Abstract
Key points
GABA is an inhibitory transmitter but can sometimes produce paradoxical excitatory effects through synaptic networks.
We found a novel GABA‐mediated excitation within a single retinal cell. It involves a chain of events from receptor stimulation to the sequential modulation of two associated channels, resulting in enhanced neuroexcitability.
GABAB receptor activation selectively suppresses N‐type calcium channels.
The BK‐type potassium channels are exclusively linked to the N‐type calcium channel.
Thus, stimulation of GABAB receptors suppresses an outward current, increasing the excitatory range of single neurons.
Abstract
GABAB receptors (GABABRs) suppress voltage‐gated calcium channels and activate G‐protein coupled potassium channels (GIRK and TREK channels), both mechanisms serving to inhibit neurons. In isolated rat retinal spiking neurons, GABABRs produce both actions but the net effect is to enhance excitatory signals. This is because GABABRs selectively suppress N‐type calcium channels, which in turn are specifically linked to BK channels. Consequently, when GABABRs are stimulated there is a reduction in outward current, allowing neurons to extend their level of depolarization. Whereas many retinal neurons use L‐type channels to stimulate vesicle fusion, the suppression of N‐type channels augments dynamic range without affecting transmitter release.
Key points
GABA is an inhibitory transmitter but can sometimes produce paradoxical excitatory effects through synaptic networks.
We found a novel GABA‐mediated excitation within a single retinal cell. It involves a chain of events from receptor stimulation to the sequential modulation of two associated channels, resulting in enhanced neuroexcitability.
GABAB receptor activation selectively suppresses N‐type calcium channels.
The BK‐type potassium channels are exclusively linked to the N‐type calcium channel.
Thus, stimulation of GABAB receptors suppresses an outward current, increasing the excitatory range of single neurons.
Abbreviations
- BK channels
big conductance potassium channels
- GABAR
GABA receptor
- GABAAR
alpha‐subunit containing ionotropic GABA receptor
- GABABR
B‐type metabotropic GABA receptor
- GABAcR
rho‐subunit containing ionotropic GABA receptor
- GIRK
G‐protein coupled inward rectifying potassium channels
- GTPγS
guanosine 5′‐O‐[gamma‐thio]triphosphate
- NBA
neurobasal A culture medium
- SK channels
small conductance potassium channels
- TPMPA
(1,2,5,6‐tetrahydropyridin‐4‐yl)methylphosphinic acid
- TEA
tetraethylammonium chloride
- TREK
two‐pore‐domain containing potassium channels
Introduction
GABA is the pre‐eminent inhibitory neurotransmitter in the CNS, acting on both ionotropic and metabotropic receptors. Dysfunction of this transmitter system is associated with a number of neuropathologies such as insomnia, epilepsy and anxiety as well as psychiatric disorders such as depression and schizophrenia. In these cases the disease states arise because suppression within the GABAergic pathway leads to elevated excitation.
Although the ionotropic alpha‐subunit containing ionotropic GABA receptor (GABAAR) with its associated chloride channel is the dominant mediator of inhibition, the B‐type metabotropic GABA receptor (GABABR) performs a complementary role through G‐protein regulation of voltage‐gated calcium channels, inward rectifying potassium channels (GIRKs) and the two‐pore‐domain TREK‐2 potassium channels (Bowery et al. 2002; Deng et al. 2009; Padgett & Slesinger, 2010). These two GABA systems can produce reinforcing inhibition and act synergistically through their kinetics, mechanisms and amplification (Barnard et al. 1998).
However, the ionotropic GABAR can induce an excitatory effect in the nervous system by suppressing a second inhibitory pathway, a mechanism referred to as cross inhibition. The GABABR can also be excitatory, acting presynaptically to suppress ionotropic GABA and glycine inhibitory pathways. By suppressing presynaptic calcium channels, GABABR autoreceptors reduce GABA release. Cross‐over to block glycine release can produce excitation by disinhibition. An example is found in rabbit retina, where release of acetylcholine is suppressed by GABAARs but enhanced by release of GABABRs (Friedman & Redburn, 1990; Massey et al. 1997). This GABABR pathway is reported to act by inhibiting glycinergic circuits (Neal & Cunningham, 1995). Here, we describe a postsynaptic mechanism by which GABABRs can augment excitation.
The GABABR is prominent in rat retina, constituting approximately one‐quarter of all GABARs, found throughout the inner plexiform layer and in horizontal cells in distal retina (Koulen et al. 1998). There is a notable lack of GABABRs on glutamatergic bipolar cells, but they are found on inhibitory amacrine cells presynaptic to the bipolar cells. This is a potential site for excitatory disinhibition.
The pharmacology and mechanisms of action of GABABRs are varied in vertebrate retina. Baclofen, the prototype agonist, suppresses voltage‐gated calcium currents in fish retinal ganglion cells (Bindokas & Ishida, 1991). However, in fish bipolar cells, baclofen is ineffective while cis‐aminocrotinic acid inhibits voltage‐gated calcium currents (Heidelberger & Matthews, 1991; Matthews et al. 1994). In amphibian retinal ganglion cells baclofen suppresses N‐type but enhances L‐type calcium channels (Shen & Slaughter, 1999), while cis‐aminocrotonic acid suppresses L‐type channels (Zhang et al. 1997). In this study the focus was on the baclofen‐sensitive GABABR.
GABABRs potentiate excitatory signals in amphibian ganglion cells. Light‐evoked ganglion cell excitatory postsynaptic potentials are enhanced by exogenously applied baclofen (Bai & Slaughter, 1989; Pan & Slaughter, 1991). GABABR antagonists reveal two endogenous GABABR‐mediated mechanisms that enhanced excitatory postsynaptic currents. One is a direct increase in bipolar cell glutamate release and a second is glycinergic pathway disinhibition. In mammalian retina, where direct regulation of bipolar cells seems absent, we explored other possible direct mechanisms that might enhance excitatory signals in ganglion cells. One such direct process is GABABR regulation of voltage‐gated channels on ganglion cells that yields a postsynaptic increase in excitability.
