Abstract
Glutamate receptor‐mediated recruitment of GABAergic inhibitory interneurons is a critical determinant of network processing. Early studies observed that many, but not all, interneuron glutamatergic synapses contain AMPA receptors that are GluA2‐subunit lacking and Ca2+ permeable, making them distinct from AMPA receptors at most principal cell synapses. Subsequent studies demonstrated considerable alignment of synaptic AMPA and NMDA receptor subunit composition within specific subtypes of interneurons, suggesting that both receptor expression profiles are developmentally and functionally linked. Indeed glutamate receptor expression profiles are largely predicted by the embryonic origins of cortical interneurons within the medial and caudal ganglionic eminences of the developing telencephalon. Distinct complements of AMPA and NMDA receptors within different interneuron subpopulations contribute to the differential recruitment of functionally divergent interneuron subtypes by common afferent inputs for appropriate feed‐forward and feedback inhibitory drive and network entrainment. In contrast, the lesser‐studied kainate receptors, which are often present at both pre‐ and postsynaptic sites, appear to follow an independent developmental expression profile. Loss of specific ionotropic glutamate receptor (iGluR) subunits during interneuron development has dramatic consequences for both cellular and network function, often precipitating circuit inhibition–excitation imbalances and in some cases lethality. Here we briefly review recent findings highlighting the roles of iGluRs in interneuron development.
Abbreviations
- AMPAR
AMPA receptor
- CB
calbindin
- CCK
cholecystokinin
- CGE
caudal ganglionic eminence of the ventral telencephalon
- iGluR
ionotropic glutamate receptor
- KAR
kainate receptor
- MGE
medial ganglionic eminence of the ventral telencephalon
- NMDAR
NMDA receptor
- OLM
oriens lacunosum‐moleculare
- P
postnatal day
- PV
parvalbumin
- VIP
vasoactive intestinal peptide
Although comprising only ∼15% of the total cortical and hippocampal neuronal population, inhibitory interneurons provide almost all inhibitory neurotransmission to downstream targets via the neurotransmitter γ‐aminobutyric acid (GABA). This extraordinarily diverse neuronal population plays key roles in regulating both local and long‐range circuits by forming extensive axonal arborizations with exquisite target selectivity. Interneurons can directly control subthreshold voltage‐dependent conductances and regulate both Na+‐ and Ca2+‐dependent action potential initiation via targeting of GABA release to perisomatic and dendritic compartments (Miles et al. 1996). In addition, interneurons have the capacity to entrain the activity of large neural ensembles (Ben‐Ari et al. 1989; Leinekugel et al. 1998; Klausberger et al. 2003; Bartos et al. 2007; Allen & Monyer, 2015). Historically, cortical and hippocampal interneurons have been classified based on their somatic location, dendritic arborization, axonal targeting, electrophysiological properties, biochemical profiles, and in recent years developmental origins (Freund & Buzsaki, 1996; Cauli et al. 1997; Kawaguchi & Kubota, 1997; Monyer & Markram, 2004; Petilla Interneuron Nomenclature et al. 2008; Tricoire et al. 2011; Welagen & Anderson, 2011). These features when used together can help identify and define the divergent roles of the myriad subtypes of inhibitory interneurons. More recently the advent of genetic approaches and emergence of numerous transgenic mouse lines, which label interneuron subtypes, has accelerated interneuron research (i.e. Figs 1 A and B, and 3 A and C) (Taniguchi et al. 2011; Madisen et al. 2012).
Figure 1. AMPARs on interneuron subtypes show embryonic origin‐specific properties .
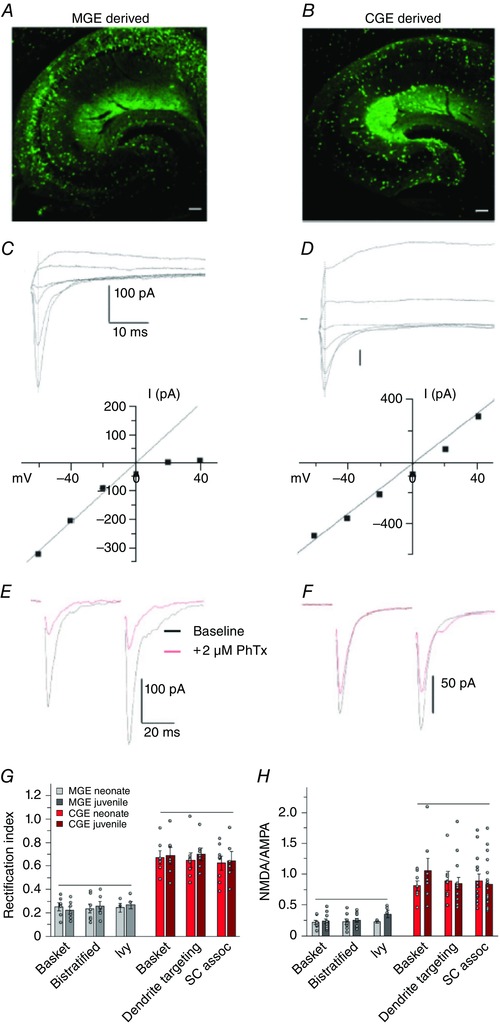
The vast majority of hippocampal interneurons originate from two distinct embryonic regions, the MGE and CGE. MGE‐ (A) and CGE‐ (B) derived cohorts of inhibitory interneurons are shown here labelled with eGFP expression driven by Nkx2.1 or 5ht3ra promoter activity, respectively. C and D, glutamatergic synapses onto MGE‐ and CGE‐derived interneurons differ in their synaptic properties. Traces are representative of Schaffer collateral‐evoked total glutamate receptor (AMPAR and NMDAR)‐mediated EPSCs recorded in voltage clamp configuration from MGE‐derived (C) and CGE‐derived (D) interneurons of the CA1 stratum radiatum, at holding potentials ranging from −60 mV to +40 mV. The vertical line that crosses the raw traces indicates the time point for the extraction of the AMPAR‐mediated fast component of the EPSCs. The plot of these data reveals an inwardly rectifying current–voltage relationship for AMPARs at synapses onto cells derived from the MGE (C) and an essentially linear current–voltage relationship for AMPARs onto CGE‐derived interneurons (D). At positive holding potentials Schaffer collateral–MGE interneuron EPSCs exhibit only a relatively small fast NMDAR component indicating that the contribution of NMDARs at these synapses is low (C and H). In contrast NMDAR‐mediated EPSCs at Schaffer collateral–CGE synapses are relatively large and slow (D and H). E and F, AMPARs on MGE‐ and CGE‐derived interneurons show differential sensitivity to block by extracellular polyamines. The traces show AMPAR‐mediated EPSCs before (black) and after (red) application of philanthotoxin (PhTx) for representative recordings from MGE‐derived (E) and CGE‐derived (F) interneurons. PhTx blocks AMPAR‐mediated currents in a use‐dependent manner in MGE‐derived interneurons to a greater extent compared to CGE‐derived interneurons verifying the Ca2+‐permeable properties of AMPARs at this synapse. G and H, graphs summarizing a detailed developmental analysis of the NMDAR‐ and AMPAR‐mediated EPSCs. The current–voltage relationship remains unchanged across two developmental time points (postnatal day P6–9 and P17–21) indicating that the AMPAR subunit composition is not susceptible to developmental regulation (G). Across a similar developmental age range NMDA/AMPA receptor ratios are low (∼1:5) in three morphologically distinct subtypes of MGE‐derived interneurons in CA1 and ∼1 in three distinct CGE‐derived interneuron subtypes. Reproduced from Matta et al. (2013) with permission from the authors.
Figure 3. Genetic deletion of NMDARs reveals control of both synaptic and anatomical properties .
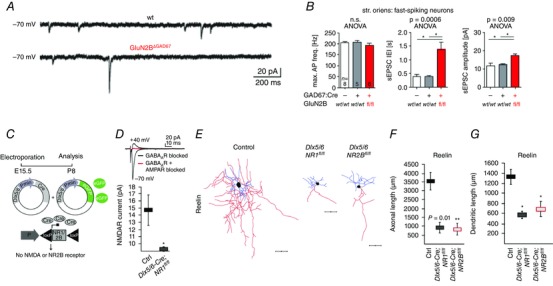
A and B, genetic deletion of the GluN2B subunit of NMDARs results in abnormal maturation of glutamatergic synapses in hippocampal interneurons. In the transgenic line the gene coding for GluN2B is flanked with loxP cassettes and deleted upon breeding with GAD67:Cre mice. A, representative traces of spontaneous EPSCs from wild‐type (wt; upper trace) and GluN2B knockout (GluN2BΔGAD67; lower trace) fast spiking GAD67‐positive interneurons in stratum oriens. In GluN2B knockout interneurons, there is an apparent reduction in spontaneous events, as well as large amplitude events indicating that the number of active synapses has decreased while more AMPARs are incorporated into those remaining. B, graphs show the group data for analysis of the firing properties of the wild‐type and knockout interneurons (left), interspike intervals of spontaneous (s)EPSCs as representation of event frequency (IEI, middle), and mean amplitude of events (right). C–G, loss of either all NMDARs or only the GluN2B subunit alone in cortical interneurons results in abnormal anatomy. C, the genetic strategy to create the conditional knockout animals. Both transgenic animal and virus technology were used to knock out all or the GluN2B‐rich subgroup of NMDARs in CGE‐derived cortical interneurons. The genes coding for GluN1 or GluN2B were deleted in Dlx5/6‐expressing interneurons of targeted knockin mice in which the genes coding for GluN1 or GluN2B are flanked with loxP cassettes. Upon electroporation of viruses that carry the Cre‐expressing sequence downstream of Dlx5/6 promoter, glun1 or glun2b gene deletions occur. (E, embryonic day.) D, the traces are representative evoked EPSCs at −70 mV (inward current) and +40 mV (outward current, black trace and no current, red trace) holding potentials show the loss of NMDAR‐mediated currents (red trace) in GluN1‐knockout interneurons. The graph below shows the group data. E, reconstructions of reelin‐positive interneurons of wild‐type (left), GluN1‐ (middle) and GluN2B‐ (right) knockout mice. Both GluN1 and GluN2B loss result in shrinkage of dendrites and axons in RE‐positive cortical interneurons and GluN2B loss is enough to create this anatomical abnormality. Scale bars, 50 μm. Axons are shown in red, dendrites in blue. The data are summarized with the graphs for axonal (F) and dendritic (G) length analysis of the RE‐positive interneuron morphology. A and B are reproduced with permission from Kelsch et al. (2014). C–G are reproduced with permission from De Marco Garcia et al. (2015).
