Abstract
Thousands of mothers are at risk of transmitting mitochondrial diseases to their offspring each year, with the most severe form of these diseases being fatal [1]. With no cure, transmission prevention is the only current hope for decreasing the disease incidence. Current methods of prevention rely on low mutant maternal mitochondrial DNA levels, while those with levels close to or above threshold (>60%) are still at a very high risk of transmission [2]. Two novel approaches may offer hope for preventing and treating mitochondrial disease: mitochondrial replacement therapy, and CRISPR/Cas9. Mitochondrial replacement therapy has emerged as a promising tool that has the potential to prevent transmission in patients with higher mutant mitochondrial loads. This method is the subject of many ethical concerns due its use of a donor embryo to transplant the patient’s nuclear DNA; however, it has ultimately been approved for use in the United Kingdom and was recently declared ethically permissible by the FDA. The leading-edge CRISPR/Cas9 technology exploits the principles of bacterial immune function to target and remove specific sequences of mutated DNA. This may have potential in treating individuals with disease caused by mutant mitochondrial DNA. As the technology progresses, it is important that the ethical considerations herein emerge and become more established. The purpose of this review is to discuss current research surrounding the procedure and efficacy of the techniques, compare the ethical concerns of each approach, and look into the future of mitochondrial gene replacement therapy.
Keywords: CRISPR-Cas systems, mitochondrial diseases, mitochondrial replacement therapy, nuclear transfer, gene editing, assisted human reproduction, ethics
Introduction
Mitochondrial diseases are among the most devastating inheritable diseases, with the most severe forms causing death shortly after birth [1]. While there is currently no cure for mitochondrial diseases, advances in science have provided avenues for prevention of disease transmission. Preimplantation genetic diagnosis (PGD), one such method of mitochondrial disease prevention, is a technique that screens cells taken from in vitro fertilization (IVF) embryos prior to uterine implantation to select for healthy embryos. Armed with the knowledge of the mitochondrial genotype, parents can make informed decisions about the pregnancy. Specifically, PGD has been shown to significantly increase the probability of producing healthy offspring for heteroplasmic carriers of mitochondrial DNA (mtDNA) mutations. However, PGD is not a valuable test for all patients. While it provides information about the mtDNA, it offers no intervention when the mtDNA is found to be mutant, as is often the case with homoplasmic carriers or heteroplasmic carriers close to the disease threshold [2]. For this reason, the field of mitochondrial disease prevention will be limited until other interventions are ready for clinical use.
In recent years, a novel method of mtDNA replacement therapy (MRT) has emerged as a promising approach to preventing mitochondrial disease transmission [3]. This technique uses an enucleated donor embryo as a cytoplasmic environment for the nuclei of parent germ cells, thereby preventing the transmission of mitochondria from the parent cells. Here, we discuss recent findings, results, and efficacies of the three main variations of MRT being studied. While MRT shows much potential, there are serious ethical concerns surrounding this approach, which may put its future on dubious ground.
Additionally, we will discuss the recent studies of CRISPR/Cas9 as an effective method of editing genes and treating disease in animal models, and apply these findings to potential curative or therapeutic future treatments of mitochondrial disease.
Background
Mitochondrial disease genetics
Mitochondrial disorders encompass a wide array of genetic mutations and resulting disease characteristics, ranging in severity from asymptomatic to fatal within the first few years of life [4,5]. There is no cure for mitochondrial disease, so treatment is mostly palliative and symptomatic. The wide-ranging manifestations and clinical presentations of these disorders has made it difficult to ascertain the true prevalence and impact on the population. Recent epidemiological studies with detailed pedigree analysis have determined that mitochondrial disease is more common than previously thought, with a prevalence of 16.5 per 100,000 children and young adults, making it one of the most common inherited neuromuscular disorders [6].
mtDNA is exclusively maternally inherited. Various mutations of mtDNA result in mitochondrial dysfunction, which can have serious consequences for tissues with high metabolic demand, such as the brain, heart, muscle, and central nervous system. Mitochondrial dysfunction often manifests as multi-organ disease. The wide array of clinical features of these diseases can include: central and peripheral nervous system abnormalities including seizures, stroke-like episodes, deafness, and visual impairment; myopathies such as external ophthalmoplegia and proximal limb myopathy; hepatic stenosis and liver failure; renal dysfunction including tubular acidosis and Fanconi syndrome; gastrointestinal disturbances such as gastroparesis and pancreatitis; and finally, endocrine dysfunctions including the hyposecretion of thyroid, parathyroid, and hypothalamic hormones, as well as insulin and adrenaline [4,7-9]. Mothers carrying the mutated mtDNA can be either homoplasmic (containing only mutated mtDNA), in which case the symptoms are usually severe, or heteroplasmic (containing both mutated and wild-type mtDNA), where patients present with symptoms ranging from nearly nonexistent to moderate. While the clinical features of mitochondrial disease are vast, the severity of phenotypic effects depends on the proportion of mitochondria that carry the mutation [4,10].
While a mother may present as asymptomatic because she contains just a small percentage of mutant mtDNA, she only passes on a small number of mtDNA molecules to her offspring; therefore, there is a chance that she will pass on a vastly different proportion of mutant mtDNA. Large shifts in this proportion from mother to offspring, also known as genetic bottlenecking of mtDNA, can result in phenotypic effects if the threshold level of mutant mtDNA is met [11]. The threshold level is the minimum critical number of mitochondria with mutant mtDNA required to cause mitochondrial dysfunction in a tissue. This number is usually between 70-90%, and is determined by the metabolic need of the tissue [11].
