Abstract
Interleukin-6 (IL-6), one of the most important inflammatory cytokines, plays a pivotal role in metastasis and stemness of solid tumors. However, the underlying mechanisms of IL-6 in HCC metastasis remain unclear. In the present study, we demonstrated that stemness and metastatic potential of HCC cells were significantly enhanced after IL-6 stimulation. IL-6 could induce expression of osteopontin (OPN), along with other stemness-related genes, including HIF1α, BMI1, and HEY1. Block of OPN induction could significantly abrogate the effect of IL-6 on stemness and metastasis of HCC cells. Furthermore, IL-6 level was positively correlated with OPN in HCC. Patients with high plasma IL-6 or OPN level had poorer prognosis. In multivariate analysis, IL-6 and OPN were demonstrated to be independent prognostic indicators for HCC patients, and their combination had a better prognostic performance than IL-6 or OPN alone. Collectively, our findings indicate that IL-6 could enhance stemness and promote metastasis of HCC via up-regulating OPN expression, which can be a potential therapeutic target for combating HCC metastasis, and the combination of IL-6 and OPN serves as a promising prognostic predictor for HCC.
Keywords: Liver cancer, cancer stem cell, inflammatory factor, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancies and the second leading cause of death from cancer worldwide, about 50% of newly diagnosed cases occur in China [1]. During the past decades, although many progresses have been achieved in the clinical managements of HCC, its prognosis remains dismal, which is mainly due to the high probabilities of metastasis and recurrence [2]. Therefore, elucidating the metastatic mechanisms and exploring new strategies to block the metastatic cascade are critical to improve HCC prognosis.
Our previous work has demonstrated that IL-6 is a direct target of miRNA-26a, and plays an important role in HCC metastasis [3]. As one powerful functional inflammatory cytokine in tumor biology, IL-6 is also able to modulate cancer stem cell (CSC) properties of HCC and other types of tumor [4-6]. And, IL-6 is found to contribute at least in part to the mystery for gender disparity of HCC incidence [7,8]. In addition, several studies have illustrated that IL-6 can activate the signal transducers and activators of transcription 3 (stat3) signaling pathway, which drives cancer progression and metastasis, as well as tumorigenesis and CSC expansion [9-11]. Reports have summarized that cancer stem cells might be the core players in cancer metastasis [12,13].
Osteopontin (OPN) is a secretary extracellular matrix protein that is high expressed in numerous human cancers [14]. Our previous works have demonstrated that OPN is a significant negative prognostic indicator for patients with HCC [15,16], and plays pivotal roles in HCC metastasis [17-20]. OPN derived from tumor microenvironment also could promote HCC metastasis [21]. Recent studies reported that OPN functioned as a key player in stemness maintenance via interacting with CD44, one of its receptors [22,23].
In the present study, we found that plasma IL-6 level was higher in patients with advanced stage of HCC, and was associated with recurrence and metastasis. IL-6 could promote cancer stem cell traits and metastasis and up-regulate the stemness related genes, including OPN, in HCC. Furthermore, through both in vitro and in vivo functional studies, as well as validation in a large cohort of clinical specimens, we confirmed that IL-6 is an upstream regulator of OPN and promotes HCC metastasis and stemness via up-regulating OPN expression, and IL-6 in combination with OPN might serve as a promising prognostic indicator and potential therapeutic target for HCC.
Materials and methods
Patients, follow-up, and clinical specimens
A total of 353 patients who received curative resection for HCC at authors’ institutes from January 2006 to December 2008 were enrolled in this study. None of them received any preoperative cancer treatment. The clinical samples were collected from patients after obtaining informed consent in accordance with a protocol approved by the Ethics Committee of Fudan University (Shanghai, China). Plasma samples were collected before surgery and stored at -80°C until further processing.
The patients were followed-up after surgical treatment until April 2013, with a median follow-up time of 40.7 months (range, 2-85 months). Serum alpha-feta protein (AFP) level and liver ultrasonography were monitored every 2 months during the first year postoperatively and at least every 4 months thereafter. Computed tomography (CT) or magnetic resonance imaging (MRI) scan was performed every 6 months or when recurrence was suspected. All these examinations were performed by independent doctors without knowing this study.
The overall survival (OS) was calculated from the date of operation to the date of death or to the date of last follow-up. The disease-free survival (DFS) was calculated from the date of resection to the date when tumor recurrence was diagnosed, if recurrence was not diagnosed during the period of study, the cases were censored on the date of death or the last date of follow-up.
Cell lines
HCC cell lines HCC-LM3, MHCC97-H and MHCC97-L were established at the Liver Cancer Institute, Fudan University. They have genetically identical backgrounds and stepwise increasing metastatic potentials [24]. The Huh7, Hep3B and HepG2 cell lines were purchased from the Shanghai cell bank, Chinese Academy of Sciences. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone) supplemented with 10% fetal bovine serum (FBS) (Gibco) and maintained in a humidified incubator with 5% CO2 at 37°C.
Concentration/time dependent stimulation
To assess the effects of IL-6 on OPN expression, recombinant IL-6 (Peprotech) at different concentrations of 1, 5, 10, 50, 100 ng/ml was added in the medium of MHCC97-L and HepG2 cells, and processed cells were incubated for 24 hours before protein and RNA extraction, and supernatant collection. Phosphate buffered solution (PBS) was used as control.
50 ng/ml recombinant IL-6 was added in the medium of MHCC97-L and HepG2 cells, and the cells were incubated for 1, 12, 24, 36 and 48 hours before protein and RNA extraction, and supernatant collection. PBS was used as control.
Sphere formation and in vitro migration and invasion assays
Sphere formation was performed by plating 1,000 cells per well into 6-well ultra-low attachment plate (Corning Incorporated Life Sciences) in serum-free DMEM/F12 medium (Gibco), supplemented with B27 (1:50; Invitrogen), N2 (1:100; Invitrogen), 20 ng/ml bFGF and 10 ng/ml EGF (Peprotech). Cells were incubated in a 5% CO2 incubator at 37°C for 1 week. For passaging of primary spheres to secondary spheres, 0.25% trypsin (Gibco) was used, and 1,000 cells were re-seeded into 6-well ultra-low attachment plate for another week. The number of tumor spheres per-well was counted under an inverted microscope (×100 or ×40, Olympus).
The migratory and invasive ability of HCC cells were determined by using 24-well transwell chambers, with upper and lower culture compartments separated by polycarbonate membranes with 8 µm pores (BD Pharmingen). The bottom chamber was filled with DMEM containing 10% FBS as a chemoattractant. Cells, 5×104 cells for migration (without prepared matrigel) and 10×104 cells for invasion (with prepared matrigel), in serum-free medium were seeded into the upper chamber and maintained at 37°C in a humidified incubator containing 5% CO2. Cells that migrated to the underside of the membrane were stained with crystal violet, imaged, and counted with light microscope (×100, Leica).