Interneurons pace and control the activity of neural circuits by leveraging excitation with high spatiotemporal precision. To do this, they must ‘sense’ the ongoing activity of the neuronal ensembles in which they are embedded and respond in a timely fashion. To this end interneurons of the cortex receive synaptic and non‐synaptic glutamatergic input from many different sources: locally via principal neurons distributed across different subfields as well as long‐range subcortical afferent pathways. Thus, recruitment of cortical and hippocampal interneurons often depends on the convergence of several excitatory inputs. For example, in the CA1 hippocampus interneurons can potentially receive input from up to five distinct glutamatergic sources, the axons of CA3, CA2 and CA1 pyramidal cells, as well as entorhinal and thalamic afferents, depending on their somato‐dendritic position (Miles, 1990; Han et al. 1993; Blasco‐Ibanez & Freund, 1995; Freund & Buzsaki, 1996; Maccaferri & McBain, 1996; Ceranik et al. 1997; McMahon & Kauer, 1997; Vida et al. 1998; Chevaleyre & Siegelbaum, 2010). Excitatory inputs onto interneurons are often larger in magnitude than equivalent inputs onto neighbouring principal neurons and in certain interneurons a single excitatory synaptic event is sufficient to trigger action potential firing (Miles, 1990). However, the physiological and pharmacological properties of excitatory input onto different interneuron subtypes is known to vary, giving the first indication of differential receptor subunit expression across different interneuron subtypes (Iino et al. 1990, 1996; McBain et al. 1992; McBain & Dingledine, 1993; Jonas et al. 1994; Isa et al. 1996). Until recently little was known concerning the rules and mechanisms regulating the expression of specific iGluR subtypes within particular interneuron subgroups and importantly what the functional significance of receptor variation in a given neuronal network was. In this review we present an overview of some of the differential and unique roles played by iGluRs in both synaptic transmission and circuit integration of interneurons within cortical networks.
Glutamate receptor diversity
Ionotropic glutamate receptors are divided into three subtypes: α‐amino‐3‐hydroxyl‐5‐methyl‐4‐isoxazole‐propionate (AMPA) receptors, N‐methyl‐d‐aspartate (NMDA) receptors and kainate (KA) receptors. Each receptor subtype comprises distinct subunits that confer specific biophysical properties to the assembled receptors. Expression of several subunits and splice variants of glutamate receptor subtypes have been investigated in different types of neurons in the entire brain and have been extensively reviewed by Traynelis et al. (2010).
AMPA receptors
AMPA receptors (AMPARs) are composed of homomers or heteromers (Nakanishi et al. 1990; Rosenmund et al. 1998) of different combinations of four subunits, GluA1–4 (Keinanen et al. 1990). In general, GluA2 subunit‐containing AMPARs are Ca2+ impermeable whereas the other subunits, in the absence of GluA2, combine to form Ca2+‐permeable AMPARs (Hollmann et al. 1991; Verdoorn et al. 1991). In the hippocampus, initial evidence for AMPA‐preferring receptors that differed from the ‘traditional’ receptors observed on principal cells came from work by Iino and colleagues who demonstrated kainate‐induced currents in unidentified cultured cells that exhibited sigmoidal current–voltage (I–V) relationships indicative of GluA2‐lacking, Ca2+‐permeable AMPA receptors (Iino et al. 1990; Ozawa et al. 1991). McBain & Dingledine (1993) then demonstrated similar inwardly rectifying synaptic AMPARs on CA3 stratum radiatum interneurons. Subsequent reports using in situ hybridization and single cell reverse transcription (RT)‐PCR combined with electrophysiology showed that in cortical interneurons GluA2 expression is limited, consistent with the presence of inwardly rectifying Ca2+‐permeable AMPA receptors (Jonas et al. 1994; Geiger et al. 1995; Audinat et al. 1996). However, the picture was complicated by subsequent reports of GluA2‐containing Ca2+‐impermeable AMPARs in vasoactive intestinal peptide (VIP)‐positive bitufted interneurons (Rozov et al. 2001) and calbindin‐ or parvalbumin‐ (CB and PV) positive multipolar bursting neocortical cells (Blatow et al. 2003). Thus, synapses onto inhibitory interneurons could express either GluA2‐lacking, Ca2+‐permeable AMPARs or GluA2‐containing, Ca2+‐impermeable AMPARs (Fig. 1). However the rules dictating differential AMPAR subunit composition amongst distinct interneuron cohorts remained elusive. Of particular interest, in some cases, Ca2+‐impermeable and Ca2+‐permeable AMPAR complexes were shown to exist in the same interneuron (Toth & McBain, 1998; Sambandan et al. 2010). Indeed interneurons of the CA3 stratum lucidum localized Ca2+‐permeable AMPARs at synapses innervated by mossy fibre inputs and Ca2+‐impermeable AMPARs at synapses from CA3 associational fibres revealing a remarkable afferent input specificity to synaptic AMPAR composition in individual cells (Toth & McBain, 1998).
Preferred combinations of AMPAR subunits have functional consequences as subunit composition dictates ion selectivity and kinetic properties (Hume et al. 1991; Geiger et al. 1995; Koh et al. 1995; Traynelis et al. 2010). For example, GluA2‐containing AMPARs possess a lower single channel conductance with slower gating properties relative to other AMPARs that lack the GluA2 subunit (Lomeli et al. 1994; Mosbacher et al. 1994; Swanson et al. 1997) and incorporation of GluA4 subunits dramatically increases channel kinetics (Swanson et al. 1997). GluA4 expression is high, while GluA2 expression is low in fast spiking PV‐positive hippocampal interneurons (Geiger et al. 1995; Lambolez et al. 1996; Angulo et al. 1997; Pelkey et al. 2015) resulting in excitatory postsynaptic currents (EPCS) on PV interneurons with high Ca2+ permeability and an extremely rapid time course (Geiger et al. 1995, 1997; Lawrence & McBain, 2003) compared to EPSCs observed onto principal neurons (Hestrin, 1993). In addition, two alternatively spliced isoforms (flip and flop) of each AMPAR subunit exist. The flop isoform yields receptors with more rapid gating properties and is the preferred isoform of AMPAR subunits in interneurons (Bochet et al. 1994; Geiger et al. 1995; Angulo et al. 1997). The expression of rapidly gating Ca2+‐permeable AMPARs in fast spiking interneurons is an important requirement for their ability to provide temporally precise control of network activity (for review see Bartos et al. 2007; Hu et al. 2014). Indeed fast‐spiking PV‐positive cortical interneurons show a correlation between action potential timing and miniature (m)EPSC half‐width underscoring the importance of precise subunit expression for temporal precision in interneuron network dynamics (Helm et al. 2013). Moreover, findings in mice with genetically altered AMPAR subunit expression in interneurons have demonstrated the importance of specific subunit combinations for interneuron‐mediated network entrainment (Table 1). For example, GluA2 overexpression in GAD67‐positive interneurons altered the firing properties of the mutant interneurons and significantly disrupted long‐range oscillation synchrony (Fuchs et al. 2001). In another study from the same group (Fuchs et al. 2007), elimination of either GluA1 or GluA4 subunits specifically in PV‐positive interneurons compromised gamma frequency network oscillations and hippocampal working memory. The neuronal pentraxin 2/neuronal pentraxin receptor (NPTX2 and NPTXR), which belong to the neuronal pentraxin family, are expressed at glutamatergic synapses onto interneurons and cluster AMPARs (Xu et al. 2003; Sia et al. 2007). Mice with NPTX2/NPTXR loss of function exhibit severely reduced GluA4 expression in PV interneurons with concomitant alterations in gamma oscillations, sharp wave ripples measured in vivo, as well as impairing hippocampal working memory (Pelkey et al. 2015).
Table 1.
