Abstract
Introduction:
This study aims to investigate the effects of acupuncture stimulation on the radial artery’s pressure pulse wave, along with various hemodynamic parameters, and to explore the possible underlying mechanism of pulse diagnosis in healthy participants in their twenties.
Methods and analysis:
This study is a prospective, single-arm, exploratory clinical study. A total of 25 healthy participants, without regard to gender, in their twenties will be recruited by physicians. Written informed consent will be obtained from all participants. The participants will receive acupuncture once at ST36 on both sides. The radial arterial pulse waves will be measured on the left arm of the subjects by using an applicable pulse tonometric device (KIOM-PAS). On the right arm (appearing twice), electrocardiogram (ECG), photoplethysmogram (PPG), respiration and cardiac output (CO) signals, will be measured using a physiological data acquisition system (Biopac module), while the velocity of blood flow, and the diameter and the depth of the blood vessel will be measured using an ultrasonogram machine on the right arm (appearing twice). All measurements will be conducted before, during, and after acupuncture. The primary outcome will be the spectral energy at high frequencies above 10 Hz (SE10-30Hz) calculated from the KIOM-PAS device signal. Secondary outcomes will be various variables obtained from the KIOM-PAS device, ECG, PPG, impedance cardiography modules, and an ultrasonogram machine.
Discussion:
The results of this trial will provide information regarding the physiological and the hemodynamic mechanisms underlying acupuncture stimulation and clinical evidence for the influence of acupuncture on the pressure pulse wave in the radial artery.
Ethics and dissemination:
This study was approved by the Institutional Review Board (IRB) of Kyung Hee University’s Oriental Medical Center, Seoul, Korea (KOMCIRB-150818-HR-030). The study findings will be published in peer-reviewed journals and presented at national and international conferences.
Trial registration number:
This trial was registered with the Clinical Research Information Service (CRIS) at the Korea National Institute of Health (NIH), Republic of Korea (KCT0001663), which is a registry in the World Health Organization’s (WHO’s) Registry Network.
Keywords: acupuncture, protocol, radial artery’s pressure pulse wave
1. Introduction
Acupuncture was originally a technique employed in Korean medicine, and its efficacy has been proven by several clinical researches [1]. The World Health Organization (WHO) has publicly acknowledged that 80% of 129 countries now recognize the use of acupuncture [2]. According to the 2011 National Statistical Office survey of the Republic of Korea, 77.5% of Koreans have been treated using Korean medicine, in which acupuncture (48%) is the most frequently employed treatment [3].
One of the physiological reactions associated with acupuncture is Deqi [4, 5]. The Emperor’s Inner Canon (Huangdi Neijing) described Deqi as follows: “If there is no Deqi with one acupuncture needle insertion, keep inserting it until you generate one. If Deqi is generated after insertion of the acupuncture needle, remove it, and do not insert it again. Because acupuncture needles of different shapes have their own purposes and because it should be used according to its therapeutic purposes, the key to acupuncture treatment is Qizhi which brings about the therapeutic effect of acupuncture” [6]. Bae reported that Qizhi is a synonym for Deqi, which refers to the acupuncture sensation in a recent Korean medical theory. However, the Qizhi mentioned in the Emperor’s Inner Canon referred to changes in a pulse wave, and did not relate to the sensation occurred by acupuncture manipulation. Therefore, according to the Emperor’s Inner Canon, observing changes in the pulse wave before and after acupuncture treatment can help predict the effectiveness of acupuncture in the patients [7]. However, objective evidence is required to prove this theory.
The pulse waves measured from Chon (Cun), Gwan (Guan), and Cheok (Chi), which are three adjacent regions along the radial artery used for pulse diagnosis in traditional Korean medicine (TKM), can reveal information on the balance of Qi and blood and on the homeostasis of the body and various organ systems [8]. Changes in the pulse wave and blood flow characteristics observed before and after acupuncture treatment can be used to objectively evaluate the efficacy of acupuncture. Hence, scientific observational studies are required to understand the physiological and the hemodynamic mechanisms of acupuncture.
In previous studies on the effects of acupuncture, Boutouyrie et al [9] observed changes in the hemodynamic characteristics by using ultrasound and found significant differences in the blood vessel diameter between the sensitized patient group and the native patients group after applying sham acupuncture and real acupuncture. Sandberg et al [10] reported that the Deqi stimulation group showed significantly elevated skin and muscle blood flow compared to the control group after acupuncture at ST36. Takayama et al [11-12] confirmed that blood flow dropped during acupuncture stimulation and contradistinctively rose after 180 seconds of acupuncture stimulation at LS3. Huang et al [13] found that the spectral energy of the pulse wave frequency in dyspepsia patients showed a significant increase after acupuncture stimulation. The effects of acupuncture are reported to be hemodynamic changes, such as those in the heart rate variability (HRV), high blood pressure effect, cerebral blood flow, and finger photoplethysmography (PPG) after acupuncture [14-17]. In Korea, the first such study was performed in 2012 that observed changes in the pulse rate of healthy subjects before and after acupuncture treatment using a pulse wave detector [18]. However, studies on acupuncture effects with pulse wave are still rare and fundamental aspects of acupuncture effects on pulse wave need to be investigated.