Establishment of in vivo tumor models
For the assessment of tumor initiation abilities, 1,000 cells were suspended in 100 μl of PBS (Hyclone) and Matrigel (BD Pharmingen) mix (1:1) and implanted subcutaneously into the armpit of 4- to 6-week-old NOD/SCID female mice. Tumor formation was monitored weekly.
For tumor metastasis assay, xenografts were established by subcutaneously implanting 5×106 cells into male nude mice (BALB/c nu/nu) that were 4-6 weeks old. Then subcutaneous tumors were removed and dissected into 1 mm3 sections, which were planted into the liver of nude mice to establish orthotopic implantation tumor models. Mice were sacrificed after 6 weeks. Tumors, livers, and lungs were removed, fixed in formalin, and embedded in paraffin. Consecutive sections were made for each lung tissue block and stained with hematoxylin and eosin. The number of lung metastasis was calculated and evaluated independently by two pathologists. All experimental procedures involving animals were approved by The Animal Care and Use Committee of Fudan University, China.
Western blotting
Total protein was extracted by lysing cells in RIPA buffer containing protease inhibitor. Protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking with 5% non-fat milk in TBS-T, membranes were incubated with the primary antibody. The following antibodies were used: anti-IL-6 (1:1000, proteintech), anti-OPN (1:1000, proteintech), anti-E-cadherin (1:1000, CST), anti-N-cadherin (1:1000, CST), anti-β-actin (1:2000, Epitomics), anti-GAPDH (1:2000, CST). Protein bands were detected by using Image Acquisition using ImageQuant™ LAS 4000 (GE Healthcare Life Sciences).
RNA isolation, reverse-transcription, and quantitative real-time polymerase chain reaction (qPCR)
RNA of cell lines was isolated using Trizol reagent (Invitrogen). RNA was quantified using a Nanodrop ND-1000 (Thermo Fischer Scientific). Complementary DNA synthesis was performed using PrimeScript reverse transcriptase reagent kit (Takara) according to the manufacturer’s directions.
Real-time PCR was performed using SYBR Green PCR Master Mix (DBI Bioscience) and ABI PRISM 7900 Sequence Detection System (Applied Biosystems). Results were normalized to β-actin for mRNA measurement. Fold change was calculated by the 2-ΔΔCt method where ΔΔCt=ΔCt (Target)-ΔCt (Reference). All the primers were listed in Table 1.
Table 1.
Primer sequences of HCC stemness-related genes
| Primer name | Sense | Anti-sense |
|---|---|---|
| IL-6 | CAATCTGGATTCAATGAGGAGAC | CTCTGGCTTGTTCCTCACTACTC |
| OPN | CTCCATTGACTCGAACGACTC | CAGGTCTGCGAAACTTCTTAGAT |
| OCT4 | GGTTCTATTTGGGAAGGTATTCAG | TGGTTCGCTTTCTCTTTCGG |
| Nanog | CTCTCCTCTTCCTTCCTCCA | GGTCTTCACCTGTTTGTAGCTG |
| SOX2 | TGGACAGTTACGCGCACAT | CGAGTAGGACATGCTGTAGGT |
| HIF1α | ATCCATGTGACCATGAGGAAATG | TCGGCTAGTTAGGGTACACTTC |
| BMI1 | CCACCTGATGTGTGTGCTTTG | TTCAGTAGTGGTCTGGTCTTGT |
| ABCG2 | CAGGTGGAGGCAAATCTTCGT | ACCCTGTTAATCCGTTCGTTTT |
| CK19 | ACCAAGTTTGAGACGGAACAG | CCCTCAGCGTACTGATTTCCT |
| NOTCH1 | GAGGCGTGGCAGACTATGC | CTTGTACTCCGTCAGCGTGA |
| KLF4 | CAGCTTCACCTATCCGATCCG | GACTCCCTGCCATAGAGGAGG |
| CD44 | CTGCCGCTTTGCAGGTGTA | CATTGTGGGCAAGGTGCTATT |
| CD90 | ATCGCTCTCCTGCTAACAGTC | CTCGTACTGGATGGGTGAACT |
| CD133 | AGTCGGAAACTGGCAGATAGC | GGTAGTGTTGTACTGGGCCAAT |
| CD117 | CGTGGGCGACGAGATTAGG | CTTCTTTCCCATACAAGGAGCG |
| CD24 | CTCCTACCCACGCAGATTTATTC | AGAGTGAGACCACGAAGAGAC |
| EPCAM | AATCGTCAATGCCAGTGTACTT | TCTCATCGCAGTCAGGATCATAA |
| TCF3 | ACGAGCGTATGGGCTACCA | GTTATTGCTTGAGTGATCCGGG |
| TCL | ACTTGCTCGGACTGTATGACA | CCGTGTTGGGGTAGGAGAGT |
| β-catenin | AGCGCCGTACGTCCATGGGTG | GTTCACAGAGGACCCCTGCAGC |
| HEY1 | GTTCGGCTCTAGGTTCCATGT | CGTCGGCGCTTCTCAATTATTC |
| C-MYC | GTCAAGAGGCGAACACACAAC | TTGGACGGACAGGATGTATGC |
Enzyme linked immunosorbent assay (ELISA)
Plasma IL-6 and OPN levels in patients with HCC were assessed using the Human IL-6 ELISA Ready-SET-Go! ®kit (eBioscience) and Human Osteopontin Platinum ELISA kit (eBioscience), according to the manufacturer’s instructions.
Vectors and cell transfections
Expression vector mediated by lentivirus for human IL-6 was constructed. The sequence of IL-6 was amplified from cDNA library via specific primers: forward primer-5’-ATGAACTCCTTCTCCACAAGCGCC-3’, reward primer-5’-TTATGCCGAAGAGCCCTCA-3’. Then harvested DNA was inserted into pCDH-GFP expression vector (System Biosciences).
Expression vector for OPN and shRNA for IL-6 and OPN were constructed as previously described, as well as methods of cell transfections [3,25].
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences Version 16.0 (SPSS16.0) and Graphpad Prism® 5.0 software. The χ 2 test, Student’s t test and One Way ANOVA were used for comparison between groups. The correlation was determined by Pearson analysis. Kaplan-Meier survival analyses were used to estimate the prognostic value, and the log-rank test was used to assess the survival differences. Univariate and multivariate Cox regression analysis were performed to evaluate differences of all possible factors in the risk of death and recurrence. P<0.05 were considered statistically significant.
