Abstract
Despite recent advances in surgical technique and treatment strategies for esophageal cancer (EC), to effectively manage the advanced (metastatic or disseminated) and recurrent EC still remain a great challenge. The aim of this study was to determine the feasibility of using intra-esophagus radiofrequency hyperthermia to enhance local HSV-TK/ganciclovir-mediated suicide gene therapy of an innovative animal models with orthotopic esophageal squamous cancers. Human esophageal squamous cancer (ESCa) cells were labeled with lentivirus/luciferase. ESCa cells and nude rats with orthotopic ESCa were divided into in four groups (n = 6/group) and treated with: i) combination therapy of MR imaging-heating-guidewire-mediated radiofrequency hyperthermia ((RFH, 42°C) plus local HSV-TK/GCV; ii) HSV-TK/GCV alone; iii) RFH alone; and (iv) phosphate-buffered saline (PBS). Bioluminescence optical imaging and transcutaneous ultrasound imaging were used to follow up bioluminescence signal and size changes of tumors among different groups over two weeks, which were correlated with subsequent histology. We demonstrated that combination therapy of RFH with gene therapy resulted in the lowest cell proliferation (37.5±8.6%, P<0.0001), rendered the smallest relative tumor volume (0.90±0.15, P<0.01), and relative bioluminescence optical imaging photon signal intensity (0.81±0.17, P<0.01) of orthotopic esophageal cancers, compared with groups treated with gene therapy alone, RFH alone and PBS. Our study indicated that intra-esophageal radiofrequency hyperthermia could enhance the HSV-TK-mediated effect on esophageal squamous cancers.
Keywords: Radiofrequency hyperthermia, gene therapy, esophageal squamous cancers
Introduction
Esophageal cancer is the sixth leading cause of cancer-related mortality with an estimated 455,800 new esophageal cancer cases and 400,200 deaths occurred each year worldwide [1,2]. According to National Cancer Institute, there will be almost 17,000 new cases of esophageal cancer in the United States in 2015 and approximately 16,000 people will die of the disease. The prognosis for patients with locally advanced esophageal cancer treated with the standard approaches of surgery or chemo-radiotherapy is poor [3-5]. In the United States, the overall 5-year survival rate for patients with esophageal adenocarcinoma is <20% [6].
Oesophagectomy is still considered to be the standard procedure for all malignancies without lymph-node metastases in many institutions [7,8]. However, at the time of the diagnosis of esophageal cancer, more than 50 percent of patients have unresectable tumors [1]. Some patients may not be candidates for surgery because of other underlying medical illnesses, which would increase the risk of death during or shortly after surgery. A combination therapy of chemotherapy with radiotherapy is now a standard of care in the nonsurgical management of locally advanced esophageal cancer [3]. However, combining chemotherapy and radiation treatments increases the likelihood and severity of side effects, while radiation can cause side effects from sunburn-like skin reactions, painful or difficult swallowing to severe damages to nearby organs, such as heart, lung and spinal cord. Although chemotherapy can offer the benefits of palliating symptoms in many patients, survival is rarely exceeding one year [1]. The primary reason for this is the resistance of esophageal malignancies to chemotherapeutics, which diminishes the usefulness of chemotherapy in management of esophageal malignancies [9,10]. Thus, it is desirable to develop new alternative technologies for treating unresectable and chemo-resistant esophageal cancers.
Gene therapy is one of the promising approaches to treat malignancies, and over 2200 clinical trials of gene therapies have being conducted worldwide so far (http://www.wiley.com/legacy/wileychi/genmed/clinical/). Among various gene therapeutic strategies, HSV-TK-mediated suicide gene therapy, followed by intravenous administration of ganciclovir (GCV), has been extensively studied [11]. HSV-tk gene in tumor cells can not only phosphorylate certain nucleoside analogs (e.g. ganciclovir, an antiherpetic drug), thus converting them from non-toxic to toxic DNA replication inhibitors, but also generate “bystander effect” to induce the death of neighboring untransfected cells [12]. HSV-tk gene therapy had been investigated for treating various neoplasms, such as HCC and colon carcinoma [13], glioma [14], and non-small cell lung cancer [15]. However, a critical weakness with systemic HSV-tk/GCV therapy is its low rate of gene delivery into tumor and thereby low gene transfection/expression, which is one of the major hurdles to the clinical application of suicide gene therapy [16]. Attempts to solve this problem have been made by increasing the concentration of HSV-tk genes, which can consequently cause substantial and undesirable toxicities to other vital organs.