This study is a prospective, single-arm, exploratory clinical study to investigate the effects of acupuncture stimulation on the radial artery pressure pulse wave in healthy participants in their twenties. The results of this study will be used as a reference for further studies on the effectiveness of acupuncture on various diseases, sypmtoms, ages and genders, and for establishing scientific evidences and clinical usefulness of pulse diagnosis in TKM.
2. Methods and Analysis
2.1. Study design
We will conduct a prospective, single-arm, exploratory clinical study to observe the effect of acupuncture stimulation on the radial artery’s pressure-pulse wave in healthy young participants. Because, this clinical study will be performed using a single-arm evaluation, the method of randomization and blinding do not have to be taken into consideration.
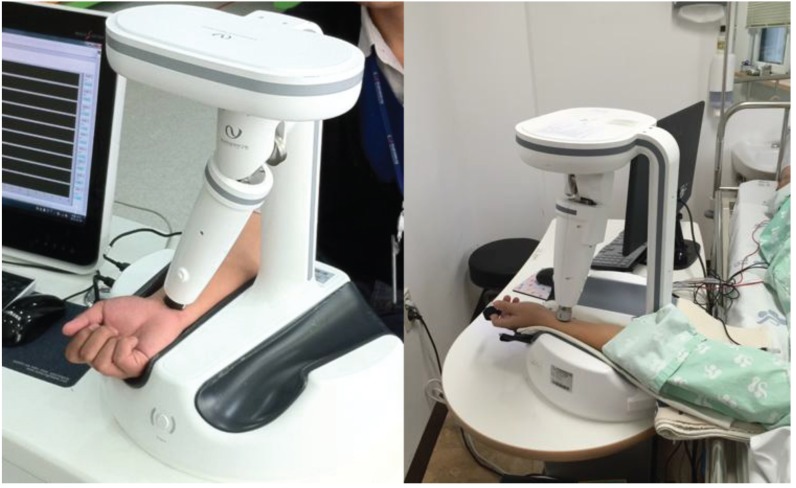
The study will be carried out at the Korean Medicine Clinical Trial Center, Kyung Hee University’s Oriental Medical Center, Seoul, South Korea., from October 12, 2015, to October 11, 2016. Healthy men and women in their twenties will be recruited, with a target sample size of 25 participants, through advertisements on hospital websites and on bulletin boards. Those responding to the advertisements will be given full information about the study, including the method of acupuncture and the instrument measuring process. Those interested in participation will be guided through the informed consent process. After informed written consent has been obtained, the participants will be asked to answer screening questions and undergo electrocardiography and a pregnancy test to determine their eligibility. If eligible, a study researcher will administer the baseline questionnaire after which the interviewer will schedule the study procedure (Fig. 1). Participant will be categorized by age and sex. Vital signs and relevant personal medical histories will be recorded. Each participant will receive one session of acupuncture over the course of this study.
Fig. 1. Applicable pulse tonometric device (KIOM-PAS).
The inclusion criteria are as follows: (1) age 20 to 30 years; (2) ability to communicate about one’s physical condition and to fill out a questionnaire; (3) ability to participate voluntarily; (4) clearly understanding of the purpose and the characteristics of the clinical trial; (5) provision of informed written consent form.
Participants will be excluded from the study if one or more of the following criteria are fulfilled: (1) use within one month of medications, such as blood pressure depressants, hypoglycemic agents, sleeping drugs, tranquilizers, antithrombotic agents, antiplatelet agents, anticoagulants, and hormone drugs that were prescribed for an internal medicine, surgical, or psychiatric diagnosis; (2) pregnancy or lactation; (3) student of Korean Medicine College or Korean medical doctor; (4) acupuncture treatment within the last four months; (5) physical characteristics that preclude assessments using the applicable pulse tonometric device and the ultrasonogram machine; (6) a history of heart disease or transplant of devices such as pacemakers; (7) participation in other clinical trials within the last three months; (8) communication disorder; (9) drug addiction or alcohol abuse; (10) conditions where acupuncture might not be safe, such as an allergy to metal; (11) refusal to participate in the trial or to provide informed written consent form; (12) exclusion at the investigator’s discretion.
The medical history of the participants, including their current medication status, surgical history, the presence of other diseases, and the results of electrocardiography and pregnancy tests, will be recorded at baseline. Data for lifestyle factors, including exercise, smoking, caffeine intake, and alcohol consumption, will also be documented as will hypertension, height, weight, and demographic information. Licensed Korean medicine doctors (KMDs) are specialists or residents of Korean medicine with more than two years of clinical experience and six years of education. All participating KMDs will take part in an educational course to ensure that they strictly adhere to the study protocol. All will be familiar with administering the study treatments and will undergo intensive, customized training to ensure that they have a full understanding of the “acupuncture” procedure, including details such as the acupuncture points and depths (Table 1).