Results
Plasma IL-6 level is associated with tumor progression and post-operative tumor relapse of HCC
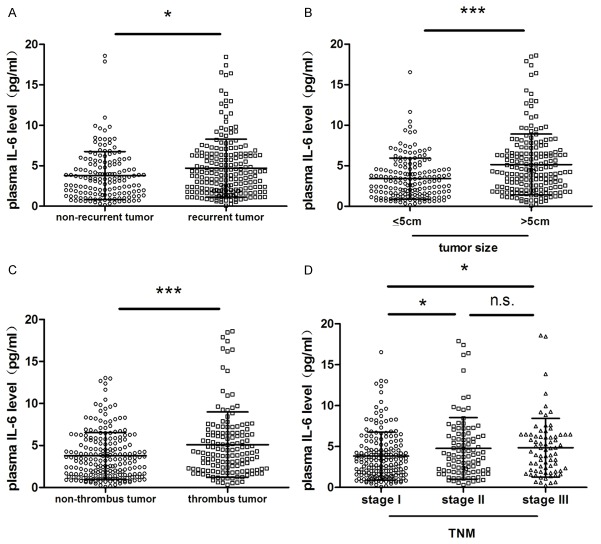
The preoperative plasma IL-6 levels and their association with clinicopathological features were assessed in 353 HCC patients. The IL-6 levels in patients who were found to have postoperative tumor recurrence during follow-up after HCC resection were significantly higher compared to those without tumor recurrence (P<0.05, Figure 1A). The patients with large HCC (larger than 5 cm in diameter) had much higher IL-6 levels than that in those patients with small HCC (less than 5 cm in diameter) (P<0.001, Figure 1B). Moreover, plasma IL-6 level was obviously increased in patients with tumor thrombus in comparison to those without tumor thrombus (P<0.001, Figure 1C). Similarly, the patients with middle and advanced stages (TNM stage II/III) of HCC had much higher plasma IL-6 levels than those patients with early stage (TNM stage I) HCC (P<0.05) (Figure 1D). Furthermore, according to the cut-off value of plasma IL-6 level (4.16 pg/ml), we divided these patients into two groups, high IL-6 group (n=147) and low IL-6 group (n=206), and found significant differences between these two groups in tumor capsule (P<0.05), tumor size (P<0.001), tumor thrombus (P<0.05) and TNM stage (P<0.05) (Table 2).
Figure 1.
Association of plasma IL-6 level with clinical characteristics of HCC. Plasma IL-6 level was significantly higher in patients with (A) recurrent HCC, (B) larger tumor size (diameter over 5 centimeter), (C) and tumor thrombus. (D) Plasma IL-6 level was significantly higher in patients with TNM stage II or III HCC than in those with stage I HCC, and no significant difference was observed between stage II and stage III patients. The expression of IL-6 level in plasma was determined by ELISA. *P<0.05; ***P<0.001; n.s. no significance.
Table 2.
Relationship between plasma IL-6 level and clinicopathologic features
| Variable | Plasma IL-6 level (pg/ml) | ||||
|---|---|---|---|---|---|
|
| |||||
| High (n=147) | Low (n=206) | ||||
|
|
|||||
| No. of patients | % | No. of patients | % | P | |
| Gender | 0.064 | ||||
| Female | 24 | 16 | 20 | 10 | |
| Male | 123 | 84 | 186 | 90 | |
| Age (years) | 0.276 | ||||
| ≤50 | 59 | 40 | 71 | 34 | |
| >50 | 88 | 60 | 135 | 66 | |
| HBsAg | 0.947 | ||||
| Negative | 26 | 18 | 37 | 18 | |
| Positive | 121 | 82 | 169 | 82 | |
| HBcAb | 0.500 | ||||
| Negative | 16 | 11 | 18 | 9 | |
| Positive | 131 | 89 | 188 | 91 | |
| Cirrhosis | 0.951 | ||||
| No | 16 | 11 | 22 | 11 | |
| Yes | 131 | 89 | 184 | 89 | |
| ALT (U/L) | 0.750 | ||||
| ≤75 | 126 | 86 | 179 | 87 | |
| >75 AFP (ng/mL) | 21 | 14 | 27 | 13 | 0.704 |
| ≤20 | 45 | 31 | 67 | 33 | |
| >20 | 102 | 69 | 139 | 67 | |
| Tumor size (cm) | <0.001 | ||||
| ≤5 | 51 | 35 | 117 | 57 | |
| >5 | 96 | 65 | 89 | 43 | |
| Tumor number | 0.977 | ||||
| Single | 124 | 84 | 174 | 84 | |
| Multiple | 23 | 16 | 32 | 26 | |
| Tumor capsule | 0.032 | ||||
| None | 98 | 67 | 114 | 55 | |
| Complete | 49 | 33 | 92 | 45 | |
| Tumor thrombus | 0.018 | ||||
| No | 78 | 53 | 135 | 66 | |
| Yes | 69 | 47 | 71 | 34 | |
| Tumor differentiation | 0.781 | ||||
| I+II | 100 | 68 | 143 | 69 | |
| III+IV | 47 | 32 | 63 | 31 | |
| TNM stage | 0.018 | ||||
| I | 64 | 44 | 116 | 56 | |
| II+III | 83 | 56 | 90 | 44 | |
| BCLC stage | 0.762 | ||||
| 0+A | 118 | 80 | 168 | 82 | |
| B+C | 29 | 20 | 38 | 18 | |
Abbreviations: HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B core antibody; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; TNM, tumor-node-metastasis; BCLC, Barcelona Clinic Liver Cancer. Statistical analysis: Chi-Square.
IL-6 promotes cancer stem cell traits and epithelial-mesenchymal transition of HCC cells
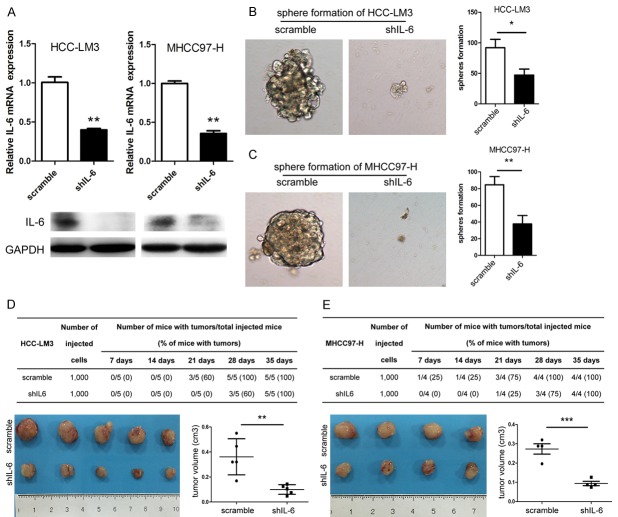
To investigate the effects of IL-6 on the stem cell properties in HCC, we constructed two stably IL-6 knockdown (KD) cell lines with HCC-LM3 and MHCC97-H (Figure 2A). After IL-6 KD, the capabilities of sphere formation of HCC-LM3 (P<0.05) and MHCC97-H (P<0.01) cells were significantly decreased (Figure 2B, 2C). After subcutaneous implantation into NOD-SCID mice, tumor initiation was significantly delayed for about one week in both HCC-LM3 and MHCC97-H cells with IL-6 KD (approximately 4 weeks for IL-6 KD vs. 3 weeks in controls) (Figure 2D, 2E). At the fifth week after tumor cell implantation, the average tumor volumes of IL-6 KD groups were significantly smaller than that of the control groups (P<0.001 in HCC-LM3 and P<0.01 in MHCC97-H, respectively) (Figure 2D, 2E).
