Abstract
Natural polyphenol compound curcumin has been found to exhibit its anticancer activity in a variety of human malignancies including pancreatic cancer (PC). However, the underlying mechanism has not been fully understood. Accumulating evidence has demonstrated that Skp2 (S-phase kinase associated protein 2) plays an oncogenic role in the development and progression of human cancers. In this study, we aim to explore the molecular basis of curcumin-induced cell growth inhibition in PC cells.Multiple methods such as CTG assay, Flow cytometry, clonogenic assay, wound healing assay, Transwell invasion assay, Western blotting, and transfection were performed to validate the oncogenic role of curcumin in PC cells. We found that curcumin suppressed cell growth, clonogenic potential, migration and invasion, and induced cell apoptosis and cell cycle arrest. Moreover, we observed thatover-expression of Skp2 significantly promoted cell growth, whereas down-regulation of Skp2 with siRNAs inhibited cell growth. The molecular basis of curcumin-mediated cell growth inhibition we identified is that curcumin significantly suppressed Skp2 expression and subsequently induced p21 expression. These findings suggested thattargeting Skp2 by curcumin could be a promising therapeutic strategy for the treatment of PC patients.
Keywords: Curcumin, pancreatic cancer, Skp2, invasion, proliferation
Introduction
Pancreatic cancer (PC) is one of the most aggressive and lethal forms of human malignancies worldwide [1]. PC is the fourth leading cause of cancer-related deaths with an approximately annual incidence of 46,420 and mortality of 39,590 in the United States in 2014 [2]. In China, PC is the seventh deadliest disease with an overall 5-year survival rate of 4.1% and a median survival time of 3.9 months [3]. The PC patients are always diagnosed at an advanced stage and effective targeted therapy is extremely limited. It has been well known that the majority of the PC patients become intractable to standard chemotherapeutic drugs such as gemcitabine and 5-FU (5-fluorouracil) or their combinations [4]. Thus, there is an urgent need to explore the underlying molecular mechanisms of PC pathogenesis and to develop efficient pharmacological agents for the treatment.
Skp2 (S-phase kinase associated protein 2) is a crucial component of the SCF (Skp1-Cullin1-F-box) type of E3 ubiquitin-ligase complexes involved in cell cycle progression through degradation of its ubiquitination targets [5]. Noteworthy, accumulated evidence has demonstrated that Skp2 plays a critical role in the development and progression of human cancers including PC [6].Skp2 is a bona fide proto-oncoprotein and exerts its oncogenic activity by targeting and degrading its ubiquitination targets such as p21 [7], p27 [8], p57 [9], E-cadherin [10], and FOXO1 [11]. Consistent with this notion, Skp2 plays a key role in regulating cell growth,apoptosis, differentiation, cell cycle progression and metastasis [12]. One study has shown that acetylated by p300, Skp2 is localized in cytoplasm and subsequently enhances cell migration via degradation of E-cadherin [10,13].Lin et al. reported that Akt directly phosphorylates Skp2, leading to promotion of cell proliferation and tumorigenesis [14]. They also proved that inactivation of Skp2 suppresses tumorigenesis [15]. Moreover, Skp2 isover-expressed and correlated with poor prognosis in a variety of human cancers, including PC [12,16], prostate cancer [12], breast cancer [17,18], nasopharyngeal carcinoma [19], and glioma [20]. Remarkably, over-expression of Skp2 is associated with the extent of lymph node metastasis, higher histological grade, and poorer patient outcome in PC patients [16]. Schuler et al. further demonstrated that Skp2 confers resistance of PC cells towards TRAIL (tumor necrosis factor-related apoptosis-inducing ligand)-induced apoptosis [21]. NotablySkp2 activates Akt ubiquitination, glycolysis, herceptin sensitivity and tumorigenesis [22]. Strikingly, pharmacological inactivation of Skp2 ubiquitin ligase restricts cancer stem cell traits and cancer progression [23] and tumorigenesis [24]. Altogether, these findings indicated thatinactivation of Skp2 could be a promising approach for better management of human cancer patients.
Curcumin is a natural polyphenol compound derived from turmeric (Curcuma longa). A growing body of evidence implicates that curcumin exhibits multiple activities such as antioxidant, anti-inflammatory, anti-diabetic, antiviral, antifungal, antibacterial, wound-healing and neuroprotective properties [25]. Over the last two decades, a number of studies have proved that curcumin has anticancer effects against a variety of tumors both in vitro and in vivo [26]. More importantly, in contrast with conventional cytotoxic drugs, curcumin has minimal toxicity and is safety at high dose by human clinical trials [27,28]. Curcumin exerts anticancer effects, both alone and in combination with other anticancer drugs (e.g. gemcitabine, 5-FU, and oxaliplatin), by modulating a variety of molecular targets. To date, more than 30 molecular targets have been identified, including NF-κB (nuclear factor-κB), Akt, Notch, mTOR (mammalian target of rapamycin), and Hedgehog [26,29,30]. Although numerous studies have indicated curcumin’s anticancer effects, the underlying mechanism has not been fully understood.
Therefore, in the current study, we explored whether high-level Skp2 was responsible for cell growth, clonogenic ability, migration, invasion, apoptosis and cell cycle arrest. We also determined whether curcumin exhibited its anticancer activity against PC cell lines via inactivation of Skp2. We found that Skp2 was critically involved in PC tumorigenesis. A significantly down-regulation of Skp2 after curcumin treatment was observed, resulting in up-regulation of p21, which could lead to restraint of tumorigenesis. These findings suggest that inhibition of Skp2 by curcumin could be an imperative approach for the treatment of PC.
Materials and methods
Cell culture and reagents
Human PC cell lines Patu8988 and Panc-1 were obtained from ATCC and maintained in DMEMsupplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in a 5% CO2 atmosphere at 37°C. Primary antibodies against Skp2, β-actin and the secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).Anti-p21 and p27 antibodies were purchased from Cell Signaling Technology. Lipofectamine 2000 was purchased from Invitrogen. Curcumin (CAS number 458-37-7, 99.5% purity) was obtained from Sigma-Aldrich (St. Louis, MO). Curcumin was dissolved in DMSO to make a 30 mM stock solution and was added directly to the medium at different concentrations. Cells were treated with 0.1% DMSO as the control group. CellTiter-Glo Luminescent Cell Viability Assay (CTG, Promega) was carried out by following the manufacture’s instruction.
