Abstract
Pyruvate dehydrogenase A1 (PDHA1) serves as a gate-keeper enzyme link between glycolysis and the mitochondrial citric acid cycle. The inhibition of PDHA1 in cancer cells can result in an increased Warburg effect and a more aggressive phenotype in cancer cells. This study was conducted to investigate the expression of PDHA1 in ovarian cancer and the correlation between PDHA1 expression and the prognosis of patients. The PDHA1 protein expression in 3 ovarian cancer cell lines (OVCAR-3, SKOV-3 and ES-2) and 248 surgically removed ovarian carcinoma samples was immunocytochemically examined. Statistical analyses were performed to evaluate the correlations between PDHA1 expression and the clinicopathological characteristics of the patients as well as the predictive value of PDHA1. The results showed the presence of variable expression of PDHA1 in the three ovarian cancer cell lines. Of the 248 ovarian cancer tissue specimens, 45 cases (18.1%) were negative in tumor cells for PDHA1, 162 cases (65.3%) displayed a low expression level, and 41 cases (16.5%) had a relatively high PDHA1 staining. The expression of PDHA1 was associated with the histological subtype (P=0.004) and FIGO stage (P=0.002). The median OS time in the PDHA1 negative group, low expression group and high expression group were 0.939 years, 1.443 years and 9.900 years, respectively. The median PFS time in the above three groups were 0.287 years, 0.586 years and 9.900 years, respectively. Furthermore, the high expression of PDHA1 in ovarian carcinoma cells was significantly associated with better OS and PFS by statistical analyses. Multivariate analyses showed that PDHA1 expression was also an independent prognostic factor for higher OS in ovarian cancer patients (HR=0.705, 95% CI 0.541-0.918, P=0.01). Our study indicated that the decreased expression of PDHA1 might be an independent prognostic factor in unfavorable outcomes.
Keywords: Pyruvate dehydrogenase A1, ovarian carcinoma, prognosis, immunohistochemical analysis
Introduction
Epithelial ovarian cancer accounts for 25% of all the malignancies of the female genital tract, and it remains the most lethal gynecologic malignancy worldwide [1]. Most women with ovarian carcinoma are diagnosed at an advanced stage, and the 5-year survival is less than 30% for patients diagnosed in advanced stages [2]. Despite aggressive treatment, most ovarian cancer patients develop recurrent cancer, and cancer metastasis is one of the leading causes of death [3]. Thus, novel biomarkers for early diagnosis and effective therapeutic treatment will significantly improve the current treatment and prognosis of ovarian cancer.
The Warburg effect, also known as aerobic glycolysis, is one of the characteristics of tumor cells. Recent studies have shown that normal differentiated cells prefer to obtain energy through mitochondrial oxidative phosphorylation (OXPHOS); most cancer cells preferentially perform glycolysis, even when sufficient oxygen exists, which is a phenomenon called aerobic glycolysis [4,5], probably because of rapid tumor growth [6]. This unique aerobic glycolysis might provide cancer cells with numerous selective advantages, including adaptation to hypoxia, resistance to mitochondria-mediated apoptosis, acidification of the tumor microenvironment and increased tumor invasion and metastasis ability [7,8]. Some studies have correlated the glycolytic state of tumor cells with cancer aggressiveness, which is an important link to the Warburg effect [9].
Pyruvate dehydrogenase (PDH) functions as a gatekeeper in glucose metabolism by oxidatively decarboxylating pyruvate to produce acetyl-CoA for the TCA cycle, and pyruvate can come from glycolysis or from other pathways. Therefore, this enzyme plays an important role in the metabolic node, which separates pyruvate between aerobic and anaerobic metabolisms [10]. Pyruvate decarboxylation catalyzed by pyruvate dehydrogenase E1 (PDHA1), consisting of two α and two β subunits is considered to be the rate-limiting step. The main subunit of E1 is the E1α subunit, which is encoded by the pdha1 gene. It is known that pdha1 hemizygous loss-of-function mutations in human males result in severe lactic acidosis [11-13]. In cancer cells, inhibition of PDHA1 activity by the over-expression of pyruvate dehydrogenase kinase (PDK) leads to reduced flow of glucose-derived pyruvate into the TCA cycle. As a result, the cells switch their energy metabolism from mitochondrial glucose oxidation to cytoplasmic glycolysis [14,15]. It has been demonstrated that tyr-301 phosphorylation inhibits pyruvate dehydrogenase and promotes the Warburg effect in leukemia cells [16]. It was also reported that the Warburg effect might occur in part due to up-regulation of PDK activity and therefore inhibition of PDH or by direct inactivation of PDHA1 in cancer cells [17]. Therefore, PDHA1 serves as a key enzyme linking glycolysis and the mitochondrial TCA cycle. We have found that PDHA1 gene knockout forces cells to undergo glycolysis, and the cells show more malignant features with the up-regulation of cell stemness in the LNcaP human prostate cancer cell line [18].