Previous studies have demonstrated that controlled hyperthermia can enhance gene transfection/expression [17,18]. Our recent studies have confirmed that radiofrequency hyperthermia (RFH) can enhance gene transfection and transduction efficiency in metabolic active tissues, such as atherosclerosis [19]. Based on these experience, in this project we aimed to challenge a critical clinical problem, poor prognosis of those patients with esophageal cancers. We attempted to develop an alternative to chemotherapy, a completely new interventional oncology technique, named “radiofrequency heat-enhanced direct intratumoral gene therapy of esophageal malignancies”. As the initial step, the present study focused on establishing the “proof-of-principle” of the new concept, RFH could enhance the efficiency of HSV-TK/GCV-mediated gene therapy on esophageal cancers.
Materials and methods
Study design
The present study was carried out in two phases: (a) in-vitro experiments to confirm RFH-enhanced gene therapeutic efficacy of HSV-tk/GCV on human esophageal cancer cells; and (b) in-vivo feasibility validation of using optical and ultrasound imaging to monitor RFH-enhanced HSV-tk/GCV gene therapeutic efficacy on rat models with orthotopic esophageal cancer xenografts.
In-vitro experiments
Cells and RFH-enhanced gene therapy
For the purpose of using molecular imaging to evaluate the therapeutic effect, human esophageal cancer cells (T.T) were first transfected with luciferase (Luc)/red fluorescence protein (RFP) gene/lentivirus, to create Luc/RFP-positive esophageal cancer cells according to the protocol provided by the manufacturer (GeneCopoeia Inc., Rockville, MD). Luc/RFP-positive cells were sorted out using fluorescence-activated cell sorting technique (Aria II, Becton Dickinson, Franklin Lakes, NJ). Then , these cells were cultured in Delbecco’s modified Eagle’s medium/F12 (DMEM/F12, 1:1) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), and incubated with a humidified 5% CO2 atmosphere at 37°C. Cells were cultured in 4-chamber cell culture slides (Nalge Nunc International, Rochester, NY, USA). Radiofrequency hyperthermia was performed as described in the literature [19,20]. Briefly, RFH was carried out by attaching a 0.022-inch MR imaging-heating-guidewire (MRIHG) under the bottom of chamber 4 of the chamber slide. The MRIHG was connected to a custom RF generator for heating. A 400 µm fiber optical temperature probe (PhotonControl, Burnaby BC, Canada) was placed in the chamber for measuring the temperature. HSV-tk/lentiviral vector and 3rd generation lenti-combo packing mix were purchased from Applied Biological Materials Inc (Richmond, BC, Canada). HSV-tk gene expression in cells was quantified by qRT-PCR.
Cells were divided into different groups with various treatments, including (a) RFH-enhanced HSV-tk/lentivirus (using a MOI of 20) gene transduction at approximately 42°C for 30 mins, followed by 3-day GCV exposure (100 mmol/L); (b) HSV-tk/lentivirus followed by GCV treatment alone; (c) RFH followed by GCV treatment; (d) phosphate buffered saline (PBS) followed by GCV treatment. The lentiviral transduction was carried out according to manufacturers’ instructions (Applied Biological Materials Inc, Richmond, BC, Canada). Subsequently, cell proliferations in different groups were evaluated by MTS assay 72 hours after the GCV treatment. Briefly, MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) agent was added to the cell chambers and incubated for 4 h. The absorbance was measured using a microplate reader at 490 nm. The relative cell proliferation of each cell group was determined using the equation Acontrol-Atreated/Acontrol, where A is the absorbance. Then, cells on slides were washed twice with PBS, fixed in 4% paraformaldehyde, counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA), and then imaged with a laser confocal microscope (A1R; Nikon, Tokyo, Japan). The experiments for each cell group were repeated six times.
Cell apoptosis assay
The percentages of viable, apoptotic and necrotic cells were quantified by flow cytometry using Annexin V-APC/PI staining kit (BD Biosciences) according to the manufacturer’s protocol. Cells were stained with Annexin-V/APC- and PI in binding buffer along with appropriate controls. Total number of Annexin V- and PI-positive cells were counted using a FACScan flow cytometer (BD FACSCantoTM II). The data was analyzed using the FlowJo software version 10 (TreeStar, Inc.).
Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted using All Prep DNA/RNA Mini kit (Qiagen, Inc., Valencia, USA). RT-qPCR was performed using an Mx3005PTM real time PCR system (Stratagene, La Jolla, USA) with the TaqMan EZ RT-PCR kit, according to manufacturer’s protocol (Applied Biosystems, Foster City, CA). We used forward primer 5’-GGTGATGACCTCTGCCCAGAT-3’, reverse primer 5’-TGTGAGGAGCCAGAACAGCAT-3’, and TaqMan(R) MGB Probe 6FAM-TGG GAA TGC CCT ATG C-MGBNFQ. The following PCR parameters were used: initial UNG activation at 50°C for 2 min, RT reaction at 60°C for 30 min, UNG deactivation at 95°C for 5 min, then 40 cycles of denaturation at 94°C for 15 sec and anneal extend at 60°C for 1 min. After the final cycle, RT-qPCR products were analyzed by MxPro-Mx3005P software (Stratagene, La Jolla, USA). The ΔCt data were collected and -ΔΔCt was calculated using the following formula: -ΔΔCt = ΔCt of HSV-TK gene group -ΔCt of the HSV-TK gene +RFH group. The relative expression of the target gene was calculated using 2-ΔΔCt [21].
In-vivo experiments
Animal model
The animal protocol was approved by our Institutional Animal Care and Use Committee.
Twenty-four nude rats, weighted 180-220 g (Charles River Laboratories, San Diego, CA), were used to create innovative animal models with orthotopic esophageal cancers, which were performed using an ultrasound imaging-guided minimally invasive approach. Briefly, the nude rats were positioned supine on the surgical table and a 0.035-inch guidewire was transorally introduced into esophagus, and then a custom micro-catheter was advanced into the cervical esophagus over the guidewire. After withdrawing the guidewire, a custom micro-coaxial needle that had a curved tip was positioned into the target esophagus through the micro-catheter. Under real-time ultrasound imaging guidance, the curved tip of the needle punctured into the cervical esophagus wall with a controlled penetration depth of 3 mm, where 5×106-1×107 Luc/RFP-positive esophageal cancer cells in 100-µl Matrigel were injected into the target esophageal region.
RFH-enhanced gene therapy
When the volume of tumors reached around 5 mm3, the animals were anesthetized with 1-3% isoflurane in 100% oxygen. Six rats in each of four study groups were treated by (a) HSV-TK lentiviral particles plus RFH, which was carried out by intratumoral injection of approximately 108 HSV-TK lentiviral particles in a total of 50 µL PBS, followed by RFH at approximately 42°C for 30 mins; (b) HSV-TK lentiviral particles therapy without RFH; (c) RFH alone; and (d) PBS to serve as control. HSV-TK lentiviral particles was directly injected into the esophageal cancer mass through the intraesophageal agent delivery needle under real-time ultrasound imaging guidance. Immediately after the HSV-TK lentiviral particles delivery, intraesophageal RFH was generated by inserting a 0.022-inch MRIHG into the esophagus with its heating spot centered at the tumor. A fibrotic temperature probe was placed in the targeted esophagus, in parallel to MRIHG, to monitor the temperature. The intraesophageal RF heating was controlled at around 42°C for 30 minutes. One day after the gene injection, GCV at a daily dose of 50 mg/kg was intraperitoneally administered for 14 consecutive days.
Post-treatment following up with optical imaging and ultrasound imaging
Optical imaging was performed on a Bruker In-Vivo Xtreme Imaging Systems (Bruker Corp., Billerica, MA). Each animal was imaged at day 0 before treatment and days 7 and 14 after the treatments. Animals were anaesthetized with 1%-3% isoflurane (Piramal Healthcare, Andhra Pradesh, India) in 100% oxygen through a nose cone. Optical images were achieved 20 minutes after intraperitoneal injection of Pierce D-Luciferin at 150 mg/Kg (ThermoFisher Scientific, Rockford, IL). Signal intensity was quantified using the Burker MI software. Relative signal intensity (RSI) was calculated by using the equation: RSI = SIDn/SID0, where SI is signal intensity, Dn represents days after treatment, and D0 is the day before treatment. Ultrasound imaging was performed to follow up the tumor growth (Sonosite Inc, Bothel, WA) at day 0 before treatment and days 7 and 14 after the treatment. The axial (X) and longitudinal (Y) diameters of tumors, as well as tumor depths (Z) were measured on the ultrasound images at the maximal tumor dimensions. The volume of each tumor mass was calculated according to the equation of volume = X*Y*Z*π/6. Data was expressed as relative tumor volume (RTV) by using the following equation: RTV = VDn/VD0, where V is tumor volume, Dn represents days after treatment, and D0 is the day before treatment. We followed up the tumors for only 14 days post-treatments. This was because our institutional IACUC did not approve a longer period of follow up since the tumor xenografts might grow up over 10 percent of the animal body weight in the control animal groups as the following up time lasted.