Cell viability assay
The Patu8988 and Panc-1 cells (4×103) were seeded in a 96-well plate. After an overnight culture, cells were treated with different concentrations of curcumin for 48 h and 72 h. At the end of treatment period, 20 μL of reagent CTG was added to each well. Mix contents for 2 minutes on an orbital shaker to induce cell lysis. Allow the plate to incubate for 10 minutes at room temperature and then detect the omitted luminescence using a plate reading luminometer.
Clonogenic assay
In order to examine the survival of cells treated with curcumin, Patu8988 and Panc-1 cells were plated (3×105 per well) in a 6-well plate and incubated overnight. After 72 h exposure to different concentrations of curcumin, the viable cells were counted and seeded into 60 mm dishes in a range of 1,000 cells per plate. The cells were then incubated for 21 days at 37°C in a humidified 5% CO2 atmosphere. All the colonies were stained with 2% crystal violet.
Wound healing assay
Patu8988 and Panc-1 cells were seeded in a 6-well plate at the concentration of 2×106 cells per well.After cells converged almost 100%, absorbed the supernatant carefully and scratched the cells with a yellow pipette tips. The wound was generated and then washed the cells with PBS. Addedmedium containing curcumin to the cells and incubated for 20 h. The scratched area was photographed with an Olympus microscope at 0 h and 20 h, respectively.
Cell apoptosis analysis
The apoptotic cells were detected with Annexin V-FITC/PI apoptosis detection kit (Biouniqure, China). Briefly, PC cells were incubated in 6-well plate overnight and treated with various concentrations of curcumin for 48 h. Cells wereharvested by centrifugation, washed with PBS, and then resuspended in 500 μl of binding buffer with5 μl Propidium iodide (PI) and 5 μl FITC-conjugated anti-Annexin V antibody. All the samples were kept in the dark for 15 min at room temperature. Apoptosis was analyzed using a flow cytometer (BD, USA).
Cell cycle analysis
Exponentially growing PC cells were seeded in the 6-well plate and incubated overnight. The cells were then treated with curcumin and cultured for 48 h. At the end of treatment period, cells were collected and fixed with ice-cold 70% (v/v) ethanol and kept at4°C overnight. The cells were collected and washed with ice-cold PBS. Then, the cell pellets were re-suspended at1×106 cells/ml in PBS and incubated with0.1 mg/ml RNase I and 50 mg/ml Propidium iodide (PI) at 37°C for 30 min. DNA contents were determined with a flow cytometer (BD, USA).
Transwell invasion assay
Cell invasive capacity of Patu8988 and Panc-1 was performed using Transwell Filter (8 μm pore size, Corning) with Matrigel (BD Biosciences). Briefly, PC cells treated with curcumin or Skp2 transfection or combination were transferred in each upper chamber in 200 μL of serum-free medium. And 500 μL of complete medium was added into each bottom chamber with the same concentration of curcumin. After incubation for 24 h, the cells in the upper chamber were removed, and the invaded cells in the membrane were stained with Wright’s-Giemsa. The stained cells were photographed and counted under a light microscope in at least six randomly-selected fields.
Transfection
PC cells were seeded into 6-well plates and transfected with Skp2 cDNA or Skp2 siRNA or empty vector using lipofectamine 2000 by following the manufacture’s instruction. Skp2 siRNA oligonucleotides were purchased from GenePharma (Shanghai, China): sense 5’-GGA GUG ACA AAG ACU UUG UTT-3’; antisense 5’-ACA AAG UCU UUG UCA CUC CTT-3’. After the indicated periods of incubation, the cells were subjected to further analysis as described under the results sections.
Western blotting analysis
The harvested PC cells were washed by PBS and lysed with cell lysis buffer (Cell Signaling, Danvers, MA). The protein concentrations were tested by BCA Protein Assay kit (Thermo Scientific, MA). Equal amount of protein samples were prepared and fractionated by electrophoresis in Sodium Dodecyl Sulfonate (SDS)-polyacrylamide gel and then transferred onto a Polyvinylidene Fluoride (PVDF) membrane. Appropriateprimary antibodies were added and then incubated at 4°C overnight. The membranes were washed 3 times with TBST and then incubated with second antibody at room temperature for 1 h. The protein bands were subsequently detectedby electrochemiluminescence (ECL) assay.
Statistical analysis
All data analyses were conducted using GraphPad Prism 4.0 (Graph Pad Software, La Jolla, CA). Statistical comparisons were performed using the Student t test. Results are expressed as means ± SD. P values < 0.05 were considered statistically significant.
Results
Curcumin suppressed cell proliferation
In order to determine whether curcumin treatment suppresses cell growth in PC cells, CTG measurement was carried out to test the growth viability in Patu8988 and Panc-1 cells treated with various concentrations of curcumin for 48 hours and 72 hours, respectively. We found that curcumin significantly suppressed cell growth in a time- and dose-dependent manner in both Patu8988 and Panc-1 cells (Figure 1A). Moreover, the 50% inhibitive concentration (IC50) of curcumin was examined. After a 72 h treatment, the IC50 values of Patu8988 and Panc-1 cells were found to around 10 μM and 15 μM, respectively (Figure 1A). Therefore, we selected 10 μM and 15 μM of curcumin for Patu8988 cells treatment and 15 μM and 20 μM for Panc-1 cells in the following studies.
Figure 1.
