Abstract
Nasopharyngeal carcinoma (NPC) is highly incident in southern China. Metastasis is the major cause of death in NPC patients. Concurrent chemoradiotherapy (CCRT) has been accepted as standard in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma (NPC). However, induction chemotherapy (IC) also has benefits in this disease, especially in the patients with certain high-risk factors such as bulky and/or extensive nodal disease. It has been presented that adding IC to CCRT might be a reasonable approach and need more work to confirm. The optimal chemotherapeutic regimen combined with radiotherapy has not been determined so far. It is important to explore high effective and low toxic chemotherapy for the patients. In the multicenter prospective study, 223 patients with locoregionally advanced untreated NPC were randomized into experimental group and control group. The patients received two cycles of induction chemotherapy (IC) with docetaxel (DOC) plus nedaplatin (NDP) in experimental group every 3 weeks, followed by IMRT concurrent with weekly NDP for six cycles, and NDP was replaced by cisplatin (CDDP) in control group. More patients in experimental group could receive full courses of IC and concurrent chemoradiotherapy (CCRT) (P=0.013). There was no significant difference between the two groups in the percentage of reduction of GTVnx and GTVnd after IC (P=0.207 and P=0.107) and CR rate three months after completion of chemoradiotherapy (P=0.565 and P=0.738). With a mean follow-up of 35.1 months, no statistically significant difference in the 3-year OS, LRFS, RRFS, DMFS, and PFS was found. During IC, more patients suffered vomiting in control group (P=0.001). During CCRT, grade 3/4 neutropenia/thrombocytopenia were more common in experimental group (P=0.028 and P=0.035); whereas, severe anemia and vomiting were more common in control group (P=0.0001 and P=0.023). In conclusions, patients with locoregionally advanced NPC showed good tolerance and compliance with a manageable toxicity profile to the regimen of IC with DOC plus NDP followed by concomitant NDP and IMRT, which is as effective as the regimen of DOC plus CDDP as IC followed by concomitant CDDP and IMRT. This trial is registered at ClinicalTrials.gov (NCT 01479504).
Keywords: Nasopharyngeal carcinoma, docetaxel, nedaplatin, toxicity, efficacy
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant disease of the head and neck in Southern China and Southeast Asia, with an annual incidence of 15-50 cases per 100,000 [1]. Radiotherapy is the primary treatment modality for NPC, achieving a 5-year overall survival (OS) of 90% and 84% for early stage I and IIA disease, respectively [2]. However, the majority of patients with NPC present with locoregionally advanced disease, and the results for these patients are unsatisfactory [3]. As we and other researchers previously reported that with the advent of IMRT, local and regional controls have been substantially improved, and distant metastasis has become main patterns of relapse and cause of death [4,5]. Due to the apparent chemosensitivity of the disease, the role of chemotherapy, mainly platinum-based, was evaluated either before RT, as induction chemotherapy (IC), or concomitantly with radiotherapy (CCRT). CCRT has proved its superiority to radiotherapy alone and become the standard treatment in locoregionally advanced NPC [6-8]. However, despite the validation of this finding in a number of randomized studies [6-9], IC followed by CCRT has received a considerable attention. A number of phase II studies of IC followed by CCRT showed an excellent local and/or regional control rate in patients with locoregionally advanced NPC [10-12]. A meta-analysis by OuYang et al. [13] indicated that IC enhanced 3-year OS rate by 5.13% and reduced DM rate. Similar results were found in other studies [10,14]. Besides, CCRT may not be adequate for the patients with certain high-risk factors especially with bulky and/or extensive nodal disease resulting in higher potential for metastasis [15]. Consequently, adding IC to CCRT may be a reasonable approach and need more work to confirm.
The optimal chemotherapeutic regimen combined with radiotherapy has not been determined so far. The combination of 5-fluorouracil (5-FU) and cisplatin (CDDP) has been widely applied as standard regimen in NPC for many years. However, its clinical use has been limited by its potent toxicities in recent years [16]. CDDP is strongly nephrotoxic, and requires extensive hydration with saline, and 5-FU requires continuous intravenous infusion for 120 h and can cause severe oral mucositis. Other regimens that have better antitumor effects and lower toxicity are needed. Nedaplatin (NDP) is the second-generation platinum complex that was developed in Japan to relieve the side effects of CDDP, including digestive symptoms and renal toxicity, and to enhance its antitumor effects [17]. In clinical practice, it has been suggested that NDP is as effective as CDDP, or more effective than CDDP in patients with squamous cell carcinomas [18], and does not cause severe digestive symptoms or renal toxicity [19]. Taxanes has also shown considerable activity in the management of NPC [10,20,21]. Although the combination of taxanes and NDP has shown promising efficacy for head and neck cancer [22,23], there’re few trails based on multicenter study reporting the results of this regimen for NPC.
Building upon this information, we designed a multicenter, prospective, and randomized phase II clinical trial to compare the efficacy and toxicities of a regimen of docetaxel (DOC) plus nedaplatin (NDP) as IC followed by concomitant NDP with intensity-modulated radiotherapy (IMRT) and a regimen of DOC plus cisplatin (CDDP) as IC followed by concomitant CDDP with IMRT in patients with locoregionally advanced NPC.
This trial is registered at ClinicalTrials.gov (NCT 01479504).