In order to diagnose mitochondrial disease, a very detailed family history is required to seek out subtle clues demonstrating a maternal line of inheritance [11]. Due to the variable expression of these conditions, it may not always be obvious that a woman is a carrier of a mtDNA mutation. Advances in research and medicine over the past several decades have allowed the complete sequence of human mtDNA to be elucidated [5]. Genetic testing for the most common mutations in mtDNA are now widely available in diagnostic laboratories and a simple blood test can reveal the most common mutations associated with the most prevalent clinical syndromes. Despite these advances in detecting mtDNA mutations, it is still difficult to provide women with accurate preconception genetic counseling. While many large-scale deletions are infrequent, point mutations and heteroplasmy are much more common and make it difficult to predict transmission [12]. A heteroplasmic female carrier of a mtDNA mutation could pass on to offspring the same proportion of mutant to wild-type mtDNA, a higher proportion, or even a lower proportion [11]. Due to the unpredictability of transmission, it is not possible to offer accurate preconception advice to women who carry heteroplasmic disease-causing point mutations. Given the lack of curative treatments for mitochondrial diseases and the limitations of genetic counseling and prenatal diagnoses, research has focused on preventing the transmission of mutated mtDNA. While this is an improvement, it is still inadequate for dealing with mitochondrial disease, and future research must focus on developing curative and therapeutic treatments.
CRISPR/Cas9
The possibilities of gene editing expanded vastly in 2012 upon the discovery of the CRISPR/Cas9 complex, which is short for “clustered regularly-interspaced short palindromic repeats” and “CRISPR-associated protein 9”, respectively [13]. Prokaryotes have CRISPR segments of DNA containing short repetitions of base sequences that allow bacterial cells to record DNA sequences of viruses that it has been exposed to. This enables cells to bolster the immune function of progeny. The Cas9 enzyme then functions to seek out those bits of viral DNA and cut them out of sequence. Importantly, the complex is programmable, allowing scientists to target and remove specific bits of DNA with great precision. The cutting-edge discovery of the CRISPR/Cas9 technology has propelled scientists forward in attempts to treat or cure diseases caused by single-gene mutations [13]. Applications of the CRISPR/Cas9 complex to mitochondrial disease have yet to be fully explored.
MRT: a novel approach
Candidates for MRT
After birth, there are currently no curative therapies for diseases caused by mtDNA mutations. While mild disease as a result of mtDNA mutations in mothers does not seem to have a profound effect on fertility, there is a significant risk of the defect being passed to the child. According to data from the Medical Research Council (MRC) Mitochondrial Disease Cohort U.K. in 2011, it was estimated that 2473 women were at risk for transmitting mtDNA disease nationally. Based on national fertility rates, it was estimated that the average number of births per year among the high risk women is 152 in the United Kingdom [14]. Using midyear 2012 data for women in the same age group in the United States, it was extrapolated that 12,423 women are at risk for transmitting mitochondrial disease, resulting in nearly 778 births per year in the United States that could potentially benefit from treatment [14]. In the absence of transmission prevention, the increasing number of affected females will cause an even greater number of individuals to be affected in the next generation.
As previously mentioned, mothers can be either homoplasmic or heteroplasmic carriers of mitochondrial disease. Mitochondrial disease is usually seen in individuals carrying mutant mtDNA loads of greater than 60% [3]. Homoplasmy presents a greater challenge, due to the complete lack of healthy wild-type mtDNA. For heteroplasmic individuals, in vitro mtDNA therapy is thought to be effective, though perhaps more so in younger mothers. Current research suggests that as women age, they acquire an increasing number of de novo mutations in germline tissues [15]. The level of heteroplasmy can also change drastically from one oocyte to another due to genetic drift and a lack of uniform separation of cytoplasmic material during oogenesis in the germline bottleneck phenomenon. This can lead to a mother who has a relatively low fraction of mutated mtDNA and few symptoms giving birth to an offspring with a larger fraction of mutated mtDNA and a more severe phenotypic expression [15]. It can be concluded that those with the most to gain from potential mtDNA therapies are women of advanced maternal age who carry low levels of mutant mtDNA in their eggs, though there has been much controversy surrounding the proposed treatment [3]. More recently, however, it has been suggested that MRT will still be able to benefit women close to or above disease threshold, in contrast to PGD, which requires most of the mtDNA to be wild-type [1]. Therefore, while MRT has been shown to prevent transmission in mothers with low mutant mtDNA, new advancements may make it possible for homoplasmic carriers to conceive without transmission, a feat no other method has come close to achieving.
Techniques for MRT
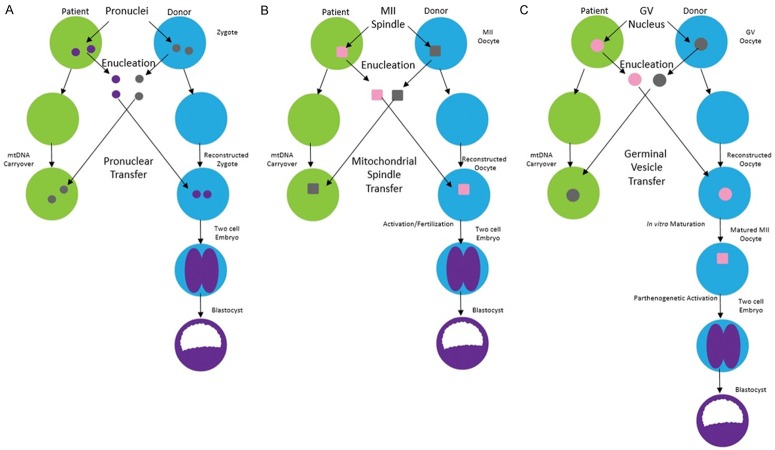
Due to the complexities of altering oocyte mtDNA content in vitro, there are currently three primary procedures for accomplishing this daunting task; metaphase II spindle-chromosome complex (MII-SCC) transfer, pronuclear (PN) transfer, and germinal vesicular (GV) transfer (Figure 1). In MII-SCC transfer, the mature oocyte containing mutant mtDNA is progressed to metaphase II where the chromosomal material is arranged along the metaphase plate. Subsequently it can be harvested and implanted into a healthy, enucleated donor oocyte (Figure 1A). This technique allows for the newly constructed oocyte to be fertilized by a viable sperm after the transfer occurs, but due to the nebulous nature of the spindle complex, carries the risk of extracting more cytoplasm and increasing the amount of mutated mtDNA that is concomitantly transferred [16]. PN transfer is the process by which the pronuclei, the nuclei of the sperm and oocyte before they fuse inside the oocyte, are removed from the parent zygote and are placed in a donor zygote that was previously fertilized and subsequently enucleated [3] (Figure 1B). This technique allows for the extraction of the two, well-defined pronuclei after the sperm has been introduced into the oocyte, potentially reducing the amount of cytoplasm that is transferred with the pronuclei and decreasing the carryover of mutated mtDNA [3]. With this data in mind, PN, rather than MII-SCC, transfer may be the technique worth pursuing in future studies in order to achieve a better outcome in those with higher levels of mutant mtDNA. While it is difficult at this point to compare MII-SCC and PN transfer techniques due to lack of meaningful concomitant research using the same animal model, both have their advantages from a technical standpoint and perhaps, more importantly, show great promise.