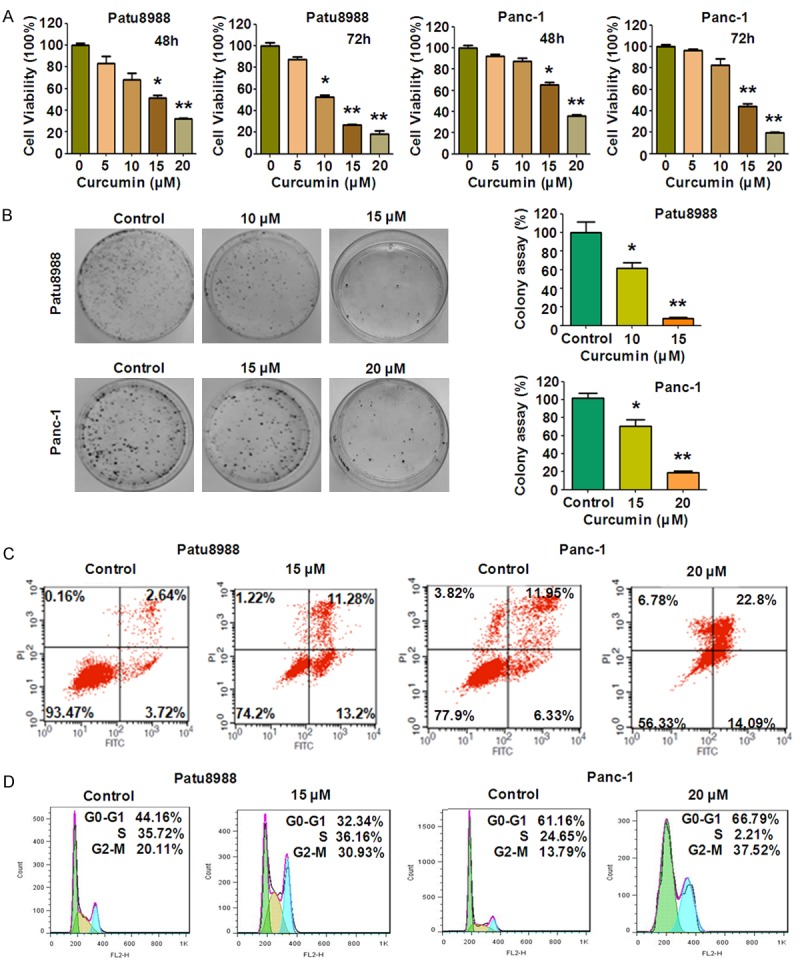
Effect of curcumin on PC cell growth, apoptosis, and cell cycle. A. Effect of curcumin on PC cells growth was detected by CTG assay after treatment with curcumin for 48 and 72 h. *P < 0.05, **P < 0.01, compared to the control groups (DMSO treatment). B. Left panel, Colony formation viability of PC cells treated with curcumin was evaluated by clonogenic assay. Right panel, quantitative results are illustrated for left panel. *P < 0.05, **P < 0.01 vs control. X axis: concentrations of curcumin. Y axis: percentage of colony numbers in control groups. C. Cell apoptosis in PC cells treated with curcumin was accessed by Flow cytometry. D. Curcumin induced PC cell cycle arrest at G2-M Phase.
Curcumin inhibited colony formation
We further confirmed the effects of curcumin on cell growth by clonogenic assay. Compared with control, curcumin treatment caused a significant inhibition of colony formation in both PC cells in a dose-dependent manner (Figure 1B). In accordance with CTG assay (Figure 1A), the results from clonogenic assay indicate that curcumin inhibited cell survival in both Patu8988 and Panc-1 cells.
Curcumin induced apoptosis
Next, we measured apoptotic cells using Annexin V-FITC/PI apoptosis detection kit. Patu8988 cells were treated with 15 μM curcumin for 48 hours and Panc-1 cells were treated with 20 μM curcumin. As shown in flow cytometer data, the percentage of apoptotic cells increased from 6.36% in the control to 24.48% and from 18.28% in control cells to 36.89% in curcumin-treated Patu8988 and Panc-1 cells (Figure 1C), respectively. These results indicated that curcumin treatment caused a statistically evident increase of apoptotic cells, leading to notable cell growth inhibition in both PC cell lines.
Curcumin induced cell cycle arrest
To detect whether curcumin abolished cell cycle progression, PI staining and flow cytometry assay were performed in both PC cells treated with curcumin for 48 hours. As a result, a typical G2/M arrest pattern was identified. The G2/M phase fraction increased from 20.11% in control cells to 30.93% in curcumin-treated Patu8988 cells (Figure 1D). Similar G2/M arrest was found in curcumin-treated Panc-1 cells(Figure 1D). These findings revealed thatcurcumin treatment could induce obvious G2/M phase arrest in PC cells.
Curcumin inhibited cell migration and invasion
Wound healing assay and Transwell assay were conducted to examinewhether curcumin inhibited the motility of the PC cells. Compared with control group, our wound healing assay showed thatcurcumin significantly inhibited the migration of Patu8988 and Panc-1 cells(Figure 2A). Transwell assay furtherdemonstrated that curcumin treatment inhibited the invasion of PC cells transit from the matrigel-coated membrane. Moreover, we found that curcumin inhibited cell migration and invasion of both PC cells in a dose-dependent manner(Figure 2B). These results clearly suggest that curcumin possesses an anti-invasive function in PC cells.
Figure 2.