Table. 1. STRICTA (Standards for Reporting Interventions in Clinical Trials of Acupuncture).
| Item | Details | |
|---|---|---|
| 1. Acupuncture rationale | (a) Style of acupuncture | Traditional Korean medicine theory |
| (b) Reasoning for treatment provided (based on historical context, literature sources and consensus methods) | Acupuncture related articles (published trials) [23-24], ‘WHO Standard Acupuncture Point Location in the Western Pacific Region’ [25] | |
| (c) Extent to which treatment was varied | Two predefined acupoints | |
| 2. Details of needling | (a) Number of needle insertions per patient per session | Two needles |
| (b) Names (or location if no standard name) of points used (uni/bilateral) | Bilateral zusanli (ST36) | |
| (c) Depth of insertion | 10.0 mm (subcutaneous) | |
| (d) Response sought (for example, de qi or muscle twitch response) | Deqi sensation (penetrating, sharp, aching and painful sensations when penetrating the skin, spreading and lumpish sensation around the acupuncture site) | |
| (e) Needle stimulation (for example, manual, electrical) | Bidirectional rotation with the needle at 90° relative to the skin in 18 seconds to induce Deqi sensation | |
| (f) Needle-retention time | 20 minutes | |
| (g) Needle type (diameter, length and manufacturer or material) | 0.16 mm × 40.0 mm, stainless steel, disposable acupuncture needle (Seirin Co. Ltd., Shizuoka, Japan) | |
| 3. Treatment regimen | (a) Number of treatment sessions | Once |
| (b) Frequency and duration of treatment sessions | Once a day | |
| 4. Other components of treatment | (a) Details of other interventions administered to the acupuncture group |
Not done |
| (b) Setting and context of treatment, including instructions to practitioners and information and explanations to patients | Not done | |
| 5. Practitioner background | (a) Description of participating acupuncturists | Korean medical doctors who are specialists or resident of Korean medicine, with more than two years of clinical experience and six years of education |
| 6. Control or comparator interventions |
(a) Rationale for the control or comparator in the context of the research question with sources that justify this choice | Not Applicable (single-arm study) |
| (b) Precise description of the control or comparator if sham acupuncture | Not Applicable (single-arm study) |
2.2. Sample size
The sample size was determined based on the difference in spectral energy from 13 to 50 Hz (SE13-50Hz) between before and after acupuncture stimulation, as described previously by Huang et al [13]. The characteristic of SE13-50Hz is very similar to that of SE10-30Hz measured by using our tonometric device, because the variation in the spectral energy at frequencies above 10Hz is only 0.9% of the total variation [19]. The formula to estimate the sample size is as follows:
where α is a significance level (type I error), β is a type II error, μD is the mean difference in the SE13-50Hz between before and after acupuncture stimulation, σD is the variance of the difference, and z_(1-α/2) or z_(1-β) is the (1-α/2)th or the (1-β)th quartile of the standard normal distribution, respectively. We acquired μD and σD from the result of Huang’s study [13]. The mean difference μD is -3.38. The variance of the change in SE13-50Hz was estimated based on the following formula:
where n is the sample size reported previously [20, 21], and t0 is the t-value given by the degree of freedom and reported P-value. The sample size used previously was 30, and the P-value for the difference in SE13-50Hz between before and after acupuncture stimulation was reported as 0.0029. Thus the value of t0 is found to be -3.3526 by using the inverse function of the t distribution. Applying the described information and the above formula gives a σD value of 32.3958. The estimated sample size of the study is 25, with a 5% type I error, an 80% and a 5% drop rate.
2.3. Study procedures
All study procedures will be conducted at the Korean Medicine Clinical Trial Center, Kyung Hee University’s Oriental Medical Center, Seoul, South Korea.
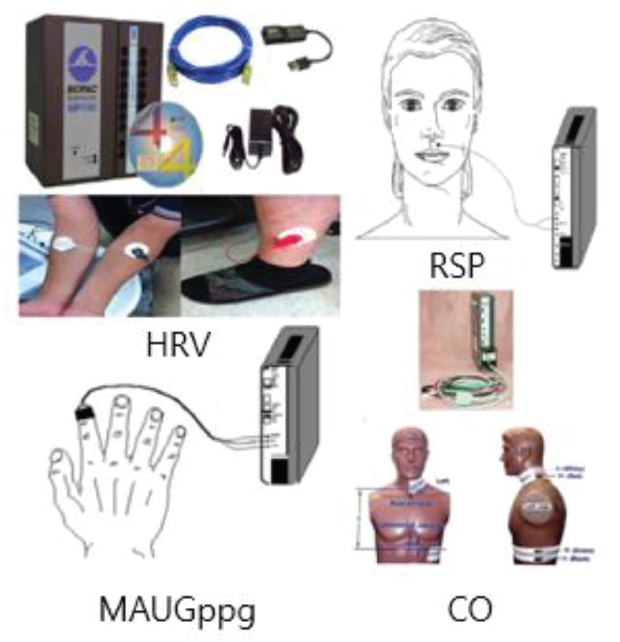
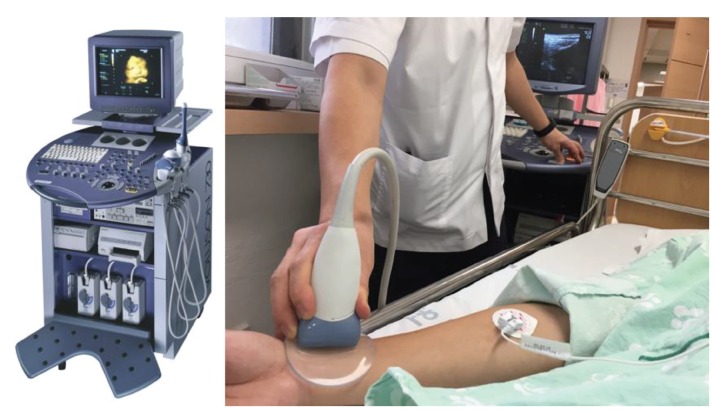
The findings from the global physical activity questionnaire (GPAQ) and the credibility/expectancy questionnaire (CEQ) will be recorded before engagement of treatment-measurement protocol. After the questionnaires have been completed, the subjects will be given a 20-minute resting session. After the rest, the spectral energy at 10 - 30 Hz (SE10-30Hz), the spectral energy at 0 - 10 Hz (SE0-10Hz), the pulse power index (PPI), the pulse depth index (PDI), and the pulse volume index (PVI) will be measured on the left wrist of the subjects with by using an applicable pulse tonometric device (KIOM-PAS, Korea Institute of Oriental Medicine, Daejeon, Korea) (Fig. 1). The HRV, the mean area under the curve of the PPG signal (MAUCppg), the cardiac output (CO), and the respiration (RSP) will be measured using a physiological data acquisition system (Biopac module, Biopac MP150, Biopac Systems Inc., USA) (Fig. 2) while the velocity of blood flow, and the diameter and the depth of the blood vessel will be measured using an ultrasonogram machine (Voluson 730 Pro, GE Healthcare Austria GmbH & Co OG, Austria) (Fig. 3) on the right wrist the before, during, and after acupuncture. The environmental temperature and moisture will be maintained at 20 ─ 21°C and 40% ─ 45 %, respectively. All measurements will be conducted 6 times in total (once 5 minutes before the needle insertion, 3 times in 20 minutes during the needle retention, and twice during 14 minutes after needle removal). After the measurements have been completed, the participants will be given the acupuncture sensation questionnaire (ASQ) (Table 2).