Histologic correlation/conformation
Tumors were harvested at day 14 after treatments. Tumor tissues were embedded in O.C.T compound, frozen in liquid nitrogen, kept frozen at -80°C, and then cryosectioned at 10-µm slices for apoptosis staining. Level of apoptosis was determined by a terminal deoxynucleotidyl transferase dUTP nick end labeling assay (TUNEL) using ApopTag Plus Peroxidase In Situ Apoptosis Detection kit, according to the manufacturer’s instruction (EMD Millipore Corporation, Temecula, CA).
RNA preparation and RT-Qpcr
Tumor tissues from the HSV-TK/GCV-treated group and the HSV-TK/GCV/RFH treated group were collected. HSV-TK gene expression level in tumor tissues was quantified by qRT-PCR as described in the in-vitro experiment.
Statistical analysis
Statistical software (SPSS, Version 19.0; Chicago, III) was used for all data analyses. The non-parametric Mann-Whitney U test was used to compare (i) relative proliferation rates among different cell groups; (ii) relative signal intensity as well as (iii) relative tumor volumes at different time points among different animal groups; and (iv) HSV-TK gene expression rates at end points among various animal groups. P value of less than 0.05 was considered significant difference.
Results
RFH-enhanced gene therapeutic effect on esophageal cancer cells
Of the in-vitro experiments, MTS assay demonstrated the lowest cell viabilities in the group treated with a combination of HSV-TK/lentivirus-GCV plus RFH, compared to those of control groups with HSV-TK/lentivirus-GCV, RFH-GCV and PBS-GCV alone (37.5±8.6% vs 50.9±7.7% vs 92.5±10.6% vs 100±10.1%, P<0.0001) (Figure 1A). Confocal microscopy showed much more cells were killed after the treatment of combination therapy than the other three groups (Figure 1B), which was consistent with the results of MTS assay. Cell apoptosis analysis demonstrated that 22.2% more apoptotic and necrotic cells in the combination therapy group than other control groups with different therapy alone (Figure 1C).
Figure 1.
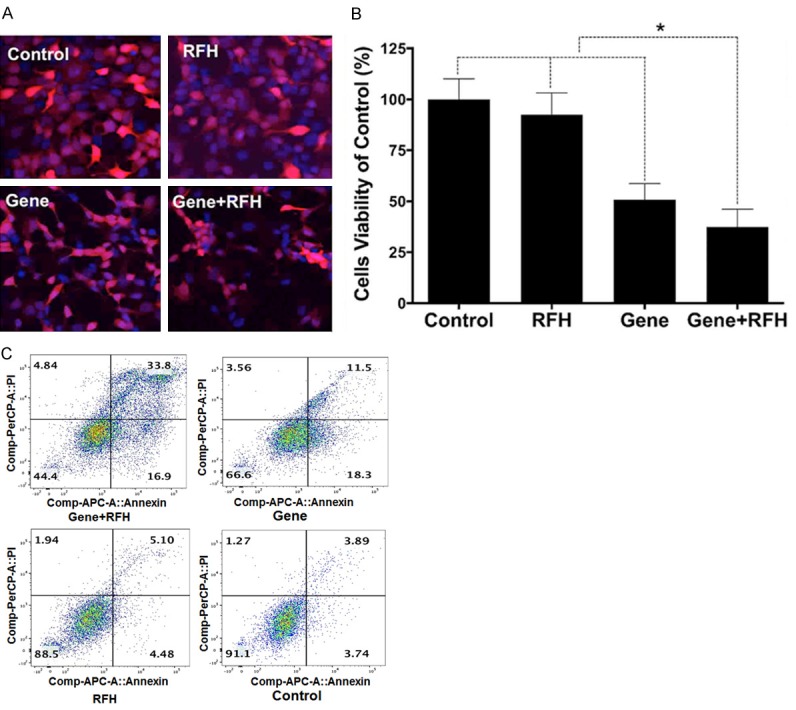
A. Confocal microscopy shows a lowest number of cells survived in combination treatment (Gene+RFH). B. Results of MTS assay show that the lowest cell viability in the group receiving combination treatment (Gene+RFH), compared with those of control groups (*P<0.05). C. Apoptosis analysis shows much more apoptotic and necrotic cells in combination therapy group (Gene+RFH), compared to that of three control groups.
Successful creation of the rat models with orthotopic esophageal cancers
All 24 rat models with orthotopic esophageal cancers were successfully created. These esophageal cancers could be easily visualized and the sizes of these tumors could be precisely measured by ultrasound imaging at three dimensions (Figure 2A). These esophageal cancers could also be detected, in vivo, by bioluminescence optical imaging, manifesting as brightly red-orange-colored signals (Figure 2B).