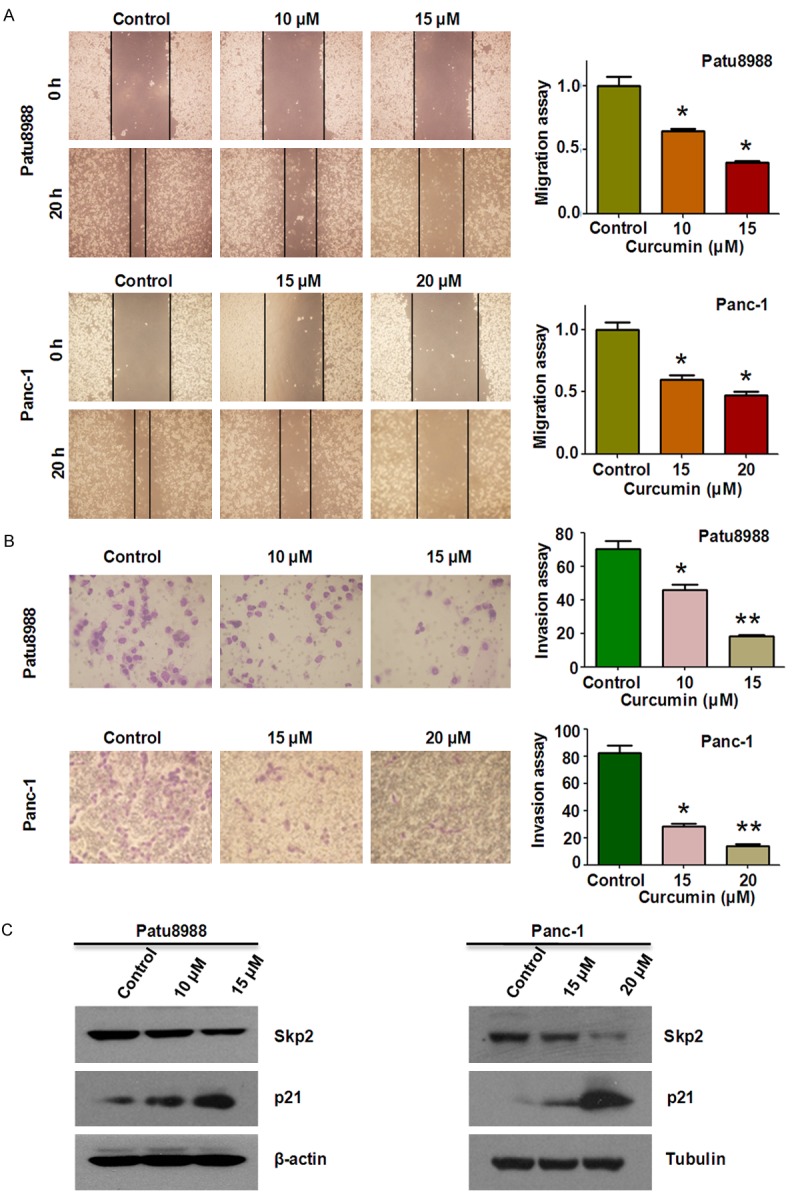
Curcumin inhibited cell migration and invasion in PC cells. A. Left panel, the inhibitory effect of curcumin on PC cell migration was detected using wound healing assay in Patu8988 cells and Panc-1 cells. Right panel, quantitative results are illustrated for left panels. *P < 0.05, vs control (DMSO treatment). B. The inhibitory effect of curcumin on PC cell invasion was detected by Transwell chambers assay in Patu8988 cells and Panc-1 cells. Right panel, quantitative results are illustrated for left panel. *P < 0.05, **P < 0.01 vs control. C. The expression levels of Skp2 and p21 were determined by Western blotting analysis in Patu8988 and Panc-1 cells after curcumin treatment.
Curcumin down-regulated Skp2 expression
Recently, it has been reported that Skp2 plays its oncogenic roles in tumorigenesis [31]. To further investigate the underlying molecular mechanism of curcumin-mediated anticancer activities, alterations in Skp2 were examined using Western blotting analysis in PC cells treated with curcumin. As shown in Figure 2C, Skp2 wasmarkedly down-regulated in both patu8988 and Panc-1 cells after curcument treatment. Consistent with the note that p21 was one of Skp2 downstream targets, we furtherconfirm whether curcumn-mediatedinactivation of Skp2 could impact the expression of p21 in PC cells. As a result, we found that curcumin treatment significantly enhanced the accumulation of tumor suppressor p21 in both PC cell lines (Figure 2C). Our findings suggest that curcumin exerts its anticancer activities at least partly throughinactivation of Skp2 and subsequent up-regulation of its target in PC cells.
Over-expression of Skp2 rescued curcumin-induced cell growth inhibition and apoptosis
In order to detect whether curcumin exerts its anticancer effects through inhibition of Skp2 in PC cells, Patu8988 and Panc-1 cells were transfected with Skp2 cDNA or empty vector as control. We found that re-expression of Skp2 promoted cell growth (Figure 3A). Moreover, over-expression of Skp2 partly abrogated curcumin-induced cell growth inhibition in both PC cells(Figure 3A).Next, we measured whether over-expression of Skp2 could reverse curcumin-induced apoptosis. Indeed, we found that over-expression of Skp2 significantly reduced percentage of apoptotic cells in Patu8988 cells (Figure 3B). Moreover,Skp2 cDNA transfection decreased curcumin-induced apoptosis in both PC cells (Figure 3B). These results suggest that curcumin-induced apoptosis could be partly due to down-regulation of Skp2 in PC cells.
Figure 3.