Figure 1.
Schematic of three different transfer methods for MRT. A. In pronuclear transfer, fertilization occurs first and the resulting zygote’s male and female pronuclei is transferred to another enucleated zygote. B. In spindle transfer, the nuclear genetic material from an oocyte containing mutated mtDNA is transferred over to an enucleated oocyte containing healthy mtDNA and is subsequently fertilized. C. In germinal vesicle transfer, the nuclear genetic material from an oocyte containing mutated mtDNA is harvested earlier in the cell cycle, transferred over to a donor oocyte, and then matured in vitro prior to fertilization and implantation (adapted from Mitalipov et al., 2014; Neupane, 2014).
Though these two methods are the most predominant in the current literature, it is worth mentioning that GV transfer, the process of transferring a healthy, immature oocyte nucleus to an otherwise healthy donor egg, is yet another proposed way to reduce the amount of inviable mtDNA but there have been fewer studies on it [17].
GV transfer is done during the arrested prophase of meiosis I, when two copies of each of the 23 pairs of chromosomes are present. Because of the large size of the nucleus at this stage, it is easier to successfully harvest in the lab. The reconstructed oocyte is then matured in vitro prior to fertilization. The maturation of the oocyte in vitro allows for surveillance of meiosis defects. This may specifically confer benefits on women of advanced maternal age, who are thought to have reduced pregnancy rates associated with aneuploidy, or the uneven distribution of chromosomes during meiosis II [18]. However, during the harvest of the GV, small amounts of residual ooplasm may be transferred as well, thus opening the possibility of mutated mtDNA being present in the newly reconstructed oocyte [19]. In a 2014 study by Neupane et al., all three techniques for MRT were compared in the same animal model. Results showed that the GV transfer method was equally as successful as its counterparts in excluding mtDNA, with an average level of mtDNA carryover of less than 2% [20].
Additional studies regarding the comparison of these techniques should be pursued to determine the indication and use guidelines for each prior to clinical trials.
Efficacy
In order to assess the efficacy of these various modes of treatment, most research focuses on two main quality indicators, namely the level of maternal mtDNA carryover and the developmental potential of the reconstructed oocyte. Percentages of mtDNA carryover less than 3% are usually considered satisfactory as there will be no phenotypic changes observed and the chance for recurrence in future generations is relatively low. However, rates of mtDNA carryover greater than 5% increased the possibility of another family member exhibiting the mitochondrial disease in later generations [21]. The standard for the developmental potential of the reconstructed oocyte varies among studies, with some considering the progression of the fertilized egg to the blastocyst stage a success while others consider a viable, live offspring as their benchmark.
Two separate studies in 2014 focusing on MRT in mice both yielded positive results. A recent study by Wang et al. showed that MRT allowed for normal development of progeny within the normal weight range like that of the control group [22]. The same study also showed that most offspring exhibited low levels of mtDNA carryover (under 6.88%), with the notable exception being the progeny coming from PN transfer, which showed heteroplasmy in the wide range of 5.55%-39.8%. A second study by Neupane et al. produced similar results with regard to developmental potential showing that embryonic development up to the blastocyst stage was nearly identical in both MII-SCC and PN transfer and the quality of the blastocyst itself was comparable to the controls. With regard to mtDNA carryover, 21 out of 25 cases showed no detectable mtDNA carryover and the remaining four cases showed only small amounts of mtDNA (<3% heteroplasmy) [20]. While GV transfer was shown to be as effective as PN and MII-SCC transfer in terms of excluding mutated maternal mtDNA, it lagged in efficacy. Despite the success in transferring GV nuclei, embryonic development faltered during the in vitro maturation process and all of the samples failed to reach the blastocyst stage [20].
In a 2009 study, Tachibana et al. evaluated the efficacy of MRT using MII-SCC transfer in primates, garnering positive results [17]. The study reported heteroplasmy levels in the newly reconstructed oocytes of less than 3%, which was the lowest threshold that could be detected by their lab [17]. Once the oocytes were fertilized and matured to the blastocyst stage, they were implanted in a female surrogate. Of the six females receiving implantations, three became pregnant and at the time of publication, two of the mothers had given birth, a pair of twins and a singleton. All three babies were healthy with pregnancy parameters, such as birth weight and the length of gestation, within normal ranges for the species [17]. 3-year follow up studies of the offspring showed normal growth, development, and overall health as compared to age-matched controls, thus providing “convincing evidence that oocyte manipulation and mtDNA replacement procedures are compatible with normal development” [16].
Trials using human oocytes in 2010 were largely successful, with the mean mtDNA carryover staying well under 2% for all embryos, which is consistent with the controls [3]. No substantial difference was found in the amount of mtDNA carryover between MII-SCC and PN transfer, suggesting that both approaches are equal in efficacy [3]. Due to ethical considerations, it is difficult to assess the developmental potential of these human embryos beyond the blastocyst stage. It is also worth noting that many studies cite concerns about whether the amount of heteroplasmy found in samples of blastocyst and trophectoderm are representative of the embryo as a whole.