However, the expression status of PDHA1 in ovarian cancer and the relationship of PDHA1 expression with the progression and prognosis of patients remain unknown. In this study, the variable expression of PDHA1 in three ovarian cancer cell lines was first verified, and its expression in a series of ovarian cancers was then examined in consideration of its correlations with clinical pathological parameters and overall survival (OS) and progression-free survival (PFS). Our results demonstrated that loss of or low PDHA1 expression might be considered as a marker of tumor aggressiveness, and decreased expression of PDHA1 in ovarian cancer was predictive of unfavorable outcomes.
Methods and materials
Ethics statement
This study was approved by the Regional Committee for Medical Research Ethics South of Norway (S-06277a), the Social- and Health Directorate (06/3280) and the Data Inspectorate (06/5345).
Cell Lines
Three ovarian cancer cell lines, ES-2, SKOV-3, OVCAR-3 (from American Type Culture Collection, Wesel, Germany), were maintained in our laboratory. The ES-2 line was derived from a patient with ovarian clear cell carcinoma. The SKOV3 cell line was derived from malignant ascites of ovarian adenocarcinoma patients. The OVCAR3 cell line came from the ovary of an ovarian adenocarcinoma patient. All of the cells were cultivated in PRMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified 5% CO2 incubator.
Clinical samples
A total of 248 surgically removed ovarian carcinoma samples were randomly enrolled in this study. All of the patients were diagnosed and operated at the Norwegian Radium Hospital, Oslo University Hospital, from 1983 to 2000. The ages of the patients at diagnosis ranged from 19 to 89 years old, with a median of 58 years. The patients were followed up until January 1, 2012. All of the patients were clinically staged using the criteria of the International Federation of Gynecology and Obstetrics (FIGO) [19]. The primary tumors were histologically graded as well, moderately or poorly differentiated according to WHO recommendations, by two of the authors (J.M. and Z.S.) [20]. Disease progression was determined based on the definitions provided by the Gynecologic Cancer Intergroup [21].
Immunocytochemistry (ICC) and immunohistochemistry (IHC)
Cytoblocks were prepared for ICC. For each cell line, the cells at 80% confluence were harvested by mechanical scraping, and the cells in suspension were centrifuged at 2000 rpm for 5 minutes before the supernatant was discarded. The cells were rinsed twice with phosphate-buffered saline (PBS) to delete further the dead cells or cell organelles. Four drops of plasma and 2 drops of thrombin were added to the sedimentation after the supernatant was discarded, and the contents were carefully mixed by rotating the tube for one minute before coagulation occurred. Buffered formaldehyde at a concentration of 4% was added to the coagulation for cell fixation. The coagulated mass was then wrapped in linen paper, loaded into a labeled cassette, and placed in 4% buffered formaldehyde. The material was paraffin-embedded to make a cytoblock before being cut into 4-μm paraffin sections for ICC.
Paraffin-embedded ovarian carcinoma specimens were cut into 4-μm thick sections. The Dako Envision FLEX+ system (K8012; Dako, Glostrup, Denmark) and the Dako Autostainer were used for IHC. Paraffin sections were deparaffinized, and epitopes were unmasked in PT-link with high PH target retrieval solution (Dako), and then they were blocked with peroxidase blocking (Dako) for 5 minutes. The slides were incubated with PDHA1 antibody (Pyruvate Dehydrogenase [C54G1] Rabbit mAb, #3205, Cell Signaling Technology) for 30 minutes at room temperature, followed by rabbit linker (Dako), according to the resource of the primary antibody, for 15 minutes and HRP for 30 minutes at room temperature. The slides were then stained with 3,3,9-diaminobenzidine tetrahydrochloride (DAB) for 10 minutes, and they were counter-stained with hematoxylin, dehydrated, and mounted in Richard-Allan Scientific Cytoseal XYL (Thermo Scientific, Waltham, MA, USA). Already known PDHA1-positive tissue was used as a positive control in the same procedure. The same positive control slide was used as a negative control after incubation with the same concentration of non-immune rabbit/mouse IgG, replacing the primary antibody.
IHC scoring system
The Allred scoring system [22,23] was used for evaluating PDHA1 expression levels in ovarian carcinoma tissues. The intensity of the immunohistochemical staining was scaled from 0 to 3, and the percentage of immunostained cells was scaled from 0 to 5 (Table 1). The sum of the intensity score and percentage score was considered the total score, which ranged from 0 to 8. The slide was regarded as PDHA1 negative, low expression and high expression when the total scores were 0, 1 to 6 and 7 to 8, respectively. The morphology evaluation and immunostaining judgment were re-confirmed by two pathologists.