Materials and methods
Patient characteristics
A total of 223 patients with primary histopathologically-confirmed NPC at five centers in Guangxi Zhuang Autonomous Region participated in the study between November 2011 and November 2012. Inclusion criteria were as follows: histologically proven NPC (World Health Organization type I, II, and III); stage III-IVb according to the 2009 AJCC Staging System; age 18~65 years; Karnofsky performance status ≥70; serum creatinine ≤1.6 mg/dl and serum bilirubin ≤1.5 mg/dl; white blood cell ≥4,000/mm3, platelet ≥100,000/mm3, and hemoglobin ≥12.0 g/dl for male, >11.0 g/dl for female; no uncontrolled medical or psychiatric disease. Patients diagnosed with or treated for other malignances were excluded in the study. Initial work-up included clinical and laboratory examinations, computed tomography (CT) and/or magnetic resonance imaging (MRI) of the head and neck region, endoscopy with histological confirmation, chest x-ray or CT, abdominal ultrasound or CT and bone scan for exclusion of distant metastases. Written informed consent was obtained for all patients.
Intensity-modulated radiotherapy
Immobilization and simulation
Patients were immobilized in tailored-made thermoplastic mask from head to shoulders, with the head in a neutral position. Intravenous contrast-enhanced CT using slice thickness of 3 mm from the skull vertex to 2 cm below the head of clavicles was performed for planning. The CT data were imported to treatment planning system for treatment design.
Target delineation
The target volumes were defined as follows: the primary gross volume (GTVnx) and the involved lymph nodes (GTVnd) included all known gross disease as determined by the imaging, clinical, and endoscopic findings. For the clinical target volume 1 (CTV1) a margin of 0.5-1 cm was added manually to the GTVnx. CTV2 was defined as CTV1 plus 0.5-1 cm margin, including the bilateral uninvolved regional nodes (retro- and parapharyngeal nodes, cervical nodes Level II, III, and upper portion of level V limited to inferior body of the cricoid bone). PTVnx, PTVnd, PTV1, and PTV2 were generated by adding 0.5 cm margin to GTVnx, GTVnd, CTV1, and CTV2, respectively. Care was taken to ensure at least 5-mm gap was present between the PTVs and the skin. The contoured critical structures included the brain stem, chiasm, optic nerves, spinal cord, temporal lobes, eyes, lens, parotid glands, oral cavity, larynx, mandible, and temporomandibular joints (TMJs).
Treatment planning and delivery
The treatment technique was split-field IMRT, which was delivered via seven fixed-gantry angles with step-and-shoot treatment techniques. The total doses were prescribed to the median of the target volume and usually the 95% isodose surrounded the PTV. The prescribed radiation doses delivered to PTVnx, PTVnd, PTV1, and PTV2 were 68-74 Gy, 68-70 Gy, 60-64 Gy, and 50-56 Gy, respectively, in 30-33 fractions. The lower neck region was irradiated separately to a total dose of 50 Gy at 2.0 Gy per fraction, using an anterior-posterior (AP) portal. The dose constrains to critical structures were within the tolerance according to the RTOG 0225 protocol, and any efforts were made to meet the criteria as closely as possible. The dose constraints for critical structures were listed as follows: The dose constraints to brainstem, optic chiasm, optic nerves, were 54 Gy, respectively. The dose constraints to temporal lobes, mandible, TMJs and 1 mL of the cervical spinal cord were 60, 65, 65 and 45 Gy, respectively. The dose constraints to 50% of the volume values of the left and right parotid glands were 30 Gy, respectively. The mean dose constraints to inner/middle ears, Eyes, glottic larynx were 50, 35 and 45 Gy. The maximum dose constraints to lens and tongue were 9 and 45 Gy, respectively.
Chemotherapy
Patients were randomized into experimental group and control group. In the experimental group, induction chemotherapy comprised two cycles of DOC at 65 mg/m2 on day 1 and NDP at 80 mg/m2 on day 1, repeated every 3 weeks, followed by IMRT concurrent with NDP at a dose of 40 mg/m2 every week for six cycles. In the control group, induction chemotherapy comprised two cycles of DOC at 65 mg/m2 on day 1 and DDP at 80 mg/m2 on day 1, repeated every 3 weeks, followed by IMRT concurrent with DDP at a dose of 40 mg/m2 every week for six cycles. As anti-emetic agents, the combination of a steroid and ondansetron hydrochloride or granisetron hydrochloride was administered before chemotherapy. Prophylactic use of recombinant granulocyte colony-stimulating factor was not allowed. When a WBC count <3000/mm2 or a platelet count <100000/mm2 was obtained at the scheduled date of drug administration, chemotherapy was postponed and radiation therapy was performed. When hematological data obtained two weeks after radiotherapy did not meet the inclusion criteria, the next cycle of chemotherapy was withdrawn. When the WBC count decreased to <1000/mm2 or the platelet count decreased to <25000/mm2 after chemotherapy, doses of both docetaxel and NDP or DDP were decreased by 25% at the next cycle. In addition, the dose of DDP only was decreased by 25% when serum creatinine levels >1.5 mg/dl were noted.
Patient evaluation and follow-up
All patients were evaluated once a week during treatment. The first assessment of tumor response was performed 3 months after the completion of chemoradiotherapy by physical examination, flexible nasopharyngoscopy and magnetic resonance imaging (MRI) for the head and neck. Then patients were follow-up every 3 months during the first 2 years, every 6 months from 3 to 5 years, and every 1 year thereafter. Acute and late morbidity were assessed according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Physical examination, chest X-ray, abdomen ultrasound, fiber nasopharyngoscopy, and laboratory analysis were performed at each follow-up. Magnetic resonance imaging (MRI) for the head and neck was performed every 6 months after the first assessment of tumor response. For patients with suspicious distant metastasis on physical examination, computed tomography (CT) for the chest and abdomen and bone scintigraphy were obtained to confirm the metastasis.