Methods
Ethical approval
Experiments were performed on cultured neurons from 2‐ to 8‐day‐old Sprague‐Dawley (Rattus norvegicus) rat pups. Female rats and 2‐day‐old pups were obtained from Harlan Laboratories (Indianapolis, IN, USA) and housed at the University at Buffalo Comparative Medicine‐Laboratory Animal Facilities. Rats were housed at 21°C on a 12 h dark/light cycle. Handling and use of animals was performed in accordance with USA Animal Welfare Act, National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal Care Guidelines of the State University of New York. Protocols and procedures involving animals were approved by the University at Buffalo Institutional Animal Care and Use Committee. The investigators understand, and the experiments abide by, the ethical principles checklist of the Journal of Physiology.
The dissociation procedure was as follows: pups were anaesthetized with halothane (2‐bromo‐2‐chloro‐1,1,1‐trifluoroethane; Sigma‐Aldrich, St Louis MO, USA) then decapitated. Eyes were enucleated, placed in cold Hanks Balanced Salt Solution (Life Technologies, Grand Island, NY, USA) and the retina was isolated. The retina was transferred to a centrifuge tube containing NBA culture media (neurobasal A, 2% B‐27 nutritional supplement, 1% penicillin streptomycin and 0.5 mm l‐glutamine) along with 20 U ml−1 papain (Worthington Biochemical, Lakewood Township, NJ, USA). The retina was incubated in a water bath at 35°C for 20–40 min, shaken gently every 10 min. The retina was transferred to a 15 ml conical tube containing 2 ml of NBA and gently triturated using a fire‐polished glass Pasteur pipette. An additional 5 ml of fresh NBA media along with 0.5 ml of BSA was added to the solution before plating on 35 mm untreated culture dishes. The cells were allowed to recover for a minimum of 3 days at 37°C and 5% CO2.
Electrophysiological techniques
Electrophysiological recordings were made in whole cell mode using an Axon Instruments Multiclamp 700B Amplifier, Axon Digidata 1322A digitizer and pCLAMP 10 software (Molecular Devices, Sunnyvale, CA, USA), Electrodes were pulled from borosilicate glass pipettes of OD 1.5 mm, ID 0.87 mm (Friedrich & Dimmock, Millville, NJ, USA) and had an average resistance of 7 MΩ. The standard bath solution contained: 130 mm tetraethylammonium chloride (TEA), 1 mm MgCl2, 10 mm BaCl2, 10 mm Hepes and 10 mm D‐glucose adjusted to pH 7.4 with NaOH. The pipette solution contained: 135 mm potassium gluconate, 5 mm KCl, 1 mm MgCl2, 10 mm EGTA, 10 mm Hepes, 2 mm Mg‐ATP and 0.5 mm GTP adjusted to pH 7.2 with KOH for general recordings, and 140 mm CsMeSO3, 4 mm MgCl2, 10 mm EGTA, 10 mm Hepes, 2 mm Mg‐ATP and 0.5 mm GTP adjusted to pH 7.2 with CsOH for calcium current recordings. All experiments were performed at room temperature.
Drugs and reagents
Culture media were purchased from Life Technologies, pharmaceuticals from Tocris Bioscience (Ellisville, MO, USA) and standard chemicals from Sigma‐Aldrich.
Statistics
Statistical comparisons were calculated using Minitab software for Student's t tests. Paired t tests were used to compare electrical responses before and during drug application from a series of cell experiments, and unpaired t tests were used when comparing mean experimental responses from a population of cells.
Results
GABABRs in third‐order neurons
The effects of baclofen and GABA were tested in isolated rat retinal third‐order neurons: amacrine and ganglion cells. Neurons were identified by their morphology and the presence of prominent sodium currents during voltage steps under control conditions. Based on the large amplitude of the inward, voltage‐activated sodium current (>1 nA) it was assumed that most of the recordings were from ganglion cells. After identification, neurons were bathed in an extracellular sodium‐free Ringer solution that contained 130 mm TEA‐Cl, which blocked most of the potassium current and eliminated the inward sodium current. The remaining current was a voltage‐gated inward calcium current. The identification of this calcium current was ascertained by application of cadmium. Application of 10 μm GABA suppressed this inward current, as well as inducing an outward current that probably represented a ligand‐gated chloride current (Fig. 1 A, green trace). If the ligand‐gated chloride channels, GABAARs and GABACRs, were blocked by picrotoxin and (1,2,5,6‐tetrahydropyridin‐4‐yl)methylphosphinic acid (TPMPA), respectively, then application of GABA reduced the inward calcium channel current without eliciting an outward current (Fig. 1 A, red trace). Picrotoxin and TPMPA alone did not affect the inward calcium current. GABA alone or GABA in the presence of picrotoxin and TPMPA produced a similar suppression of the calcium current (Fig. 1 B). Note that GABACRs in rat, unlike many other species, are not effectively blocked by picrotoxin (Feigenspan et al. 1993).
Figure 1. GABAB inhibits a voltage‐gated calcium current .