Table 1.
The criteria of Allred scoring system used for evaluating PDHA1 expression in the ovarian carcinoma cells in our study
| 1. The criteria of intensity scoring system | ||||||
| Intensity Score | 0 | 1 | 2 | 3 | ||
| Negative | Weak | Moderate | Strikingly positive | |||
| 2. The criteria of percentage scoring system | ||||||
| Percentage Score | 0 | 1 | 2 | 3 | 4 | 5 |
| <1% | 1-10% | 11-33% | 34-66% | 67-100% | ||
| 3. Total Scorea | ||||||
| 0 | 1-6 | 7-8 | ||||
| Negative | Low | High | ||||
The total score was obtained by adding the percentage score to intensity score.
It ranges from 0 to 8.
Statistical analyses
SPSS software (version 18.0) was used for data analyses. Associations between categorical variables were assessed by the Chi-square test (Pearson’s and linear-by-linear, as appropriate). Survival analysis was estimated using the Kaplan-Meier method, and groups were compared with the log-rank test. For all of the analyses, associations were considered to be significant if the P value was P<0.05.
Results
Variable expression of PDHA1 in ovarian cancer cell lines
Cytoplasmic PDHA1 was detected by ICC in all three of the following ovarian cancer cell lines: ES-2, SKOV-3 and OVCAR-3 (Figure 1). Comparatively, it was discovered that the ES-2 cell line was weakly positive (Figure 1), the SKOV-3 cell line was moderately positive (Figure 1), and the OVCAR-3 cell line was strongly positive for PDHA1 expression (Figure 1).
Figure 1.
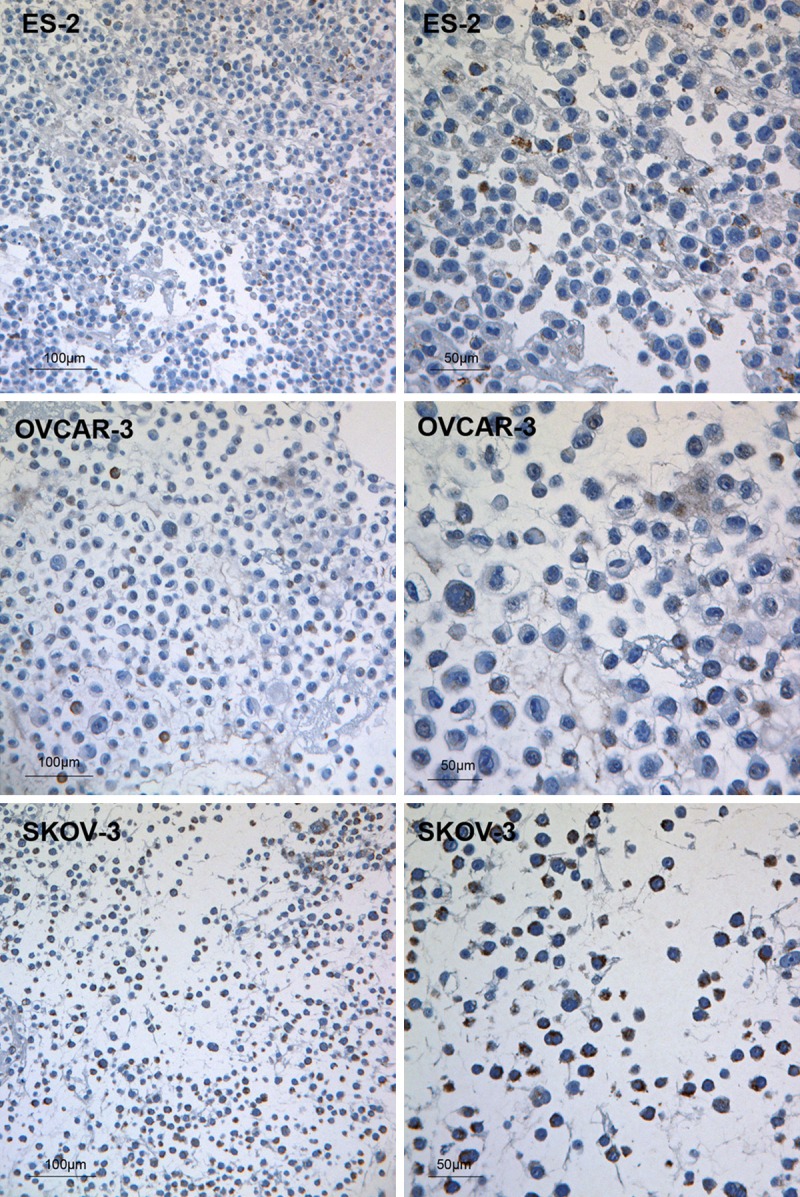
PDHA1 protein expression in ovarian cancer cell lines. Weakly positive for the PDHA1 antibody in the ES-2 cells; Moderately positive for the PDHA1 antibody in the OVCAR-3 cells; Strongly positive for the PDHA1 antibody in the SKOV-3 cells. The scale bars are indicated on the Figure.