Statistical analysis
The primary end point for this trial was toxicity during IC and CCRT, and the secondary endpoints were overall survival (OS), local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), distant metastasis-free survival (DMFS) and progression-free survival (PFS). OS was calculated from the date of entry into the study to the date of death or the last follow-up visit. LRFS, RRFS, DMFS and PFS was calculated from the date of entry into the study to the date of local recurrence, regional recurrence, distant metastasis occurrence, and the first physical or radiographic evidence of disease progression, death, or the last follow-up visit, respectively.
The present study was done on an ‘intent-to-treat’ basis and thus, all eligible patients were included in the analysis. The sample size was calculated according to the previous report [24] that the incidence of grade 3/4 nausea/vomiting in the experimental group treated with nedaplatin for patients with locoregionally advanced NPC and the control group treated with cisplatin were 2% (1/50) and 14% (7/50), respectively. For a two-sided test with α and β errors of 0.05 and 0.10, respectively, 93 patients were required per group. After adjusting for a 10% rate of dropout or loss to follow-up, 102 patients were required per group.
Descriptive statistics were used to summarize the patient characteristics. The estimated OS, LRFS, RRFS, DMFS and PFS rates were calculated using the Kaplan-Meier method, and differences between survival curves were assessed using the log-rank test. Univariate analysis was performed using log-rank test to identify parameters associated with treatment outcome, and multivariate analyses using Cox regression. A P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Between November 2011 and November 2012, 223 eligible patients were randomly assigned to experimental group (113 patients) or to control group (110 patients). The two groups were well balanced in all patient characteristics and tumor factors. Table 1 lists the pretreatment patient demographic and clinical tumor characteristics.
Table 1.
Patient characteristics
| Characteristic | Experimental group (N=113) | Control group (N=110) | P |
|---|---|---|---|
| Age (Years) | 0.792 | ||
| Median | 45.05 | 45.32 | |
| Range | 28~65 | 23~65 | |
| Gender | 0.788 | ||
| Male | 89 | 85 | |
| Female | 24 | 25 | |
| Histology | 0.625 | ||
| WHO I | 2 | 2 | |
| WHO II | 6 | 4 | |
| WHO III | 105 | 104 | |
| T stage | 0.679 | ||
| T1 | 1 | 1 | |
| T2 | 12 | 10 | |
| T3 | 46 | 51 | |
| T4 | 54 | 48 | |
| N stage | 0.74 | ||
| N0 | 5 | 7 | |
| N1 | 29 | 32 | |
| N2 | 66 | 54 | |
| N3 | 13 | 17 | |
| AJCC stage grouping | 0.721 | ||
| III | 52 | 50 | |
| IV | 61 | 60 |
Treatment compliance
In the control group, 2 patients could not receive CCRT for elevation of aminotransferase accompanied with replication of HBV-DNA and lung metastasis after two cycles of IC (each in one patient). 89 patients (78.8%) in the experimental group and 69 (62.7%) patients in the control group could receive the full courses of IC and CCRT (x 2=2.487, P=0.013) (Table 2).
Table 2.
Chemotherapy delivery
| Chemotherapy | Number of patients n (%) | x2 | P | |
|---|---|---|---|---|
|
| ||||
| Experimental group | Control group | |||
| Induction | ||||
| 1 course | 0 | 0 | ||
| 2 courses | 113 (100) | 110 (100) | ||
| Concurrent | 2.487 | 0.013 | ||
| 0 course | 0 (0) | 2 (1.8) | ||
| 4 courses | 6 (5.3) | 12 (10.9) | ||
| 5 courses | 18 (15.9) | 27 (24.6) | ||
| 6 courses | 89 (78.8) | 69 (62.7) | ||
Efficacy and survival
In the experimental group, GTVnx reduced from 63.29 cm3 to 46.17 cm3 after IC, and the percentage of reduction was 27.31% (27.31±8.36%), while in the control group, GTVnx reduced from 63.48 cm3 to 47.26 cm3, with the reduction ratio of 25.91% (25.91±8.22%). There was no significant difference between the two groups. Similarly, no significant difference in the percentage of reduction of GTVnd between the two groups was found. Table 3 shows the volume change of GTVnx and GTVnd after IC. 104 cases (92%) of complete response (CR) and 9 cases (8%) of partial response (PR) were confirmed three months after completion of chemoradiotherapy in the experimental group, versus 97 cases (89.8%) of CR and 11 cases (10.2%) of PR in the control group. There was no significant difference in CR rate between the two groups (Table 4).
Table 3.
The volume change of GTVnx and GTVnd before and after IC
| GTVnx | GTVnd | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Before IC (cm3) | After IC (cm3) | Reduced volumn (cm3) | Percentage of reduction (%) | Before IC (cm3) | After IC (cm3) | Reduced volumn (cm3) | Percentage of reduction (%) | |
| Experimental group | 63.29±20.45 | 46.17±16.72 | 17.12±6.74 | 27.31±8.36 | 17.87±11.40 | 9.12±6.79 | 8.75±5.69 | 45.91±15.98 |
| Control group | 63.48±21.26 | 47.26±17.78 | 16.22±6.17 | 25.91±8.22 | 17.93±12.20 | 10.23±9.28 | 7.70±4.22 | 41.80±14.50 |
| t | 1.045 | 1.265 | 1.556 | 1.619 | ||||
| P | 0.297 | 0.207 | 0.121 | 0.107 | ||||
Table 4.