List of studies that used transgenic/knockout animals to study the significance of iGluR subunits in interneurons
| iGluR | Genetic | Interneuron subtype | ||||||
|---|---|---|---|---|---|---|---|---|
| subunit | modification | (mouse driver line) | Cellular physiology | Morphology | Cell number | Network | Behaviour | Reference |
| GluA1 | Knockout | PV (PV‐Cre) | Decreased AMPAR‐mediated EPSCs.Altered firing. | N/A | N/A | Altered gamma frequency network oscillations. | Impaired spatial working memory, novel object recognition and response to spatial changes. | (Fuchs et al. 2007) |
| GluA2 | Overexpression | GAD67 | Altered firing properties. | N/A | N/A | Disrupted long‐range oscillation synchrony. | — | (Fuchs et al. 2001) |
| GluA3 | — | — | — | — | — | — | — | — |
| GluA4 | Knockout | Global | Decreased AMPAR‐mediated EPSCs.Altered firing. | N/A | N/A | Gamma frequency network oscillations altered. | Impaired spatial working memory. Novel object recognition and response to spatial changes. | (Fuchs et al. 2007) |
| GluN1 | Knockout | PV (PV‐Cre) | N/A | N/A | No change in hippocampus. | Hippocampal theta oscillations reduced and gamma oscillations increased, gamma‐theta oscillation coupling altered. | Impaired object recognition and spatial memory. | (Korotkova et al. 2010) |
| Knockout | Ppp1r2 (a protein phosphatase, expressed mostly in inhibitory interneurons) (Ppp1r2‐Cre). | N/A | N/A | N/A | Increased firing rate of pyramidal neurons in cortex. Reduced synchronous activity. | Symptoms of schizophrenia: novelty‐induced hyperlocomotion, mating and nest‐building deficits, anhedonia‐like and anxiety‐like behaviours observed. Impaired social memory, spatial working memory and prepulse inhibition. | (Belforte et al. 2010) | |
| Knockout | Dlx5/6 (Dlx5/6‐Cre introduced with viral infection). | N/A | Reduced dendrites and axons of cortical RE interneurons.No morphological change in VIP interneurons. | N/A | N/A | N/A | (De Marco Garcia et al. 2015) | |
| GluN2A | Knockout | Dlx5/6 (Dlx5/6‐Cre introduced with viral infection) | N/A | No change | N/A | N/A | N/A | (De Marco Garcia et al. 2015) |
| GluN2B | Knockout | GAD67 | Reduced AMPAR‐mediated mEPSC frequency, increased amplitude. | No change in str. oriens. | Normal PV cell number in barrel cortex. | Fatal neonatal seizures. | Reduced locomotor activity over time from birth to early death. | (Kelsch et al. 2014) |
| Knockout | Olfactory granule cells(local deletion with viral infection). | Reduced excitatory synaptic contacts in proximal dendrite. Decreased frequency and amplitude of spontaneous excitatory postsynaptic current (sEPSCs). | Normal | Increased cell death of adult born granule cells. | N/A | N/A | (Kelsch et al. 2012) | |
| Knockout | Dlx5/6 (Dlx5/6‐Cre introduced with viral infection) | N/A | Reduced dendrites and axons of cortical RE interneurons.No change in VIP interneurons. | N/A | N/A | N/A | (De Marco Garcia et al. 2015) | |
| GluN2C | — | — | — | — | — | — | — | — |
| GluN2D | Knockout | Global | No change in decay τ of NMDAR‐mediated EPSCs after ifenprodil treatment in hippocampal interneurons. | N/A | N/A | N/A | N/A | (von Engelhardt et al. 2015) |
| GluN3A | — | — | — | — | — | — | — | — |
| GluN3B | — | — | — | — | — | — | — | — |
| GluK1 | Knockout | Global | Kainate can still activate an inward current insensitive to AMPAR blockers in CA1 str. radiatum interneurons, and increased spontaneous inhibitory postsynaptic current (sIPSC). Frequency in CA1 pyramidal cell (PC) similar to wild‐type. | N/A | N/A | N/A | N/A | (Mulle et al. 2000) |
| Knockout | Global | Kainate did not increase sIPSC amplitude and frequency in CA3 str. radiatum interneurons, but reduced eIPSCs similar to wild‐type. | N/A | N/A | Enhanced kainate‐induced gamma oscillations. | N/A | (Fisahn et al. 2004) | |
| GluK2 | Knockout | Global | Kainate can still activate an inward current in CA1 str. radiatum interneurons. Kainate leads to a rapid and reversible decrease in the amplitude of eIPSCs in CA1 pyramidal neurons, similar to wild‐type. | N/A | N/A | N/A | N/A | (Mulle et al. 2000) |
| Knockout | Global | Kainate did not activate an inward current in CA3 str. radiatum interneurons, and increased sIPSC amplitude and frequency in PC, similar to wild‐type, but did not change eIPSCs. | N/A | N/A | Disrupted kainate‐induced gamma oscillations. | N/A | (Fisahn et al. 2004) | |
| GluK1/2 | Knockout | Global | Kainate‐activated inward current is eliminated in CA1 str. radiatum interneurons, and does not increase sIPSC frequency, and does not affect eIPSC amplitude in CA1 PC. | N/A | N/A | N/A | N/A | (Mulle et al. 2000) |
| NPTX2 (NARP) | Knockout | Global (PV interneurons studied). | Activity‐dependent scaling of excitatory input on hippocampal PV interneurons is lost. | N/A | N/A | Decreased spontaneous firing rate of PV interneurons. Mice became hypersensitive to kindling‐induced seizures. | N/A | (Chang et al. 2010) |
| Knockout | Global (PV interneurons studied). | Connectivity between layerII/III pyramidal neurons to PV interneurons reduced as well as the release sites and probability. | N/A | N/A (Note: cortical macroorganization normal.) | Increased neuronal excitability in visual cortex.Impaired ocular dominance plasticity (critical period plasticity). Visual acuity normal: experience‐dependent enhancement of the VEPs (visually evoked potentials) contralateral bias and experience‐dependent enhancement of VEP amplitudes in response to high‐frequency visual stimulation persist. | N/A | (Gu et al. 2013) | |
| NPTX2/ NPTXR | Knockout | Global (PV interneurons studied). | Lost or severely reduced GluA4 expression in hippocampal PV interneurons. Reduced AMPA/NMDA current ratio along with amplitude and frequency of sEPSCs in PV interneurons. Increased decay τ of sEPSCs. | Basket cell dendritic morphology normal. | N/A | Reduced feedforward inhibition of hippocampal pyramidal neurons reduced.Increased time window of giant depolarizing potential occurrence. Impaired gamma oscillations recorded from acute slices or awake animal brains. Impaired sharp wave ripples in vivo. Enhanced epileptic activity. | Impaired hippocampal working memory. | (Pelkey et al. 2015) |
In cortical and hippocampal circuits, excitatory synaptic inputs either facilitate or depress in response to repetitive input (Reyes et al. 1998). In neuronal networks, paired‐pulse depression (PPD) may help interneurons operate as coincidence detectors and permit firing at early time points in a train of ongoing activity, whereas paired‐pulse facilitation (PPF) provides a mechanism to detect ongoing high frequency activity. PPD is common at synapses between pyramidal neurons and perisomatic targeting interneurons, such as pyramidal–multipolar PV interneuron synapses in neocortex (Reyes et al. 1998) and CA1 pyramidal–PV basket cell, –bistratified cell, and –cholecystokinin (CCK) cell synapses in hippocampus (Ali et al. 1998). In contrast PPF is observed at synapses between pyramidal neurons and dendrite targeting interneurons, such as CA1 pyramidal–oriens lacunosum‐moleculare (OLM) interneuron synapses (Ali & Thomson, 1998; Reyes et al. 1998). Both types of plasticity are largely determined by presynaptic release properties. However, postsynaptic factors such as AMPAR desensitization and polyamine block can contribute to short‐term plasticity. GluA2‐lacking Ca2+‐permeable AMPARs are tonically blocked by endogenous intracellular polyamines at more positive membrane potentials producing their characteristic inwardly rectifying I–V relationship (Bowie & Mayer, 1995; Kamboj et al. 1995; Koh et al. 1995). Relief of this polyamine block is both use and voltage dependent (Bowie & Mayer, 1995; Rozov et al. 1998) and in interneurons either reduces the magnitude of paired‐pulse depression or produces a facilitating response (Rozov & Burnashev, 1999). The degree of facilitation that results from relief of polyamine block is enhanced at more depolarized membrane potentials and presents a mechanism that can boost subthreshold excitatory inputs (Toth et al. 2000) providing a novel mechanism for synaptic gain.
NMDA receptors
Like AMPARs, NMDA receptors (NMDARs) are also remarkably diverse in their molecular composition, with various biophysical and pharmacological properties determined by the exact combination of subunits and splice variants incorporated into the native complex (McBain & Mayer, 1994; Traynelis et al. 2010). NMDARs are obligate heterotetramers with GluN1 being required for functional channels. Compared to AMPARs, NMDARs have much slower activation and deactivation kinetics and consequently their synaptic activation typically triggers a slower secondary component of synaptic input prolonging the window for temporal summation of excitatory events. Within NMDARs, GluN2A subunits promote the fastest kinetics while addition of GluN2B, GluN2C and GluN2D subunits slows channel kinetics with GluN1–GluN2D diheteromeric channels being the slowest (∼2 s decay τ) (Vicini et al. 1998). NMDAR subunit expression varies across different brain regions, shows developmental regulation, and can be altered in disease states (Watanabe et al. 1992; Monyer et al. 1994; Akbarian et al. 1996; Purcell et al. 2001).