PDHA1 expression in human ovarian cancer tissues
The typical diffuse cytoplasmic immunohistochemical staining of PDHA1 was variably detected in the ovarian carcinoma cells in all of the ovarian primary tumor samples (Figure 2). The endothelial cells of the blood vessels and stromal cells were positive for PDHA1. The immunostaining was limited to the cytoplasm. Of the 248 ovarian cancer tissue specimens, 45 cases (18.1%) were negative in tumor cells for PDHA1, 162 cases (65.3%) displayed a low expression level, and 41 cases (16.5%) had relatively high PDHA1 staining (Table 2). Generally, the tumor cells from well-differentiated carcinomas tended to show high expression for PDHA1, and those from poorly differentiated carcinomas tended to express low PDHA1. Compared with the variable PDHA1 expression in tumor cells, PDHA1 expression in the stromal cells and endothelial cells was generally positive.
Figure 2.
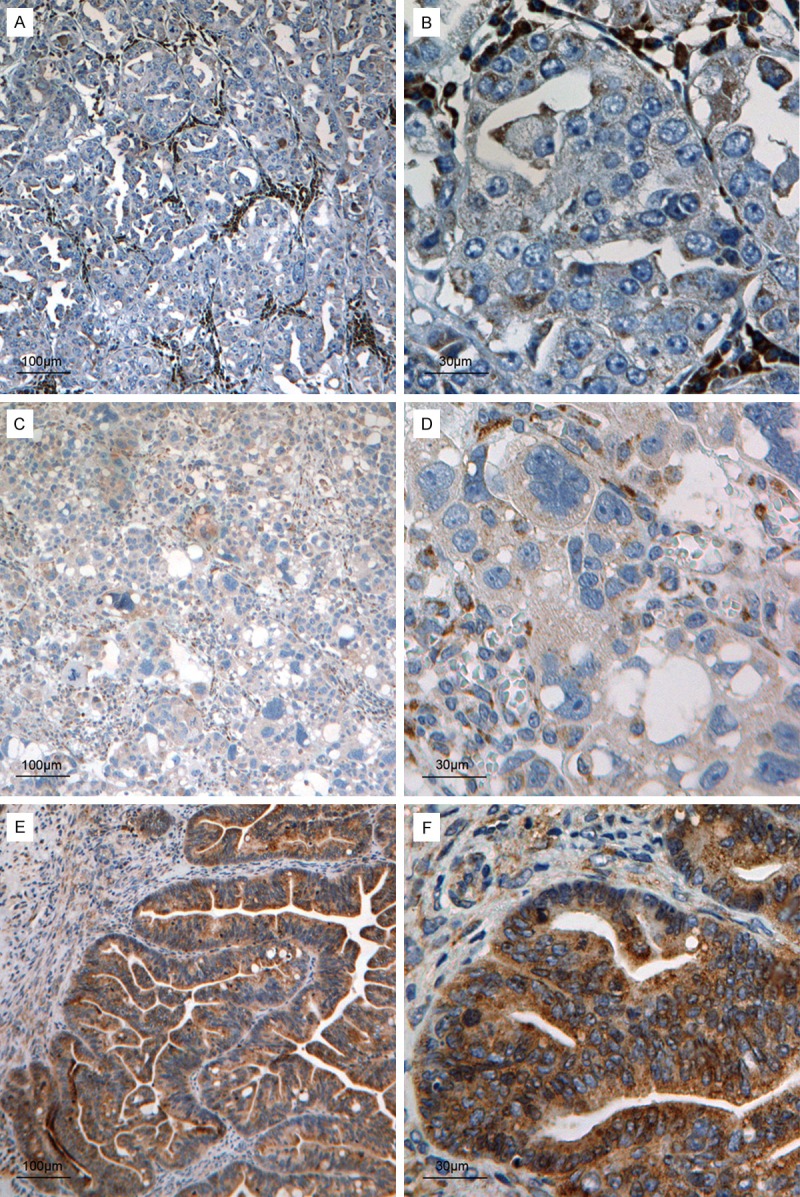
Variable PDHA1 expression in ovarian carcinoma cells. Negative PDHA1 protein expression in a poorly differentiated ovarian carcinoma (A and B); (Note: The stromal and endothelial cells surrounding the tumor cells are positive for the PDHA1 antibody). Weakly positive PDHA1 protein expression in a poorly differentiated ovarian carcinoma, in which stromal and endothelial cells are also positive for the PDHA1 antibody (C and D); Strongly positive PDHA1 protein expression in a well-differentiated ovarian carcinoma, in which tumor cells show strong cytoplasmic immunoreaction, and the stromal cells and endothelial cells in the tumor are also positive but relatively weakly (E and F). The scale bars are indicated on the Figure.