Short-time effect three months after completion of chemoradiotherapy
| Effect of NP | x2 | P | Effect of LN | x2 | P | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CR | PR | CR | PR | |||||
|
|
|
|||||||
| n (%) | n (%) | |||||||
| Experimental group | 104 (92.0) | 9 (8.0) | 0.331 | 0.565 | 102 (90.3) | 11 (9.7) | 0.112 | 0.738 |
| Control group | 97 (89.8) | 11 (10.2) | 96 (88.9) | 12 (11.1) | ||||
The median follow-up time was 35.1 months (range 26-41 months). 221 (99.1%) of the 223 patients were assessable for response. 44 patients had developed treatment failure. Of the 44 patients, 10 (5 in the experimental arm and 5 in the control arm) had primary recurrence, 4 (2 versus 2) had regional nodal recurrence, 3 (1 versus 2) had primary and regional nodal recurrence, and 27 (13 versus 14) had distant metastasis, respectively. Among them, 2 (1 in each arm) had developed primary recurrence and distant metastasis, and 4 (3 versus 1) had developed regional nodal recurrence and distant metastasis. 13 patients (6 versus 7) had developed distant metastasis in a single organ: 5 cases (3 versus 2) in bone, 4 cases (2 versus 2) in lung, 4 cases (1 versus 3) in liver. 8 patients (3 versus 5) had developed multi-organ metastasis. For all patients, the 3-year OS, LRFS, RRFS, DMFS, and PFS rates were 86.8%, 91.8%, 92.7%, 85.9%, and 76.2%, respectively. There was no significant difference between the two groups (3-year OS rate: 87.5% vs. 85.9%, P=0.877; LRFS: 91.9% vs. 91.7%, P=0.699; RRFS: 92.5% vs. 92.8%, P=0.843; DMFS: 86.7% vs. 85.1%, P=0.702; PFS: 77.5% vs. 74.9%, P=0.512).
Prognostic factors
To identify which factors affected patient outcome, we performed univariate and multivariate analyses to evaluate the prognostic value of age (≤45 years old versus >45 years old), gender, histology, T stage, and N stage, treatment method (Table 5). Results showed that the 3-year OS, RRFS, DMFS and PFS of patients with advanced N-stage were inferior to those of early N-stage (3-year OS, 81.0% vs. 98.1%, P=0.003; RRFS, 88.8% vs. 100%, P=0.014; DMFS, 79.5% vs. 98.6%, P=0.001; PFS, 67.1% vs. 94.3%, P=0.0001). By using multivariate Cox analysis, only N stage was independent prognostic predictors of OS (P=0.013), DMFS (P=0.009) and PFS (P=0.001). Figures 1, 2 and 3 show Kaplan-Meier curves for 3y-OS, DMFS and PFS according to N stage, respectively.
Table 5.
Effect of prognostic factors on survival in univariate analyses
| Factor | 3-year OS (%) | P | 3-year LRFS (%) | P | 3-year RRFS (%) | P | 3-year DMFS (%) | P | 3-year PFS (%) | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | 0.659 | 0.247 | 0.263 | 0.632 | 0.916 | |||||
| Male | 83.0 | 92.7 | 91.0 | 84.9 | 75.5 | |||||
| Female | 88.0 | 88.8 | 97.8 | 89.0 | 78.3 | |||||
| Age years | 0.978 | 0.239 | 0.299 | 0.735 | 0.328 | |||||
| ≤45 | 86.4 | 90.2 | 90.8 | 85.2 | 74.0 | |||||
| >45 | 87.3 | 94.0 | 95.3 | 86.9 | 79.3 | |||||
| Histology | 0.415 | 0.643 | 0.422 | 0.149 | 0.433 | |||||
| WHO I | 100 | 100 | 80.0 | 100 | 80.0 | |||||
| WHO II | 80.0 | 88.9 | 100 | 88.0 | 88.9 | |||||
| WHO III | 86.3 | 91.7 | 92.9 | 86.3 | 76.2 | |||||
| T stage | 0.591 | 0.226 | 0.897 | 0.703 | 0.965 | |||||
| T1-2 | 87.5 | 100.00 | 90.0 | 86.4 | 76.8 | |||||
| T3-4 | 86.5 | 91.2 | 92.8 | 86.0 | 76.3 | |||||
| N stage | 0.003 | 0.217 | 0.014 | 0.001 | 0.000 | |||||
| N0-1 | 98.1 | 95.6 | 100 | 98.6 | 94.3 | |||||
| N2-3 | 81.0 | 89.9 | 88.8 | 79.5 | 67.1 | |||||
| Regimen | 0.877 | 0.689 | 0.848 | 0.702 | 0.512 | |||||
| Experimental group | 87.5 | 92.0 | 92.6 | 86.7 | 77.5 | |||||
| Control group | 85.9 | 91.7 | 92.8 | 85.1 | 74.9 |
Figure 1.