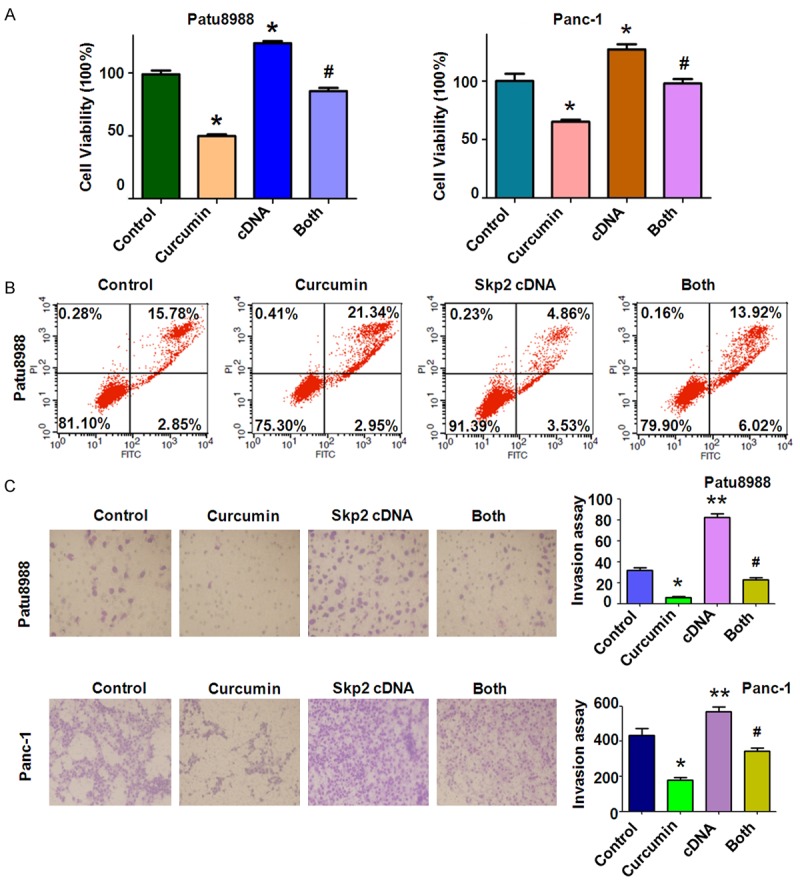
Overexpression of Skp2 promoted cell proliferation and inhibited apoptosis in PC cells. A: The effect of Skp2 overexpression in combination with curcumin treatment on PC cell growth was detected by CTG assay. Control: control cDNA (pcDNA 3.1); cDNA: Skp2 cDNA; Both: Skp2 cDNA+Curcumin. B: Cell apoptosis was accessed by Flow cytometry. *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 cDNA transfection. C. Left panel, the PC cells invasion was detected after Skp2 cDNA transfection and curcumin treatment. Right panel, Quantitative results are illustrated for left panel. *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 cDNA transfection.
Over-expression of Skp2 enhanced cell motility in PC cells
In order to verify the contribution of Skp2 to cell motility and invasiveness in PC cell lines, we performed wound healing assay and Transwell assay to examine the migration and invasion potential of Skp2 cDNA tranfected cells. We found that Skp2 cDNA tranfection triggered the migration and invasion abilities in Patu8988 cells (Figures 3C, 4A). Notably, over-expression of Skp2 abrogated the inhibitory effects of curcumin on cell migration and invasion in PC cells. We further identified that over-expression of Skp2 counteractedactivation of p21 induced by curcumin to a certain degree (Figure 4B, 4C).
Figure 4.
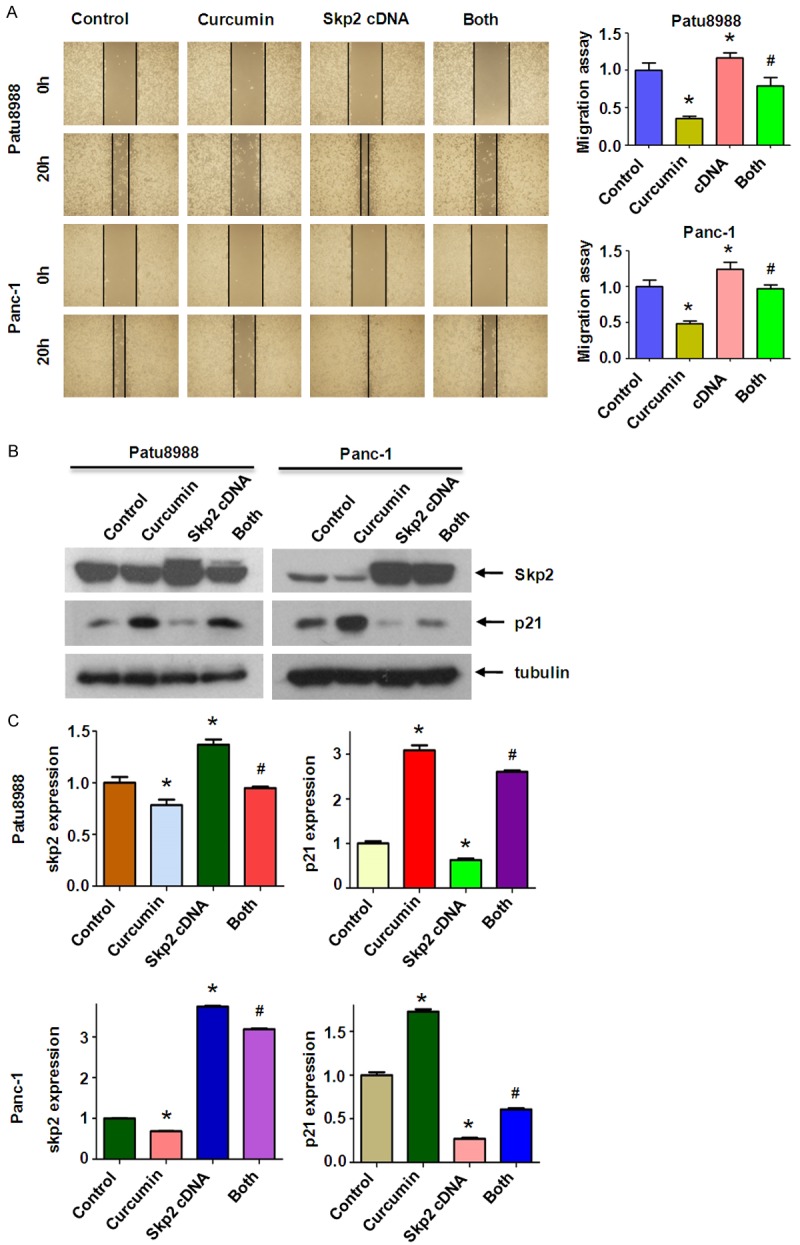
Overexpression of Skp2 enhanced PC cells migration. (A) Left panel, the PC cells migration after Skp2 cDNA transfection and curcumin treatment was detected by wound healing assay. Control: control cDNA (pcDNA 3.1); cDNA: Skp2 cDNA; Both: Skp2 cDNA+Curcumin. Right panel, Quantitative results are illustrated for left panel. *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 cDNA transfection. (B) The expression of Skp2 and p21 was measured in Skp2 cDNA transfected PC cells treated with curcumin. (C) Quantitative results are illustrated for (B). *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 cDNA transfection.