Table 2.
The associations of PDHA1 expression levels in ovarian carcinoma and the clinical and pathologic characteristics
| PDHA1 expression in ovarian carcinoma | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Total N | Negative | Low expression | High expression | P value | ||
| Age (years old) | 0.195 | |||||
| ≤39 | 16 | 2 (12.5%) | 9 (56.2%) | 5 (31.2%) | ||
| 40-49 | 41 | 4 (9.8%) | 26 (63.4%) | 11 (26.8%) | ||
| 50-59 | 64 | 11 (17.2%) | 44 (68.8%) | 9 (14.1%) | ||
| 60-69 | 72 | 17 (23.6%) | 43 (59.7%) | 12 (16.7%) | ||
| ≥70 | 39 | 10 (25.6%) | 26 (66.7%) | 3 (7.7%) | ||
| Missing | 16 | |||||
| Histological subtype | 0.004** | |||||
| Serous carcinoma | 163 | 36 (22.1%) | 112 (68.7%) | 15 (9.2%) | ||
| Mucinous carcinoma | 18 | 1 (5.6%) | 9 (50.0%) | 8 (44.4%) | ||
| Endometrioid carcinoma | 19 | 2 (10.5%) | 9 (47.4%) | 8 (42.1%) | ||
| Clear cell carcinoma | 11 | 0 (0%) | 9 (81.8%) | 2 (18.2%) | ||
| Mixed epithelial tumor | 11 | 2 (18.2%) | 6 (54.5%) | 3 (27.3%) | ||
| Undifferentiated tumor | 5 | 1 (20.0%) | 3 (60.0%) | 1 (20.0%) | ||
| Unclassified tumor and others | 21 | |||||
| FIGO Stage | 0.002** | |||||
| I | 28 | 1 (3.6%) | 15 (53.6%) | 12 (42.9%) | ||
| II | 18 | 3 (16.7%) | 9 (50.0%) | 6 (33.3%) | ||
| III | 117 | 19 (16.2%) | 84 (71.8%) | 14 (12.0%) | ||
| IV | 79 | 22 (27.8%) | 49 (62.0%) | 8 (10.1%) | ||
| Not staged or missing | 6 | |||||
| Histological Grade | 0.074 | |||||
| Well | 19 | 3 (15.8%) | 10 (52.6%) | 6 (31.6%) | ||
| Moderate | 62 | 10 (16.1%) | 40 (64.5%) | 12 (19.4%) | ||
| Poor | 133 | 30 (20.1%) | 90 (65.4%) | 13 (14.5%) | ||
| Not graded or missing | ||||||
P<0.01.
Clinicopathological correlation
As summarized in Table 2, it was found that decreased or negative expression of PDHA1 was significantly associated with a high FIGO stage and histological subtype (P<0.05), but not with age or differentiation grade (P>0.05). The ages at diagnosis were divided into five groups for the association analyses: ≤39, 40-49, 50-59, 60-69 and ≥70 years old. There were no significant differences among the five different age groups when the expression of PDHA1 in the tumor samples was considered (P=0.195, linear-by-linear association). In our study, it was discovered that highly positive PDHA1 protein expression in tumors was significantly associated with early FIGO stage (P=0.002, linear-by-linear association, Table 2). Well-differentiated carcinomas were regarded to have low malignant potential, and conversely, poorly differentiated carcinomas were regarded to have high malignant potential in ovarian carcinomas. Nevertheless, in our current study, we did not find any correlation between differentiation grade and PDHA1 protein expression (P=0.74), which was different from the general impression when we reviewed the slides. There were significant differences in PDHA1 expression levels in tumor cells between each histological subtype, with the highest in mucinous carcinoma and endometrioid carcinoma (P=0.004, linear-by-linear association, Table 2).
Because ovarian serous carcinoma is the most common histosubtype of the ovarian cancer, we also enrolled the largest sample size for this carcinoma. Moreover, the PDHA1 expression was closely correlated with the histosubtype. Therefore, we further analyzed the PDHA1 expression in the serous carcinomas. Of the 167 ovarian serous cancer tissue specimens, 35 cases (21.5%) were negative in tumor cells for PDHA1, 113 cases (68.9%) displayed a low expression level, and 15 cases (9.1%) had relatively high PDHA1 staining (Table 3). It was found that decreased or negative expression of PDHA1 was significantly associated with a high FIGO stage (P=0.014) and a poor differentiation grade (P=0.048), but not with age (P=0.172).