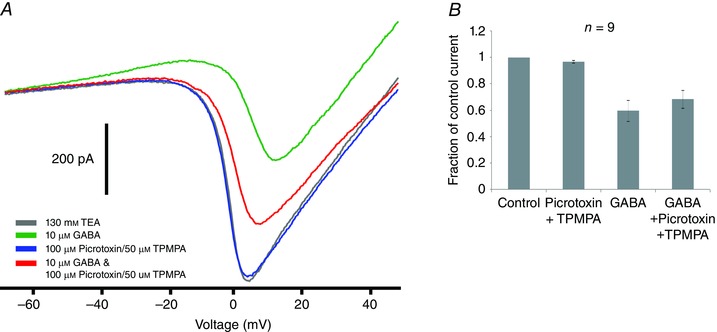
A, whole cell currents recorded from third‐order neuron in response to voltage ramp (−70 to +50 mV) (grey trace). Application of 10 μm GABA decreased the inward calcium current and elicited a sustained outward current (green trace). Application of 100 μm picrotoxin and 50 μm TPMPA (GABAA and GABAC antagonists, respectively) alone (blue trace) and with 10 μm GABA (red trace). B, summary of similar experiments in nine cells. GABA alone suppressed the calcium current by 41 ± 8% (P < 0.01) and by 32 ± 7% (P < 0.01) in the presence of picrotoxin and TPMPA.
Baclofen (10 μm), a potent GABABR agonist (Bowery et al. 1980), suppressed the inward calcium current without eliciting the outward current produced by GABA (Fig. 2 A, green trace). On average, 10 μm baclofen was more effective than 10 μm GABA, but this difference was not statistically significant and the experiments were not performed on the same cells. The action of baclofen was blocked by 20 μm CGP 55845, a GABABR antagonist, while CGP 55845 alone had no effect on the calcium channel current (Fig. 2 C). In previous studies, baclofen had an unexpectedly high IC50 of 100 μm in isolated salamander ganglion cells, although this was also shown to be an artifact due to elevated internal calcium induced by the dissociation process (Shen & Slaughter, 1999). In contrast to amphibians, baclofen was a potent agonist on isolated rodent retinal neurons, with an IC50 of 117 ± 20 nm and a Hill coefficient of 1.7 ± 0.5 (Fig. 2 B). This result is in line with previous reports of baclofen sensitivity in mammalian tissue (Bowery et al. 1983; Shen & Johnson, 1997).
Figure 2. Pharmacology of the GABAB receptor effect .
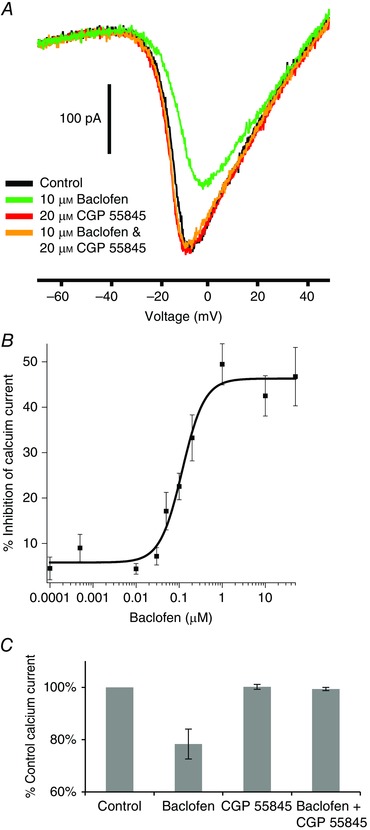
A, baclofen reduces the inward calcium current (green trace vs. black trace). Baclofen in the presence of CGP 55845 (orange trace), a GABAB antagonist, did not suppress the calcium current. CGP 55845 alone (red trace) had no effect on the calcium current. B, the suppression produced by various concentrations of baclofen was normalized to the effect of 50 μm baclofen and the results fitted to the Hill equation. The IC50 was 117 ± 20 nm and the Hill coefficient was 1.7 ± 0.5. C, summary of the experiments shown in A on six neurons. Baclofen produced a reduction of 32 ± 6% (P < 0.01) in the inward calcium current. There was no statistical difference between control, CGP 55845, and baclofen + CGP 55845.
Calcium channel subtypes
The types of calcium channel regulated by baclofen vary with the tissue. Retina is unusual in that it has a high proportion of L‐type calcium channels. There are several antagonists used to suppress L‐type calcium channels: nifedipine, verapramil, nimodipine and SR 33805. When each of these antagonists was individually tested at a concentration of 20 μm (except verapamil at 100 μm), they suppressed voltage‐gated calcium channels in some third‐order neurons but not in others. Several studies use higher concentrations of antagonists, often 50–100 μm, presumably to overcome this apparent ineffectiveness (Maruyama et al. 1997). However, high concentrations risk a possible loss in selectively. As an alternative, we made a cocktail that included all four antagonists, each at a concentration of 20 μm (with verapamil at 100 μm). This antagonist cocktail was effective in consistently blocking a portion of the voltage‐gated calcium current in all the third‐order neurons tested (Fig. 3 A). Comparable observations were made using voltage steps (data not shown). In a set of nine cells in which 10 μm baclofen suppressed 27 ± 3% (P < 0.01) of the total inward current under control conditions, baclofen in the presence of the cocktail reduced 43 ± 6% (P < 0.01) of the calcium current. There was a significant difference in the percentage suppression, but the absolute amplitudes suppressed were equivalent. We interpret this to indicate that the antagonist cocktail is suppressing L‐type calcium channels and baclofen is suppressing a different calcium channel subtype.
Figure 3. Calcium channel subtypes affected by GABAB receptor activation .