Figure 2.
IL-6 knockdown (KD) impaired tumor-initiating properties of HCC cells. (A) IL-6 expression in HCC-LM3 (left) and MHCC97-H (right) with IL6 KD was significantly decreased both at mRNA and protein levels compared with control. The number of 2° tumor spheres of (B) HCC-LM3 and (C) MHCC97-H cell lines with IL-6 KD was decreased as compared with the control (magnification ×100). 1,000 cells of (D) HCC-LM3 and (E) MHCC97-H transfected with shIL-6 or scrambled shRNA were subcutaneously implanted into NOD/SCID mice per site to test their tumorigenicity. Tumorigenic ability of HCC cells was compromised with a delayed tumor initiation and reduced tumor volume after IL-6 was down-regulated. *P<0.05; **P<0.01; ***P<0.001.
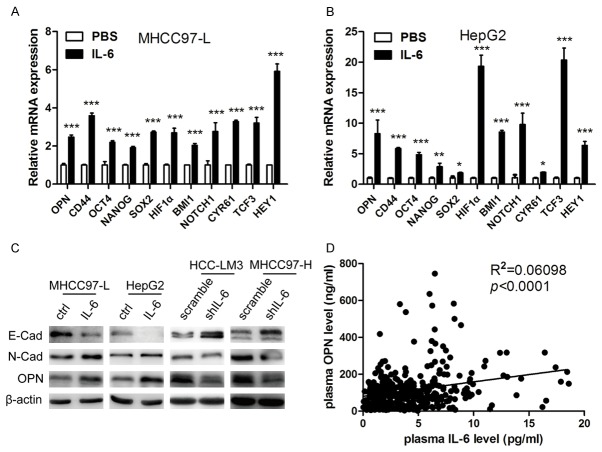
In order to further evaluate the effects of IL-6 on the stemness of HCC cells, we detected the alterations in the expression levels of 21 genes that are reported to be related to HCC stemness [4,26,27] in HCC cells treated with recombinant human IL-6 (Table 1). After IL-6 stimulation, 11 genes including OPN, CD44, OCT4, NANOG, SOX2, HIF1α, BMI1, NOTCH1, CYR61, TCF3 and HEY1 were up-regulated in both MHCC97-L and HepG2 cell lines (Figure 3A, 3B). In addition, decreased expression of epithelial marker E-cadherin concomitant with significantly increased mesenchymal marker N-cadherin were found in MHCC97-L and HepG2 after IL-6 treatment (Figure 3C, left panel). On the other hand, IL-6 KD resulted in increased E-cadherin level and significantly decreased levels of N-cadherin in HCC-LM3 and MHCC97-H cell lines (Figure 3C, right panel).
Figure 3.
Effects of IL-6 on stemness-related genes expression in HCC cells and relationship of IL-6 and OPN levels in clinical samples. The expression of stem cell-related genes (OPN, CD44, OCT4, NANOG, SOX2, HIF1α, BMI1, NOTCH1, CYR61, TCF3 and HEY1) were significantly up-regulated in (A) MHCC97-L and (B) HepG2 cells stimulated by IL-6. (C) Significant changes of typical EMT markers expression, E-cadherin and N-cadherin, were observed both in MHCC97-L and HepG2 after IL-6 treatment and HCC-LM3 and MHCC97-H with IL-6 KD. (D) Plasma OPN levels were positively correlated with plasma IL-6 levels in 353 HCC patients. *P<0.05; **P<0.01; ***P<0.001.
IL-6 positively correlated with OPN in plasma of HCC patients
Since above findings indicate that IL-6 treatment increases the expression levels of 11 stemness-related genes, including OPN, and accumulating studies have demonstrated that OPN plays a pivotal role in HCC metastasis [18,20,26], we further investigated the correlation between IL-6 and OPN in clinical samples. Plasma IL-6 and OPN concentration of 353 HCC patients was assessed by ELISA, and plasma IL-6 levels were positively correlated with plasma OPN levels (P<0.001) (Figure 3D).
IL-6 is an upstream regulator of OPN
Given that a significant positive correlation between IL-6 and OPN in HCC patients, as well as the increased expression of OPN after IL-6 treatment, was observed, we further validated whether IL-6 was an upstream regulator of OPN. Using different concentration gradient of IL-6 to stimulate MHCC97-L and HepG2 cells, we observed a dose-dependent increase of cellular OPN both at mRNA and protein levels, as well as the secreted OPN level in cell culture supernatant (Figure 4A-F). Furthermore, these effects could be detected at each time-point (Figure 4G-L). However, up- or down-regulation of OPN in HCC cell lines had no significant effect on IL-6 expression (Figure 5A, 5B). Moreover, the up-regulation of OPN stimulated by IL-6 was abrogated when IL-6 was withdrawn (Figure 5C, 5D).
Figure 4.
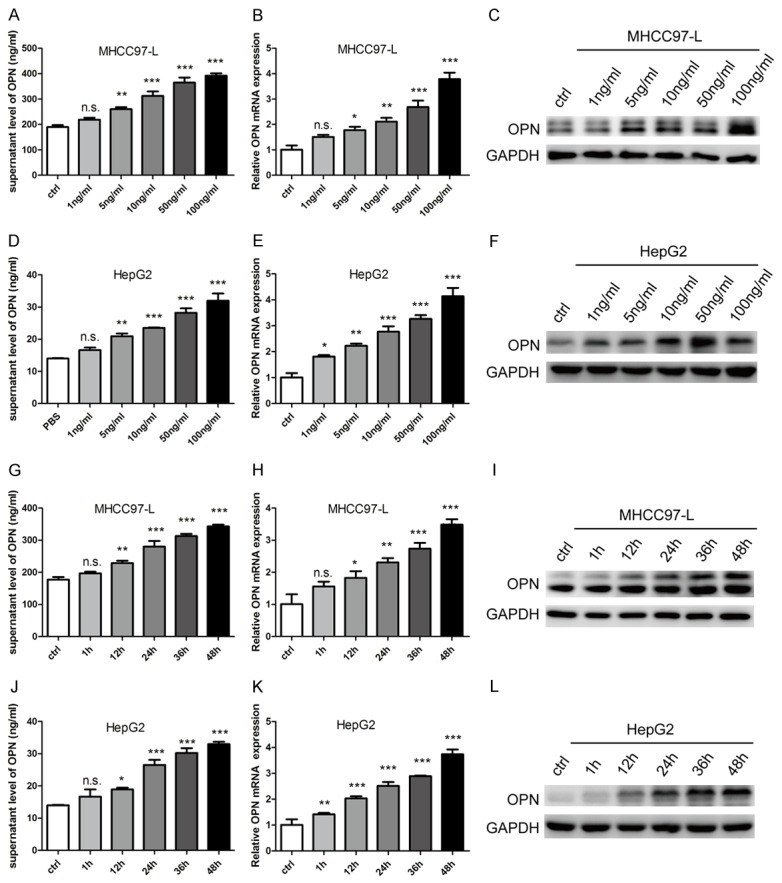
IL-6 up-regulates OPN in HCC cells in dose- and time-dependent manner. Dose-dependent increasing of OPN in cell supernatant (A and D), mRNA level (B and E) and protein level (C and F) was detected after stimulating by different concentration of IL-6 in (A-C) MHCC97-L cells and (D-F) HepG2 cells. Time-dependent increasing of OPN in cell supernatant (G and J), mRNA level (H and K) and protein level (I and L) was detected after stimulating by different concentration of IL-6 (G-I) in MHCC97-L cells and (J-L) HepG2 cells. *P<0.05; **P<0.01; ***P<0.001; n.s. no significance.
