Cellular Physiology of Neurons, 2nd edn Harvard University Press, Cambridge, MA, USA. [Google Scholar]
- Fain GL & Dowling JE (1973). Intracellular recordings from single rods and cones in the mudpuppy retina. Science 180, 1178–1181. [DOI] [PubMed] [Google Scholar]
- Fain GL, Hardie R & Laughlin SB (2010). Phototransduction and the evolution of photoreceptors. Curr Biol 20, R114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC & Koutalos Y (2001). Adaptation in vertebrate photoreceptors. Physiol Rev 81, 117–151. [DOI] [PubMed] [Google Scholar]
- Fu Y, Kefalov V, Luo DG, Xue T & Yau KW (2008). Quantal noise from human red cone pigment. Nat Neurosci 11, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross OP, Pugh EN Jr & Burns ME (2012). Calcium feedback to cGMP synthesis strongly attenuates single‐photon responses driven by long rhodopsin lifetimes. Neuron 76, 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Hanson SM, Song X, Vishnivetskiy SA & Gurevich EV (2011). The functional cycle of visual arrestins in photoreceptor cells. Prog Retin Eye Res 30, 405–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger S & Hepler JR (2002). Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev 54, 527–559. [DOI] [PubMed] [Google Scholar]
- Kaupp UB & Seifert R (2002). Cyclic nucleotide‐gated ion channels. Physiol Rev 82, 769–824. [DOI] [PubMed] [Google Scholar]
- Kefalov V, Fu Y, Marsh‐Armstrong N & Yau KW (2003). Role of visual pigment properties in rod and cone phototransduction. Nature 425, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC & Yau KW (2005). Breaking the covalent bond – a pigment property that contributes to desensitization in cones. Neuron 46, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI, Mehta M, Tserentsoodol N, Postlethwait JH & Rebrik TI (2013). EML1 (CNG‐modulin) controls light sensitivity in darkness and under continuous illumination in zebrafish retinal cone photoreceptors. J Neurosci 33, 17763–17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Hirano T & Kakinuma M (1991). Molecular cloning and sequence analysis of cDNA and genomic DNA for the human cone transducin α subunit. FEBS Lett 291, 245–248. [DOI] [PubMed] [Google Scholar]
- Liu P, Osawa S & Weiss ER (2005). M opsin phosphorylation in intact mammalian retinas. J Neurochem 93, 135–144. [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Herrmann R, Finkelstein S, Reidel B, Skiba NP, Deng WT, Jo R, Weiss ER, Hauswirth WW & Arshavsky VY (2010). Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light. J Neurosci 30, 6815–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J (2007). Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406, 365–382. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Chen C, Simon MI & Pugh EN Jr (2000). Mice lacking G‐protein receptor kinase 1 have profoundly slowed recovery of cone‐driven retinal responses. J Neurosci 20, 2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Harosi FI, Makino CL & Crouch RK (2001). A visual pigment expressed in both rod and cone photoreceptors. Neuron 32, 451–461. [DOI] [PubMed] [Google Scholar]
- Majumder A, Pahlberg J, Muradov H, Boyd KK, Sampath AP & Artemyev NO (2015). Exchange of cone for rod phosphodiesterase 6 catalytic subunits in rod photoreceptors mimics in part features of light adaptation. J Neurosci 35, 9225–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W, Miyagishima KJ, Yao Y, Soreghan B, Sampath AP & Chen J (2013). Functional comparison of rod and cone Gαt on the regulation of light sensitivity. J Biol Chem 288, 5257–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH (2012). The neuronal organization of the retina. Neuron 76, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Fain GL, Murphy RL & Lamb TD (1990). Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol 420, 447–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Murphy RL, Fain GL & Lamb TD (1988). Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature 334, 67–69. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA & Swaroop A (2001). Nrl is required for rod photoreceptor development. Nat Genet 29, 447–452. [DOI] [PubMed] [Google Scholar]
- Morshedian A & Fain GL (2015). Single‐photon sensitivity of lamprey rods with cone‐like outer segments. Curr Biol 25, 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradov H, Boyd KK, Kerov V & Artemyev NO (2007). PDE6 in lamprey Petromyzon marinus: implications for the evolution of the visual effector in vertebrates. Biochemistry 46, 9992–10000. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Tamura T & Yau KW (1991). Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol 97, 413–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K & Yau KW (1988). Calcium and light adaptation in retinal rods and cones. Nature 334, 69–71. [DOI] [PubMed] [Google Scholar]
- Nakatani K & Yau KW (1989). Sodium‐dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol 409, 525–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle B & Robinson PR (2007). The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies. Cell Mol Life Sci 64, 2917–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov S, Lamb TD & Pugh EN Jr (2000). The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light‐adapted salamander rod photoresponse. J Gen Physiol 116, 795–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN Jr & Craft CM (2008). Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron 59, 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J & Pugh EN Jr (2006). Physiological features of the S‐ and M‐cone photoreceptors of wild‐type mice from single‐cell recordings. J Gen Physiol 127, 359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong OC, Yamane HK, Phan KB, Fong HK, Bok D, Lee RH & Fung BK (1995). Molecular cloning and characterization of the G protein γ subunit of cone photoreceptors. J Biol Chem 270, 8495–8500. [DOI] [PubMed] [Google Scholar]