Fig. 2. Physiological data acquisition system (Biopac module).
HRV, heart rate variability; RSP, respiration; MAUCppg, mean area under the curve of the photoplethysmogram signal; CO, cardiac output.
Fig. 3. Ultrasonogram machine.
Table. 2. Schedule for study procedures.
| Visit | Visit 1 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | |||||||||||||||||||
| Measurement | ||||||||||||||||||||
| Before Measurement | Before Acupuncture (-5 min) | During Acupuncture (0 – 20 min) | After Acupuncture (20 – 34 min) |
After Measurement | ||||||||||||||||
| Questionnaire | ||||||||||||||||||||
| GPAQ | • | |||||||||||||||||||
| CEQ | • | |||||||||||||||||||
| ASQ | • | |||||||||||||||||||
| Applicable pulse tonometric device (KIOM-PAS) | ||||||||||||||||||||
| SE10-30Hz | • | • | • | • | • | • | ||||||||||||||
| SE0-10Hz | • | • | • | • | • | • | ||||||||||||||
| PPI | • | • | • | • | • | • | ||||||||||||||
| PDI | • | • | • | • | • | • | ||||||||||||||
| PVI | • | • | • | • | • | • | ||||||||||||||
| Physiological data acquisition system (Biopac module) | ||||||||||||||||||||
| HRV | • | • | • | • | • | • | ||||||||||||||
| RSP | • | • | • | • | • | • | ||||||||||||||
| MAUC ppg | • | • | • | • | • | • | ||||||||||||||
| CO | • | • | • | • | • | • | ||||||||||||||
| Ultrasonogram machine | ||||||||||||||||||||
| Velocity of blood flow | • | • | • | • | • | • | ||||||||||||||
| Diameter of blood vessel | • | • | • | • | • | • | ||||||||||||||
| Depth of blood vessel | • | • | • | • | • | • | ||||||||||||||
GPAQ, global physical activity questionnaire; CEQ, credibility/expectancy questionnaire; ASQ, acupuncture sensation questionnaire; SE10-30Hz, spectral energy at 10 - 30 Hz; SE0-10Hz, spectral energy at 0 - 10 Hz; PPI, pulse power index; PDI, pulse depth index; PVI, pulse volume index; HRV, heart rate variability; RSP, respiration; MAUCppg, mean area under curve of photoplethysmogram; CO, cardiac output.