Table 3.
The associations of PDHA1 expression levels in ovarian serous carcinoma and the clinical and pathologic characteristics
| PDHA1 expression in serous carcinoma | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Total N | Negative | Low expression | High expression | P value | ||
| Age (years old) | 0.172 | |||||
| ≤39 | 9 | 1 (11.1%) | 8 (88.9%) | 0 (0%) | ||
| 40-49 | 26 | 3 (11.5%) | 20 (76.9%) | 3 (11.5%) | ||
| 50-59 | 47 | 10 (21.3%) | 33 (70.2%) | 4 (8.5%) | ||
| 60-69 | 52 | 12 (23.1%) | 33 (63.5%) | 7 (13.5%) | ||
| ≥70 | 24 | 8 (33.3%) | 15 (62.5%) | 1 (4.2%) | ||
| Missing | 6 | |||||
| FIGO Stage | 0.014* | |||||
| I | 7 | 0 (3.6%) | 5 (71.4%) | 2 (28.6%) | ||
| II | 4 | 1 (25.0%) | 2 (50.0%) | 1 (25.0%) | ||
| III | 93 | 17 (18.3%) | 68 (73.1%) | 8 (8.6%) | ||
| IV | 59 | 17 (28.8%) | 38 (64.4%) | 4 (6.8%) | ||
| Not staged or missing | 1 | |||||
| Histological Grade | 0.048* | |||||
| Well | 9 | 1 (11.1%) | 5 (55.6%) | 3 (33.3%) | ||
| Moderate | 44 | 9 (20.5%) | 31 (70.5%) | 4 (9.0%) | ||
| Poor | 100 | 24 (22.2%) | 71 (70.0%) | 5 (7.8%) | ||
| Not graded or missing | 11 | |||||
P<0.05.
Decreased PDHA1 expression in ovarian carcinoma cells was associated with unfavorable survival
There were a total of 232 valid cases with full information for the analyses of OS and PFS. The median OS time in the PDHA1-negative group, low expression group and high expression group were 0.939 years, 1.443 years and 9.900 years, respectively. The median PFS time in the above three groups were 0.287 years, 0.586 years and 9.900 years, respectively. Furthermore, high PDHA1 expression in ovarian carcinoma cells was significantly associated with better OS and PFS by statistical analyses (P<0.001 for both, Kaplan-Meier method, Figure 3A, 3B). There were a total of 158 valid cases with full information for the analyses of OS and PFS for serous carcinoma. The median OS time in the PDHA1 negative group, low expression group and high expression group of serous carcinoma were 0.988 years, 1.443 years and 4.802 years, respectively. The median PFS time in the above three groups were 0.476 years, 0.586 years and 2.185 years, respectively. Furthermore, high PDHA1 expression in the tumor cells of the serous ovarian carcinomas was significantly associated with better OS and PFS by statistical analyses (P=0.011 and 0.011, Kaplan-Meier method, Figure 3C, 3D).
Figure 3.
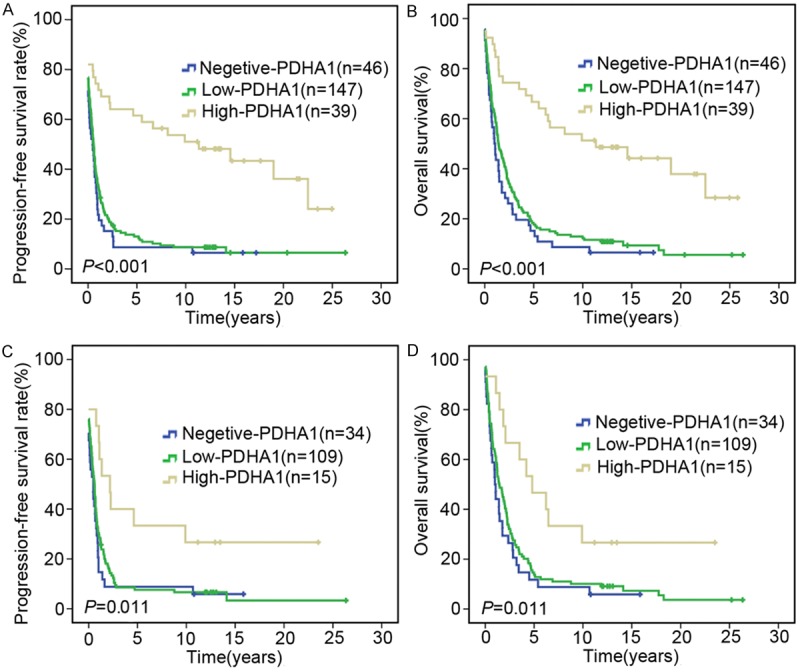
Survival probabilities. A. Survival plots disclose that higher levels of PDHA1 expression in tumor cells in ovarian carcinomas have a better OS than the low expression and negative groups (P<0.001). B. High levels of PDHA1 expression in tumor cells in ovarian carcinomas are significantly associated with high PFS probabilities (P<0.001). C. Survival plots show that high levels of PDHA1 expression in tumor cells in ovarian serous carcinomas have a better OS than the low expression and negative groups (P=0.011). D. High expression level of PDHA1 in tumor cells in ovarian serous carcinomas is significantly associated with high PFS probability (P=0.011).