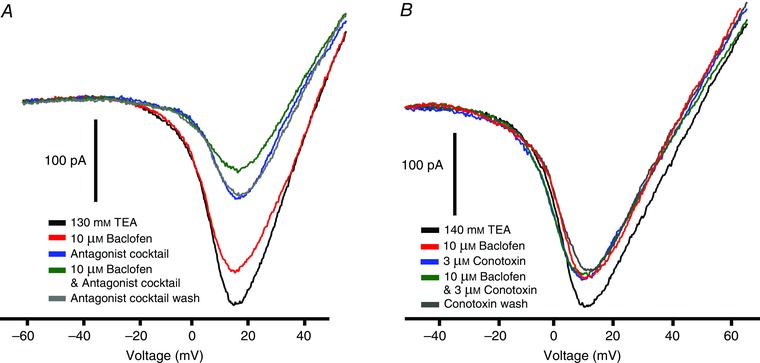
A, baclofen (10 μm) suppressed the ramp‐generated inward calcium current (red vs. black traces). A ‘cocktail’ of L‐type calcium channel blockers (20 μm nifedipine, 100 μm verapramil, 20 μm nimodipine and 20 μm SR33805) reduced the inward current (blue trace) and the addition of baclofen to this cocktail produced an additional reduction in the calcium current (green trace). Extracellular calcium instead of barium was used as the charge carrier in this experiment. B, using the same protocol as in A, 10 μm baclofen reduced the inward current (red trace), 3 μm omega conotoxin GVIA (N‐type calcium channel blocker) reduced the recovered inward current (blue trace) but baclofen in the presence of omega conotoxin (green trace) had no additional effect.
Another high‐voltage activated calcium channel that has been identified in third‐order retinal neurons is the N‐type, which can be blocked by omega conotoxin GVIA. As shown in Fig. 3 B, 3 μm conotoxin suppressed a portion of the inward calcium channel current. Baclofen (10 μm) suppressed a similar portion of the inward current in the cell illustrated, although in many cells it reduced slightly less inward current than did omega conotoxin. Unlike the effects of the L‐type calcium channel blocker cocktail, omega conotoxin completely occluded the effect of baclofen. In nine cells tested, omega conotoxin suppressed 30 ± 6 % (P < 0.01) of the inward current, while baclofen suppressed slightly less, 22 ± 3 % (P < 0.01). The action of omega conotoxin and baclofen in combination was no greater than the effect of omega conotoxin alone. Overall, this indicates that baclofen suppression of voltage‐gated calcium channels is almost exclusively due to its action on N‐type calcium channels.
GABABRs suppress N‐type channels by a direct interaction between the channel and the G‐protein. The following experiments support that mechanism in retinal neurons. First, the non‐hydrolysable guanosine 5′‐O‐[gamma‐thio]triphosphate (GTPγS) fully occluded the effect of baclofen in all 13 cells tested, demonstrating that this is a G‐protein‐mediated action (Fig. 4 A, D). Second, the effect of baclofen was voltage‐dependent (Fig. 4 B). G‐proteins suppress N‐type calcium channels by direct binding that is voltage‐dependent and reduced at positive voltages (Bean, 1989). Thus, a depolarizing prepulse to +60 mV reduced the baclofen‐induced suppression of the calcium current. In the presence of baclofen, the calcium current during the first step to −10 mV was reduced by 44 ± 5%, while for the second step to −10 mV, after the prepulse, was reduced by 19 ± 3%. Third, although the GABABR can stimulate Gs, forskolin stimulation of the protein kinase A pathway did not reduce baclofen's inhibition of the calcium channel (Fig. 4 C, D), suggesting this was not the operational mechanism. Overall, the baclofen suppression of calcium current in retinal neurons has the hallmarks of a direct G‐protein suppression of N‐type channels.
Figure 4. GABAB receptor second messenger pathway .
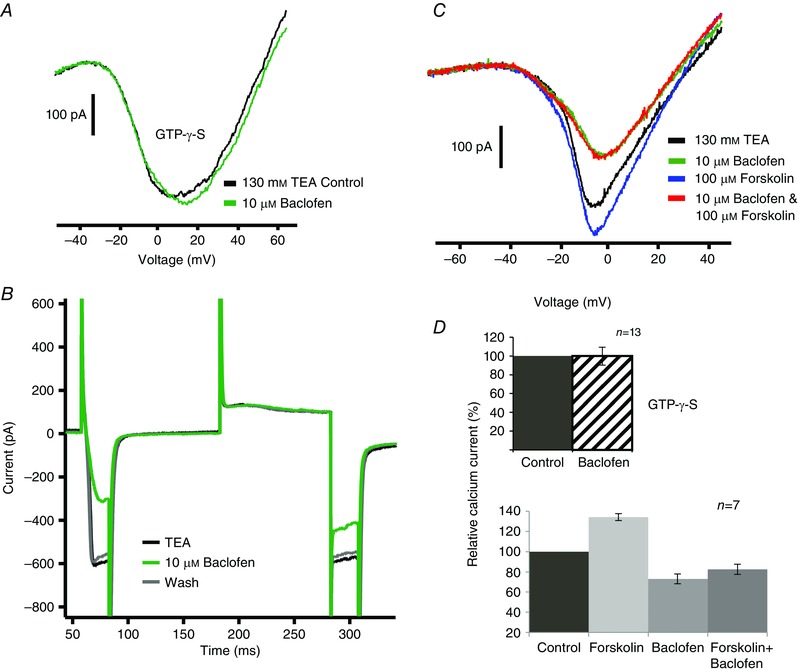
A, baclofen did not reduce the inward current when 0.3 mm GTPγS, a G‐protein activator, was included in the pipette solution. B, a depolarizing prepulse reduced the effect of baclofen on the calcium current. The voltage protocol was a depolarizing step to −10 mV from the holding potential of −70 mV, then a return to −70 mV, then a depolarizing step to +60 mV followed by again stepping to −10 mV and finally returning to −70 mV. Note that baclofen reduced the inward current more in response to the first −10 mV step than the second. C, forskolin (100 μm) increased the inward calcium current (blue vs. black trace) but 10 μm baclofen still reduced the calcium current (red trace). D, summary of the experiments illustrated in A (13 cells) and C (7 cells). There was no statistically significant effect produced by baclofen in the presence of GTPγS. In seven cells, forskolin increased the calcium current by 34 ± 3%. Compared to the control calcium current, baclofen alone reduced the calcium current by 26 ± 6% (P < 0.05), while baclofen in the presence of forskolin reduced the current by 18 ± 5% (P < 0.01).