In research, three species (human, mice and primates) have been shown a very high rate of efficacy for both MII-SCC and PN transfers [23]. Trials were successful in producing offspring, whether in the form of a viable blastocyst (human embryos) or a viable neonate (mice and primate embryos), with levels of heteroplasmy ranging from less than 2% in humans [3] and as high as 6.88% in mice [22]. With the exception of the 2014 Wang et al. study, which found higher rates of heteroplasmy in the PN transfer than MII-SCC transfer, all studies consistently showed that while both techniques are successful, lower levels of heteroplasmy were found in PN transfer. This is most likely due to the lower rates of cytoplasm transfer in PN transfer. At less than 2%, human embryo trials showed the lowest rates of mtDNA carryover, thus supporting further research into the efficacy of MRT in human, full-term trials. Furthermore, additional research on PN transfer should be pursued in order to fine-tune the technique to prevent transmission in patients having higher mutant mtDNA loads.
CRISPR/Cas9
The CRISPR/Cas9 technology has exploded in popularity because of its affordability and potential applications in curing genetic disease. In the last few years, several animal studies have been published demonstrating its powerful gene editing capabilities. In a 2014 study by Yin et al., CRISPR/Cas9 mediated gene-editing was demonstrated to be possible in adult animals, and was curative for the hereditary, single-gene mutation condition tyrosinemia, despite changing only 6% of cells [24]. This is reason enough to be curious about the curative potential for both heteroplasmic and homoplasmic mtDNA mutation carriers - while the gene-editing may not affect all mtDNA, it may modify enough to bring the individual below the disease threshold, conferring therapeutic benefits.
Other studies have examined the effectiveness of CRISPR/Cas9 in treating single gene disorders such as Duchenne muscular dystrophy, as well as eye conditions like retinitis pigmentosa and Leber congenital amaurosis (LCA) [25,26]. Corporate research companies such as Editas suggest that the first human clinical trials using CRISPR/Cas9 will aim to treat LCA and may begin as early as 2017. The eye presents an ideal testing location, as it is contained, immunologically isolated from the rest of the body, easily monitored externally, and can be measured using established standards of function. CRISPR/Cas9 technology has the potential for use in diseased individuals, as well as IVF embryos prior to implantation. In the case of diseased individuals, CRISPR/Cas9 offers the “promise” of reducing the mutation load, which may subsequently reduce symptoms and the burden of disease. Application of CRISPR/Cas9 therapy with adult patients is less controversial than its use with embryos, as adults are able to provide informed consent. Furthermore, many patients with incurable diseases and compromised quality of life are eager to participate in clinical trials of new therapies, as there are no viable alternative treatments and it offers hope for a cure [27].
In a 2015 study by Liang et al. that was the first of its kind, CRISPR/Cas9 was used on tripronuclear (3PN) zygotes to better understand its effects in preimplantation embryos [28]. While targeting the β-globin gene (HBB), the CRISPR/Cas9 complex also produced off-target effects. Additionally, the efficacy of homologous recombination directed repair of HBB was low and produced mosaic embryos. In several of the 3PN zygotes, there were high rates of DNA repair using endogenous sequences instead of the therapeutic template [28]. This is a major obstacle that must be overcome if the CRISPR/Cas9 system is to be used therapeutically.
CRISPR/Cas9 editing of embryonic mtDNA may appeal as a more socially-acceptable alternative to “three-parent IVF”. Instead of combining the genetics of three individuals, this technique may enable a couple to conceive without requiring donor genetic material. Another advantage of trying to treat mitochondrial disease before implantation is the small number of cells; it may be easier to ensure a reduced mutation load if the embryo is in the 8- or 16-cell stage than when there are exponentially more cells after birth.
While the CRISPR/Cas9 technology has great potential in treating genetic disease, more research is needed before the science community begins human clinical trials. Researchers must overcome a number of obstacles, such as the reaction of the human immune system, efficient modes of delivery, determining how to ensure that a corrected copy of DNA is inserted into the sequence, safeguarding against Cas9 proteins cutting at incorrect loci, and understanding and controlling off-target effects [26].
Ethical concerns
Like many potential treatments before it, MRT raises several ethical concerns, both in theory and practice. These concerns have become barriers to furthering research and developing clinical studies to test the efficacy of MRT in humans. Issues from risks to donors and children, as well as the fear that this would lead us down a slippery slope to “designer” babies, have raised important questions that must be addressed before this technology can be applied.
Tri-Parental offspring: definition and consequences
MRT has often been controversially termed “three-parent IVF” with the resultant offspring being called tri-parental offspring by the media and the scientific community at large. Prior to MRT being developed, the term tri-parental offspring had only been applied to a form of bacterial conjugation and therefore a proper scientific definition has not been established for humans [29]. It is also worth mentioning that donor eggs have commonly been used with assisted reproductive technology to help women without competent oocytes become pregnant. In those cases, offspring contain genetic material from the oocyte donor and the father, but are also influenced in utero by epigenetic factors from the carrier mother. Despite the molecular contribution of three individuals to the offspring, this has not been dubbed “three-parent IVF”.
Those that claim that MRT should not be considered three-parent-IVF point to the fact that mtDNA codes only 37 genes (13 proteins, 22 tRNAs, and 2 rRNAs) making up just 0.1% of the total DNA [30]. Furthermore, mtDNA haplotypes have little variance amongst the human population, which deepens the argument for those claiming that the name three-parent-IVF is a harmful misnomer [30]. Cytoplasm donors are considered as organ donors, and ethical researchers concluded that mitochondrial donation “does not indicate, either biologically or legally, any notion of the child having either a third parent or second mother” especially when socioeconomic and cultural definitions of parenthood are taken into account [29,30,32]. Furthermore, just as organ donors have no claim on the donated organs, cytoplasm donors have no claim on the donated organelles and by extension any children that receive those organelles.