Figure 2.
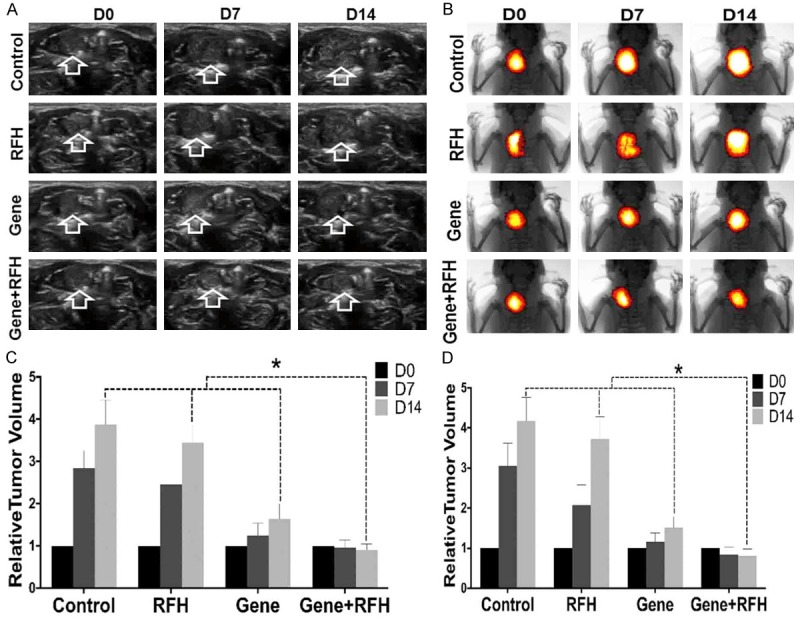
Ultrasound imaging (A) and Optical/x-ray imaging (B) follow-up of rat esophageal cancers (arrows on A and yellow-red colors on B) in four different animal groups with various treatments. (C) Quantified analysis shows the significant decreased relative tumor volume in the combination therapy group (Gene+RFH) at day 14, compared with those of other three control animal groups, which is consistent with the drop of photon intensities on optical images (D).
RFH-enhanced gene therapeutic effect on rat orthotopic esophageal cancer xenografts
The in vivo experiment on rats showed that the HSV-TK gene therapy plus RFH significantly inhibited the growth of tumors. Fourteen days after the treatment, the average relative tumor volume was the smallest in the gene therapy plus RFH group, as compared to those in the HSV-TK-only group (0.90±0.15 vs 1.65±0.35, P = 0.0004) and RF hyperthermia-only group (0.90±0.15 vs 3.44±0.44, P<0.0001). RFH did not inhibit tumor growth, as compared to the tumor growth in the control group (relative tumor volume: 3.44±0.44 vs 3.88±0.58, P = 0.065) (Figure 2C). Optical imaging demonstrated a decrease of relative photon intensities in the group with gene therapy plus RFH compared with the control groups with gene therapy alone (0.81±0.17 vs 1.51±0.28, P = 0.0041), RF hyperthermia-alone (0.81±0.17 vs 3.73±0.55, P<0.0001) and PBS (0.81±0.17 vs 4.18±0.58, P<0.0001) (Figure 2D). The findings of both ultrasound imaging and optical imaging correlated well with subsequent histologic confirmation, which displayed more apoptotic cells in the combination therapy group than those of three control groups (Figure 3).
Figure 3.
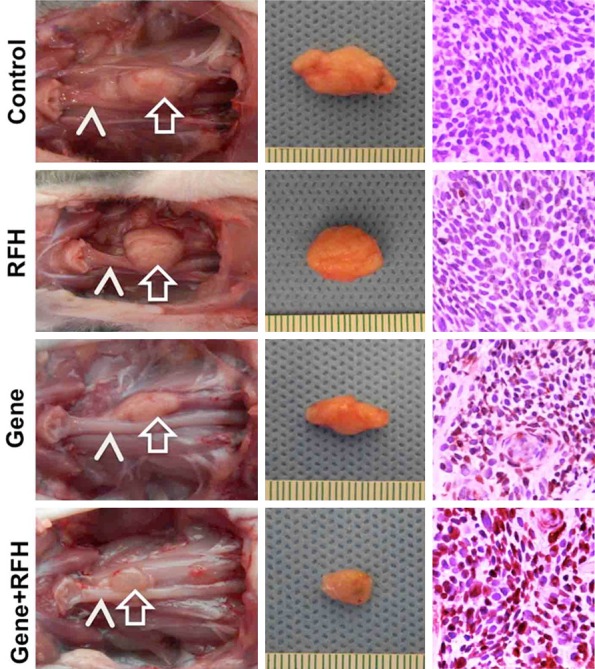
Representative tumors harvested from four different animal groups showed the smallest tumor size treated by combination therapy (Gene+RFH) in comparison with other three treatments. The photographs of harvested specimens show the tumors (arrows) adhere to the esophaguses (arrow heads). Apoptosis analysis by TUNEL staining demonstrates more apoptotic cells (brown dots) in the combination therapy group (Gene+RFH) than the three control groups (20×).