Indirect suppression of potassium current
When experiments were performed in normal extracellular Ringer solution, instead of the high TEA solution used to isolate calcium channel currents, the most evident effect of baclofen was to reduce the voltage‐dependent outward current (Fig. 5 A). The reduction of the outward current was blocked by a GABABR antagonist CGP 55845. It might be expected that the potassium current would increase if the inward calcium current was suppressed. The reduced outward current could be due to a direct block of potassium channels mediated by GABABRs, but the only reported direct effect of GABABRs is to activate G‐protein coupled inward rectifying potassium (GIRK) and two‐pore‐domain containing potassium (TREK) currents. Therefore, it is more likely that metabotropic GABARs are indirectly affecting calcium‐activated potassium channels linked to the N‐type channel. This link has been reported previously and the next series of experiments were designed to examine this pathway.
Figure 5. Effect of GABAB receptor activation on outward potassium current .
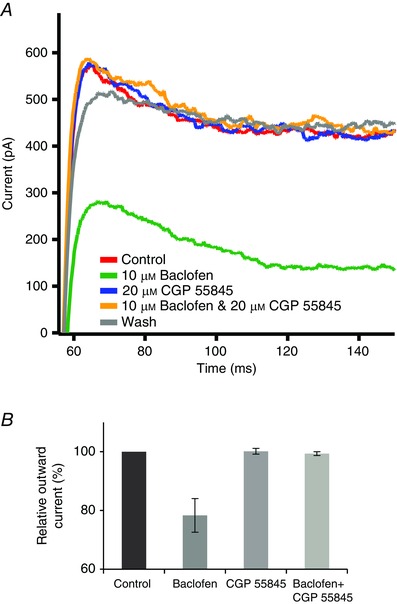
A, depolarizing step to +10 mV evoked an outward potassium current (red trace) that was reduced by 10 μm baclofen (green trace). While 20 μm CGP 55845 did not reduce the outward current (blue trace), it blocked the effect of baclofen (yellow trace). B, summary of these experiments on six cells. Baclofen alone suppressed the outward current by 22 ± 6% (P < 0.01 compared to control). There was no statistically significant difference between the control outward current and the outward currents in the presence of CGP 55845 alone or in baclofen plus CGP 55845.
Demonstrating this link, cadmium block of voltage‐gated calcium channels suppressed a portion of the outward potassium current and baclofen produced no further suppression of the potassium current in the presence of cadmium (Fig. 6 A, C). The big conductance (BK) subtype of the calcium‐activated potassium channels has been reported in retinal ganglion cells, and thus was a likely candidate. Low concentrations of TEA were used to distinguish between BK and small conductance potassium (SK) channels. At a concentration of 1 mm, TEA preferentially inhibits BK channels (80%) over SK channels (20%) (Grissmer et al. 1994). Application of 1 mm TEA suppressed the outward potassium current (Fig. 6 B, C) and the additional application of 10 μm baclofen did not further reduce the outward current.
Figure 6. The GABAB receptor‐sensitive outward current is linked to calcium channels and BK channels .
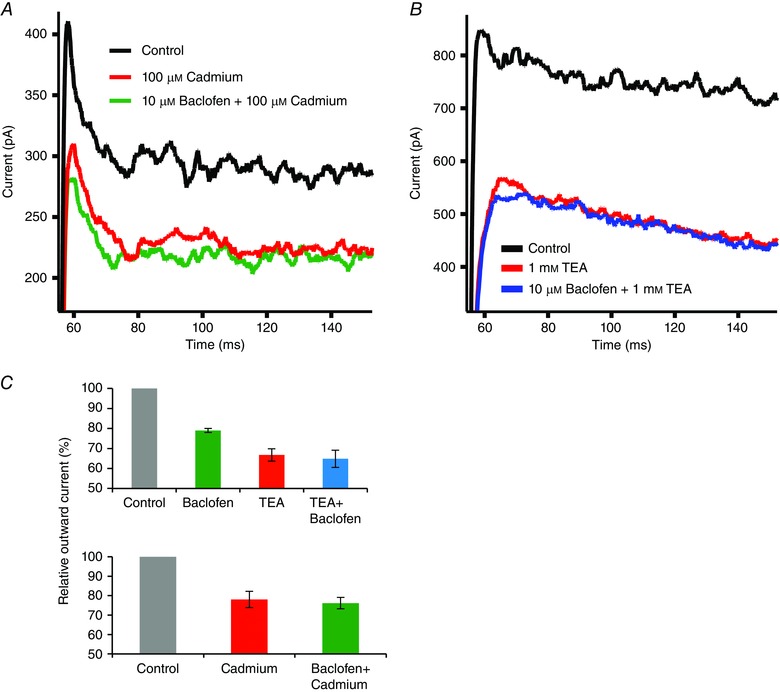
A, an outward current was elicited by a step from −70 to +10 mV (black trace). This was repeated in the presence of 100 μm cadmium (red trace), which blocks voltage‐gated calcium channels. Further addition of 10 μm baclofen had no additional effect (green trace). This indicates that baclofen suppresses a calcium‐dependent potassium current. B, a step from −70 to +10 mV produced an outward current (black trace) that was reduced by 1 mm TEA, a selective BK channel blocker (red trace). In the presence of TEA, baclofen did not further reduce the outward current (blue trace). C, data summarizing the effects of TEA (n = 9) or cadmium (n = 9) with, or without, baclofen. Baclofen reduced the outward current by 21 ± 1% (P < 0.01 compared to control), TEA alone reduced the outward current by 33 ± 3% (P < 0.01 compared to control), while TEA plus baclofen reduced the outward current by 35 ± 4% (P < 0.01 compared to control). Cadmium reduced the outward current by 22 ± 4%, while the addition of baclofen in the presence of cadmium produced no additional effect on outward current.