Additionally, oocyte donors are routinely required to sign various release and consent forms relinquishing all parental claims to the offspring, prior to donation. Furthermore, despite the 50% contribution of the donor to the genetic material of the offspring, there has not been a single case in the U.S. where an egg donor has later been granted parental status following formally giving up rights to eggs and resulting offspring [33]. Based on this precedent, and the incrementally smaller fraction of genetic material donated, cytoplasm donors should have no realistic expectation of parental claim.
On the other side of the argument, the existence of any traceable genetic material at all, even just 0.1%, may be considered significant enough to warrant the importance of the donor as a third parent in the equation. Because a person’s identity is shaped by who they are and how they interact with the world, a disease that person may carry also forms part of that identity. As successfully avoiding passing on a mitochondrial disease changes a person’s life, the addition of a mtDNA donor in IVF may drastically change the potential identity of a person. For this reason, it is argued that the third person’s genetic material is much more important than what detractors say and cannot be simply dismissed as providing just 0.1% of the genetic material [34]. However, we believe that the donated mtDNA would promote a positive change of identity in terms of health outcomes, rather than create a negative change in the form of the identity crisis of having three parents. While a definition of what makes a three-parent offspring has not been agreed upon, it is reasonable to conclude that socially, legally, and 99.9% genetically, the parents that are receiving the oocyte donation are responsible for the child.
Harm to cytoplasm donors
When looking at the risk-benefit ratio for donors, ethical issues arise because the ratio appears skewed. The benefits to the donor are a positive feeling from altruism and financial payment (which is controversial in and of itself during studies) with many more harms. Risks to those donating include daily hormonal injections and added stress from the relatively invasive procedure. The hormonal injections can lead to side effects such as pain, nausea, organ damage, infertility, hemorrhage, and cancers [34]. The process of hormone injection and several oocyte retrievals also raises the risk for ovarian hyperstimulation syndrome, which can result in death in rare cases [31]. While normally a risk-benefit ratio such as this can be overlooked when trying to bring a healthy baby into the world, it becomes harder to do so when a person is taking the risk for someone else’s reproductive goals rather than their own [34]. Despite this, it can be argued that as long as the donor is aware of all of these risks and consents to the procedure knowing that the donated material may be used to conceive a child, then it is ethical. Additionally, as knowledge of the practice expands, siblings, relatives, or close friends may wish to donate oocytes specifically to a couple. These donors especially may derive more benefit from supporting the reproductive goals of their loved ones, further shifting the risk-benefit ratio. Donors have their own reasons for donating their oocytes to such causes and they are able to better understand their own risk-benefit ratios after having the MRT technique explained to them in full.
Impact on offspring and future generations
Another ethical obstacle for starting MRT trials is that there is very little long term research into how such a procedure will affect the offspring and subsequent generations. For example, MRT can “affect highly co-ordinated mitochondrial-nuclear allelic interactions that have become optimized over evolutionary time” [31]. This may require matching donor oocytes to compatible patients. Additionally, possible epigenetic changes are also a concern, as mtDNA replacement’s effects on epigenetic programming are still not known. Long term research may be the only way to understand downstream effects on subsequent generations; however, long term research will not be able to provide evidence-based guidance for best practices in a reasonable time frame. The concerns of epigenetic influence, allelic interactions, and health of subsequent generations should be investigated with further research using animal models to glean more information.
Moving this research from the bench to the clinic requires the determination of a favorable risk-benefit ratio. However, it is difficult to determine when a favorable ratio is reached due to several unknown factors that cannot be properly measured at the bench and could only be potentially elucidated when the research is moved to the clinic. Furthermore, unlike other potential studies, it is not the parent that faces the risk, but the unborn child [35]. The response to this may be to just start human trials, as the unknowns of risk-benefit are what first time trials are about. Nevertheless, the risk to children and future generations may be argued to be too great, especially when there are alternatives available, such as adoption, egg donation, and prenatal diagnosis paired with abortion [34]. However, offering alternatives such as adoption and egg donation provide little consolation to high risk parents wishing to conceive genetically related offspring, and also constitutes disregard for patient autonomy.
The challenge of determining risk-benefit ratio is best addressed by using more animal models and following at least two future generations of those animals. If the animal models show that the procedure is safe and without downstream effects, then human trials following the children throughout their lives should be the next step, with the consent of all parties involved.
Slippery slope and harms to society
By far, one of the biggest ethical concerns for MRT is the “slippery slope” argument and the fear of playing God by changing mtDNA; that it is only a matter of time before we start changing nuclear DNA and eventually start creating “designer” babies. By their very nature, MRT techniques change the heritable genetic information of an individual, and many critics believe that tampering with germline genetic information is unethical. Some may argue that this would deprive future generations of their right to receive an unmanipulated gene pool. The fear is that once society reaches the point where altering mtDNA is not an issue, it will not be long until we pursue other germline modifications to enhance humans and affect the gene pool; it will seem like a small step rather than a large one. This, however, is an example of an inherently weak “slippery slope” argument [36]. It is not reasonable to believe that preventing mitochondrial disease via MRT will inevitably lead to the pursuit of germline modifications for enhancement of healthy embryos. Like many arguments of this nature, it is speculative and can be staved off via internal and external monitoring systems so that labs keep within the proper bounds [31,34,37].
Benefitting the few
With all the controversies facing it, perhaps the procedure would get more support if it affected a larger number of lives. In the U.K. for example, the number of women with mitochondrial defects is 3,500. This number can be further divided as mitochondrial defects can be due just a mtDNA mutation or a combination of mtDNA and nuclear DNA mutations. There are disagreements within the scientific community about how many patients would benefit from the therapy. While Ishii estimates that mtDNA replacement IVF could save only about 10 children per year in the U.K., Gorman et al. put the estimate at 152 births per year in the U.K. When considering the bioethical tenet of beneficence, the procedure is worth pursuing if it can improve lives, even if only helping a small number of people [14,31,38,39].