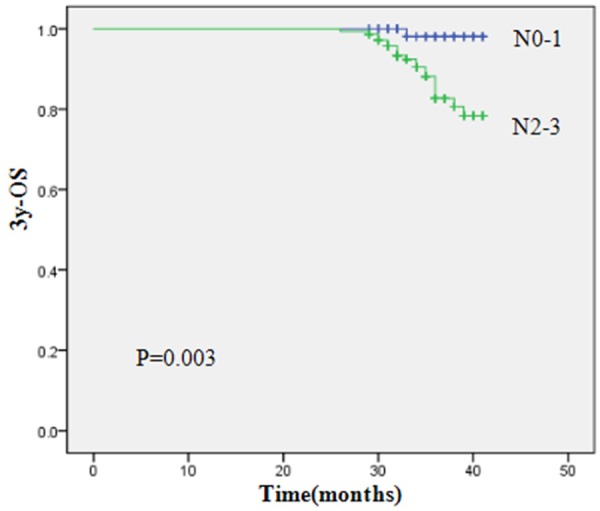
Kaplan-Meier curves for 3y-OS according to N stage.
Figure 2.
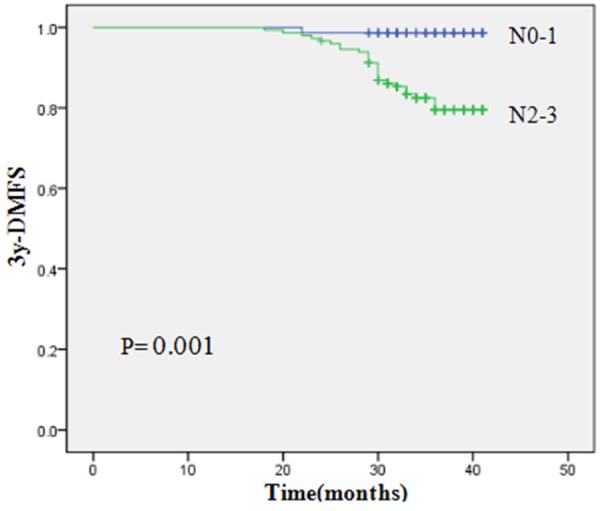
Kaplan-Meier curves for 3y-DMFS according to N stage.
Figure 3.

Kaplan-Meier curves for 3y-PFS according to N stage.
Acute and late toxicities
The most frequently observed acute toxicities from IC were neutropenia and vomiting. No Grade 4 acute toxicities were observed. In the experimental group, 41 patients (36.3%) had grade 1 neutropenia, 15 (13.3%) had grade 2, and 6 (5.3%) had grade 3. While 41 patients (37.3%) in the control group had grade 1 neutropenia, 11 (10.0%) had grade 2, and 4 (3.6%) had grade 3. There was no significant difference between the two groups (P=0.401). More patients suffered grade 3 vomiting in the control group than in the experimental group (13 versus 6, P=0.001). Although anemia from IC was infrequently seen and not serious, more patients suffered grade 1/2 anemia in the control group than in the experimental group (34 versus 16, P=0.005). Table 6 shows the frequency of the acute toxicities from IC by type and grade. The incidence of toxic effects from CCRT is shown in Table 7. The most common acute hematologic toxicities from CCRT were neutropenia, anemia and thrombocytopenia. Grade 3/4 neutropenia and thrombocytopenia were more common in the experimental group (P=0.028 and P=0.035), while severe anemia was more common in the control group (P=0.000). The most common acute non-hematologic toxicities from CCRT were vomiting, mucositis, dermatitis, and xerostomia. Apart from vomiting, which was more common in the control group (P=0.023), the rates of all other non-hematologic toxicities were similar in both groups. On the other hand, no severe liver or renal function damage was observed. No treatment-related deaths occurred.
Table 6.
Frequency of acute toxicities from IC by type and grade
| Toxicity | Experimental group n (%) | Control group n (%) | z | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||
| Neutropenia | 51 (45.1) | 41 (36.3) | 15 (13.3) | 6 (5.3) | 0 (0) | 54 (49.1) | 41 (37.3) | 11 (10.0) | 4 (3.6) | 0 (0) | 0.841 | 0.401 |
| Anemia | 97 (85.8) | 10 (8.8) | 6 (5.3) | 0 (0) | 0 (0) | 76 (69.1) | 28 (25.5) | 6 (5.5) | 0 (0) | 0 (0) | 2.820 | 0.005 |
| Thrombocytopenia | 106 (93.8) | 7 (6.2) | 0 (0) | 0 (0) | 0 (0) | 105 (95.5) | 5 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0.544 | 0.586 |
| Vomiting | 35 (31.0) | 35 (31.0) | 37 (32.7) | 6 (5.3) | 0 (0) | 14 (12.7) | 35 (31.8) | 48 (43.6) | 13 (11.8) | 0 (0) | 3.377 | 0.001 |
| Hepatotoxicity | 107 (94.7) | 6 (5.3) | 0 (0) | 0 (0) | 0 (0) | 98 (89.1) | 10 (9.1) | 2 (1.8) | 0 (0) | 0 (0) | 1.557 | 0.119 |
| Nephrotoxic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
Table 7.