RT-qPCR analysis further showed that HSV-TK gene copy numbers of cells and rat esophageal cancer tissues of the combination therapy group were 1.79±0.30 and 1.37±0.23 fold higher than the gene therapy alone group (P = 0.0007 and 0.0058) (Figure 4).
Figure 4.
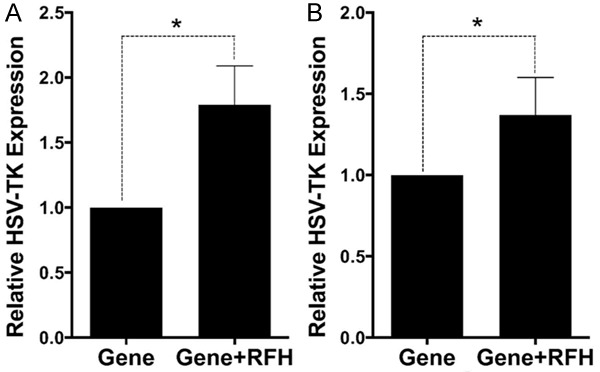
qRT-PCR quantitative analysis of HSV-TK gene expression in cells (A) and tumor tissues (B). RFH could enhance HSV-TK gene expression level in both HCC cells and mice tumors.
Discussion
In this study, we investigated the possibility of using unablative hyperthermia at the temperature of approximately 42°C to enhance intratumoral HSV-TK/GCV-mediated gene therapy of esophageal cancers. The findings of our study show that, (a) RF hyperthermia can increase HSV-TK gene expression level in human esophageal cancer cell lines and esophageal cancer tumors, as manifested by decreased cell viabilities in in-vitro experiments as well as shrunken tumor volumes and decreased optical signal intensities of treated tumors in the in-vivo experiments, (b) RF hyperthermia can been specifically delivered to esophageal tumor through a transesophageal approach using MRIHG, thus providing substantial localized therapeutic hyperthermia, (c) under ultrasound imaging guidance, it is feasible to locally inject therapeutic gene into esophageal cancers through an intra-esophageal approach, (d) it is feasible to use ultrasound and bioluminescence optical imaging to follow up the response of orthotopic human esophageal cancer xenografts to gene therapy.
Gene transfection relies on the use of a genetically modified viral or non-viral vectors that mediates the transfer of therapeutic genes into cells, allowing their transient or stable expression. Selecting an efficient method to boost the gene transfection and expression is the key step when promoting the therapeutic effect on genetically manipulated cells. The combination of hyperthermia with gene therapy, in which hyperthermia increases the transgene expression, representing a promising strategy [22]. Our study established “the proof of principle” that RF hyperthermia can enhance not only HSV-TK gene expression in esophageal cancer cells, but also the therapeutic effect of HSV-TK/GCV on both esophageal cancer cells and esophageal cancer tumors. The explanation of hyperthermia-enhanced gene transfection/expression may lie in the potential mechanisms that hyperthermia can fracture tissues, increase the cell membrane permeability and the cell metabolism, and therefore increase the gene expression [18,23-25].
To date, HSV-TK/GCV therapies have been performed via an intratumoral gene delivery approach for treating easily accessible solid tumors, such as liver cancer, kidney cancer and lung cancer. However, for deep-seated esophageal cancers, no technique is available to facilitate the delivery of high dose of therapeutic gene materials into tumors. Systemic administration of therapeutic genes not only poses the potential risk of toxicities to vital organs, such as brain and heart, but also lacks the capability of delivering sufficient therapeutic genes to the targets. Furthermore critical problem with systemic gene administrations is the low transfection/transuction efficiency and thereby low expression of therapeutic genes at the targets. Many studies have focused on developing new strategies to enhance the therapeutic effect of HSV-TK/GCV system, such as stem cell-based gene targeted delivery [26,27], tumor-selective enhancer-promoters based strategy [28], as well as combination with other chemotherapeutic drugs [29].