The medical community has considered the ethical aspects of researching and treating rare disease, which highlights the conflict between beneficence and distributive justice. Based on utilitarian theories of justice, some argue that society should allocate resources in a way that maximizes benefit to the greatest number of people, and thus rare diseases are not worth the investment [38]. “On the other hand, many would uphold that society has a moral obligation not to abandon individuals who have had the bad luck to be affected by a serious but rare condition for which no treatment exists” [38]. Several professional bodies in the medical community, such as the Royal College of Physicians, have acknowledged a professional duty to promote the advancement of medical knowledge and push the envelope of current medical treatment. Ultimately, the medical community has a basic moral commitment to not abandon those in need of “highly specialized health care…even in resource constrained settings” [38]. It can be argued that the pursuit of MRT and related gene therapies is a responsibility that falls upon physicians and researchers as stewards of medicine [39].
CRISPR/Cas9 vs. MRT
CRISPR/Cas9 avoids the pitfalls and ethical concerns seen with MRT since it does not require a third person to donate any form of genetic material. This avoids the three-parent dilemma previously mentioned. Additionally, the CRISPR/Cas9 process edits the mtDNA at the blastocyst stage of the embryo, the same stage where preimplantation diagnosis is being performed.
Safety issues
As a new technology, CRISPR/Cas9 has many obstacles to overcome, especially before it can be used on human embryos. For this reason, voluntary moratoriums have been proposed on the use of CRISPR/Cas9 on human embryo until safety concerns can be assuaged through testing on animal embryos. However, these moratoriums are voluntary and thus there is no enforcement to follow it [40]. Many in the science community believe that the 2015 study by Liang et al. violated the spirit of this moratorium by using human 3PN zygotes [41].
The biggest safety concern is precision. As Lander points out, the Liang et al. study done on human tripronuclear zygotes resulted in numerous problem such as incomplete editing, inaccurate editing, and off-target mutations due to the inaccuracy of CRISPR/Cas9 [42]. This is due to the system potentially altering DNA locations other than the target, resulting in inactivation of essential genes, activation of pro-oncotic genes, or rearrangement of chromosomes. Additionally, CRISPR/Cas9 therapy may carry the risk of genetic mosaicism, if it is unable to affect all cells uniformly [43]. The solution to that may be to increase the amount of nuclease used, as it may increase how many mutated genes are corrected; however, the end result could instead increase alteration of the wrong gene sequences [44].
It is reasonable to posit that until the technology has become safer and more precise, human embryo studies should not be done. Like all new techniques, CRISPR/Cas9 will only grow more refined as research progresses. Once the precision of CRISPR/Cas9 comes close to that of other techniques approved for use on human embryos, it would become acceptable from a safety standpoint to move forward with human embryo studies.
Are we ready to change the germline?
As of now, CRISPR/Cas9 has only been used in humans on somatic cells. In addition to the aforementioned safety concerns about using CRISPR/Cas9 on germline cells, there are also ethical considerations to changing the germline. Some believe that without knowing the downstream effects for future generations, it is unethical and too risky to proceed. However, since mitochondria account for such a small amount of a person’s genetic material, the ramifications of changing the mtDNA would probably not be as significant as changing chromosomal DNA. Alternatively, because only females pass on their mitochondria to the next generation, a proposed solution to avoid future generations inheriting altered mtDNA is to only alter the mtDNA of male embryos. While this proposed solution offers a way to proceed without changing the germline, it denies the potentially curative technology to families and female embryos on the basis of sex. This violates the Code of Ethics promulgated by the American Medical Association, which states that physicians cannot refuse to care for potential patients based on gender or other criteria that constitute “invidious discrimination” [45].
As illustrated by Darnovsky and Lanphier et al., some believe that once the medical community starts using the CRISPR/Cas9 technology for therapeutic reasons, such as with mitochondrial disease, it would be logical to expand to other medical uses, like removing identified genes for fatal conditions [37,44]. In addition to those objectively beneficial uses of CRISPR/Cas9, it could be reasonably argued that removing risk genes for cancers and other conditions would be a benefit to society in the long run. However, once the scientific community begins to feel comfortable altering genes that do not have an immediate impact on the vitality of an embryo, progressing to non-therapeutic genetic enhancements may follow, leading to “designer” babies [44]. This is especially concerning since the effects of these changes are not know for future generations that inherit these genes.
The biggest worry is that it will lead to a second coming of the eugenics movement, which was defined by its false ideas that criminal activity and low intelligence were inherited genetically, an idea the Nazi movement perpetuated to the extreme. If CRISPR/Cas9 were to become common practice to edit genes for genetic enhancement, the fear is that eventually those that choose not to or cannot afford the technology would be stigmatized [43]. As with MRT, the best solution for this would be monitoring and laws against using CRISPR/Cas9 for enhancements.
Future of IV mitochondrial genetic therapy
In 2011, the U.K. government asked the Human Fertilization and Embryology Authority (HFEA) to determine the efficacy and safety of mitochondrial gene replacement therapy. The HFEA concluded that there is no evidence that supports either maternal spindle transfer or pro-nuclear transfer to be unsafe, and that this method is suitable for specific circumstances in which patients would like to produce genetically-related offspring without transfer of mitochondrial disease [46]. On February 3, 2015 the U.K. was the first country to approve MRT [47]. BBC news reported that by November 24, 2015 clinics would be able to apply for licenses to perform IV mitochondrial replacement and this technique could be performed before the new year [47]. This major change in legislature has been widely debated due to the aforementioned ethical concerns; 11 days after the House of Lords voted to approve three-parent IVF, a minority stronghold attempted to block the change in legislature [47]. Although the attempt failed, this quick response shows the magnitude of the controversy surrounding this technique. In the upcoming year, this change in legislature will not only cause a push for more research regarding the safety and efficacy of IV mitochondrial replacement before clinic licensing for the procedure takes place, but will also pave the way for additional research on applying this technique in the clinic. Because MRT is now legal, more donor oocytes and/or zygotes will be used for this research. The supply of donor eggs can be obtained through good will or perhaps an exchange program, in which those undergoing MRT will donate some of their eggs to MRT research, although donation raises ethical issues and requires more regulations to be set [46]. Following in the footsteps of the U.K., the US has demonstrated more willingness to consider the technique for use, as is evident by the US Food and Drug Administration (FDA) recently mentioning the use of enucleated donor embryos to solve problems of infertility among older women [48]. While it continues to stir up ethical concerns, change in U.K. legislature allowing MRT has the potential to revolutionize the way we treat both mitochondrial disease transmission and infertility.