- Picones A & Korenbrot JI (1992). Permeation and interaction of monovalent cations with the cGMP‐gated channel of cone photoreceptors. J Gen Physiol 100, 647–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LR (2011). Guanylyl cyclase structure, function and regulation. Cell Signal 23, 1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN Jr & Lamb TD (1993). Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1141, 111–149. [DOI] [PubMed] [Google Scholar]
- Pugh EN Jr & Lamb TD (2000). Phototransduction in vertebrate rods and cones: molecular mechanism of amplification, recovery and light adaptation In Handbook of Biological Physics, pp. 183–255. Elsevier, Amsterdam. [Google Scholar]
- Rebrik TI, Botchkina I, Arshavsky VY, Craft CM & Korenbrot JI (2012). CNG‐modulin: a novel Ca‐dependent modulator of ligand sensitivity in cone photoreceptor cGMP‐gated ion channels. J Neurosci 32, 3142–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingruber J, Holcman D & Fain GL (2015). How rods respond to single photons: Key adaptations of a G‐protein cascade that enable vision at the physical limit of perception. Bioessays 37, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F & Baylor DA (1996). Molecular origin of continuous dark noise in rod photoreceptors. Biophys J 71, 2553–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F & Baylor DA (2000). Origin and functional impact of dark noise in retinal cones. Neuron 26, 181–186. [DOI] [PubMed] [Google Scholar]
- Sakmar TP & Khorana HG (1988). Total synthesis and expression of a gene for the α‐subunit of bovine rod outer segment guanine nucleotide‐binding protein (transducin). Nucleic Acids Res 16, 6361–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Chen J & Kefalov VJ (2011). Role of guanylyl cyclase modulation in mouse cone phototransduction. J Neurosci 31, 7991–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Onishi A, Imai H, Chisaka O, Ueda Y, Usukura J, Nakatani K & Shichida Y (2007). Physiological properties of rod photoreceptor cells in green‐sensitive cone pigment knock‐in mice. J Gen Physiol 130, 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP & Baylor DA (2002). Molecular mechanism of spontaneous pigment activation in retinal cones. Biophys J 83, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Matthews HR, Cornwall MC, Bandarchi J & Fain GL (1999). Light‐dependent changes in outer segment free Ca2+ concentration in salamander cone photoreceptors. J Gen Physiol 113, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F & Hurley JB (2005). Recoverin improves rod‐mediated vision by enhancing signal transmission in the mouse retina. Neuron 46, 413–420. [DOI] [PubMed] [Google Scholar]
- Schultze M (1866). Zur Anatomie und Physiologie der Retina. Archiv für mikroskopische Anatomie 2, 175–286. [Google Scholar]
- Shi G, Yau KW, Chen J & Kefalov VJ (2007). Signaling properties of a short‐wave cone visual pigment and its role in phototransduction. J Neurosci 27, 10084–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y & Matsuyama T (2009). Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci 364, 2881–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuart NG, Haitin Y, Camp SS, Black KD & Zagotta WN (2011). Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide‐gated ion channels. Nat Commun 2, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN Jr & Arshavsky VY (2002). Massive light‐driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron 34, 95–106. [DOI] [PubMed] [Google Scholar]
- Tachibanaki S, Arinobu D, Shimauchi‐Matsukawa Y, Tsushima S & Kawamura S (2005). Highly effective phosphorylation by G protein‐coupled receptor kinase 7 of light‐activated visual pigment in cones. Proc Natl Acad Sci USA 102, 9329–9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibanaki S, Tsushima S & Kawamura S (2001). Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc Natl Acad Sci USA 98, 14044–14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto N, Tachibanaki S & Kawamura S (2009). High cGMP synthetic activity in carp cones. Proc Natl Acad Sci USA 106, 11788–11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinberg F, Wang T, Chen J & Kefalov V (2015). Na+/Ca2+, K+ exchangers 4 and 2 are required for the rapid light response recovery and normal light adaptation of cones. Invest Ophthalmol Vis Sci 56, E‐Abstract 1713. [Google Scholar]
- Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM & Osawa S (2001). Species‐specific differences in expression of G‐protein‐coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci 21, 9175–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XH, Dizhoor AM & Makino CL (2014). Membrane guanylyl cyclase complexes shape the photoresponses of retinal rods and cones. Front Mol Neurosci 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Morris L, Thapa A, Ma H, Michalakis S, Biel M, Baehr W, Peshenko IV, Dizhoor AM & Ding XQ (2013). cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J Neurosci 33, 14939–14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R & Liebman PA (1978). Light‐activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem 253, 8902–8909. [PubMed] [Google Scholar]
- Zhang X, Wensel TG & Kraft TW (2003). GTPase regulators and photoresponses in cones of the eastern chipmunk. J Neurosci 23, 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS & Yau KW (2002). The heteromeric cyclic nucleotide‐gated channel adopts a 3A:1B stoichiometry. Nature 420, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]