Frequency of acute toxicities from CCRT by type and grade
| Toxicity | Experimental group n (%) | control group n (%) | z | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||
| Neutropenia | 5 (4.4) | 20 (17.7) | 38 (33.6) | 44 (38.9) | 6 (5.3) | 5 (4.6) | 27 (25.0) | 45 (41.7) | 28 (25.9) | 3 (2.8) | 2.203 | 0.028 |
| Anemia | 59 (52.2) | 35 (31.0) | 12 (10.6) | 5 (4.4) | 2 (1.8) | 30 (27.8) | 32 (29.6) | 26 (24.1) | 15 (13.9) | 5 (4.6) | 4.475 | 0.0001 |
| Thrombocytopenia | 38 (33.6) | 35 (31.0) | 19 (16.8) | 18 (15.9) | 3 (2.7) | 51 (47.2) | 29 (26.9) | 15 (13.9) | 11 (10.2) | 2 (1.9) | 2.107 | 0.035 |
| Vomiting | 2 (1.8) | 7 (6.2) | 48 (42.5) | 56 (49.6) | 0 (0) | 0 (0) | 2 (1.9) | 38 (35.2) | 68 (63.0) | 0 (0) | 2.270 | 0.023 |
| Hepatotoxicity | 87 (77.0) | 22 (19.5) | 4 (3.5) | 0 (0) | 0 (0) | 79 (73.1) | 24 (22.2) | 5 (4.6) | 0 (0) | 0 (0) | 0.675 | 0.500 |
| Nephrotoxic | 110 (97.3) | 3 (2.7) | 0 (0) | 0 (0) | 0 (0) | 104 (96.3) | 4 (3.7) | 0 (0) | 0 (0) | 0 (0) | 0.444 | 0.657 |
| Mucositis | 0 (0) | 18 (15.9) | 62 (54.9) | 33 (29.2) | 0 (0) | 0 (0) | 15 (13.9) | 57 (52.8) | 36 (33.3) | 0 (0) | 0.701 | 0.483 |
| Dermatitis | 2 (1.8) | 82 (72.6) | 24 (21.2) | 5 (4.4) | 0 (0) | 4 (3.7) | 75 (69.4) | 25 (23.1) | 4 (3.7) | 0 (0) | 0.082 | 0.935 |
| Xerostomia | 3 (2.7) | 24 (21.2) | 84 (74.3) | 2 (1.8) | 0 (0) | 3 (2.8) | 21 (19.4) | 82 (75.9) | 2 (1.9) | 0 (0) | 0.278 | 0.781 |
The most common late toxicity for 221 patients (113 patients in the experimental group and 108 patients in the control group) who survived for more than 2 years was xerostomia. After treatment, 21.2% of patients in the experimental group had grade 1, 74.3% had grade 2, and 1.8% had grade 3 xerostomia. 19.4% of patients in the control group had grade 1 xerostomia, 75.9% had grade 2, and 1.9% had grade 3. There was no significant difference between the two groups (P=0.781). However, the severity of xerostomia decreased over time. At 24 months after treatment, only 10.6% of patients in the experimental group and 13.9% of patients in the control group had grade 2 xerostomia (P=0.926), and none had grade 3 or 4 xerostomia. Other late toxicities observed including subcutaneous fibrosis, hearing impairment, trismus, cranial nerve palsy, temporal lobe necrosis, vision loss, dysphagia. Table 8 shows the frequency of the late toxicities by type and grade.
Table 8.
Frequency of late toxicities by type and grade
| Toxicity | Experimental group | Control group | z | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||
| Xerostomia | 16 (14.2) | 85 (75.2) | 12 (10.6) | 0 (0) | 0 (0) | 18 (16.7) | 75 (69.4) | 15 (13.9) | 0 (0) | 0 (0) | 0.093 | 0.926 |
| Subcutaneous fibrosis | 40 (35.4) | 63 (55.8) | 10 (8.8) | 0 (0) | 0 (0) | 39 (36.1) | 62 (57.4) | 7 (6.5) | 0 (0) | 0 (0) | 0.318 | 0.750 |
| Hearing impairment | 79 (69.9) | 32 (28.3) | 2 (1.8) | 0 (0) | 0 (0) | 79 (73.1) | 28 (25.9) | 1 (0.9) | 0 (0) | 0 (0) | 0.562 | 0.574 |
| Trismus | 106 (93.8) | 5 (4.4) | 2 (1.8) | 0 (0) | 0 (0) | 100 (92.6) | 6 (5.6) | 2 (1.9) | 0 (0) | 0 (0) | 0.352 | 0.724 |
| Cranial nerve palsy | 112 (99.1) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 106 (98.1) | 2 (1.9) | 0 (0) | 0 (0) | 0 (0) | 0.620 | 0.536 |
| Temporal lobe necrosis | 112 (99.1) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 105 (97.2) | 3 (2.8) | 0 (0) | 0 (0) | 0 (0) | 1.053 | 0.292 |
| Vision loss | 111 (98.2) | 2 (1.8) | 0 (0) | 0 (0) | 0 (0) | 104 (96.3) | 4 (3.7) | 0 (0) | 0 (0) | 0 (0) | 0.882 | 0.378 |
Discussion
In this Phase II trial, we have demonstrated that a regimen of induction chemotherapy with DOC and NDP, followed by IMRT with concurrent NDP in experimental group, results in similar efficacy to the regimen in control group. Besides, more patients in the experimental group could receive the full courses of IC and CCRT mainly dueing to lower incidence of gastrointestinal reaction than that in the control group. Accordingly, we can come to the conclusion that the patients in the experimental group were better in compliance.