Figure 5.
IL-6 is an upstream regulator of OPN. (A) OPN KD in MHCC97-H cells showed little influence on IL-6 expression both at mRNA and protein level. (B) Overexpression of OPN in HepG2 cells showed little influence on IL-6 expression both at mRNA level and protein level. The up-regulation of OPN stimulated by IL-6 was abolished when IL-6 was withdrawn both at mRNA level and protein level in (C) MHCC97-L and (D) HepG2 cells. *P<0.05; **P<0.01; ***P<0.001; n.s. no significance.
IL-6 enhances HCC stemness and promotes metastasis through OPN induction
To further determine whether IL-6 promoted HCC stemness by inducing OPN expression, we first adopted tumor sphere formation assay, in OPN-KD MHCC97-L or HepG2 cells stimulated by IL-6. OPN KD significantly antagonized the enhanced ability of tumor sphere formation (Figure 6A and 6B) and the up-regulation of three stemness-related genes including HIF1α, BMI1 and HEY1 (Figure 6C and 6D), induced by IL-6 stimulation in MHCC97-L and HepG2 cells. Next, we established MHCC97-L cell lines stably overexpressing IL-6 (OE), and MHCC97-L-IL-6 cells stably transfected with shOPN or scrambled shRNA (Figure 6E). Then 1,000 cells of each group were injected subcutaneously into NOD-SCID mice. The tumor of IL-6 OE group initiated about two weeks ahead compared with control group (approximately 2 weeks for IL-6 OE vs. 4 weeks for controls). However, the stimulatory effects of IL-6 on tumor initiation were abrogated after OPN KD (Figure 6F). At six weeks after tumor cell implantation, the average tumor volumes of IL-6 OE group were significantly larger than that of the control group and OPN KD group (Figure 6F).
Figure 6.
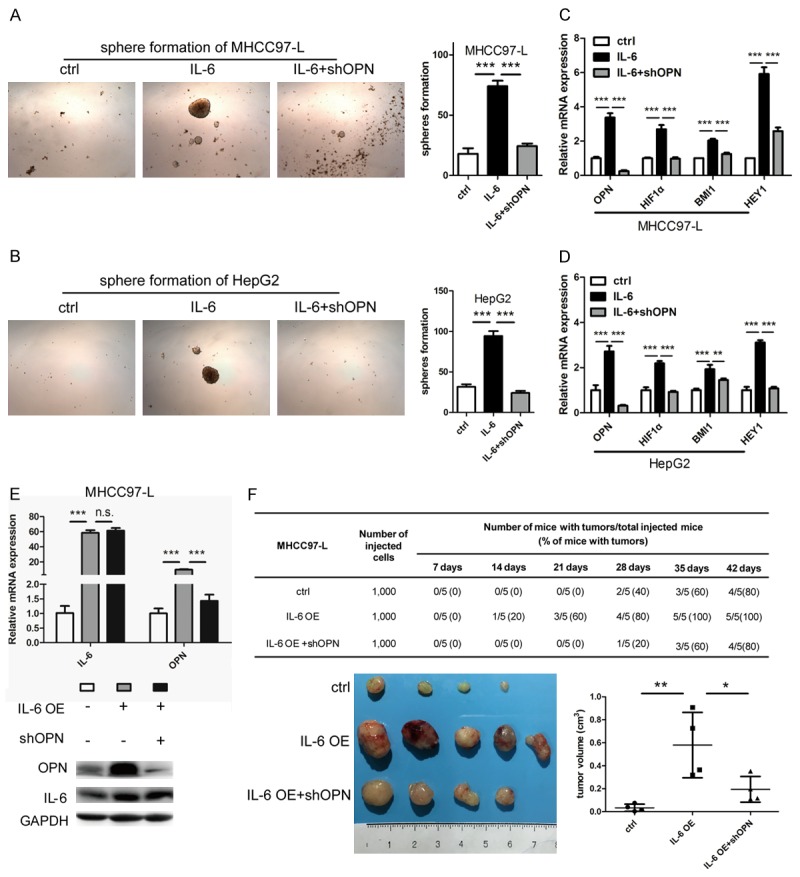
The roles of OPN in the HCC stemness mediated by IL-6. Increasing numbers of sphere formation could be observed after (A) MHCC97-L and (B) HepG2 treated by IL-6, which were abrogated by OPN KD (magnification ×40). Relative expression of stem cell-related genes (OPN, HIF1α, BMI1, and HEY1) were up-regulated in IL-6 treated (C) MHCC97-L and (D) HepG2 cells compared with control and OPN KD counterparts. (E) Expression of IL-6 was significantly increased in MHCC97-L cells by IL-6 overexpression (OE), followed with up-regulation of OPN which was abrogated by OPN knockdown both at mRNA and protein levels. (F) 1,000 MHCC97-L-IL-6 cells transfected with shOPN and their controlled counterparts were subcutaneously implanted into NOD-SCID mice to test their tumorigenicity. Tumorigenic ability of HCC cells was significantly enhanced when IL-6 was overexpressed. However, the stimulatory effects of IL-6 on tumor initiation were inhibited by OPN KD. *P<0.05; **P<0.01; ***P<0.001; n.s. no significance.
As we have previously confirmed that OPN play important roles in promoting HCC metastasis [18,20], we further explored that whether IL-6 promotes HCC metastasis via up-regulating OPN. We treated MHCC97-L and HepG2 cells with OPN neutralizing antibody and IL-6. Treatment with OPN neutralizing antibody was able to significantly abrogate the promoting effects on the migratory and invasive abilities of HCC cells induced by IL-6 (Figure 7A-D), and to block the stimulatory effects of IL-6 on in vivo tumor growth (Figure 7E) and lung metastasis (Figure 7F) in orthotopic nude mice models. In addition, after IL-6 stimulation, E-cadherin expression was down-regulated and N-cadherin up-regulated in MHCC97-L and HepG2 cells, and these effects were eliminated in OPN KD cells (Figure 7G).
Figure 7.