Initially, NMDARs were considered to minimally contribute to fast synaptic transmission in interneurons relative to their role in most principal cells (McBain et al. 1992; McBain & Dingledine, 1993; Monyer et al. 1994; He et al. 1998). However, more detailed analysis by various groups (Standaert et al. 1996; Lei & McBain, 2002; Matta et al. 2013; De Marco Garcia et al. 2015; von Engelhardt et al. 2015) showed that NMDAR contribution and subunit composition in interneurons varied based on interneuron subtype and interestingly appeared to correlate with the properties of AMPARs expressed in the same cell (McBain & Dingledine, 1993; Lei & McBain, 2002; Matta et al. 2013). For example, hippocampal mossy fibre–interneuron synapses expressing GluA2‐lacking, Ca2+‐permeable AMPARs typically contain GluN2B‐rich NMDARs with slow decay kinetics. Conversely, mossy fibre–interneuron synapses that comprise GluA2‐containing, Ca2+‐impermeable AMPARs tend to coexpress NMDARs with relatively faster decay kinetics and larger amplitudes (Lei & McBain, 2002).
Unlike principal cells, Ca2+ influx into interneurons can potentially occur via both AMPA and NMDA receptors, depending on the iGluR subunit composition within a given synapse. As a general rule, in the CA1 hippocampus, interneuron synapses that comprise Ca2+‐permeable AMPARs (Fig. 1 C, E and G) express low levels of synaptic NMDARs as reflected by a low NMDA/AMPA receptor‐mediated current amplitude ratio (∼1:5; Fig. 1 C and H) (Matta et al. 2013). In contrast, interneurons dominated by Ca2+‐impermeable AMPARs (Fig. 1 D and F) have larger NMDAR‐mediated currents with higher NMDA/AMPA ratios (∼1:1; Fig. 1 D and G) (Lei & McBain, 2002; Matta et al. 2013; De Marco Garcia et al. 2015).
Similar to AMPARs, NMDAR subunit expression also varies among interneuron subtypes. Both GluN2A and GluN2B subunits of NMDARs are expressed in GABAergic interneurons. GluN2A‐containing or GluN2B‐containing NMDARs can be identified based on decay time constant and subunit‐selective NMDAR antagonists (i.e. ifenprodil, Ro 25‐6981, NVP‐AAM077) (Fig. 2). In juvenile rat brain, Ca2+‐permeable AMPARs at mossy fibre–CA3 interneuron synapses have NMDARs with slow kinetics and are strongly blocked by ifenprodil, consistent with a high GluN2B subunit expression. In contrast, mossy fibre–CA3 interneuron synapses comprising Ca2+‐impermeable AMPARs typically have NMDARs with faster kinetics and less sensitivity to ifenprodil suggesting a lower GluN2B and higher GluN2A content (Lei & McBain, 2002). CA1 interneurons of mice at comparable ages typically express NMDARs with fast kinetics (Fig. 2 B and E) and low ifenprodil sensitivity (Fig. 2 B and F) (i.e. GluN2A‐containing) at synapses containing Ca2+‐permeable AMPARs and NMDARs with slower kinetics (Fig. 2 D and E) and high ifenprodil sensitivity (Fig. 2 D and F) (i.e. GluN2B‐containing) at synapses expressing Ca2+‐impermeable AMPARs (Matta et al. 2013). Thus, different rules for particular subunit expression may exist across different synapses onto different interneuron subtypes, or as we shall discuss below NMDAR subunit expression can show differential developmental regulation, which depends on interneuron identity. Compared to GluN2A and GluN2B, very little is known about GluN2C and GluN2D subunit expression in interneurons. In situ hybridization showed the presence of GluN2D in cortical and hippocampal PV‐, somatostatin (SOM)‐, CB‐ and calretinin (CR)‐positive interneurons as well as in VIP‐positive irregular spiking interneurons (Monyer et al. 1994; Porter et al. 1998; von Engelhardt et al. 2015). Indeed, blocking NMDAR activity by ifenprodil increased the decay time constant of the synaptic currents in wild‐type mice at only early ages, whereas the same treatment did not change the decay kinetics of the currents in GluN2D knockout mice suggesting early expression of GluN2D by some hippocampal interneurons (Table 1) (von Engelhardt et al. 2015). The expression of slow gating GluN2B‐ and GluN2D‐dominated NMDARs early in development probably provide a wider temporal window for synaptic integration potentially important for network maturation, whereas GluN2A expression at later ages enhances the precision of synaptic responses.
Figure 2. NMDAR subunit expression in MGE‐derived hippocampal interneurons show developmental regulation .
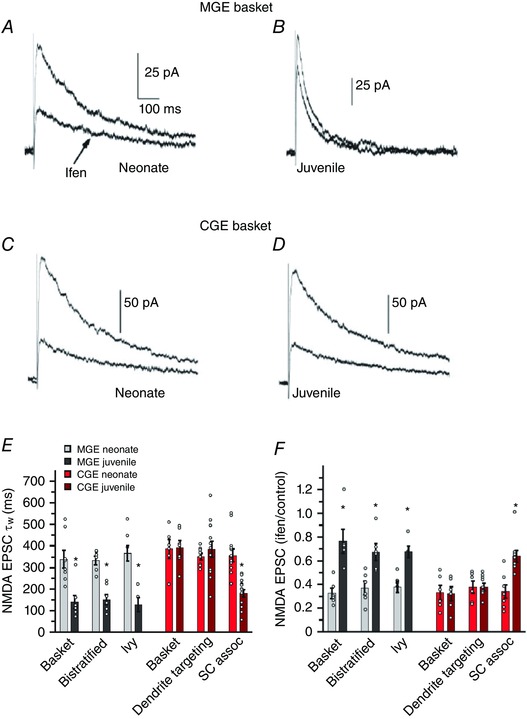
A–D, channel gating of NMDARs accelerates and loses GluN2B antagonist (ifenprodil) sensitivity in MGE‐derived interneurons whereas NMDARs in CGE‐derived interneurons have properties that persist through development. The traces are representative evoked NMDAR‐mediated EPSCs recorded at +40 mV holding potential from MGE‐derived basket cells (A and B) and CGE‐derived basket cells (C and D) from a neonate (A and C) and a juvenile (B and D) upon Schaffer collateral stimulation in the absence (upper traces) or presence (lower traces) of ifenprodil. In both MGE‐ and CGE‐derived basket cells of neonate (A and C), the peak amplitude of the current is markedly reduced indicating GluN2B‐rich NMDARs in these interneurons. Similar antagonist application is unable to reduce evoked current in MGE‐derived basket cell of juvenile indicating a significant loss of GluN2B subunits from NMDAR pool in this synapse. Juvenile NMDAR currents also possess faster kinetics than neonate consistent with a GluN2B–GluN2A switch (B). In contrast ifenprodil block is significant in CGE‐derived basket cell across both developmental time points indicating that NMDA receptor subunit expression does not undergo developmental regulation in CGE‐derived interneurons (D). E, graph summarizes the detailed analysis of the NMDAR EPSC decay kinetics weighted time constant (τw) for both neonate and juvenile MGE‐ and CGE‐derived interneurons at two different developmental time points, neonate (P6–9) and juvenile (P17–21). F, graph summarizes the detailed analysis of the developmental regulation of ifenprodil sensitivity expressed as the ratio of the NMDAR EPSC peak amplitude measured in the presence of ifenprodil divided by the control NMDA EPSC peak amplitude. Reproduced from Matta et al. (2013) with permission from the authors.
Although somewhat controversial, NMDARs have also been posited to exist on the presynaptic terminals of various synapses including those between pyramidal cells and interneurons, where they are thought to regulate synaptic transmission and information flow (Duguid, 2013) in a target‐specific manner. For example, in layer V of developing mouse visual cortex, activation of presynaptic NMDARs modulates release probability between pyramidal and Martinotti cells but not at pyramidal to basket cell synapses (Buchanan et al. 2012). Similarly, activation of presynaptic NMDA receptors facilitates inhibition at multipolar interneuron to pyramidal cell synapses but not at bitufted to pyramidal cell synapses (De‐May & Ali, 2013) in neocortical layers II–V.
Despite the relatively small contribution of NMDARs to synaptic transmission in a number of interneuron subtypes (e.g. PV‐positive interneurons) physiological roles have been described at cellular (Lei & McBain, 2002; Kelsch et al. 2012, 2014; Matta et al. 2013), network (Korotkova et al. 2010; Kelsch et al. 2014) and behavioural levels (Belforte et al. 2010). In synapses containing either high or low NMDAR/AMPAR ratios, pharmacological blockade of NMDARs decreased action potential firing in response to stimulus trains by decreasing temporal summation of excitatory events (Lei & McBain, 2002). At Ca2+‐impermeable mossy fibre–interneuron synapses, which exhibit high NMDAR/AMPAR ratios, a train of stimuli triggers multiple action potentials at each stimulus followed by a large late NMDAR‐dependent depolarizing envelope that persisted long after the stimulus. In contrast, in synapses with low NMDAR/AMPAR ratios, EPSPs triggered only single action potential firing with no substantial after‐depolarizing phase (Lei & McBain, 2002). In the MGE‐derived hippocampal interneurons of newborn mice, blocking GluN2B similarly modulated EPSP‐driven action potential firing (Matta et al. 2013).