PDHA1 expression in ovarian carcinoma is an independent risk factor for overall survival
Multivariate analyses were performed using the Cox proportional hazards model for all of the significant variables in the univariate analysis. Table 4 shows the analyses, adjusted for age, FIGO stage, differentiation grade, histological type and PDHA1 expression covariates. FIGO stage was an independent risk factor for overall survival in ovarian carcinomas (P<0.05). Moreover, high levels of PDHA1 expression were also an independent prognostic factor for longer OS (HR=0.705, 95% CI 0.541-0.918, P=0.01).
Table 4.
Unvariate and multivariate analysis for OS in a total of 232 valid ovarian carcinoma patients
| Unvariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristics | HR | CI (95%) | P value | HR | CI (95%) | P value |
| PDHA1 expression | 0.526 | 0.420-0.658 | 0.000*** | 0.705 | 0.541-0.918 | 0.01* |
| Age group | 1.354 | 1.202-1.525 | 0.000*** | 1.178 | 1.037-1.338 | 0.012* |
| Differentiation grade | 1.552 | 1.229-1.961 | 0.000*** | 1.318 | 1.029-1.687 | 0.029* |
| FIGO stage | 1.922 | 1.619-2.281 | 0.000*** | 1.615 | 1.303-2.002 | 0.000*** |
| Histological subtype | 0.923 | 0.838-1.107 | 0.104 | 0.993 | 0.903-1.092 | 0.889 |
Note: HR: Hazard ratio; 95% CI: 95% confidence interval.
P<0.05;
P<0.001.
Discussion
To evaluate the expression status of the PDHA1 protein in ovarian cancers, we first verified the protein expression in three commercial ovarian cancer cell lines, including ES-2, SKOV-3 and OVCAR-3. The specificity of the antibody was evaluated with both positive and negative controls. We discovered that the PDHA1 protein expression in the ES-2 cell line was low/weak. The ovarian carcinoma patients involved in our present study were diagnosed and verified with several subtypes by pathology, including the following: serous carcinoma, mucinous carcinoma, endometrioid carcinoma, clear cell carcinoma, mixed epithelial tumor undifferentiated tumor and others. We found that there were significantly lower levels of PDHA1 protein expression in the clear cell carcinoma group, consistent with the results for the ES-2 cell line. It is known that clear cell ovarian carcinoma is associated with poor clinical outcomes compared with other subtypes of epithelial ovarian cancers [24], and the low PDHA1 protein expression in the ES-2 and the clear cell carcinomas in our current study might indicate that the aggressive features of clear cell carcinoma occur at least in part due to low PDHA1 protein expression. Low levels of PDHA1 protein expression should result in decreased OXPHOS and increased glycolysis in tumors, which are typical results of the Warburg effect. However, this finding should be explained with care due to the limited number of clear cell carcinoma samples involved in this study.
To the best of our knowledge, this study was the first with a large series and long follow-up of patients with ovarian cancer to investigate the relationships of PDHA1 expression. We evaluated the PDHA1 protein expression by IHC in a series of 248 ovarian cancer samples and found no or low PDHA1 protein expression in tumors significantly associated with shorter progressive-free and overall survival.
A striking discovery in tumor metabolism was the finding by Warburg in 1920s, the so-called Warburg effect, which describes the increased utility of glycolysis over oxidative phosphorylation by tumor cells for their energy requirements, even under physiological oxygen conditions [25]. The important function of aerobic glycolysis in tumor progression has been recognized [26,27], although the molecular mechanisms leading to this phenotype and its functional significance in tumor development remain unknown. PDHA1 is a mitochondrial key rate-limiting enzyme for pyruvate conversion to acetyl-coA, and plays an important role in the TCA cycle. Inhibition of PDHA1 activity decreases mitochondrial OXPHOS and promotes tumor aerobic glycolysis in tumor cells [14,28]. Nevertheless, the expression status in ovarian cancer was largely unknown. Therefore, we chose to study the PDHA1 expression in a series of 248 ovarian carcinomas to determine the clinicopathological correlations and survival associations.