2.4. Interventions
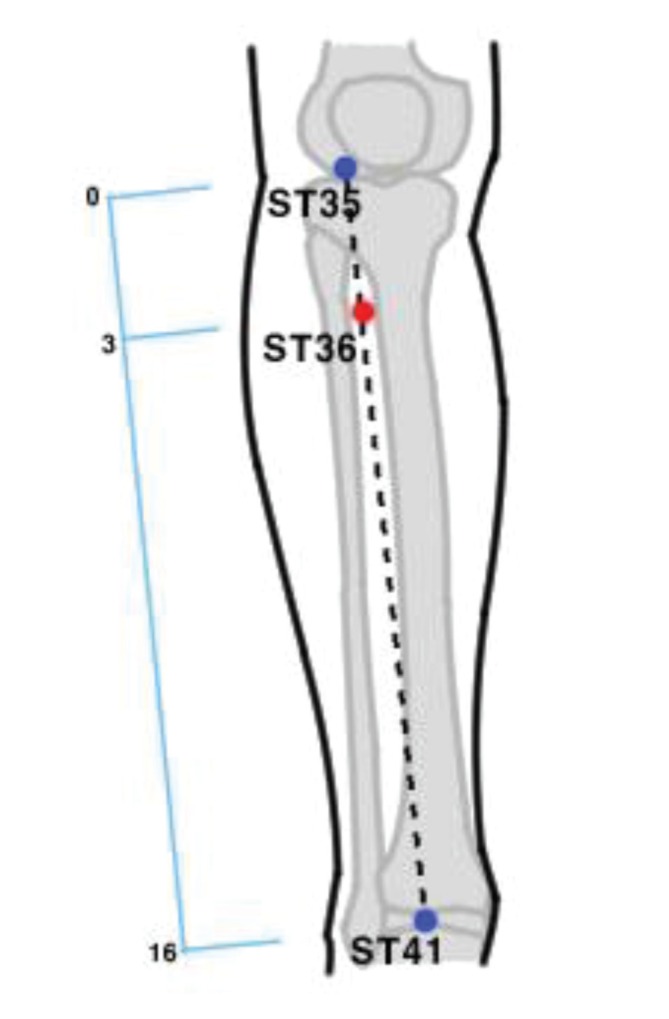
The participants will receive acupuncture once at ST36 on both sides. A previous study found that acupuncture stimulation at ST36 can improve various features of the biomarkers of the cardiovascular system, such as the baroreflex function and various hemodynamic parameters [22, 23], thereby indicating that acupuncture stimulation at ST36 can affect cardiovascular parameters including the pulse wave and the heart rate. The ST36 acupuncture points will be localized according to the ‘WHO Standard Acupuncture Point Location in the Western Pacific Region’ protocol (Fig. 4) [24]. KMDs will perform the interventions using disposable acupuncture needles (Seirin Co. Ltd., Shizuoka, Japan) which are stainless steels of the size of 0.16 mm × 40.0 mm. The Deqi sensation will be induced by bidirectional rotation of the needles by 90° around an axis perpendicular to the surface of the skin in 18 seconds, after which the needles will stay in place for 20 minutes before removal (Table 2). Any changes in medical history or vital signs and any reports of adverse events will be recorded.
Fig. 4. ST36 acupuncture points.
2.5. Devices and measurement technique
The radial pulse signal will be measured on the left wrist of the subjects with a applicable pulse tonometric device (KIOM- PAS, Korea Institute of Oriental Medicine, Daejeon, Korea) (Fig. 1). This device consists of a main body with an arm holder and a sensing body attached to a mobile actuator. A pulse detection sensor, which is composed of 7 piezoresistive unit sensors within 9 × 9 mm², is located at the actuator tip. An example of measuring a radial pulse using the KIOM-PAS is illustrated in Fig. 1. In this study, the technician will measure the pulse signals at the Gwan (Guan) location [25].
Other Physiological data related to the HRV, RSP rate, PPG and CO will be obtained using an integrated Biopac module a (MP150, Biopac Systems Inc., USA) (Fig. 2). More specifically, the HRV signal will be obtained using an electrocardiogram module (ECG100C), the RSP signal will be obtained using a thermistor (SKT100C), the mean area under the curve of PPG (MAUCppg) signal will be obtained using a PPG module (PPG100C), and the CO signal will be obtained by an impedance cardiography module (NICO100C).
The blood flow and the diameter and depth of the artery will be obtained on the right wrist by an ultrasonogram machine (Volusion 730 Pro, GE Medical, USA) (Fig. 3). The arterial diameter and the depth will be measured in the B mode and the blood flow velocity will be measured in the Color Doppler mode. The angle of incidence for the ultra-sonogram will be kept at 60° in the Color Doppler Mode and at 20° in the B mode. A gel pad (BLUEMTECH, Korea) will be used for ease of measurement.
2.6. Outcome measures
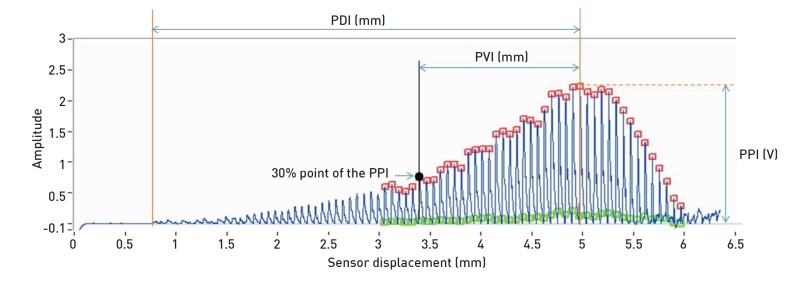
The primary outcome measure will be the SE10-30Hz. From the radial pulse signal obtained for 60 seconds under constant hold-down pressure at which pulse amplitude is maintained its maximum, power spectral density (PSD) will be calculated. The integrated value of the high frequency regime of PSD above 10 Hz will be defined as the spectral energy of the range of 10 - 30 Hz. The first six secondary outcomes will be as follows: (1) the SE0-10Hz, for which the spectral energy in the range of 0 - 10 Hz will be calculated from the PSD; (2) PPI is defined as the highest pulse amplitude of the pulse signal obtained by a continuously evolved tonometric mechanism [26]; (3) PDI is defined as the sensor displacement between the contact point of the sensor to the skin and the point at which the PPI is obtained (Fig. 5). (4) The pulse volume index (PVI) is defined as the depth window between the 30% point of the PPI and the PPI (Fig. 5). (5) HRV will be calculated by the ECG signal. More specifically, we calculate the R-R interval (NN), the standard deviation of the NN (SDNN), the root mean squared standard deviation (RMSSD), low and high frequency spectral power density (LF, HF) etc. [27]. (6) RSP For which a fast-response surface temperature thermistor will be attached to the philtrum region of participants to measure temperature changes in inspirations and exhalations through the nose. RSP rates will be calculated using the flows of such temperature change signals.