An instructive role for NMDAR activity in the circuit integration of developing interneurons is suggested by the finding that genetic deletion of GluN2B selectively within interneurons leads to a reduction in the frequency of AMPAR‐mediated mEPSCs in hippocampal interneurons (Fig. 3 A and B; Table 1) (Kelsch et al. 2014). This decreased excitatory drive promoted hippocampal seizures and subsequent lethality (Table 1) (Kelsch et al. 2014). Selective ablation of NMDARs later in development in PV‐positive interneurons alters theta and gamma oscillations indicating a critical role for NMDARs in PV–interneuron‐mediated circuit entrainment (Table 1) (Korotkova et al. 2010). These same mice, together with another transgenic model in which loss of NMDARs is more widespread among interneurons (Table 1) (Belforte et al. 2010), also showed impairments in spatial and short‐ and long‐term memory tests. Furthermore, loss of NMDARs in cortical GABAergic interneurons at early postnatal ages triggered several behavioural deficits associated with schizophrenia including psychomotor agitation, anhedonia, reduced prepulse inhibition of acoustic startle, deficits in nesting/mating, and social withdrawal (Belforte et al. 2010) supporting the NMDAR hypofunction theory of schizophrenia (for reviews see Coyle, 1996, 2012).
Kainate receptors
Of the ionotropic glutamate receptors, kainate receptors (KARs) on inhibitory interneurons have received much less attention and consequently little clarity exists regarding their expression profiles and functional role(s). KARs are homo‐ or heteromeric tetramers composed of GluK1 (GluR5) (Bettler et al. 1990), GluK2 (GluR6) (Egebjerg et al. 1991), GluK3 (GluR7) (Bettler et al. 1992), GluK4 (KA1) or GluK5 (KA2) subunits (Werner et al. 1991; Herb et al. 1992; Sakimura et al. 1992; for review see Traynelis et al. 2010). KAR subunits are expressed widely in brain (Wisden & Seeburg, 1993) and interneurons are particularly enriched with both GluK1, GluK2 and GluK4 (Bureau et al. 1999; Paternain et al. 2000; Fisahn et al. 2004; Lein et al. 2007) where they act to regulate both pre‐ and postsynaptic activity (for review see Carta et al. 2014). Both pharmacological and genetic tools have been used to dissect the roles of particular KAR subunits in interneurons; however, conflicting results have often arisen (Table 1) (Bureau et al. 1999; Mulle et al. 2000; Fisahn et al. 2004; Maingret et al. 2005) due in part to poor receptor‐selective pharmacology and the non‐selective targeting of specific subpopulations of hippocampal interneurons, which probably show different KAR subunit expression profiles (Bureau et al. 1999; Maingret et al. 2005; Wondolowski & Frerking, 2009).
Evoked or spontaneous synaptic events mediated by KARs on inhibitory interneurons have been reported in only a small number of studies (Cossart et al. 1998; Frerking et al. 1998; Cossart et al. 2002; Frerking & Ohliger‐Frerking, 2002; Wondolowski & Frerking, 2009). In most of these studies the KAR‐mediated component of the synaptic event appears as a small slow tail current in the EPSC waveform reminiscent of that seen on principal cells. The clearest example of a pure KAR‐mediated synaptic event was demonstrated in recordings of spontaneous synaptic activity in interneurons of the CA1 stratum oriens, primarily OLM cells (Cossart et al. 2002; Goldin et al. 2007). In these studies quantal synaptic events mediated by KARs were observed which were slow (decay τ ∼10 ms) compared to pure AMPA‐mediated synaptic events and represented a sizeable fraction of the total spontaneous EPSC population (∼30%). This study was the first to suggest that excitatory synapses onto interneurons may target KARs and AMPARs to distinct synaptic compartments. Attempts by others to replicate these observations, however, have proven difficult. Oren et al. (2009) in contrast concluded that the vast majority of spontaneous EPSCs in stratum oriens OLM cells observed at negative holding potentials arose via AMPA receptor activation with no evidence for KAR‐mediated events (see also Wondolowski & Frerking, 2009). Indeed, evoked GluK1‐containing KAR synaptic events were observed to make only a modest contribution (∼10% of the total current) when synaptic transmission was driven by repetitive activity (Oren et al. 2009), further questioning their functional significance. Regardless, KAR‐induced inward currents triggered by exogenous agonist application have been identified in numerous inhibitory interneuron studies indicating that functional KARs reside in the postsynaptic compartment, despite the paucity of evidence for synaptic KAR events and comprise either GluK1 or GluK2 subunits (Cossart et al. 1998; Frerking et al. 1998; Paternain et al. 2000; Fisahn et al. 2004; Oren et al. 2009; Wondolowski & Frerking, 2009) or both (Porter et al. 1998; Mulle et al. 2000; Paternain et al. 2000). Activation of postsynaptic KARs by exogenous agonists depolarizes interneurons, increasing their action potential firing rate, resulting in an increased inhibitory drive onto pyramidal cells (Cossart et al. 1998; Frerking et al. 1998). KAR‐evoked inward currents are absent in CA3 stratum radiatum interneurons in GluK2 loss of function mice but persists in GluK1 knockouts consistent with a prominent role for GluK2‐containing KARs on specific populations of interneurons (Fisahn et al. 2004) while in CA1 stratum radiatum interneurons kainate could evoke inward currents in either of the single GluK1 or GluK2 loss of function mice but not in the double GluK1/GluK2 knockout (Mulle et al. 2000).
In hippocampal slices, exogenous kainate application reduces evoked IPSC (eIPSC) amplitudes and increases failures of eIPSCs onto CA1 pyramidal neurons, as well as decreasing miniature (m)IPSC frequency onto interneurons (Clarke et al. 1997; Rodriguez‐Moreno et al. 1997; Bureau et al. 1999; Cossart et al. 2001; Maingret et al. 2005) indicating a role for presynaptic KARs in the regulation of GABA release onto CA1 pyramidal neurons (however, cf. with Frerking et al. 1999; Cossart et al. 2001; Fisahn et al. 2004). Paired electrophysiological recordings between CCK‐positive interneurons and pyramidal cells, PV‐positive interneurons and pyramidal cells, and CCK‐positive‐ and PV‐positive interneurons, revealed that depression of unitary IPSCs by kainate is observed only at connections between CCK‐positive interneurons and pyramidal cells through a mechanism involving GluK1‐containing KARs (Daw et al. 2010). In a parallel study, high frequency stimulation of Schaffer collateral afferents in CA1 resulted in a small transient depression of inhibitory transmission onto pyramidal cells that arises through glutamate spillover activating presynaptic GluK1‐containing receptors on cannabinoid receptor 1 (CB1)‐positive interneuron presynaptic terminals (Min et al. 1999). This spillover mechanism appears to rely on the concerted activation of both the endocannabinoid system and presynaptic CB1 receptors and GluK1 KARs (Lourenco et al. 2010). Of particular interest transmission between CCK‐positive interneurons and their downstream targets arises through both synchronous and asynchronous transmitter release. At these synapses KAR activation depresses only the synchronous component of GABA release leaving asynchronous release unchanged (Daw et al. 2010). Thus presynaptic KAR activation may act to simultaneously reduce ‘conventional’ GABA transmission occurring via synchronous release, while preserving the influence of tonic inhibition driven by asynchronous release during periods of intense activity.
Dissecting the role(s) of KARs in shaping inhibitory interneuron activity is far from complete and will require both better pharmacological tools and targeted disruption of specific KAR subunit function in identified cohorts of inhibitory interneurons. The paucity of clear roles for synaptic KARs coupled to the small amplitudes of the conductances that have been described cast considerable doubt as to whether these receptors are performing a critical, if any, physiological role in inhibitory interneurons. However, Goldin et al. (2007) have demonstrated that KARs, but not AMPARs, on SOM‐positive OLM cells are critical for spike entrainment at theta frequencies, suggesting they are important in part for oscillatory activity.
Developmental plasticity of iGluR subunit expression
iGluR subunit expression profiles are subject to regulation through both developmental and activity dependent mechanisms in a number of cell and synapse types (Monyer et al. 1991; Liu & Cull‐Candy, 2000; Kelsch et al. 2012, 2014; Matta et al. 2013; von Engelhardt et al. 2015). Three (GluA1–3) of the four AMPAR subunits are expressed at embryonic stages of the developing cortex, whereas the GluA4 subunit appears only postnatally (Bettler et al. 1990; Monyer et al. 1991; Geiger et al. 1995; Pelkey et al. 2015). Although the rectification index of AMPARs changes at Ca2+‐permeable AMPAR synapses of neonatal cortical pyramidal neurons over development (Kumar et al. 2002; Ho et al. 2007), there is no evidence for developmental regulation of GluA2 expression in interneurons at Schaffer collateral–CA1 interneuron synapses (Fig. 1 H) (Matta et al. 2013). In contrast, GluN2 subunit expression is highly dynamic in the entire brain during development (Monyer et al. 1994). The most comprehensive and detailed analysis of glutamatergic synapse maturation in interneuron development is presented by Matta et al. (2013). In this study, interneuron populations in the hippocampal CA1 region were studied based on their origins within either the medial (MGE) (Fig. 1 A) or caudal ganglionic eminences (CGE) (Fig. 1 B) of the ventral telencephalon (Lee et al. 2010; Tricoire et al. 2011). Schaffer collateral synapses onto MGE‐derived interneurons (e.g. PV‐, SOM‐ and neuronal NO synthase (nNOS)‐positive interneurons) typically express Ca2+‐permeable, GluA2‐lacking AMPARs (Fig. 1 C, E and H) whereas CGE‐derived interneurons (CR, VIP, CCK, reelin (RE) and some nNOS interneurons) mostly express Ca2+‐impermeable, GluA2‐containing AMPARs (Fig. 1 D, F and H). These origin‐specific AMPAR profiles are consistent across a broad developmental age range and show no developmental regulation of GluA2 expression. In contrast NMDARs at synapses onto MGE‐derived interneurons switch from initially expressing NMDARs with slow kinetics and high ifenprodil sensitivity (Fig. 2; Table 1) to ifenprodil‐resistant fast NMDARs, indicating a developmental subunit switch from GluN2B‐ to GluN2A‐dominated receptors. This developmental NMDAR subunit switch can also be triggered by high frequency stimulation and such acute plasticity requires a rise in intracellular Ca2+ (perhaps through Ca2+‐permeable AMPARs) but occurs independent of either activation of NMDARs themselves or metabotropic mGluR5 (which are required for a similar subunit switch in pyramidal cell synapses; Matta et al. 2011, 2013). In contrast Schaffer collateral synapses onto almost all CGE‐derived interneuron subtypes express GluN2B‐containing NMDARs across all developmental stages tested and do not exhibit any acute plasticity following high frequency stimulation (Matta et al. 2013). Thus far no attempt has been made to address the developmental expression or profiles of KAR subunit expression on inhibitory interneurons derived from either the MGE or CGE.