Conclusion
While there is no current cure for mitochondrial disease, MRT shows great potential in prevention of transmission. Heteroplasmic carriers well below threshold will benefit the most from MRT; however, as future research progresses, this technique has the potential to be effective in those carrying high loads of mutant mtDNA. This is a ground-breaking technique because there is currently no effective method of prevention for those carrying loads close to or greater than threshold. MII-SCC and PN transfer are the primary methods of MRT, but additional studies need to be performed to highlight the differences between the two, and when is best to use each method. However, it is clear that PN transfer has potential to limit the amount of cytoplasm transferred between embryos, and is therefore a good approach to reduce risk among higher level mutant mtDNA carriers. There have been concerns that biopsied results from blastomeres in human embryos may not be representative of the entire fetus, especially as ethical concerns have prevented testing at later stages of embryonic development. Despite these concerns, safety and efficacy studies using mice, primates, and human oocytes have shown positive results, suggesting that MRT has not yet been shown to be unsafe.
While many ethical dilemmas still exist, a combination of strong research results, proper consenting methods, and strict guidelines can address those issues. Doing so will create strong oversight that could put these arguments to rest and lead to happier, healthier families.
References
- 1.Richardson J, Irving L, Hyslop LA, Choudhary M, Murdoch A, Turnbull DM, Herbert M. Concise reviews: Assisted reproductive technologies to prevent transmission of mitochondrial DNA disease. Stem Cells. 2015;33:639–645. doi: 10.1002/stem.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellebrekers DM, Wolfe R, Hendrickx AT, de Coo IF, de Die CE, Geraedts JP, Chinnery PF, Smeets HJ. PGD and heteroplasmic mitochondrial DNA point mutations: a systematic review estimating the chance of healthy offspring. Hum Reprod Update. 2012;18:341–349. doi: 10.1093/humupd/dms008. [DOI] [PubMed] [Google Scholar]
- 3.Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, Lightowlers RN, Herbert M, Turnbull DM. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieber DS, Calvo SE, Shanahan K, Slate NG, Liu S, Hershman SG, Gold NB, Chapman BA, Thorburn DR, Berry GT, Schmahmann JD, Borowsky ML, Mueller DM, Sims KB, Mootha VK. Targeted exome sequencing of suspected mitochondrial disorders. Neurology. 2013;80:1762–1770. doi: 10.1212/WNL.0b013e3182918c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, Chinnery PF, Turnbull DM. Prevalence of mitochondrial DNA disease in adults. Ann Neurol. 2008;63:35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- 7.Arpa J, Cruz-Martinez A, Campos Y, Gutierrez-Molina M, Garcia-Rio F, Perez-Conde C, Martin MA, Rubio JC, Del Hoyo P, Arpa-Fernandez A, Arenas J. Prevalence and progression of mitochondrial diseases: a study of 50 patients. Muscle Nerve. 2003;28:690–695. doi: 10.1002/mus.10507. [DOI] [PubMed] [Google Scholar]
- 8.Hammans SR, Sweeney MG, Brockington M, Lennox GG, Lawton NF, Kennedy CR, Morgan-Hughes JA, Harding AE. The mitochondrial DNA transfer RNA(Lys)A-->G(8344) mutation and the syndrome of myoclonic epilepsy with ragged red fibres (MERRF). Relationship of clinical phenotype to proportion of mutant mitochondrial DNA. Brain. 1993;116:617–632. doi: 10.1093/brain/116.3.617. [DOI] [PubMed] [Google Scholar]
- 9.Pitceathly RD, Taanman JW, Rahman S, Meunier B, Sadowski M, Cirak S, Hargreaves I, Land JM, Nanji T, Polke JM, Woodward CE, Sweeney MG, Solanki S, Foley AR, Hurles ME, Stalker J, Blake J, Holton JL, Phadke R, Muntoni F, Reilly MM, Hanna MG, Consortium UK. COX10 mutations resulting in complex multisystem mitochondrial disease that remains stable into adulthood. JAMA Neurol. 2013;70:1556–1561. doi: 10.1001/jamaneurol.2013.3242. [DOI] [PubMed] [Google Scholar]
- 10.Chinnery PF, Andrews RM, Turnbull DM, Howell NN. Leber hereditary optic neuropathy: Does heteroplasmy influence the inheritance and expression of the G11778A mitochondrial DNA mutation? Am J Med Genet. 2001;98:235–243. doi: 10.1002/1096-8628(20010122)98:3<235::aid-ajmg1086>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. MELAS and MERRF. The relationship between maternal mutation load and the frequency of clinically affected offspring. Brain. 1998;121:1889–1894. doi: 10.1093/brain/121.10.1889. [DOI] [PubMed] [Google Scholar]
- 12.Richardson J, Irving L, Hyslop LA, Choudhary M, Murdoch A, Turnbull DM, Herbert M. Concise reviews: assisted reproductive technologies to prevent transmission of mitochondrial DNA disease. Stem Cells. 2015;33:639–645. doi: 10.1002/stem.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doudna J, editor. Jennifer Doudna: We can now edit our DNA, but let’s do it wisely. Ted Talk 2015 [video file] Retrieved from <https://www.ted.com/talks/jennifer_doudna_we_can_now_edit_our_dna_but_let_s_do_it_wisely>.