A potent and selective BK channel blocker, 100 nm iberiotoxin, reduced the outward potassium current by 24.5 ± 2.9% (P < 0.01) and occluded the effect of baclofen (Fig. 7 A). These results suggest that baclofen affects the BK channel, probably indirectly through regulation of the N‐type calcium channel.
Figure 7. The association between GABAB receptors, N‐type calcium channels, and the BK channel .
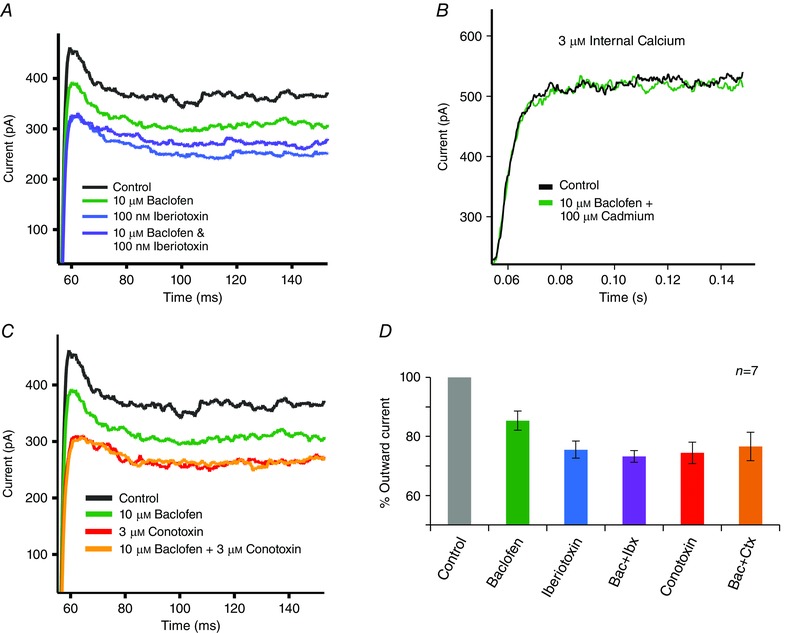
A, an outward current was elicited by a step from −70 to +10 mV (black trace). Baclofen (10 μm) reduced this outward current (green trace). After washout of the baclofen action, iberiotoxin (100 nm) produced a larger reduction of this outward current (blue trace) and occluded the effect of baclofen (purple trace). B, when internal calcium was buffered to approximately 3 μm and voltage‐gated calcium channels were blocked, then baclofen (10 μm) did not affect the outward current. C, in the same cell shown in A, omega conotoxin GVIA (3 μm) produced a reduction of outward current (red trace) and occluded the effect of baclofen (yellow trace). D, summary of the effects of iberiotoxin and omega conotoxin GVIA on the action of baclofen (n = 7). On average, 10 μm baclofen suppressed 15 ± 3% of the outward current, which was significantly less (P < 0.05) than the suppression produced by iberiotoxin or conotoxin. Iberiotoxin alone suppressed 25 ± 3% of the outward current, while with baclofen it suppressed 27 ± 2% of the current. Conotoxin alone suppressed 26 ± 2% of the outward current, while with baclofen it suppressed 24 ± 5%. The effects of iberiotoxin and conotoxin showed no statistically significant difference alone as compared to in the presence of baclofen.
Link between N‐type calcium channels and BK channels
To investigate the indirect link between BK and N‐type calcium channels, 3 μm omega conotoxin was applied to block N‐type calcium channels. Omega conotoxin reduced the outward current by 26 ± 2% (P < 0.01), similar in magnitude to iberiotoxin and 1 mm TEA (Fig. 7 C, D). While baclofen reduced the outward current under control conditions, it did not reduce the current in the presence of omega conotoxin. Both the iberiotoxin and the omega conotoxin experiments were conducted in the same cells, and the results are summarized in Fig. 7 D. While this result indicates strongly a connection between N‐type calcium channels and BK channels, it does not exclude the possibility that baclofen acts independently and separately on both the N‐type calcium channel and the BK channel. To test this possibility, the BK channel was directly activated by buffering the pipette solution to 3 μm free calcium while cadmium was applied to block the calcium channel. This uncouples the BK channel from the voltage‐gated calcium channel current. Under these conditions, 10 μm baclofen did not affect the outward potassium current (Fig. 7 B). Thus, the regulation of BK channels by GABABRs is indirect.
Metabotropic GABABRs enhance excitation
BK channels have been reported to have a role in speeding action potential repolarization and limiting membrane depolarization. To investigate the effect of GABABRs on physiological responses, neurons were current clamped at the resting membrane potential. Steps of current, ranging from 0 to 70 pA.s, were applied in 10 pA increments for durations of 300 ms. Under control conditions, incremental increases of current led to graded depolarizations. An example showing four of the seven current steps is shown in Fig. 8 A. When the current step protocol was repeated in the presence of 10 μm baclofen, there was little change in response to the initial depolarizing steps. However, as more current was applied to the cell, baclofen induced a significant increase in the depolarization. TEA (1 mm) produced an increase similar to that produced by baclofen. Baclofen increased the peak voltage response by 29 ± 5%, while TEA increased the response by 37 ± 4% (n = 6). A summary of this effect is presented in Fig. 8 B. This is in line with what would be expected when blocking BK channels. Taken together, these results imply an excitatory role for GABABRs that acts locally at single ganglion cells.
Figure 8. GABAB receptor activation enhances depolarizing voltage responses .