The role of iGluRs in development and maturation of interneurons
Embryonic expression of both AMPA and NMDA receptor subunits raises the question of whether iGluRs participate in cellular migration and anatomical maturation. Cortical interneurons originate in the ventral telencephalon and migrate tangentially through the neocortex and into the primordial hippocampus. They then move radially to reach their particular cortical or hippocampal layer of choice (Marin et al. 2010). A number of in vitro (Behar et al. 1999) and in vivo (Manent et al. 2006; Bortone & Polleux, 2009) studies have proposed a role for NMDARs (Bortone & Polleux, 2009) or AMPARs (Manent et al. 2006) in GABAergic interneuron migration based on changes in cell migration observed following pharmacological antagonism of AMPA and NMDA receptors. However, the density of PV‐positive interneurons in the somatosensory cortex of transgenic mice with selective deletion of GluN2B from GAD67‐positive interneurons was comparable to that of wild‐type mice (Table 1) (Kelsch et al. 2014). In contrast similar genetic manipulation resulted in increased cell death in the adult born granule cell population of the olfactory bulb (Table 1) (Kelsch et al. 2012), indicating that these NMDARs serve different roles for cell survival in different brain regions or developmental stages. It is evident that glutamate also serves as a chemoattractant for cellular migration (Behar et al. 1999); however, conflicting results about the functional importance of AMPA and NMDARs require additional and better approaches to the question.
Anatomical maturation of specific subpopulations of interneurons is also influenced by glutamate receptor expression (De Marco Garcia et al. 2015). Fishell and colleagues demonstrated that elimination of GluN1 and GluN2B, but not GluN2A, in cortical CGE‐derived interneurons produced cell type‐specific (RE‐positive only, but not VIP‐positive) deficits in dendritic and axonal morphology (Fig. 3 C–G; Table 1). Similarly, increased expression of fast mEPSCs as a consequence of overexpression of a postsynaptic scaffolding protein, SAP97, in cortical PV cells has been shown to be correlated with increased dendritic complexity and additionally alter passive and active membrane properties (Akgul & Wollmuth, 2013). In general, activity is considered an important factor for proper interneuron morphological maturation as blunting membrane excitability by overexpression of inwardly rectifying potassium channels stunts interneuron morphological differentiation (De Marco Garcia et al. 2011). Therefore, whether the anatomical alterations of the interneurons in these models are a result of changes in neuronal activity or removal of GluRs per se is currently unknown.
Conclusions and unanswered questions
The issues discussed in this review highlight recent research on iGluR diversity and its regulation in different interneuron subtypes. Although accumulating evidence provides some general rules for excitatory input onto interneurons, clear exceptions to these general rules exist suggesting that a complicated interplay between receptors and downstream signalling cascades operate within distinct cell populations and at discrete inputs onto individual interneurons. Of particular interest, a picture is emerging whereby receptor subunit expression and AMPA:NMDAR expression profiles within interneuron populations derived from common embryonic lineages share common rules and are regulated in a precise spatiotemporal manner by specific cellular and extracellular molecules. However, many details critical for our complete understanding of the role iGluRs play in determining interneuron function remain untested or unanswered. A short list of those we feel are the most pertinent include:
Many iGluR subunits are expressed at embryonic ages (Bettler et al. 1990; Monyer et al. 1991, 1994; Herb et al. 1992; Allen Brain Atlas; http://mouse.brain‐map.org/; Pelkey et al. 2015); however, there is a paucity of information regarding what role iGluRs play (if any) during a period when cells are migrating and incorporating into the emerging cortical circuit. Embryonic expression of iGluRs is particularly intriguing given the fact that functional glutamatergic synapse development does not occur typically until early postnatal days (Ben‐Ari, 2014). Whether these receptors detect environmental glutamate gradients and drive cellular migration during embryonic development is unknown.
Interneurons derived from the MGE or CGE require the concerted action of numerous transcription factors, (e.g. Nkx2.1, Dlx1/2/5/6, Lhx6 etc.) to determine cell fate and identity (Kessaris et al. 2014). Lineage analysis suggests that early specification of cells sets in motion a concerted programme of gene expression that will ultimately determine the cells’ function and role in the emerging neural network. For example, in PV‐positive interneurons upregulation of both kinetically fast Na+ and K+ voltage‐gated channels, the calcium‐binding protein parvalbumin, syt2, a presynaptic regulator of synaptic transmission, as well as acquisition of synapses comprising GluA1/4 heteromers and an NMDAR subunit switch all occur within a tight window during the second week of postnatal life. This suggests that the acquisition of ‘fast‐spiking’ and rapid synaptic transmission onto and from PV‐positive interneurons results from a tightly regulated and concerted gene expression programme that is held in check until postnatal week 2. How this concerted expression is achieved is at present unclear. Furthermore, one might imagine a scenario where perturbation of any one of these important proteins could impact the expression profiles of all others leading to widespread defects in a number of interrelated proteins.
The small amplitude and limited role in synaptic transmission of the NMDAR conductance at Schaffer collateral synapses onto MGE‐derived interneurons is puzzling. Indeed of all interneurons studied PV‐positive interneurons have perhaps the smallest observable NMDAR conductance (Matta et al. 2013). Of interest, loss of function of NMDAR in PV‐positive interneurons early in development has suggested an NMDAR‐dependent hypofunction model of schizophrenia (Nakazawa et al. 2012) that is not completely recapitulated when NMDARs are eliminated later on in life. This puzzling observation is hard to reconcile with the limited synaptic NMDAR currents typically triggered in these cells and suggests that alternative roles for NMDARs other than conventional synaptic transmission must exist in these cells early in development.
GluN2B and GluN2A subunits are differentially expressed during MGE‐derived interneuron maturation (with GluN2B predominating early in development) (Matta et al. 2013). One could hypothesize that a loss of GluN2B early in development would have a major impact on many aspects of cell migration and maturation into the nascent cortical circuit (whereas embryonic elimination of GluN2A would have a more limited impact). Moreover, GluN2B and GluN2A are selectively expressed at mature Schaffer collateral synapses onto CGE‐ versus MGE‐derived interneurons, respectively, suggesting distinct roles for both types of NMDARs. In addition, each of these subunits confer distinct biophysical properties on the native receptor and have preferred cytoplasmic binding partners and cell signalling cascades. Targeted elimination of particular GluN subunits on specific cohorts of interneurons is required to allow us to unravel the intricate roles played by each of these subunits in determining interneuron maturation and circuit function.
The need to identify clear role(s) for postsynaptic KARs on interneurons. Are kainate receptors functionally relevant or are they an evolutionary vestige whose limited conductance plays only a minor role in sculpting transmission onto or off interneurons?
Finally, what are the roles for GluRs on interneurons in the development of human pathological phenotypes? A number of genome‐wide association studies (GWAS) highlight GluR subunits and interacting proteins expressed on particular interneuron subtypes that may provide clues to the roles played by interneuron dysfunction in these pathologies. However, while the use of traditional global or conditional knockout strategies are helpful, they are limited in providing only a cursory understanding of diseases related to the dysregulated expression and function of these receptors in particular cell types (Yuan et al. 2015). With the emergence of advanced genome engineering technologies like CRISPR/Cas9 (Hsu et al. 2014), it will be possible (and easier) to target specific genomic DNA sequences in relevant interneuron cohorts to generate new transgenic/knockout lines within a short period of time. iGluR mutations observed in human neural circuit disorders could potentially be recapitulated in animal models to permit the targeted study of clinically relevant neurological diseases and possibly lead to the generation of specific therapeutic intervention.
Additional information
Competing interests
None declared.
Funding
This work was funded by an Intramural research award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Z1A‐HD001205‐21).
Acknowledgements
The authors would like to thank Ken Pelkey for critically reading the manuscript.