To this end, we designed and manufactured a micro-intraesophageal agent delivery/RF heating system, which could be precisely positioned into the target region of the rat orthotopic esophagus via real-time ultrasound imaging guidance. By using this micro-interventional system, we could not only precisely inject esophageal cancer cells in the esophagus wall for creating the rat model of esophageal cancers, but also locally deliver HSV-TK/lentivirus as well as RFH to enhance therapeutic effect of HSV-TK genes on esophageal cancers.
We created an innovative rat models with orthotopic esophageal cancers, which exhibits the specific pathophysiologic properties of esophageal cancers and the interactions between tumors and host organs. In addition, we labeled the esophageal cancer with luciferase gene, which enabled us to use in vivo optical imaging to follow up tumor responses to RFH-enhanced gene therapy. This animal model has several advantages: (a) high reproducibility and cost-effectiveness with precise tumor inoculation under ultrasound imaging guidance; (b) suitability for testing new intraesophageal interventional techniques; (c) relatively quick tumor growth which facilitates the evaluation of therapeutic effects of various treatments in a short period of time, using optical imaging and ultrasound imaging; and (d) easy handling in labs. Thus, we believe that the successful creation of such rat models with molecular imaging-detectable, orthotopic esophageal cancers should provide a useful animal models for basic science on esophageal malignancies.
Since this study primarily focused on the new technical development, we did not follow up the animals to the time points when the treated tumors completely disappeared. This was due to the consideration that longer follow-up period would result in the xenograft tumor masses, especially in the control animal group, most likely to exceed more than ten percent of the body weight, which was not allowed by our Institutional Animal Care and Use Committee. In addition, for proving the principle of the new concept, we used a hyperthermia temperature of 42.0°C only. Future experiments are warranted to optimize the hyperthermia temperature via a series of studies and to maximize the enhancing effect of RF hyperthermia on gene therapy.
In conclusion, the results of our study confirmed that intra-esophageal MRIHG-mediated RF hyperthermia can enhance HSV-TK/GCV-mediated suicide gene therapy on rat esophageal cancers, which may open a new avenue for effectively manage human esophageal malignancies.
Disclosure of conflict of interest
None.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Ilson DH. Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res. 2008;2:85–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, Falchi AM, Craxi A, Camma C. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88–95. doi: 10.1016/j.canep.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–1054. doi: 10.1016/j.athoracsur.2008.12.060. discussion 1054-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweigert M, Dubecz A, Stein HJ. Oesophageal cancer--an overview. Nat Rev Gastroenterol Hepatol. 2013;10:230–244. doi: 10.1038/nrgastro.2012.236. [DOI] [PubMed] [Google Scholar]
- 9.Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res. 2011;17:3029–3038. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki M, Makino T, Masuzawa T, Kurokawa Y, Miyata H, Takiguchi S, Nakajima K, Fujiwara Y, Matsuura N, Mori M, Doki Y. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer. 2011;104:707–713. doi: 10.1038/sj.bjc.6606071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerolami R, Uch R, Faivre J, Garcia S, Hardwigsen J, Cardoso J, Mathieu S, Bagnis C, Brechot C, Mannoni P. Herpes simplex virus thymidine kinase-mediated suicide gene therapy for hepatocellular carcinoma using HIV-1-derived lentiviral vectors. J Hepatol. 2004;40:291–297. doi: 10.1016/j.jhep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Hodish I, Tal R, Shaish A, Varda-Bloom N, Greenberger S, Rauchwerger A, Breitbart E, Bangio L, Ben-Shushan D, Pfeffer R, Feder B, Waitsman A, Barshack I, Goldberg I, Mazaki-Tovi S, Peled M, Harats D. Systemic administration of radiation-potentiated anti-angiogenic gene therapy against primary and metastatic cancer based on transcriptionally controlled HSV-TK. Cancer Biol Ther. 2009;8:424–432. doi: 10.4161/cbt.8.5.7589. [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Piao H, Son BR, Heo DS, Kim NK, Kim ST. Herpes simplex virus thymidine kinase and granulocyte macrophage colony-stimulating factor combination gene therapy in a murine CT26 cell colon cancer model. Cancer Gene Ther. 2004;11:570–576. doi: 10.1038/sj.cgt.7700736. [DOI] [PubMed] [Google Scholar]