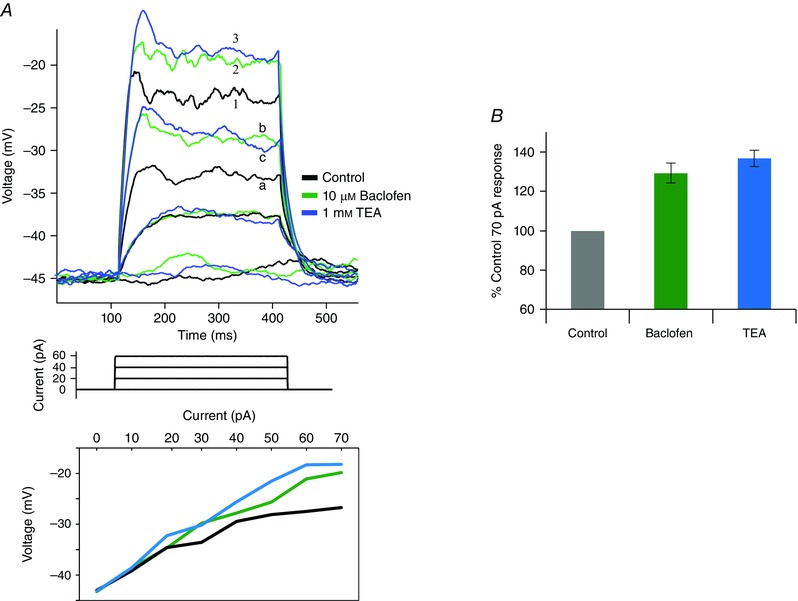
A, a neuron was current clamped (0 pA) at its resting potential and then stimulated with current steps from 0 to 70 pA in 10 pA steps. This protocol was repeated in the presence of either 10 μm baclofen or 1 mm TEA. For clarity, the responses shown in the figure are for currents of 0, 20, 40 and 60 pA. The responses to 20 pA were similar under all three conditions, but the responses to stronger currents were greater in TEA (labelled c, 3) or baclofen (b, 2) than in control (a, 1). The responses to all the current steps are plotted below. B, summary of results from six similar experiments, showing the responses to 70 pA current steps under control conditions and in the presence of 10 μm baclofen or 1 mm TEA. Baclofen increased the mean voltage response by 29 ± 5%, while TEA produced a 37 ± 4% increase.
Discussion
Is the metabotropic GABAR inhibitory?
The ionotropic GABAR is the main fast inhibitory transmitter in the CNS. Mechanistically, the GABABR is also inhibitory, activating potassium channels and suppressing calcium channels. However, functionally the GABABR often promotes excitation. GIRK stimulation produces noise suppression at resting potentials but turns off with depolarization. Presynaptic suppression of calcium channels often reduces release of inhibitory transmitters, GABA and glycine. In addition, as shown in this study, suppression of postsynaptic calcium channels can also enhance excitation. These excitatory effects of GABABRs are evident in retina, where several studies reveal that GABABRs can increase the synaptic signals in ganglion cells. In both amphibian and mammalian retinas, a GABABR‐mediated suppression of glycinergic pathways has been identified that results in increased excitation of ganglion cells (Neal & Cunningham, 1995; Song & Slaughter, 2010). In amphibian retina there also appears to be a GABABR‐induced enhancement of bipolar cell excitatory output to ganglion cells, although the mechanism is unresolved. Direct GABABR input to bipolar cells does not appear to be present in rodent retina (Koulen et al. 1998). However, the mechanism described in this study would have a similar effect, namely a direct potentiation of excitatory responses in ganglion cells.
Association between GABABRs, calcium channels and the BK channel
This study shows a selective effect of GABABRs on N‐type calcium channels. When N‐type channels were blocked, GABABR activation did not appear to affect the remaining voltage‐gated calcium currents. This is unlike amphibian retina, where GABABR activation enhanced L‐type channels in conjunction with suppression of N‐type channels (Shen & Slaughter, 1999). The regulation of N‐type channels is a membrane‐delimited control by the G‐protein itself (Bean, 1989), suggesting a close link between the GABABR and the N‐type calcium channel. The calcium influx is also likely to be spatially localized near the BK channels (Marrion & Tavalin, 1998), so the entire linkage between the receptor and the two voltage‐gated channels is localized on the cell membrane and may be designed to influence a specific synapse.
Physiology of the BK channel
In these experiments modulation of BK channels was instrumental in augmenting excitatory signals. Stimulation of BK channels is often associated with repolarization after a spike or suppression of transmitter release (Adams et al. 1982; Raffaelli et al. 2004). This large conductance channel is both voltage and calcium dependent (Cui et al. 2009). When unregulated by GABABRs, a strong depolarization activates the N‐type channel, leading to activation of a large potassium conductance that counters the depolarization. This negative feedback is eliminated by GABABRs. The BK channel is often associated with a rapid and transient hyperpolarization that truncates a spike. However, the BK channel is relatively non‐inactivating and can produce a prolonged effect on the slower, graded potentials found in retinal ganglion cells.
The BK channel has several roles in retina. In the A17 amacrine cell, a GABAergic neuron that feeds back to bipolar cells, the BK channel limits depolarization and therefore limits feedback inhibition (Grimes et al. 2009). In amphibian rod photoreceptors the BK channel counterbalances the inward calcium current, allowing calcium influx to stimulate transmitter release without inducing a depolarization at the presynaptic terminal (Xu & Slaughter, 2005). In some amphibian amacrine cells the BK channel is linked to L‐type calcium channels, producing the conventional rapid and transient outward BK current (Mitra & Slaughter, 2002 a,b).