There was no significant difference between the two groups in the percentage of reduction of GTVnx and GTVnd after IC and CR rate three months after completion of chemoradiotherapy. The 3-year OS, LRFS, RRFS, DMFS, and PFS rates in the two groups were 87.5% and 85.9%, 91.9% and 91.7%, 92.5% and 92.8%, 86.7% and 85.1%, 77.5% and 74.9%, respectively, and no significant difference was found. The treatment results are comparable with other researchers’ studies. In a recently published study by Kong et al. [11], 116 eligible patients with locoregionally advanced NPC were accrued to receive three cycles of IC with DOC, CDDP, and 5-FU every 3 weeks followed by CDDP per week concurrently with IMRT. With a median follow-up of 32.9 months, the 3-year OS were 94.8%. The 3-year PFS, DMFS, and LRFS were 78.2%, 90.5%, and 93.9%. Recently, Zhong et al. [25] reported similar good results with a regimen containing DOC and CDDP as IC 3-week cycle for 2 courses, followed by radical IMRT with concurrent CDDP every 3 weeks for 2 cycles, and 3-year OS and PFS were 94.1% and 72.7%, respectively.
We explored the prognostic value of some factors, including gender, age, T stage, N stage and regimen, and found that the N-stage was an independent prognostic factor for OS, DMFS and PFS. The results hint that effective systemic therapy for patients with advanced N-stage is demanding.
As mentioned above, the combination of taxanes and platinum has shown promising efficacy for head and neck cancer and NPC [11,22,23,25]. In the study by luo et al. of DOC plus NDP in the treatment of locally advanced NPC, clinical response rates have been comparable with that of PF [26]. Deng et al. [27] treated the patients in the experimental group with intravenous infusion of paclitaxel 135 mg/m2 and NDP 100 mg/m2 for 2 cycles every 3 weeks, followed by 2 cycles of the same chemotherapy and concurrent radiotherapy. Treatment for patients in the control group was similar to that in the experimental group, except that NDP was replaced by CDDP. Three months after the chemoradiotherapy, the incidence of neutropenia in the experimental group was significantly less than that in the control group (50%: 86.7%, P=0.02); the incidence of anemia in the experimental and control groups was 17.9% and 53.3%, with significant difference (P=0.02). The incidence of nausea and vomiting was significantly lower in the experimental group than that in the control group (14.3%: 93.3%, P<0.01). The study demonstrated that NDP and paclitaxel combined with radiotherapy is effective and tolerable. In our study, toxicity was generally manageable in both treatment arms. We obtained that neutropenia and vomiting were the most common Grade 3 adverse effects during IC course. The incidence of neutropenia in the experimental and control groups was 5.3% and 3.6%, with non-significant difference (P=0.401). The incidences of vomiting from IC and CCRT were significantly higher in the control group than that in the experimental group (P=0.001 and P=0.023), which might lead the patients in the control group more difficult to complete planned chemotherapy, and furthermore, lead to the higher incidence of anemia in the control group. Although more patients in the experimental group suffered grade 3/4 neutropenia during CCRT, all the patients could continue with the treatment without delay by use of recombinant granulocyte colony-stimulating factor. Thus, it’s explained further that patients in the experimental group show good tolerance and compliance to the regimen. On the other hand, less nephrotoxic occurred during IC and CCRT because of full hydration of CDDP, which was consistent with other reports [26,27]. As the late toxicities including subcutaneous fibrosis, hearing impairment, trismus, cranial nerve palsy, temporal lobe necrosis, vision loss and dysphagia, no significant differences were found between the two groups. The most common late toxicity was xerostomia and the severity of xerostomia decreased over time.
In conclusion, patients show good tolerance and compliance with a manageable toxicity profile to the regimen of IC with DOC plus NDP followed by concomitant NDP and IMRT, which is as effective as the regimen of DOC plus CDDP as IC followed by concomitant CDDP and IMRT. Given that the incidence of WHO Type III accounts for more than 90% of NPC in Southern China, nearly all the patients included in our study have WHO Type III NPC. Therefore, the efficacy conclusions should be limited to this histologic subtype. Our results need a longer period of follow-up to better assess these initial promising results.
Acknowledgements
This work was supported by the Key Research Program of Guangxi Health Department, China (No. Z2011074).
Disclosure of conflict of interest
None.