Effects of OPN on IL-6 promoting metastasis in vitro and in vivo. Increasing numbers of migrated (A, B) and invaded (C, D) cells could be observed after MHCC97-L and HepG2 cells treated by IL-6, which were abrogated by OPN neutralized antibody (magnification ×100). Orthotopic tumor xenografts of MHCC97-L were treated with PBS, IL-6, and IL-6 plus OPN neutralized antibody. (E) The tumor volumes and (F) number of lung metastasis were increased after IL-6 treatment and antagonized by OPN neutralized antibody. (G) After IL-6 treatment, E-cadherin expression was down-regulated and N-cadherin up-regulated in MHCC97-L and HepG2 cells, and these effects were eliminated in OPN KD cells. *P<0.05; **P<0.01; ***P<0.001.
Combination of plasma IL-6 and OPN level serves as a powerful prognostic factor for HCC patients
The clinical significances of plasma IL-6 and OPN level in HCC were further investigated in 353 patients. As mentioned above, based on the cut-off value of plasma IL-6 level (4.16 pg/ml), and OPN level (82.80 ng/ml), we divided these patients into high IL-6 group (n=147) and low IL-6 group (n=206); high OPN group (n=177) and low OPN group (n=176). The 1-, 3-, and 5-year OS rates of HCC patients in low IL-6 group were 83.1%, 63.9%, and 54.3%, respectively, which were much higher than those of patients in high IL-6 group (72.9%, 47.5%, and 33.7%, respectively; P<0.001) (Figure 8A). The 1-, 3-, and 5-year DFS rates in the low IL-6 group (69.3%,57.0%, and 47.2%, respectively) were obviously higher than that in high IL-6 group (56.7%, 38.0%, and 25.6%, respectively; P<0.001) (Figure 8B). Similarly, the 1-, 3-, and 5-year OS rates of patients in the low OPN group (91.4%, 74.3%, and 61.5%, respectively) were significantly higher than those with high plasma OPN level (67.9%, 43.6%, and 34.2%, respectively; P<0.001) (Figure 8C). The 1-, 3-, and 5-year DFS rates of the low OPN group (73.9%, 59.6%, and 49.3%, respectively) were much higher than those of the high OPN group (56.2%, 40.6%, and 29.5%, respectively; P<0.001) (Figure 8D). Since hepatitis B activity could possibly affect both prognosis and inflammatory status of HCC patients, it might become a confounding bias within our study. Through Chi-Square analysis, IL-6 was not correlated with HBcAb, an indicator of active viral replication, or alanine transferase (ALT), an enzyme released when hepatocytes destruction (Table 2). Univariate analysis showed that HBcAb and ALT had no relationship with prognosis of HCC patients (Table 3).
Figure 8.
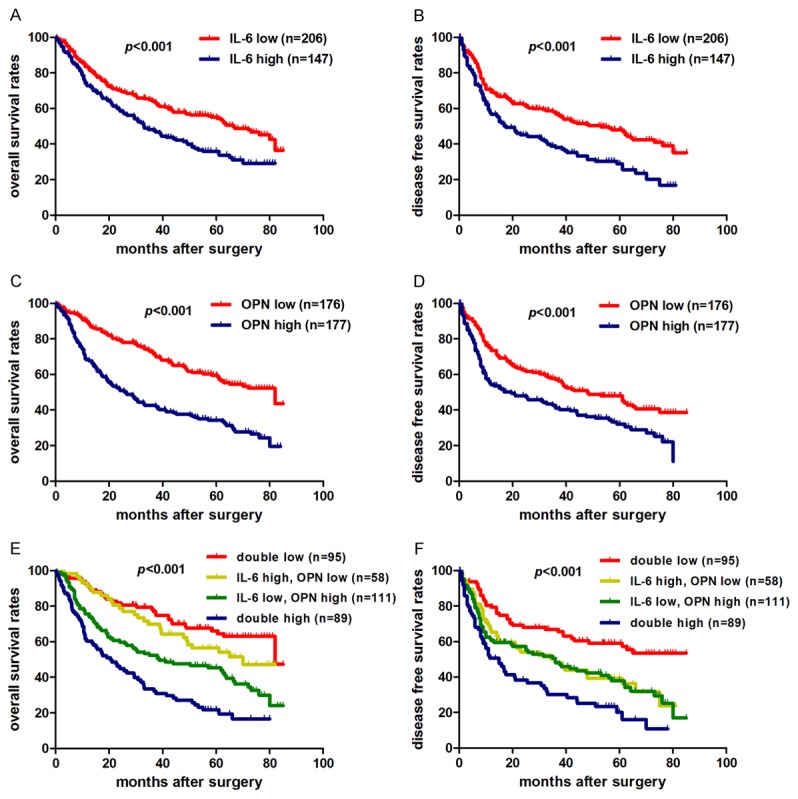
Prognostic significance of IL-6 and OPN for HCC patients. A, B. Patients with low plasma IL-6 level had significantly better overall survival (OS) and disease free survival (DFS) compared with HCC patients with high plasma IL-6 level. C, D. HCC patients with higher plasma OPN level had poorer OS and DFS. E, F. Patients with both low plasma IL-6 level and OPN level had the longest OS and DFS among the four subgroups, which were divided according to combination of IL-6 and OPN. For each cohort, different subgroups were plotted according to the cut-off values of IL-6 (4.16 pg/ml) and OPN (82.80 ng/ml).
Table 3.