Summary
The GABABR can act to enhance excitation by a presynaptic disinhibition as described previously, or by a novel postsynaptic potentiation described in this study. This potentiation depends on a cascade that starts with stimulation of G‐proteins linked with the GABABR, leading to a suppression of N‐type calcium channels, resulting in a suppression of the BK outward current. Because of the voltage dependence of both the calcium and the BK channels, the net effect is to enhance a strong depolarization with minimal effects near the resting potential of neurons.
Additional information
Competing interests
The authors have no competing interests related to this paper.
Author contributions
All work was performed in the Department of Physiology & Biophysics at the University of Buffalo School of Medicine and Biomedical Sciences, Buffalo NY, USA. J.G. was involved in the design, acquisition, analysis and interpretation, and drafting of the work shown. He approves the final version and agrees to be accountable for all aspects presented. M.M.S. was involved in the conception and design, analysis and interpretation, and drafting of the work shown. He approves the final version and agrees to be accountable for all aspects presented. Both J.G. and M.M.S., and no others, qualify for authorship.
Funding
This work was supported by National Eye Institute grant R01‐ #EY05725.
References
- Adams PR, Constanti A, Brown DA & Clark RB (1982). Intracellular Ca2+ activates a fast voltage‐sensitive K+ current in vertebrate sympathetic neurones. Nature 296, 746–749. [DOI] [PubMed] [Google Scholar]
- Bai SH & Slaughter MM (1989). Effects of baclofen on transient neurons in the mudpuppy retina: electrogenic and network actions. J Neurophysiol 61, 382–390. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN & Langer SZ (1998). International Union of Pharmacology. XV. Subtypes of gamma‐aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50, 291–313. [PubMed] [Google Scholar]
- Bean BP (1989). Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340, 153–156. [DOI] [PubMed] [Google Scholar]
- Bindokas VP & Ishida AT (1991). (–)‐Baclofen and γ‐aminobutyric acid inhibit calcium currents in isolated retinal ganglion cells. Proc Natl Acad Sci USA 88, 10759–10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI & Enna SJ (2002). International Union of Pharmacology. XXXIII. Mammalian γ‐aminobutyric acidB receptors: structure and function. Pharmacol Rev 54, 247–264. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hill DR & Hudson AL (1983). Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol 78, 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J & Turnbull M (1980). (–)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature 283, 92–94. [DOI] [PubMed] [Google Scholar]
- Cui J, Yang H & Lee US (2009). Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66, 852–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE & Lei S (2009). GABAB receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK‐2 K+ channels. Neuron 63, 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J & Wassle H (1993). Organotypic slice culture of the mammalian retina. Vis Neurosci 10, 203–217. [DOI] [PubMed] [Google Scholar]
- Friedman DL & Redburn DA (1990). Evidence for functionally distinct subclasses of γ‐aminobutyric acid receptors in rabbit retina. J Neurochem 55, 1189–1199. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE & Diamond JS (2009). BK Channels modulate pre‐ and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci 12, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD & Chandy KG (1994). Pharmacological characterization of five cloned voltage‐gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol 45, 1227–1234. [PubMed] [Google Scholar]
- Heidelberger R & Matthews G (1991). Inhibition of calcium influx and calcium current by γ‐aminobutyric acid in single synaptic terminals. Proc Natl Acad Sci USA 88, 7135–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Bettler B, Wassle H & Brandstatter JH (1998). Presynaptic and postsynaptic localization of GABAB receptors in neurons of the rat retina. Eur J Neurosci 10, 1446–1456. [DOI] [PubMed] [Google Scholar]
- Marrion NV & Tavalin SJ (1998). Selective activation of Ca2+‐activated K+ channels by co‐localized Ca2+ channels in hippocampal neurons. Nature 395, 900–905. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T & Mikoshiba K (1997). 2APB, 2‐aminoethoxydiphenyl borate, a membrane‐penetrable modulator of Ins(1,4,5)P3‐induced Ca2+ release. J Biochem 122, 498–505. [DOI] [PubMed] [Google Scholar]
- Massey SC, Linn DM, Kittila CA & Mirza W (1997). Contributions of GABAA receptors and GABAC receptors to acetylcholine release and directional selectivity in the rabbit retina. Vis Neurosci 14, 939–948. [DOI] [PubMed] [Google Scholar]
- Matthews G, Ayoub GS & Heidelberger R (1994). Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci 14, 1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P & Slaughter MM (2002. a). Calcium‐induced transitions between the spontaneous miniature outward and the transient outward currents in retinal amacrine cells. J Gen Physiol 119, 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P & Slaughter MM (2002. b). Mechanism of generation of spontaneous miniature outward currents (SMOCs) in retinal amacrine cells. J Gen Physiol 119, 355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal MJ & Cunningham JR (1995). Baclofen enhancement of acetylcholine release from amacrine cells in the rabbit retina by reduction of glycinergic inhibition. J Physiol 482, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL & Slesinger PA (2010). GABAB receptor coupling to G‐proteins and ion channels. Adv Pharmacol 58, 123–147. [DOI] [PubMed] [Google Scholar]
- Pan ZH & Slaughter MM (1991). Control of retinal information coding by GABAB receptors. J Neurosci 11, 1810–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P & Cherubini E (2004). BK potassium channels control transmitter release at CA3–CA3 synapses in the rat hippocampus. J Physiol 557, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ & Johnson SW (1997). Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. J Physiol 505, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W & Slaughter MM (1999). Metabotropic GABA receptors facilitate L‐type and inhibit N‐type calcium channels in single salamander retinal neurons. J Physiol 516, 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y & Slaughter MM (2010). GABAB receptor feedback regulation of bipolar cell transmitter release. J Physiol 588, 4937–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JW & Slaughter MM (2005). Large‐conductance calcium‐activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci 25, 7660–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shen W & Slaughter MM (1997). Two metabotropic γ‐aminobutyric acid receptors differentially modulate calcium currents in retinal ganglion cells. J Gen Physiol 110, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