References
- 1.Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29:517–26. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 2.Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, Kwong DL, Lau WH. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 3.Sanguineti G, Geara FB, Garden AS, Tucker SL, Ang KK, Morrison WH, Peters LJ. Carcinoma of the nasopharynx treated by radiotherapy alone: Determinants of local andregional control. Int J Radiat Oncol Biol Phys. 1997;37:985–996. doi: 10.1016/s0360-3016(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Wang R, Lu H, Wei B, Feng G, Li G, Liu M, Yan H, Zhu J, Zhang Y, Hu K. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: Treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112:106–111. doi: 10.1016/j.radonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22:233–244. doi: 10.1016/j.semradonc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP, Tang LL, Guo Y, Lin AH, Zeng XF, Ma J. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. 2008;71:1356–1364. doi: 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, Chua ET, Yang E, Lee KM, Fong KW, Tan HS, Lee KS, Loong S, Sethi V, Chua EJ, Machin D. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J. Clin. Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE, Ensley JF. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase 3 randomized Intergroup study 0099. J. Clin. Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Peng L, Yuan X, Hao Y, Lu Z, Chen J, Cheng J, Deng S, Gu J, Pang Q, Qin J. Concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a treatment paradigm also applicable to patients in Southeast Asia. Cancer Treat Rev. 2009;35:345–353. doi: 10.1016/j.ctrv.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, Chan I, Ahuja AT, Zee BC, Chan AT. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2009;27:242–249. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 11.Kong L, Hu C, Niu X, Zhang Y, Guo Y, Tham IW, Lu JJ. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer. 2013;119:4111–4118. doi: 10.1002/cncr.28324. [DOI] [PubMed] [Google Scholar]
- 12.Lin S, Lu JJ, Han L, Chen Q, Pan J. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10:39. doi: 10.1186/1471-2407-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OuYang PY, Xie C, Mao YP, Zhang Y, Liang XX, Su Z, Liu Q, Xie FY. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol. 2013;24:2136–2146. doi: 10.1093/annonc/mdt146. [DOI] [PubMed] [Google Scholar]
- 14.Chen YP, Guo R, Liu N, Liu X, Mao YP, Tang LL. Efficacy of the Additional Neoadjuvant Chemotherapy to Concurrent Chemoradiotherapy for Patients with Locoregionally Advanced Nasopharyngeal Carcinoma: a Bayesian Network Meta-analysis of Randomized Controlled Trials. J Cancer. 2015;6:883–892. doi: 10.7150/jca.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JC, Liang WM, Jan JS, Jiang RS, Lin AC. Another way to estimate outcome of advanced nasopharyngeal carcinoma-is concurrent chemoradiotherapy adequate? Int J Radiat Oncol Biol Phys. 2004;60:156–164. doi: 10.1016/j.ijrobp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, Lu TX, Min HQ. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phaseIII trials. J. Clin. Oncol. 2005;23:1118–1124. doi: 10.1200/JCO.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki Y, Tamura T, Eguchi K, Shinkai T, Fujiwara Y, Fukuda M, Ohe Y, Bungo M, Horichi N, Niimi S. Pharmacokinetics of (glycolate-0,0’)-diammine platinum (II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol. 1989;23:243–246. doi: 10.1007/BF00451649. [DOI] [PubMed] [Google Scholar]
- 18.Kurita H, Yamamoto E, Nozaki S, Wada S, Furuta I, Kurashina K. Multicenter phase I trial of induction chemotherapy with docetaxel and nedaplatin for oral squamous cell carcinoma. Oral Oncol. 2004;40:1000–1006. doi: 10.1016/j.oraloncology.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki Y, Amano T, Morita M, Shinkai T, Eguchi K, Tamura T, Ohe Y, Kojima A, Saijo N. Phase I study and pharmacological analysis of cisdiammine (glycolato) platinum (254-S; NSC 375101D) administered by 5-day continuous intravenous infusion. Cancer Res. 1991;51:1472–1477. [PubMed] [Google Scholar]
- 20.Wei WH, Cai XY, Xu T, Zhang GY, Wu YF, Feng WN, Lin L, Deng YM, Lu QX, Huang ZL. Concurrent weekly docetaxel chemotherapy in combination with radiotherapy for stage III and IVA-B nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2012;13:785–789. doi: 10.7314/apjcp.2012.13.3.785. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu M, Tsukuda M, Matsuda H, Horiuchi C, Taguch T, Takahashi M, Nishimura G, Mori M, Niho T, Ishitoya J, Sakuma Y, Hirama M, Shiono O. Comparison of Concurrent Chemoradiotherapy versus Induction Chemotherapy Followed by Radiation in Patients with Nasopharyngeal Carcinoma. Anticancer Res. 2012;32:681–686. [PubMed] [Google Scholar]
- 22.Uchiyama K, Iwabuchi H, Nakayama S. Phase I/II clinical trial of induction chemotherapy with nedaplatin (CDGP), docetaxel (DOC) and 5-fluorouracil (5-FU) for squamous cell carcinoma of head and neck. Gan To Kagaku Ryoho. 2007;34:43–48. [PubMed] [Google Scholar]
- 23.Kobayashi W, Teh BG, Sakaki H, Sato H, Kimura H, Kakehata S, Nagahata M. Superselective intra-arterial chemoradiotherapy with docetaxel-nedaplatin for advanced oral cancer. Oral Oncol. 2010;46:860–863. doi: 10.1016/j.oraloncology.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Cao KJ, Zhang AL, Ma WJ, Huang PY, Luo DH, Xia WX. Nedaplatin or cisplatin combined with 5-fluorouracil for treatment of stage III-IVa nasopharyngeal carcinoma: a randomized controlled study. Zhonghua Zhong Liu Za Zhi. 2011;33:50–52. [PubMed] [Google Scholar]
- 25.Zhong YH, Dai J, Wang XY, Xie CH, Chen G, Zeng L, Zhou YF. Phase II trial of neoadjuvant docetaxel and cisplatin followed by intensity-modulated radiotherapywith concurrent cisplatin in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71:1577–1583. doi: 10.1007/s00280-013-2157-2. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Lin Y, Zhou J, Li Q, Lin G, Gao Y. Clinical study of inductive chemotherapy with docetaxel plus nedaplatin followed by concurrent nedaplatin with radiotherapy for advanced nasopharyngeal carcinoma. Tumor. 2011;31:532–537. [Google Scholar]
- 27.Deng Y, Deng C, Hu H, Ren G, Yang L, Pan H. Nedaplatin or Cisplatin plus Paclitaxel Combined with radiotherapy for Locally Advanced Nasopharyngeal Carcinoma Compared. The Practical Journal of Cancer. 2011;26:175–177. [Google Scholar]