Fig. 5. Description of the PPI, PDI and PVI.
PPI, pulse power index; PDI, pulse depth index; PVI, pulse volume index.
Four more secondary outcomes are as follows: (7) MAUCppg can be used to estimate the state of heart beat activity by using the optical characteristics of in-vivo tissues in which changes in the blood flow rates and the blood vessel volumes occur due to the dilatation and the contraction of the heart. The mean waveform of the PPG wave will be measured from the starting to the ending points, as will the area under the mean waveform curve.
(8) CO is the multiplication of the stroke volume and the heart rate and will be calculated using a standard formula with an impedance cardiopraphy signal [28].
(9) The velocity of blood flow can be determined by using the Doppler effect of ultrasonic waves. Changes in the frequencies of ultrasonic waves emitted to blood vessels with flow rates and returned after being reflected will be sensed to estimate the maximum blood flow velocity and the mean blood flow velocity in the blood vessels.
(10) The diameter and depth of the radial artery will be determined. Ultrasonic waves emitted from an ultrasonogram machine will be used, and the time for the ultrasonic waves to return after being reflected in the human body will be calculated. The signals that return after being reflected will be expressed as the brightness of the images in the B mode of an ultrasonogram machine, and the pixels in the images in the B mode include distance information. Therefore, distances between two points in the images of ultrasonogram machine will be measured using information on the distance per pixel in the images of the ultrasonogram machine and will be used to estimate blood vessel diameters and depths.
The last three secondary outcomes are the results from the (11) GPAQ, (12) CEQ, and (13) ASQ.
The GPAQ was developed by the WHO for physical activity surveillance. It uses 16 questions (P1 ─ P16) to collect information on physical activity participation in three settings (or domains), as well as sedentary behavior. The domains are “activity at work,” “travel to and from places,” and “recreational activities” [29]. The GPAQ assessment will be performed before acupuncture.
The CEQ has recently been developed as measure of treatment credibility and expectancy. The questionnaire consists of one question that reflects the expectancy of acupuncture. Each participant will answer this question with a rating of 1-9 [30]. The CEQ will be assessed before acupuncture.
The ASQ was developed at the Department of Applied Korean Medicine, Kyung Hee University, Seoul, Korea, to measure acupuncture sensation (Deqi). The analysis of the interview transcripts will provide three categories: sensations during the insertion of the acupuncture needle (SIA), sensations during the manipulation of the acupuncture needle (SMA), and sensations during the time the acupuncture needle is in the skin (SM). In in-depth interviews, 33 items related to SIA, 59 to SMA, and 29 to SM will be addressed [31]. The ASQ will be assessed after acupuncture stimulation.
2.7. Statistical analysis
Statistical analysis will be performed using STATA version 13.1 (StataCorp, College Station, TX, USA) and R statistical software in the current version. The number of total participants actually enrolled and eligible participants will be described by using a flow chart. The significance level for all tests will be set to 0.05 (two-sided). Baseline characteristics of participants will be described with the available data. Categorical measures will be represented as the number of participants and percentages, and continuous variables will be summarized as means and standard deviations for normal data and as medians and interquartile ranges (25th and 75th quartiles) in the case of skewed data. The Shapiro-Wilk test will be used to verify the normality of quantitative measures. Analyses of the primary and the secondary outcomes will be conducted on a full analysis set (FAS) on the basis of the intention-to-treat (ITT) principle and on per protocol subsamples for the purpose of sensitivity analyses. The last observation carried forward (LOCF) method will be used to impute missing data.
The primary outcome is the change in SE10-30Hz between pre-acupuncture stimulation and post-acupuncture stimulation measured at the baseline and the end point, respectively. The paired two sample t-test will be used to determine the significance of the change induced by the acupuncture stimulation. The result of the analysis will be described as the mean, standard deviation, 95% confidence interval, t-value and P-value. The changes in the SE10-30Hz at six time points (before acupuncture stimulation, immediately upon needle insertion, during stimulation measured after 7 and 14 minutes of needle insertion, immediately after removal of acupuncture needle, and 7 minutes later) will be investigated using repeated measures analysis of variance (RMANOVA) as a secondary analysis. The model of RMANOVA will include covariates (sex, age, and ASQ scale effects) and interactions between sex and time effects. The model will be modified by excluding an interaction term if it is not significant. The ANOVA table for the model will be provided, as will the least-squares mean, standard error, and 95% confidence interval at each time point. The difference in time relative to the baseline (before acupuncture) will be examined using Dunnett’s test.
2.8. Data handling and adverse events
Investigators will enter the information required by the protocol on case report forms (CRFs). Non-obvious errors or omissions will be entered on data query forms, which will be returned to the investigational site for resolution. The data from all centers will be gathered and summarized with respect to demographic baseline characteristics, effectiveness, and safety observations.
Adverse events are unexpected signs, symptoms, or diseases that occur during the clinical trial. These include not only acupuncture treatment-induced side effects but also all other abnormal findings caused for any reason. All unexpected responses related to acupuncture treatment and measurement will be reported to investigators by the participants and will be examined by the investigators. Local, general, and psychological adverse events are possible as a result of acupuncture treatments. Adverse events will be evaluated by investigators and categorized as mild, moderate, or severe according to the WHO’s Draft Guidelines for Adverse Event Reporting [32] and Spilker’s criteria [33]. If serious adverse events (SAE) occur, they will be reported to the IRB and to monitors; experimental treatments will be stopped immediately and appropriate treatments will be offered. During every visit, adverse events will be reported by participants and investigated the practitioner.