Univariate and multivariate analysis of factors associated with survival and recurrence
| Overall Survival | Disease free survival | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P | HR (95% CI) | P | |
| Univariate analysis | ||||
| IL-6 (high vs. low) | 1.604 (1.209-2.127) | 0.001 | 1.662 (1.257-2.199) | <0.001 |
| OPN (high vs. low) | 2.526 (1.861-3.429) | <0.001 | 1.806 (1.354-2.408) | <0.001 |
| Sex (female vs. male) | 1.096 (0.715-1.681) | 0.673 | 1.148 (0.749-1.760) | 0.526 |
| Age (>50 vs. ≤50 years) | 0.734 (0.550-0.979) | 0.035 | 0.742 (0.557-0.987) | 0.040 |
| HBsAg (positive vs. negative) | 0.971 (0.673-1.401) | 0.876 | 1.023 (0.710-1.474) | 0.904 |
| HBcAb (positive vs. negative) | 0.891 (0.560-1.415) | 0.624 | 0.929 (0.579-1.491) | 0.760 |
| Cirrhosis (yes vs. no) | 1.043 (0.714-1.525) | 0.826 | 0.870 (0.583-1.299) | 0.497 |
| ALT (>75 vs. ≤75 U/L) | 0.817 (0.553-1.206) | 0.308 | 1.160 (0.763-1.764) | 0.488 |
| AFP (>20 vs. ≤20 ng/ml) | 1.645 (1.193-2.273) | 0.002 | 1.776 (1.292-2.445) | <0.001 |
| Tumor size (>5 vs. ≤5 cm) | 2.418 (1.799-3.249) | <0.001 | 1.843 (1.389-2.444) | <0.001 |
| Tumor number (multiple vs. single) | 1.099 (0.752-1.606) | 0.625 | 1.196 (0.823-1.738) | 0.347 |
| Tumor capsule (complete vs. none) | 1.657 (1.230-2.234) | 0.001 | 1.443 (1.081-1.926) | 0.013 |
| Tumor thrombus (yes vs. no) | 2.713 (2.042-3.605) | <0.001 | 2.089 (1.579-2.765) | <0.001 |
| Tumor differentiation* (III-IV vs. I-II) | 1.553 (1.155-2.087) | 0.004 | 1.510 (1.125-2.028) | 0.006 |
| Combination of IL-6 and OPN | ||||
| Double high vs. double low | 3.922 (2.557-6.016) | <0.001 | 2.912 (1.925-4.406) | <0.001 |
| Double high vs. (IL-6 high, OPN low) | 2.809 (1.787-4.414) | <0.001 | 1.639 (1.079-2.491) | 0.021 |
| Double high vs. (IL-6 low, OPN high) | 1.708 (1.214-2.405) | 0.002 | 1.567 (1.097-2.237) | 0.014 |
| Multivariate analysis1 | ||||
| IL-6 (high vs. low) | 1.291 (0.965-1.726) | 0.085 | 1.460 (1.091-1.954) | 0.011 |
| OPN (high vs. low) | 2.077 (1.513-2.849) | <0.001 | 1.479 (1.095-1.998) | 0.011 |
| Tumor size (>5 vs. ≤5 cm) | 1.744 (1.274-2.388) | 0.001 | 1.384 (1.023-1.873) | 0.035 |
| Tumor capsule (complete vs. none) | 1.442 (1.061-1.959) | 0.019 | 1.185 (0.879-1.598) | 0.265 |
| Tumor thrombus (yes vs. no) | 1.893 (1.392-2.573) | <0.001 | 1.518 (1.118-2.060) | 0.007 |
| Multivariate analysis2 | ||||
| Combination of IL-6 and OPN | ||||
| Double high vs. double low | 2.638 (1.645-4.229) | <0.001 | 2.169 (1.350-3.485) | 0.001 |
| Double high vs. (IL-6 high, OPN low) | 2.070 (1.276-3.360) | 0.003 | 1.178 (0.745-1.863) | 0.482 |
| Double high vs. (IL-6 low, OPN high) | 1.317 (0.925-1.876) | 0.127 | 1.264 (0.869-1.839) | 0.220 |
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B core antibody; HR, hazard ratio; CI, confidence interval.
Edmondson grade.
Multivariate analysis of IL-6, OPN, Age, AFP, Tumor size, Tumor capsule, Tumor thrombus, Tumor differentiation.
Multivariate analysis of Combination IL-6 and OPN, Age, AFP, Tumor size, Tumor capsule, Tumor thrombus, Tumor differentiation.
Univariate analysis showed that plasma IL-6 level, plasma OPN level, age, serum AFP level, tumor size, tumor capsule, tumor differentiation and vascular invasion were significantly associated with OS and DFS in HCC patients (Table 3). No prognostic significance was found in the other characteristics, including sex, HBsAg, HBcAb, liver cirrhosis, ALT of patients, for OS or DFS. Multivariate analysis showed that plasma IL-6 level, plasma OPN level, tumor size, and vascular invasion were independent prognostic indicators (Table 3). Based on the combination of plasma IL-6 and OPN levels, the patients were divided into four groups. HCC patients with low IL-6 and low OPN level had the best prognosis (OS and DFS), and those with both high IL-6 and high OPN levels had the worst prognosis (OS and DFS) (P<0.001) (Figure 6E, 6F). The combination of plasma IL-6 and OPN levels had a better prognostic performance than IL-6 or OPN alone (Table 3).
Discussion
Metastasis remains to be one of the most crucial processes in cancer progression [28]. HCC patients with high serum IL-6 level were inclined to suffer from postoperative recurrence [5]. Our previous work also showed that higher IL-6 level in HCC tissues was correlated with shorter time to recurrence after radical tumor resection [3]. In this study, we further evaluate the clinical significance of plasma IL-6 level in HCC. Consisted with previous studies, HCC patients with significantly high plasma IL-6 level were at high risk of postoperative tumor recurrence. In addition, plasma IL-6 level was positively correlated with tumor thrombus in micro-vessels. Moreover, in vitro and in vivo studies also demonstrated IL-6 could promote epithelial-mesenchymal transition and tumor metastasis. These results suggested that IL-6 plays an important role in HCC recurrence and metastasis.
Recent studies have manifested that tumor cells with activation of stemness related genes can achieve reprogramming and pathologic self-renewal [29,30], and stemness properties have also been proposed as the driving force of tumor progression [13]. It was recently reported that IL-6, secreted by tumor associated macrophages, was a crucial promoter in HCC stemness through expanding CD44 positive CSCs of HCC and promoted tumor initiation via activating stat3 pathway [4]. The mechanism of stemness maintenance mediated by IL-6 was reported that IL-6 stimulated an autocrine IGFI/IGFIR expression [5]. Consistently, we found that IL-6 KD in HCC-LM3 and MHCC97-H cells, two highly metastatic HCC cell lines, could reduce cell capacities of in vitro sphere formation and in vivo tumor initiation. IL-6 treatment could stimulate HCC stemness related genes up-regulation, including OPN.
Previous studies showed that OPN played key roles in HCC stemness and metastasis [18-20,26]. It is therefore of interest to investigate whether OPN was responsible for IL-6 promotes cancer stem cell traits. Our results indicated a concentration and time dependent increase of cellular OPN after IL-6 stimulation at mRNA and protein levels, as well as secreted OPN in cell supernatant. Meanwhile, block of OPN abrogated the effect of cancer stem cell traits stimulated by IL-6 both in vitro and in vivo. Given OPN was also a powerful promoter for HCC metastasis [17,31], we hypothesized that IL-6 could promote tumor metastasis also via OPN induction. As expected, IL-6 induced OPN high expressing, along with down-regulation of E-cadherin and increased expression of N-cadherin. In vitro migration and invasion assays and in vivo lung metastasis mice model demonstrated that IL-6 promoted tumor metastasis through inducing OPN expression. Taken together, these results suggested that IL-6 promoted cancer stem cell traits and metastasis depending on OPN expression.
Furthermore, we found that OPN up-regulation induced by IL-6 could not maintain without constant IL-6 stimulation. It was reported that IL-6 was a typical inflammatory cytokine and the inflammatory stress status could be significantly induced postoperatively [32,33]. Therefore, based on the existence of circulating tumor cells (CTCs) after radical tumor resection, and CTCs dissemination was the main reason for early HCC recurrence [34,35], we supposed that increased circulating IL-6 level postoperatively might enhance the metastatic potential of CTCs and correlate with HCC recurrence, and early recurrent potential of HCC might be weakened if postoperative circulating IL-6 level of HCC patients could be effectively controlled, which needed to be further studied.