- 14.Gorman GS, Grady JP, Ng Y, Schaefer AM, McNally RJ, Chinnery PF, Yu-Wai-Man P, Herbert M, Taylor RW, McFarland R, Turnbull DM. Mitochondrial donation--how many women could benefit? N Engl J Med. 2015;372:885–887. doi: 10.1056/NEJMc1500960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebolledo-Jaramillo B, Su MS, Stoler N, McElhoe JA, Dickins B, Blankenberg D, Korneliussen TS, Chiaromonte F, Nielsen R, Holland MM, Paul IM, Nekrutenko A, Makova KD. Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 2014;111:15474–79. doi: 10.1073/pnas.1409328111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, Gutierrez NM, Tippner-Hedges R, Kang E, Lee HS, Ramsey C, Masterson K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer R, Mitalipov S. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–631. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J. Revisiting germinal vesicle transfer as a treatment for aneuploidy in infertile women with diminished ovarian reserve. J Assist Reprod Genet. 2015;32:313–317. doi: 10.1007/s10815-014-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neupane J, Vandewoestyne M, Ghimire S, Lu Y, Qian C, Van Coster R, Gerris J, Deroo T, Deforce D, De Sutter P, Heindryckx B. Assessment of nuclear transfer techniques to prevent the transmission of heritable mitochondrial disorders without compromising embryonic development competence in mice. Mitochondrion. 2014;18:27–33. doi: 10.1016/j.mito.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Samuels DC, Wonnapinij P, Chinnery PF. Preventing the transmission of pathogenic mitochondrial DNA mutations: can we achieve long-term benefits from germ-line gene transfer? Hum Reprod. 2013;28:554–559. doi: 10.1093/humrep/des439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Sha H, Ji D, Zhang HL, Chen D, Cao Y, Zhu J. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell. 2014;157:1591–1604. doi: 10.1016/j.cell.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Amato P, Tachibana M, Sparman M, Mitalipov S. Three-parent in vitro fertilization: gene replacement for the prevention of inherited mitochondrial diseases. Fertil Steril. 2014;101:31–35. doi: 10.1016/j.fertnstert.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin H, Xue W, Chen S, Bogorad R, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T, Anderson DG. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regalado A. CRISPR gene editing to be tested on people by 2017, says Editas. MIT Technology Review. 2015 [Epub ahead of print] [Google Scholar]
- 26.Han A. Look for CRISPR/Cas9 to treat eye diseases first, scientists say. Genome Web. 2016 [Epub ahead of print] [Google Scholar]
- 27.Feenberg A. On being a human subject: interest and obligation in the experimental treatment of incurable disease. Philos Forum. 1992:23. [Google Scholar]
- 28.Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Songyang Z, Ma W, Zhou C, Huang J. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–372. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J, Alikani M. The biological basis for defining bi-parental or tri-parental origin of offspring from cytoplasmic and spindle transfer. Reprod Biomed Online. 2013;26:535–537. doi: 10.1016/j.rbmo.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Mitalipov S, Wolf DP. Clinical and ethical implications of mitochondrial gene transfer. Trends Endocrinol Metab. 2014;25:5–7. doi: 10.1016/j.tem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod Biomed Online. 2014;29:150–155. doi: 10.1016/j.rbmo.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Nuffield Council on Bioethics. Novel techniques for the prevention of mitochondrial DNA disorders: an ethical review. London: Nuffield Council on Bioethics; 2012. [Google Scholar]
- 33.Demma A, editor. Legal Information. Parents via egg donation (n.d.) Retrieved from <http://www.pved.org/delegalinfo.php>.
- 34.Baylis F. The ethics of creating children with three genetic parents. Reprod Biomed Online. 2013;26:531–534. doi: 10.1016/j.rbmo.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Bredenoord AL, Braude P. Ethics of mitochondrial gene replacement: from bench to bedside. BMJ. 2010;341:c6021. doi: 10.1136/bmj.c6021. [DOI] [PubMed] [Google Scholar]
- 36.Lode E. Slippery slope arguments and legal reasoning. Calif Law Rev. 1999:87. [Google Scholar]
- 37.Darnovsky M. A slippery slope to human germline modification. Nature. 2013;499:127. doi: 10.1038/499127a. [DOI] [PubMed] [Google Scholar]
- 38.Gericke CA, Riesberg A, Busse R. Ethical issues in funding orphan drug research and development. J Med Ethics. 2005;31:164–168. doi: 10.1136/jme.2003.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klitzman R, Toynbee M, Sauer MV. Controversies concerning mitochondrial replacement therapy. Fertil Steril. 2015;103:344–346. doi: 10.1016/j.fertnstert.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cyranoski D. Ethics of embryo editing divides scientists. Nature. 2015;519:272. doi: 10.1038/519272a. [DOI] [PubMed] [Google Scholar]
- 41.Vogel G. Embryo engineering alarm. Science. 2015;347:1301. doi: 10.1126/science.347.6228.1301. [DOI] [PubMed] [Google Scholar]
- 42.Lander ES. Brave new genome. N Engl J Med. 2015;373:5–8. doi: 10.1056/NEJMp1506446. [DOI] [PubMed] [Google Scholar]
- 43.International summit on human gene editing: a global discussion. Washington, D.C.: The National Academies Press; 2016. National Academies of Sciences, Engineering, and Medicine. [PubMed] [Google Scholar]
- 44.Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don’t edit the human germ line. Nature. 2015;519:410–411. doi: 10.1038/519410a. [DOI] [PubMed] [Google Scholar]
- 45.American Medical Association. Code of medical ethics: opinions on patient-physician relationships. Chicago: American Medical Association; 2016. [Google Scholar]
- 46.Public Health Directorate/Health Science and Bioethics Division. Draft regulations to permit the use of new treatment techniques to prevent the transmission of a serious mitochondrial disease from mother to child. Department of Health, U.K. 2014 [Google Scholar]
- 47.Gallagher J. UK approves three-person babies. BBC. 2015 [Epub ahead of print] [Google Scholar]
- 48.Cellular, tissue, and gene therapies advisory committee. Oocyte modification in assisted reproduction for the prevention of transmission of mitochondrial disease or treatment of infertility. FDA. 2014 [Google Scholar]