Depletion of Skp2 promoted curcumin-induced anti-tumor activities
To further verify the role of Skp2 in PC cells, we depleted Skp2 expression by transient transfection of Skp2 siRNA oligonucleotides. We found that depletion of Skp2 markedly inhibited cell growth (Figure 5A). Depletion of Skp2 in combined with curcumin treatment promoted cell growth inhibition to a greater degree compared with curcumin alone or siRNA transfection alone (Figure 5A). Next, we found that Skp2 siRNAs significantly promoted apoptosis of both PC cells (Figure 5B). Depletion of Skp2 enhanced curcumin-triggered apoptosis in PC cells. We further identified that down-regulation of Skp2 inhibited migration and invasion in both PC cells (Figures 5C, 6A). Notably, depletion of Skp2 combined with curcumin suppressed cell migration and invasion to maximum effects compared with siRNA treatment alone or curcumin alone (Figures 5C, 6A). We also observed that Skp2 siRNA transfection led to elevated p21 level (Figure 6B, 6C). Taken together, these results suggested that curcumin exerts its anticancer activity through down-regulation of Skp2 signaling pathway.
Figure 5.
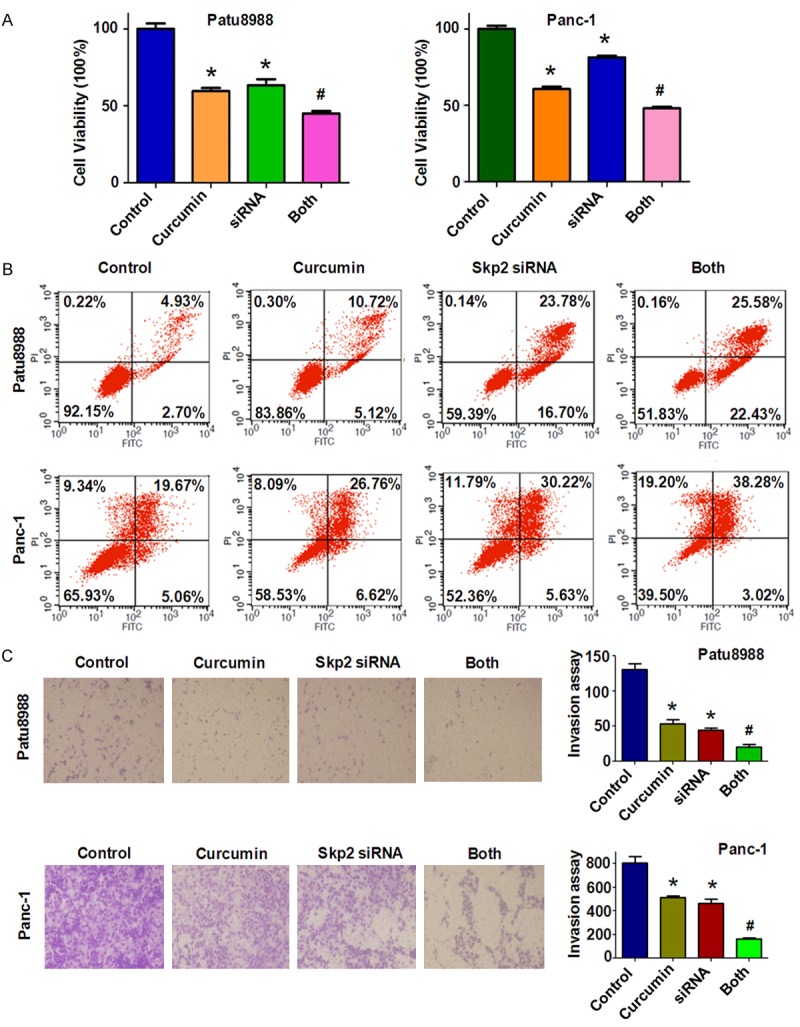
Knockdown of Skp2 inhibited cell proliferation and invasion and facilitated cell apoptosis. A: The effect of down-regulated Skp2 in combination with curcumin treatment on PC cell growth was detected by CTG assay. Control: control siRNA; siRNA: Skp2 siRNA; Both: Skp2 siRNA+Curcumin. *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 siRNA transfection. B: Cell apoptosis was accessed by Flow cytometry in PC cells treated with Skp2 siRNA and curcumin. C. Left panel, PC cells invasion was detected after Skp2 siRNA transfection and curcumin treatment. Right panel, Quantitative results are illustrated for left panel. *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 siRNA transfection.
Figure 6.