In addition, our group previously showed IL-6 and OPN were both powerful prognostic factors for HCC patients after tumor resection [3,15,16]. In this study, we found that plasma IL-6 and OPN level were also significantly associated with prognosis of HCC patients, and the combination of plasma IL-6 and OPN had a better prognostic performance than IL-6 or OPN alone. Since hepatitis B activity could possibly affect both prognosis and inflammatory status of HCC patients, it might become a confounding bias within our study. Chi-Square analysis showed that, in this HCC patients group, IL-6 was not correlated with HBcAb, an indicator of active viral replication, or alanine transferase (ALT), an enzyme released when hepatocytes destruction. Univariate analysis indicated no relationship between HBcAb/ALT and prognosis of HCC patients.
In conclusion, our results showed that plasma IL-6 level was positively correlated with tumor recurrence and metastasis of HCC. Plasma IL-6 level was an independent prognostic factor for HCC, particularly when combined with plasma OPN level. Using in vitro and in vivo studies, IL-6 was confirmed to be a powerful promoter of tumor initiation and metastasis by up-regulating OPN expression. The combination of IL-6 and OPN may be a more promising prognostic indicator and potential therapeutic target for HCC patients.
Acknowledgements
This research is supported by the National Key Basic Research Program of China (2013CB910500), China National Key Projects for Infectious Disease (2012ZX10002-012) and China National Natural Science Foundation (81372647).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–70. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 4.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang TS, Wu YC, Chi CC, Su WC, Chang PJ, Lee KF, Tung TH, Wang J, Liu JJ, Tung SY, Kuo LM, Ho HN, Ling TY, Huang YH. Activation of IL6/IGFIR confers poor prognosis of HBV-related hepatocellular carcinoma through induction of OCT4/NANOG expression. Clin Cancer Res. 2015;21:201–10. doi: 10.1158/1078-0432.CCR-13-3274. [DOI] [PubMed] [Google Scholar]
- 6.Jinushi M, Baghdadi M, Chiba S, Yoshiyama H. Regulation of cancer stem cell activities by tumor-associated macrophages. Am J Cancer Res. 2012;2:529–39. [PMC free article] [PubMed] [Google Scholar]
- 7.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 8.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, Qin LX, Man K, Lo CM, Lee J, Ng IO, Fan J, Tang ZY, Sun HC, Wang XW. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–47. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, Nguyen PM, Preaudet A, Farid R, Edwards KM, Boglev Y, Luwor RB, Jarnicki A, Horst D, Boussioutas A, Heath JK, Sieber OM, Pleines I, Kile BT, Nash A, Greten FR, McKenzie BS, Ernst M. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24:257–71. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375:51–61. doi: 10.1016/j.canlet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Lv L, Yang K. Chemotherapy targeting cancer stem cells. Am J Cancer Res. 2015;5:880–93. [PMC free article] [PubMed] [Google Scholar]
- 13.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–38. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci. 2008;13:4276–84. doi: 10.2741/3004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Ye QH, Ren N, Zhao L, Wang YF, Wu X, Sun HC, Wang L, Zhang BH, Liu YK, Tang ZY, Qin LX. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709–17. doi: 10.1007/s00432-006-0119-3. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Xu HM, Zhou HJ, Dong QZ, Zhao Y, Fu LY, Hei ZY, Ye QH, Ren N, Jia HL, Qin LX. The prognostic significance of preoperative plasma levels of osteopontin in patients with TNM stage-I of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1–7. doi: 10.1007/s00432-009-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, Ma ZC, Wu ZQ, Ye SL, Liu YK, Tang ZY, Wang XW. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–23. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 18.Dong QZ, Zhang XF, Zhao Y, Jia HL, Zhou HJ, Dai C, Sun HJ, Qin Y, Zhang WD, Ren N, Ye QH, Qin LX. Osteopontin promoter polymorphisms at locus -443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology. 2013;57:1024–34. doi: 10.1002/hep.26103. [DOI] [PubMed] [Google Scholar]
- 19.Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL, Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, Ren N, Qin LX. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008;48:1834–42. doi: 10.1002/hep.22531. [DOI] [PubMed] [Google Scholar]
- 20.Xue YH, Zhang XF, Dong QZ, Sun J, Dai C, Zhou HJ, Ren N, Jia HL, Ye QH, Qin LX. Thrombin is a therapeutic target for metastatic osteopontin-positive hepatocellular carcinoma. Hepatology. 2010;52:2012–22. doi: 10.1002/hep.23942. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Ge Z, Yang X, Luo Q, Wang C, You H, Ge T, Deng Y, Lin H, Cui Y, Chu W, Yao M, Zhang Z, Gu J, Fan J, Qin W. Hepatic stellate cells activated by acidic tumor microenvironment promote the metastasis of hepatocellular carcinoma via osteopontin. Cancer Lett. 2015;356:713–20. doi: 10.1016/j.canlet.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Pietras A, Katz AM, Ekstrom EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14:357–69. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–56. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630–6. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei J, Sheng Y, Zheng Y, Yu J, Xie L, Qin Y, Qiao P, Zhou C, Yu X, Jia H, Ren N, Zhou H, Ye Q, Qin L. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7:12997–3012. doi: 10.18632/oncotarget.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao L, Fan X, Jing W, Liang Y, Chen R, Liu Y, Zhu M, Jia R, Wang H, Zhang X, Zhang Y, Zhou X, Zhao J, Guo Y. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kappaB-HIF-1alpha pathway. Oncotarget. 2015;6:6627–40. doi: 10.18632/oncotarget.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Pan YF, Ding ZW, Yang GZ, Tan YX, Yang C, Jiang TY, Liu LJ, Zhang B, Han T, Cao D, Yang T, Yang N, Wu MC, Dong LW, Wang HY. RMP promotes venous metastases of hepatocellular carcinoma through promoting IL-6 transcription. Oncogene. 2015;34:1575–83. doi: 10.1038/onc.2014.84. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–70. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goding CR, Pei D, Lu X. Cancer: pathological nuclear reprogramming? Nat Rev Cancer. 2014;14:568–73. doi: 10.1038/nrc3781. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya SD, Mi Z, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model. Ann Surg. 2012;255:319–25. doi: 10.1097/SLA.0b013e31823e3a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takata A, Takiguchi S, Miyazaki Y, Miyata H, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Mori M, Kangawa K, Doki Y. Randomized Phase II Study of the Anti-inflammatory Effect of Ghrelin During the Postoperative Period of Esophagectomy. Ann Surg. 2015;262:230–6. doi: 10.1097/SLA.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 33.Xia F, Wang S, Chen M, Wang X, Feng X, Dong J. Protective effect of Verapamil on hepatic ischemia-reperfusion injury during hepatectomy in the cirrhotic patients with hepatocellular carcinoma. Langenbecks Arch Surg. 2009;394:1041–6. doi: 10.1007/s00423-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 34.Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–68. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 35.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]