- 14.Maatta AM, Samaranayake H, Pikkarainen J, Wirth T, Yla-Herttuala S. Adenovirus mediated herpes simplex virus-thymidine kinase/ganciclovir gene therapy for resectable malignant glioma. Curr Gene Ther. 2009;9:356–367. doi: 10.2174/156652309789753365. [DOI] [PubMed] [Google Scholar]
- 15.Chiu CC, Kang YL, Yang TH, Huang CH, Fang K. Ectopic expression of herpes simplex virus-thymidine kinase gene in human non-small cell lung cancer cells conferred caspase-activated apoptosis sensitized by ganciclovir. Int J Cancer. 2002;102:328–333. doi: 10.1002/ijc.10701. [DOI] [PubMed] [Google Scholar]
- 16.Carrio M, Romagosa A, Mercade E, Mazo A, Nadal M, Gomez-Foix AM, Fillat C. Enhanced pancreatic tumor regression by a combination of adenovirus and retrovirus-mediated delivery of the herpes simplex virus thymidine kinase gene. Gene Ther. 1999;6:547–553. doi: 10.1038/sj.gt.3300846. [DOI] [PubMed] [Google Scholar]
- 17.Takai T, Ohmori H. Enhancement of DNA transfection efficiency by heat treatment of cultured mammalian cells. Biochim Biophys Acta. 1992;1129:161–165. doi: 10.1016/0167-4781(92)90481-e. [DOI] [PubMed] [Google Scholar]
- 18.Madio DP, van Gelderen P, DesPres D, Olson AW, de Zwart JA, Fawcett TW, Holbrook NJ, Mandel M, Moonen CT. On the feasibility of MRI-guided focused ultrasound for local induction of gene expression. J Magn Reson Imaging. 1998;8:101–104. doi: 10.1002/jmri.1880080120. [DOI] [PubMed] [Google Scholar]
- 19.Du X, Qiu B, Zhan X, Kolmakova A, Gao F, Hofmann LV, Cheng L, Chatterjee S, Yang X. Radiofrequency-enhanced vascular gene transduction and expression for intravascular MR imaging-guided therapy: feasibility study in pigs. Radiology. 2005;236:939–944. doi: 10.1148/radiol.2363041021. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Zhang F, Meng Y, Wang H, Le T, Wei B, Lee D, Willis P, Shen B, Yang X. Diffusion-weighted MRI monitoring of pancreatic cancer response to radiofrequency heat-enhanced intratumor chemotherapy. NMR Biomed. 2013;26:1762–1767. doi: 10.1002/nbm.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Fang H, Chen H, Jiang X, Fang D, Wang Y, Zhu D. An artificial miRNA against HPSE suppresses melanoma invasion properties, correlating with a down-regulation of chemokines and MAPK phosphorylation. PLoS One. 2012;7:e38659. doi: 10.1371/journal.pone.0038659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walther W, Stein U. Heat-responsive gene expression for gene therapy. Adv Drug Deliv Rev. 2009;61:641–649. doi: 10.1016/j.addr.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Tang MX, Redemann CT, Szoka FC Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7:703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 24.Doukas AG, Flotte TJ. Physical characteristics and biological effects of laser-induced stress waves. Ultrasound Med Biol. 1996;22:151–164. doi: 10.1016/0301-5629(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 25.Li CY, Dewhirst MW. Hyperthermia-regulated immunogene therapy. Int J Hyperthermia. 2002;18:586–596. doi: 10.1080/0265673021000017082. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, Kim SJ, Park SH, Yang HG, Kang MC, Choi YW, Kim SM, Jeun SS, Sung YC. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res. 2013;19:415–427. doi: 10.1158/1078-0432.CCR-12-1568. [DOI] [PubMed] [Google Scholar]
- 27.Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C, Kucerova L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290:58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Leinonen HM, Ruotsalainen AK, Maatta AM, Laitinen HM, Kuosmanen SM, Kansanen E, Pikkarainen JT, Lappalainen JP, Samaranayake H, Lesch HP, Kaikkonen MU, Yla-Herttuala S, Levonen AL. Oxidative stress-regulated lentiviral TK/GCV gene therapy for lung cancer treatment. Cancer Res. 2012;72:6227–6235. doi: 10.1158/0008-5472.CAN-12-1166. [DOI] [PubMed] [Google Scholar]
- 29.Stedt H, Samaranayake H, Pikkarainen J, Maatta AM, Alasaarela L, Airenne K, Yla-Herttuala S. Improved therapeutic effect on malignant glioma with adenoviral suicide gene therapy combined with temozolomide. Gene Ther. 2013;20:1165–1171. doi: 10.1038/gt.2013.46. [DOI] [PubMed] [Google Scholar]