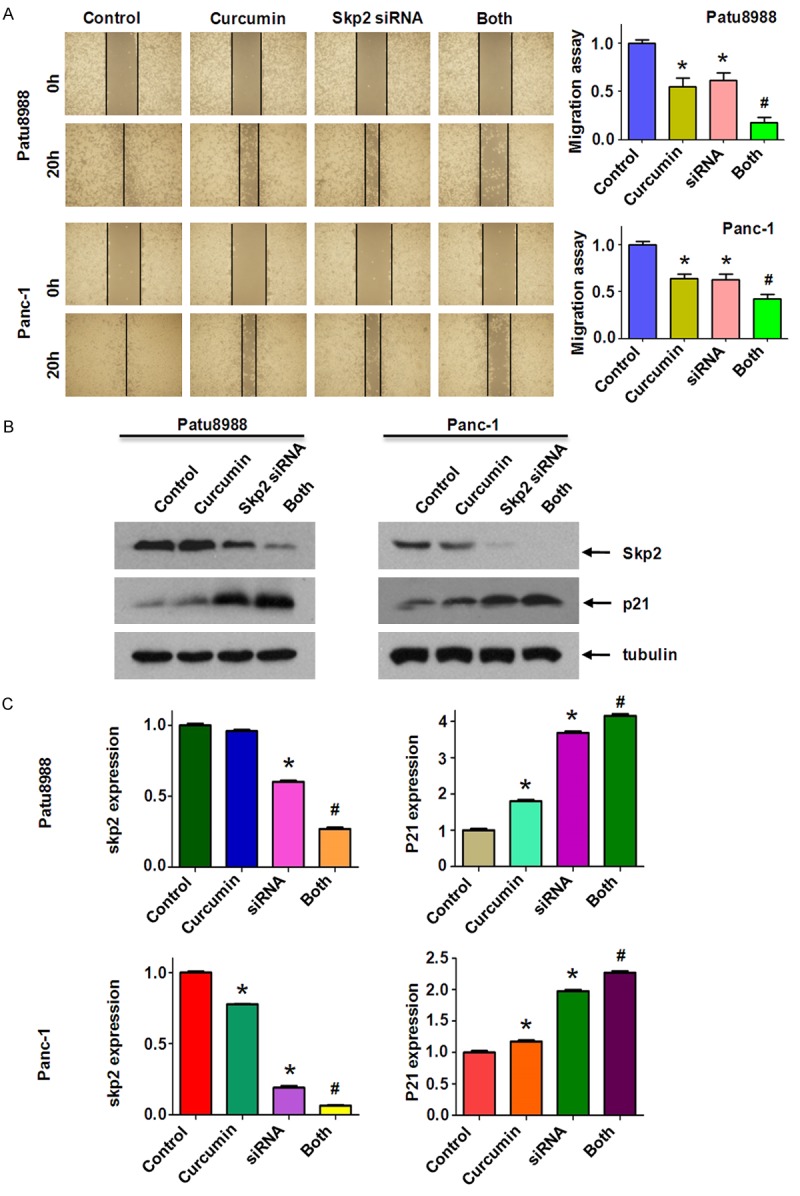
Knockdown of Skp2 inhibited PC cells migration. (A) Left panel, the PC cells migration after Skp2 siRNA transfection and curcumin treatment was detected by wound healing assay. Control: control siRNA; siRNA: Skp2 siRNA; Both: Skp2 siRNA+Curcumin. Right panel, Quantitative results are illustrated for left panel. (B) The expression of Skp2 and p21 was measured in Skp2 siRNA transfected PC cells treated with curcumin. (C) Quantitative results are illustrated for (B). *P < 0.05, **P < 0.01, compared with control; #P < 0.05 compared with curcumin treatment or Skp2 siRNA transfection.
Discussion
A growing body of evidence has demonstrated the anti-cancer effects of curcumin against PC cells [32]. Moreover, curcumin exhibits its anti-cancer effects through modulating the activity of various molecules that play important roles in cancer progression [38]. For instance, down-regulation of NF-κB by curcumin was associated with the suppression of proliferation and the induction of apoptosis in human PC cells [32]. One study has demonstrated that curcumin induced apoptosis in PC cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway [33]. Moreover, curcumin induced PC cells death via reduction of the inhibitors of apoptosis [34]. Consistent with these findings, we found that curcumin inhibited cell growth and induced apoptosis in Patu8988 and Panc-1 cells. Notably, in our present study, we also observed that curcumin suppressed cell migration and invasion. These findings suggest that curcumin could be an effective agent for the treatment of PC patients.
Recent studies have revealed the essential oncogenic function of Skp2 in pancreatic tumorigenesis [21]. Previous study has shown that down-regulation of Skp2 by ATO wasassociated with the cell growth inhibition and apoptosis in PC cells [31]. Therefore, inactivation of Skp2 could bring us considerable therapeutic benefits in treating PC patients. In fact, several specific small molecular inhibitors of Skp2 have already been developedusing in silico screens [35]. These small moleculespromoted p27 accumulation in a Skp2-dependent manner and induced cell-type specific blocks in the G1 or G2/M phases [35]. Specifically, compound CpdA inhibited Skp2 E3 ligase activity, and subsequently inducedp27 accumulationvia preventing it from recruitment to Skp2 ligase complex [36]. Additionally, SMIP0004 has been foundthrough a high-throughput screening, and was report to restore p27 due to its ability to reduce Skp2 abundance, leading to anti-proliferative activity in prostate cancer cells [37]. Compound 25, also known as SZL-P1-41, has been developed to selectively suppress Skp2 E3 ligase activity and to restrictcancer progression both in vitro and in vivo [23]. However, these inhibitors could have unexpected effects in clinical trials. Actually, inactivating Skp2 by nature agents, such ascurcumin, quercetin, lycopene, silibinin, epigallocatechin-3-gallate, and Vitamin D3, could be a safer approach for treating PC patients [38-41]. Nevertheless,further in vitro cell culture as well as in vivo mouse modeling studies should be further pursued to validate the anti-tumor effects of these above mentioned Skp2 inhibitors.
It was reported that curcumin promoted the expression of P53 through a PPARγ activation-dependent mechanism and induced hepatic stellate cell senescence by elevating the expression of senescence markers p16, p21 and Hmga1 [42]. Zhao et al. have confirmed that curcumin significantly decreased PC cell proliferation, which was associated with increased expression of the p21/CIP1, p27/KIP1 and FOXO1 by inhibition of the PI3K/Akt pathway [33]. In the present study, we validated that curcumin exerts its anticancer activity through down-regulation of Skp2 signaling pathway. Although these findings demonstrate a promising anticancer potential of curcumin, it is worth noting thatthe therapeutic use of curcumin is limited. Rapid metabolism and poor absorption of curcumin should account for this case. Therefore, it is necessary to aggrandize the bioavailable efficiency and/or improve delivery methods of curcumin to overcome the blood-brain barrier. In the present study, we revealed the anticancer activity of curcumin through suppression of Skp2 and subsequently induction of p21 in PC cells. Undoubtedly, it is necessary to determine whether curcumin exhibits its anticancer effects via inhibiting Skp2 expression in PC mouse models in vivo. In conclusion, our findings demonstrated that curcumin-mediated cell growth inhibition, apoptosis, cell cycle arrest, invasion and migration suppression in PC cells could be partly due to the down-regulation of Skp2. These results suggest that inhibiting Skp2 by curcumin could bring benefits for the treatment of PC patients.