3. Discussion
The outcome measures for this study are the changes in radial artery pressure pulse, blood vessel properties, and physiological data obtained before, during, and after acupuncture at ST36. The changes in the artery pressure pulse, properties of blood vessels, and physiological data, as measured by using the SE10-30Hz, SE0-10Hz, PPI, PDI, MAUCppg, HRV, CO, RSP, blood vessel depth, blood vessel diameter, and blood velocity, before, during, and after acupuncture at ST36 will be compared. The primary comparisons will be before acupuncture versus during acupuncture versus after acupuncture. In addition, we will record the GPAQ, CEQ, and ASQ findings.
The hypotheses to be tested are as follows: 1) the radial artery’s pressure pulse wave will differ significantly between the before, during, and after acupuncture assessments; 2) the hemodynamic parameters will differ significantly between the before, during, and after acupuncture; 3) and the radial artery’s pressure-pulse wave and the hemodynamic parameters will differ significantly between the before, during, and after acupuncture assessments according to the sensitivity of the Deqi sensation.
The results from this study will determine the efficacy and the safety of stimulation of the radial artery’s pressure pulse wave in healthy individuals in their twenties. These results can provide information regarding the physiological and hemodynamic mechanisms underlying acupuncture, as well as clinical evidence for the influence of acupuncture on the radial artery pressure pulse wave; they will also clarify whether the radial artery’s pressure pulse wave can be used to objectively evaluate the efficacy of acupuncture. The results of this trial will be available in January 2017.
This study involve healthy young participants. We expect this study to provide the clinical basis and the information that are required to assess the feasibility of a future large scale trial in patients.
Blinding and randomization will not be done in this trial, because this clinical study will be performed using a single- arm evaluation, so blinding the participants and the acupuncturists is not possible.
4. Ethics, dissemination, and status
The study protocol was approved by the Institutional Review Board of Kyung Hee University’s Oriental Medical Center, Seoul, Korea (KOMCIRB-150818-HR-030) and was registered with the Clinical Research Information Service (CRIS) at the Korea National Institute of Health (NIH), Republic of Korea (KCT0001663), which is a registry in the WHO’s Registry Network [34]. Written informed consent will be obtained from all participants in accordance with the Declaration of Helsinki. The study findings will be published in peer-reviewed journals and presented at national and international conferences. The trial is currently in the recruitment phase. Participant recruitment started in October 2015 and is expected to end in October 2016.
5. Authors’ contributions
JUK conceived the idea for the study and led protocol development. BCK, THK, JHB, HJC, JYS and JHL assisted with the study concept, study design, clinical interpretation, and manuscript drafting and finalization. THK will recruit the participants and conduct the trial. BCK planned the statistical analysis. JHL helped to conceive and design the study and critically revised the trial. JUK provided overall supervision as the primary mentor and led the team in manuscript preparation. JYS and BCK wrote the manuscript and approved the final version for publication. All authors commented upon drafts of the manuscript and approved the final version of the manuscript.
6. Registration details
This protocol was registered with the CRIS at the NIH, Republic of Korea (KCT0001663), which is a registry in the WHO’s Registry Network [19, 34].
Acknowledgments
This study was supported by the Korea Institute of Oriental Medicine (Grant no. K16021).
Footnotes
Conflict of interest The authors declare that there are no conflict of interest.
ORCID Jaeuk U. Kim. http://orcid.org/0000-0003-0408-5569.
ORCID Jun-Hwan Lee. http://orcid.org/0000-0001-5730-6869.
References
- 1.Mayer DJ. Acupuncture: an evidence-based review of the clinical literature. Annu Rev Med 2000;51:49-63. doi: 10.1146/annurev.med.51.1.49. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO traditional medicine strategy: 2014-2023. WHO Library Cataloguing in Publication; Switzerland: 2013. 78 [Google Scholar]
- 3.Lim DO, Jung MJ, Park JS. The survey of Korean medicine health care utilization and herbal medicine consumption. Korea Health Industry Development Istitute; Cheongju: 2014. 805 [Google Scholar]
- 4.Kaptchuk TJ. Acupuncture: theory, efficacy and practice. Ann Intern Med. 2002;136(5):374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 5.Chae YB, Kim YJ, Choe IH, Lim SB, Lee SJ, Lee HJ et al. [A comparison study of acupuncture sensation scale between real acupuncture and sham needle] Korean J Acupunct. 2006;23(4):85–99. Korean. [Google Scholar]
- 6.Wang B . Huangdineijing suwen. People’s Medical Publishing House; Beijing: 1963. [Google Scholar]
- 7.Bae SC, Shin SH, Kim KW. [Pulse diagnosis procedure before and after the acupuncture in Hwangjenaekyung] J Oriental Medical Classics. 2011;24(3):15–25. [Google Scholar]
- 8.Lee BK, Park YB, Kim TH. [Diagnostics of Korean medicine] Seongbosa; Gyeonggi: Korean. [Google Scholar]