Biography
Chris McBain received his BSc from the University of Aberdeen, Scotland and PhD from the University of Cambridge, England, where he worked with Ray Hill. During a postdoctoral fellowship with Raymond Dingledine at the University of North Carolina at Chapel Hill, he studied glutamate receptor function, regulation of the extracellular volume fraction and hippocampal synaptic transmission. After a brief period in the laboratory of Julie Kauer at Duke University, he joined NICHD as an Investigator within the Laboratory of Cellular and Molecular Neurophysiology. He is currently a Senior Investigator and Chief of the Laboratory of Cellular and Synaptic Neurophysiology within the NICHD. Gülcan Akgül is a postdoctoral researcher at The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) studying the role of glutamate receptors in interneuron development in hippocampus using knockout mouse models. She has a PhD in Molecular and Cell Biology from Stony Brook University, NY, USA. Her PhD studies focused on the proteins that interact with glutamate receptors at the postsynaptic density of cortical interneurons.

References
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE Jr & Jones EG (1996). Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci 16, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul G & Wollmuth LP (2013). Synapse‐associated protein 97 regulates the membrane properties of fast‐spiking parvalbumin interneurons in the visual cortex. J Neurosci 33, 12739–12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Deuchars J, Pawelzik H & Thomson AM (1998). CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol 507, 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB & Thomson AM (1998). Facilitating pyramid to horizontal oriens‐alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J Physiol 507, 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K & Monyer H (2015). Interneuron control of hippocampal oscillations. Curr Opin Neurobiol 31, 81–87. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Lambolez B, Audinat E, Hestrin S & Rossier J (1997). Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. J Neurosci 17, 6685–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audinat E, Lambolez B, Cauli B, Ropert N, Perrais D, Hestrin S & Rossier J (1996). Diversity of glutamate receptors in neocortical neurons: implications for synaptic plasticity. J Physiol Paris 90, 331–332. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I & Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8, 45–56. [DOI] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA & Barker JL (1999). Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci 19, 4449–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM & Nakazawa K (2010). Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia‐like phenotypes. Nat Neurosci 13, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ari Y ( 2014). The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience 279, 187–219. [DOI] [PubMed] [Google Scholar]
- Ben‐Ari Y, Cherubini E, Corradetti R & Gaiarsa JL (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol 416, 303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans‐Borgmeyer I, O'Shea‐Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M & Heinemann S (1990). Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron 5, 583–595. [DOI] [PubMed] [Google Scholar]
- Bettler B, Egebjerg J, Sharma G, Pecht G, Hermans‐Borgmeyer I, Moll C, Stevens CF & Heinemann S (1992). Cloning of a putative glutamate receptor: a low affinity kainate‐binding subunit. Neuron 8, 257–265. [DOI] [PubMed] [Google Scholar]
- Blasco‐Ibanez JM & Freund TF (1995). Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed‐back activation. Eur J Neurosci 7, 2170–2180. [DOI] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A & Monyer H (2003). A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38, 805–817. [DOI] [PubMed] [Google Scholar]
- Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K & Ozawa S (1994). Subunit composition at the single‐cell level explains functional properties of a glutamate‐gated channel. Neuron 12, 383–388. [DOI] [PubMed] [Google Scholar]
- Bortone D & Polleux F (2009). KCC2 expression promotes the termination of cortical interneuron migration in a voltage‐sensitive calcium‐dependent manner. Neuron 62, 53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D & Mayer ML (1995). Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine‐mediated ion channel block. Neuron 15, 453–462. [DOI] [PubMed] [Google Scholar]
- Buchanan KA, Blackman AV, Moreau AW, Elgar D, Costa RP, Lalanne T, Tudor Jones AA, Oyrer J & Sjöström PJ (2012). Target‐specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron 75, 451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Bischoff S, Heinemann SF & Mulle C (1999). Kainate receptor‐mediated responses in the CA1 field of wild‐type and GluR6‐deficient mice. J Neurosci 19, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Fievre S, Gorlewicz A & Mulle C (2014). Kainate receptors in the hippocampus. Eur J Neurosci 39, 1835–1844. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S & Rossier J (1997). Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17, 3894–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceranik K, Bender R, Geiger JR, Monyer H, Jonas P, Frotscher M & Lübke J (1997). A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J Neurosci 17, 5380–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter H, Xu D, Linden DJ, Sutula TP, McBain CJ & Worley PF (2010). Narp regulates homeostatic scaling of excitatory synapses on parvalbumin interneurons. Nat Neurosci 13, 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V & Siegelbaum SA (2010). Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico‐hippocampal loop. Neuron 66, 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D & Bleakman D (1997). A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389, 599–603. [DOI] [PubMed] [Google Scholar]
- Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben‐Ari Y & Crepel V (2002). Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35, 147–159. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C & Ben‐Ari Y (1998). GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1, 470–478. [DOI] [PubMed] [Google Scholar]
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben‐Ari Y & Bernard C (2001). Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29, 497–508. [DOI] [PubMed] [Google Scholar]
- Coyle JT (1996). The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry 3, 241–253. [DOI] [PubMed] [Google Scholar]
- Coyle JT (2012). NMDA receptor and schizophrenia: a brief history. Schizophr Bull 38, 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Pelkey KA, Chittajallu R & McBain CJ (2010). Presynaptic kainate receptor activation preserves asynchronous GABA release despite the reduction in synchronous release from hippocampal cholecystokinin interneurons. J Neurosci 30, 11202–11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco Garcia NV, Karayannis T & Fishell G (2011). Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco Garcia NV, Priya R, Tuncdemir SN, Fishell G & Karayannis T (2015). Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat Neurosci 18, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De‐May CL & Ali AB (2013). Involvement of pre‐ and postsynaptic NMDA receptors at local circuit interneuron connections in rat neocortex. Neuroscience 228, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid IC ( 2013). Presynaptic NMDA receptors: are they dendritic receptors in disguise? Brain Res Bull 93, 4–9. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Bettler B, Hermans‐Borgmeyer I & Heinemann S (1991). Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature 351, 745–748. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF & McBain CJ (2004). Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate‐induced hippocampal gamma oscillations. J Neurosci 24, 9658–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC & Nicoll RA (1998). Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1, 479–486. [DOI] [PubMed] [Google Scholar]
- Frerking M & Ohliger‐Frerking P (2002). AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci 22, 7434–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Petersen CC & Nicoll RA (1999). Mechanisms underlying kainate receptor‐mediated disinhibition in the hippocampus. Proc Natl Acad Sci USA 96, 12917–12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF & Buzsaki G (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Doheny H, Faulkner H, Caputi A, Traub RD, Bibbig A, Kopell N, Whittington MA & Monyer H (2001). Genetically altered AMPA‐type glutamate receptor kinetics in interneurons disrupt long‐range synchrony of gamma oscillation. Proc Natl Acad Sci USA 98, 3571–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN & Monyer H (2007). Recruitment of parvalbumin‐positive interneurons determines hippocampal function and associated behaviour. Neuron 53, 591–604. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Lubke J, Roth A, Frotscher M & Jonas P (1997). Submillisecond AMPA receptor‐mediated signaling at a principal neuron‐interneuron synapse. Neuron 18, 1009–1023. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P & Monyer H (1995). Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204. [DOI] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben‐Ari Y, Crepel V & Cossart R (2007). Synaptic kainate receptors tune oriens‐lacunosum moleculare interneurons to operate at theta frequency. J Neurosci 27, 9560–9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang S, Chang MC, Worley P, Kirkwood A & Quinlan EM (2013). Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 79, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Buhl EH, Lorinczi Z & Somogyi P (1993). A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local‐circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci 5, 395–410. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG & Morrison JH (1998). Synaptic coexistence of AMPA and NMDA receptors in the rat hippocampus: a postembedding immunogold study. J Neurosci Res 54, 444–449. [DOI] [PubMed] [Google Scholar]
- Helm J, Akgul G & Wollmuth LP (2013). Subgroups of parvalbumin‐expressing interneurons in layers 2/3 of the visual cortex. J Neurophysiol 109, 1600–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W & Seeburg PH (1992). The KA‐2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron 8, 775–785. [DOI] [PubMed] [Google Scholar]
- Hestrin S (1993). Different glutamate receptor channels mediate fast excitatory synaptic currents in inhibitory and excitatory cortical neurons. Neuron 11, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J, Huganir RL, Lacaille JC & McBain CJ (2007). Developmental expression of Ca2+‐permeable AMPA receptors underlies depolarization‐induced long‐term depression at mossy fiber CA3 pyramid synapses. J Neurosci 27, 11651–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Hartley M & Heinemann S (1991). Ca2+ permeability of KA‐AMPA–gated glutamate receptor channels depends on subunit composition. Science 252, 851–853. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES & Zhang F (2014). Development and applications of CRISPR‐Cas9 for genome engineering. Cell 157, 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J & Jonas P (2014). Interneurons. Fast‐spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263. [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R & Heinemann SF (1991). Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253, 1028–1031. [DOI] [PubMed] [Google Scholar]
- Iino M, Koike M, Isa T & Ozawa S (1996). Voltage‐dependent blockage of Ca2+‐permeable AMPA receptors by joro spider toxin in cultured rat hippocampal neurones. J Physiol 496, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Ozawa S & Tsuzuki K (1990). Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol 424, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Itazawa S, Iino M, Tsuzuki K & Ozawa S (1996). Distribution of neurones expressing inwardly rectifying and Ca2+‐permeable AMPA receptors in rat hippocampal slices. J Physiol 491, 719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH & Monyer H (1994). Differences in Ca2+ permeability of AMPA‐type glutamate receptor channels in neocortical neurons caused by differential GluR‐B subunit expression. Neuron 12, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT & Cull‐Candy SG (1995). Intracellular spermine confers rectification on rat calcium‐permeable AMPA and kainate receptors. J Physiol 486, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y & Kubota Y (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7, 476–486. [DOI] [PubMed] [Google Scholar]
- Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B & Seeburg PH (1990). A family of AMPA‐selective glutamate receptors. Science 249, 556–560. [DOI] [PubMed] [Google Scholar]
- Kelsch W, Li Z, Eliava M, Goengrich C & Monyer H (2012). GluN2B‐containing NMDA receptors promote wiring of adult‐born neurons into olfactory bulb circuits. J Neurosci 32, 12603–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Li Z, Wieland S, Senkov O, Herb A, Gongrich C & Monyer H (2014). GluN2B‐containing NMDA receptors promote glutamate synapse development in hippocampal interneurons. J Neurosci 34, 16022–16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Magno L, Rubin AN & Oliveira MG (2014). Genetic programs controlling cortical interneuron fate. Curr Opin Neurobiol 26, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G & Somogyi P (2003). Brain‐state‐ and cell‐type‐specific firing of hippocampal interneurons in vivo . Nature 421, 844–848. [DOI] [PubMed] [Google Scholar]
- Koh DS, Burnashev N & Jonas P (1995). Block of native Ca2+‐permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol 486, 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J & Monyer H (2010). NMDA receptor ablation on parvalbumin‐positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V & Huguenard JR (2002). A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci 22, 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B, Ropert N, Perrais D, Rossier J & Hestrin S (1996). Correlation between kinetics and RNA splicing of α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid receptors in neocortical neurons. Proc Natl Acad Sci USA 93, 1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ & McBain CJ (2003). Interneuron diversity series: containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci 26, 631–640. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling‐Leffler J, Zagha E, Fishell G & Rudy B (2010). The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30, 16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S & McBain CJ (2002). Distinct NMDA receptors provide differential modes of transmission at mossy fiber‐interneuron synapses. Neuron 33, 921–933. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA & Jones AR (2007). Genome‐wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, Ben‐Ari Y & Khazipov R (1998). Giant depolarizing potentials: the septal pole of the hippocampus paces the activity of the developing intact septohippocampal complex in vitro . J Neurosci 18, 6349–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ & Cull‐Candy SG (2000). Synaptic activity at calcium‐permeable AMPA receptors induces a switch in receptor subtype. Nature 405, 454–458. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A & Seeburg PH (1994). Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266, 1709–1713. [DOI] [PubMed] [Google Scholar]
- Lourenco J, Cannich A, Carta M, Coussen F, Mulle C & Marsicano G (2010). Synaptic activation of kainate receptors gates presynaptic CB1 signaling at GABAergic synapses. Nat Neurosci 13, 197–204. [DOI] [PubMed] [Google Scholar]
- McBain CJ & Dingledine R (1993). Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol 462, 373–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Eaton JV, Brown T & Dingledine R (1992). CNQX increases spontaneous inhibitory input to CA3 pyramidal neurones in neonatal rat hippocampal slices. Brain Res 592, 255–260. [DOI] [PubMed] [Google Scholar]
- McBain CJ & Mayer ML (1994). N‐Methyl‐d‐aspartic acid receptor structure and function. Physiol Rev 74, 723–760. [DOI] [PubMed] [Google Scholar]
- Maccaferri G & McBain CJ (1996). Long‐term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci 16, 5334–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LL & Kauer JA (1997). Hippocampal interneurons express a novel form of synaptic plasticity. Neuron 18, 295–305. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE & Zeng H (2012). A toolbox of Cre‐dependent optogenetic transgenic mice for light‐induced activation and silencing. Nat Neurosci 15, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Lauri SE, Taira T & Isaac JT (2005). Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. J Physiol 567, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Jorquera I, Ben‐Ari Y, Aniksztejn L & Represa A (2006). Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J Neurosci 26, 5901–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Valiente M, Ge X & Tsai LH (2010). Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol 2, a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz‐Clemente A, Roche KW & Isaac JT (2011). mGluR5 and NMDA receptors drive the experience‐ and activity‐dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW & McBain CJ (2013). Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 16, 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R (1990). Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea‐pig in vitro . J Physiol 428, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N & Freund TF (1996). Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16, 815–823. [DOI] [PubMed] [Google Scholar]
- Min MY, Melyan Z & Kullmann DM (1999). Synaptically released glutamate reduces γ‐aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proc Natl Acad Sci USA 96, 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B & Seeburg PH (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540. [DOI] [PubMed] [Google Scholar]
- Monyer H & Markram H (2004). Interneuron diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci 27, 90–97. [DOI] [PubMed] [Google Scholar]
- Monyer H, Seeburg PH & Wisden W (1991). Glutamate‐operated channels: developmentally early and mature forms arise by alternative splicing. Neuron 6, 799–810. [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH & Ruppersberg JP (1994). A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266, 1059–1062. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B & Heinemann SF (2000). Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28, 475–484. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Shneider NA & Axel R (1990). A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron 5, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S & Belforte JE (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62, 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P & Lamsa KP (2009). Role of ionotropic glutamate receptors in long‐term potentiation in rat hippocampal CA1 oriens‐lacunosum moleculare interneurons. J Neurosci 29, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Iino M & Tsuzuki K (1991). Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol 66, 2–11. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Herrera MT, Nieto MA & Lerma J (2000). GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci 20, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, Karlsson RM, Cameron HA, Murata Y, Colonnese MT, Worley PF & McBain CJ (2015). Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 85, 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group, Ascoli GA, Alonso‐Nanclares L, Anderson SA, Barrionuevo G, Benavides‐Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo‐Rodriguez M, Wang Y, West DC & Yuste R (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J & Audinat E (1998). Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci 10, 3617–3628. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME & Pevsner J (2001). Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57, 1618–1628. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P & Sakmann B (1998). Target‐cell‐specific facilitation and depression in neocortical circuits. Nat Neurosci 1, 279–285. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Moreno A, Herreras O & Lerma J (1997). Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern‐Bach Y & Stevens CF (1998). The tetrameric structure of a glutamate receptor channel. Science 280, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Rozov A & Burnashev N (1999). Polyamine‐dependent facilitation of postsynaptic AMPA receptors counteracts paired‐pulse depression. Nature 401, 594–598. [DOI] [PubMed] [Google Scholar]
- Rozov A, Jerecic J, Sakmann B & Burnashev N (2001). AMPA receptor channels with long‐lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci 21, 8062–8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP & Burnashev N (1998). Facilitation of currents through rat Ca2+‐permeable AMPA receptor channels by activity‐dependent relief from polyamine block. J Physiol 511, 361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Morita T, Kushiya E & Mishina M (1992). Primary structure and expression of the γ2 subunit of the glutamate receptor channel selective for kainate. Neuron 8, 267–274. [DOI] [PubMed] [Google Scholar]
- Sambandan S, Sauer JF, Vida I & Bartos M (2010). Associative plasticity at excitatory synapses facilitates recruitment of fast‐spiking interneurons in the dentate gyrus. J Neurosci 30, 11826–11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia GM, Beique JC, Rumbaugh G, Cho R, Worley PF & Huganir RL (2007). Interaction of the N‐terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron 55, 87–102. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB Jr & Young AB (1996). Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Brain Res Mol Brain Res 42, 89–102. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK & Cull‐Candy SG (1997). Single‐channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB & Huang ZJ (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K & McBain CJ (1998). Afferent‐specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1, 572–578. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips‐Tansey E & McBain CJ (2000). Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20, 8279–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ & Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X & McBain CJ (2011). A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci 31, 10948–10970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH & Sakmann B (1991). Structural determinants of ion flow through recombinant glutamate receptor channels. Science 252, 1715–1718. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB & Greyson DR (1998). Functional and pharmacological differences between recombinant N‐methyl‐d‐aspartate receptors. J Neurophysiol 79, 555–566. [DOI] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P & Buhl EH (1998). Unitary IPSPs evoked by interneurons at the stratum radiatum–stratum lacunosum–moleculare border in the CA1 area of the rat hippocampus in vitro . J Physiol 506, 755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Bocklisch C, Tonges L, Herb A, Mishina M & Monyer H (2015). GluN2D‐containing NMDA receptors‐mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Front Cell Neurosci 9, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K & Mishina M (1992). Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3, 1138–1140. [DOI] [PubMed] [Google Scholar]
- Welagen J & Anderson S (2011). Origins of neocortical interneurons in mice. Dev Neurobiol 71, 10–17. [DOI] [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinanen K, Wisden W & Seeburg PH (1991). Cloning of a putative high‐affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature 351, 742–744. [DOI] [PubMed] [Google Scholar]
- Wisden W & Seeburg PH (1993). A complex mosaic of high‐affinity kainate receptors in rat brain. J Neurosci 13, 3582–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondolowski J & Frerking M (2009). Subunit‐dependent postsynaptic expression of kainate receptors on hippocampal interneurons in area CA1. J Neurosci 29, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ & Worley P (2003). Narp and NP1 form heterocomplexes that function in developmental and activity‐dependent synaptic plasticity. Neuron 39, 513–528. [DOI] [PubMed] [Google Scholar]
- Yuan H, Low CM, Moody OA, Jenkins A & Traynelis SF (2015). Ionotropic GABA and glutamate receptor mutations and human neurologic diseases. Mol Pharmacol 88, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]