Acknowledgements
This work was supported by grant from National Natural Science Foundation of China (NSFC81572936) and a project funded the priority academic program development of Jiangsu higher education institutions.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988–8003. doi: 10.3748/wjg.v21.i26.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62:317–326. doi: 10.1136/gutjnl-2012-303588. [DOI] [PubMed] [Google Scholar]
- 5.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nature reviews. Cancer. 2014;14:233–247. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 9.Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama KI. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, Wang Z, Gygi SP, Nakayama K, Teruya-Feldstein J, Toker A, Haigis MC, Pandolfi PP, Wei W. Acetylation-dependent regulation of Skp2 function. Cell. 2012;150:179–193. doi: 10.1016/j.cell.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Gao D, Fukushima H, Inuzuka H, Liu P, Wan L, Sarkar FH, Wei W. Skp2: a novel potential therapeutic target for prostate cancer. Biochim Biophys Acta. 2012;1825:11–17. doi: 10.1016/j.bbcan.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Inuzuka H, Zhong J, Liu P, Sarkar FH, Sun Y, Wei W. Identification of acetylation-dependent regulatory mechanisms that govern the oncogenic functions of Skp2. Oncotarget. 2012;3:1294–1300. doi: 10.18632/oncotarget.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, Tempst P, Pandolfi PP. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einama T, Kagata Y, Tsuda H, Morita D, Ogata S, Ueda S, Takigawa T, Kawarabayashi N, Fukatsu K, Sugiura Y, Matsubara O, Hatsuse K. High-level Skp2 expression in pancreatic ductal adenocarcinoma: correlation with the extent of lymph node metastasis, higher histological grade, and poorer patient outcome. Pancreas. 2006;32:376–381. doi: 10.1097/01.mpa.0000220862.78248.c4. [DOI] [PubMed] [Google Scholar]
- 17.Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene. 2005;24:3448–3458. doi: 10.1038/sj.onc.1208328. [DOI] [PubMed] [Google Scholar]
- 18.Fujita T, Liu W, Doihara H, Date H, Wan Y. Dissection of the APCCdh1-Skp2 cascade in breast cancer. Clin Cancer Res. 2008;14:1966–1975. doi: 10.1158/1078-0432.CCR-07-1585. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Huang Y, Guan Z, Zhang JL, Su HK, Zhang W, Yue CF, Yan M, Guan S, Liu QQ. E3-ligase Skp2 predicts poor prognosis and maintains cancer stem cell pool in nasopharyngeal carcinoma. Oncotarget. 2014;5:5591–5601. doi: 10.18632/oncotarget.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget. 2015;6:18027–18037. doi: 10.18632/oncotarget.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuler S, Diersch S, Hamacher R, Schmid RM, Saur D, Schneider G. SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. Int J Oncol. 2011;38:219–225. [PubMed] [Google Scholar]
- 22.Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, Hazle JD, Yu D, Wei W, Sarbassov D, Hung MC, Nakayama KI, Lin HK. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, Logothetis CJ, Hung MC, Zhang S, Lin HK. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154:556–568. doi: 10.1016/j.cell.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W, Liu S, Li B, Xie Y, Adhiambo C, Yang Q, Ballard BR, Nakayama KI, Matusik RJ, Chen Z. SKP2 inactivation suppresses prostate tumorigenesis by mediating JARID1B ubiquitination. Oncotarget. 2015;6:771–788. doi: 10.18632/oncotarget.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2015;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol. 2014;20:9384–9391. doi: 10.3748/wjg.v20.i28.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Su X, Liu A, Zhang L, Yu A, Xi Y, Zhai G. Advances in clinical study of curcumin. Curr Pharm Des. 2013;19:1966–1973. [PubMed] [Google Scholar]
- 29.Beevers CS, Zhou H, Huang S. Hitting the golden TORget: curcumin’s effects on mTOR signaling. Anticancer Agents Med Chem. 2013;13:988–994. doi: 10.2174/1871520611313070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 31.Gao JK, Wang LX, Long B, Ye XT, Su JN, Yin XY, Zhou XX, Wang ZW. Arsenic Trioxide Inhibits Cell Growth and Invasion via Down- Regulation of Skp2 in Pancreatic Cancer Cells. Asian Pac J Cancer Prev. 2015;16:3805–3810. doi: 10.7314/apjcp.2015.16.9.3805. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Z, Li C, Xi H, Gao Y, Xu D. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway. Mol Med Rep. 2015;12:5415–5422. doi: 10.3892/mmr.2015.4060. [DOI] [PubMed] [Google Scholar]
- 34.Diaz Osterman CJ, Gonda A, Stiff T, Sigaran U, Valenzuela MM, Ferguson Bennit HR, Moyron RB, Khan S, Wall NR. Curcumin Induces Pancreatic Adenocarcinoma Cell Death Via Reduction of the Inhibitors of Apoptosis. Pancreas. 2016;45:101–109. doi: 10.1097/MPA.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L, Grigoryan AV, Li Y, Hao B, Pagano M, Cardozo TJ. Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chem Biol. 2012;19:1515–1524. doi: 10.1016/j.chembiol.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q, Xie W, Kuhn DJ, Voorhees PM, Lopez-Girona A, Mendy D, Corral LG, Krenitsky VP, Xu W, Moutouh-de Parseval L, Webb DR, Mercurio F, Nakayama KI, Nakayama K, Orlowski RZ. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rico-Bautista E, Yang CC, Lu L, Roth GP, Wolf DA. Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol. 2010;8:153. doi: 10.1186/1741-7007-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007;6:2696–2707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- 39.Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem. 2003;278:46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 40.Huang HC, Lin CL, Lin JK. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J Agric Food Chem. 2011;59:6765–6775. doi: 10.1021/jf201096v. [DOI] [PubMed] [Google Scholar]
- 41.Huang HC, Way TD, Lin CL, Lin JK. EGCG stabilizes p27kip1 in E2-stimulated MCF-7 cells through down-regulation of the Skp2 protein. Endocrinology. 2008;149:5972–5983. doi: 10.1210/en.2008-0408. [DOI] [PubMed] [Google Scholar]
- 42.Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu C, Bian M, Shao J, Wu L, Zheng S. Activation of PPARgamma/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2016;7:e2189. doi: 10.1038/cddis.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]