- 9.Boutouyrie P, Corvisier R, Azizi M, Lemoine D, Laloux B, Hallouin M et al. Effects of acupuncture on radial artery hemodynamics: controlled trials in sensitized and naive subjects. Am J Physiol Heart Circ Physiol. 2001;280(2):628–633. doi: 10.1152/ajpheart.2001.280.2.H628. [DOI] [PubMed] [Google Scholar]
- 10.Sandberg M, Lundeberg T, Lindberg LG, Gerdle B. Effects of acupuncture on skin and muscle blood flow in healthy subjects. Eur J Appl Physiol. 2003;90(1-2):114–119. doi: 10.1007/s00421-003-0825-3. [DOI] [PubMed] [Google Scholar]
- 11.Takayama S, Seki T, Sugita N, Konno S, Arai H, Saijo Y et al. Radial artery hemodynamic changes related to acupuncture. Explore. 2010;6(2):100–105. doi: 10.1016/j.explore.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Takayama S, Seki T, Watanabe M, Monma Y, Yang SY, Sugita N et al. Brief effect of acupuncture on the peripheral arterial system of the upper limb and systemic hemodynamics in humans. J Altern Complement Med. 2010;16(7):707–713. doi: 10.1089/acm.2009.0355. [DOI] [PubMed] [Google Scholar]
- 13.Huang CM, Chang HC, Li TC, Chen CC, Liao YT, Kao ST. Acupuncture effects on the pulse spectrum of radial pressure pulse in dyspepsia. Am J Chin Med. 2012;40(3):443–454. doi: 10.1142/S0192415X12500346. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Kim EJ, Park YC, Koh YJ, Nam DW. [Effect of acupuncture stimulation on heart rate variability in stroke patients] The Acupuncture. 2006;23(1):135–143. Korean. [Google Scholar]
- 15.Han CH, Song TW, Shin MS, Shin SH, Choi SM. [Gogoon acupuncture for hypertension] Korean J Acupunct. 2007;24(1):27–41. Korean. [Google Scholar]
- 16.Park EJ, Cho MR. [Experimental study of dadun (LR1)•shaochung (HT9)•shaofu (HT8) reinforcement in acupuncture on the improvement of cerebral hemodynamics. The Acupuncture. 2007;24(1):111–125. Korean. [Google Scholar]
- 17.Yeo KC, Yoon IA, Kim JN, Bang SP, Moon SI. [Effects of acupuncture at Palsa (BaXie) evaluated by the second derivative of photoplethysmogram waveform in hemiparetic patients after stroke] The Acupuncture. 2010;27(2):23–30. Korean. [Google Scholar]
- 18.Kim NY, Kang JH, Lee H. [The effect of sa-am lung tonifying acupuncture on radial pulse in healthy humans Subjects] The Acupuncture. 2012;29(5):17–29. Korean. [Google Scholar]
- 19.Wei LY, Lee CT, Chow P. A new scientific method of pulse diagnosis. Am J Acupunct. 1984;12(3):205–218. [Google Scholar]
- 20.Madsen MV, Gotzsche PC, Hrobjartsson A. Acupuncture treatment for pain: systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ. 2009;338:3115. doi: 10.1136/bmj.a3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linde K, Niemann K, Schneider A, Meissner K. How large are the nonspecific effects of acupuncture? a meta- analysis of randomized controlled trials. BMC Med. 2010;8:75. doi: 10.1186/1741-7015-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima JW, Hentschke VS, Rossato DD, Quagliotto E, Pinheiro L, Almeida E, Jr et al. Chronic electroacupuncture of the ST36 point improves baroreflex function and haemodynamic parameters in heart failure rats. Auton Neurosci. 2015;193:31–37. doi: 10.1016/j.autneu.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Hyun SH, Im JW, Jung WS, Cho KH, Kim YS, Ko CN et al. Effect of ST36 acupuncture on hyperventilation- induced CO2 reactivity of the basilar and middle cerebral arteries and heart rate variability in normal subjects. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/574986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Regional Office for the Western Pacific. WHO standard accupuncture point location in the Western pacific region. WHO Library Cataloguing in Publication; Switzerland: 2009. 249 [Google Scholar]
- 25.Kim JU, Lee YJ, Lee J, Kim JY. Differences in the properties of radial artery between Cun, Guan, Chi and nearby segments using ultrasonographic imaging: a pilot study on arterial eepth, diameter, and blood flow. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/381634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae JH, Jeon YJ, Kim JY, Kim JU. New assessment model of pulse depth based on sensor displacement in pulse diagnostic devices. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/938641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marek MJ, Thomas BA, John C. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 28.Yazdanian H, Mahnam A, Edrisi M, Esfahani MA. Design and implementation of a portable impedance cardiography system for noninvasive stroke volume monitoring. J Med Signals Sens. 2016;6(1):47–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Global physical activity questionnaire (GPAQ) analysis guide. World Health Organization; Switzerland: http://www.who.int/chp/steps/ resources/GPAQ_Analysis_Guide.pdf. [Google Scholar]
- 30.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/S0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ. Kyung Hee University; Seoul: 2006. Development, validity and reliability testing of a Deqi sensation questionnaire by in-depth interview. [Google Scholar]
- 32.WHO draft guidelines for adverse event reporting and learning systems. WHO Document Production Services; Switzerland: 2005. 80 [Google Scholar]
- 33.Spilker B. Quality of life and pharmacoeconomics in clinical trials. Lippincott Williams & Wilkins; Philadelphia: 1996. 1312 [Google Scholar]
- 34.International clinical trials registry platform (ICTRP) World Health Organization; Switzerland: http://www.who.int/ictrp/network/ en/. [Google Scholar]