Further analyses discovered that negative/weak PDHA1 protein expression in the 248 tumors was significantly associated with a higher FIGO stage. Therefore, we propose that the loss of or low PDHA1 expression might contribute to the development and progression of ovarian cancer. Furthermore, we analyzed the prognostic role of PDHA1 in OS and PFS among patients with ovarian cancer and found that patients with stronger PDHA1 staining had a longer survival time. The results showed that stronger PDHA1 staining was significantly associated with better OS in ovarian cancer. Therefore, our results suggested that the expression of PDHA1 is an independent prognostic factor in PFS and OS.
PDH plays central and strategic roles in the control of the use of glucose-linked substrates as sources of oxidative energy or as precursors in the biosynthesis of fatty acids by catalyzing the conversion of pyruvate into acetyl-coA. The activity of PDH depends on the integrity of a multienzyme complex, which is comprised of PDHA1 (E1), dihydrolipoamide acetyltransferase (E2) and dihydrolipoamide dehydrogenase (E3). It is also regulated by the continuous operation of competing pyruvate dehydrogenase kinase (PDK) and pyruvate dehydrogenase phosphatase (PDP) reactions [11,12,29]. PDK-1 is a Ser/Thr kinase that negatively regulates PDHA1 activity by phosphorylating the PDHA1 subunit [29]. Mounting evidence has showed the role of PDK1 in tumor mitochondrial metabolism. PDK1 expression is elevated in human liver metastases, and HIF-1α/PDK1 promotes glycolytic metabolism in liver metastatic breast cancer cells and is required for the efficient formation of liver metastasis [28]. In cancer cells, lactate dehydrogenase A had been shown to play a critical role in glycolysis by converting pyruvate into lactate, and the dependence of glycolysis has been associated with poor prognosis in several tumors [30,31]. There have also been some previous reports defining the metabolic profiles of metastatic breast cancer cells, revealing a progressive shift toward a glycolytic phenotype as normal mammary epithelial cells progressed to non-metastatic cancer, as well as further changes of significant increases in glycolytic metabolism in metastatic cells [32-34]. A previous report showed that inhibiting PDHA1 and attenuating the flux of glycolytic carbon into mitochondrial oxidation promoted anoikis resistance and metastasis, and stimulating PDHA1 in cancer cells restored their susceptibility to anoikis and impaired their metastatic potential [35]. Maintaining the glycolysis pathway might be a more important aspect of the inhibition of PDHA1. In our study, the correlation between the expression of PDHA1 and OS in ovarian cancer patients might provide important evidence that a decrease in the quantity/quality of PDHA1 is implicated in cancer metabolism in the process of ovarian cancer development. However, the mechanism remains obscure and is a subject for further study. The above studies and our research strongly suggested that a decrease in the expression and activity of PDHA1 might accompany tumor initiation, growth and progression.
Together with the literature, our current finding of decreased PDHA1 protein expression in tumors might indicate that low levels of PDHA1 expression should result in reduced PDHA1 activity and thus, in part, promote a metabolic shift toward aerobic glycolysis and decrease the dependence on mitochondrial OXPHOS. Such a metabolic shift might confer on cells a selective advantage for survival, increasing their life span and vulnerability to carcinogenesis because the cells with low levels of PDHA1 expression/activity will face more mutational insults than other normal cells. If this hypothesis is true, extensive molecular studies of PDHA1 in ovarian cancer are warranted.
In summary, we report the following two important findings in our current study: compared with the other epithelial ovarian carcinoma cell lines SKOV-3 and OVCAR3, weak PDHA1 protein expression was discovered in the ES-2 clear cell ovarian cancer cell line and tumor samples. Furthermore, negative/weak PDHA1 protein expression in ovarian carcinomas was significantly associated with higher FIGO stage and shorter PFS and OS. The above findings strongly support the potential role of PHDA1 as a prognostic marker in ovarian cancers.
Acknowledgements
This study was financially supported by grants from the National Natural Science Foundation of China (81272824), Radium Hospital Research Foundation and the Norwegian Cancer Society.
Disclosure of conflict of interest
None.
References
- 1.Eskander RN, Tewari KS. Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma--mechanistics, review of phase III randomized clinical trials, and regulatory implications. Gynecol Oncol. 2014;132:496–505. doi: 10.1016/j.ygyno.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Worley MJ Jr, Guseh SH, Rauh-Hain JA, Esselen KM, Muto MG, Feltmate CM, Berkowitz RS, Del Carmen MG, Schorge JO, Horowitz NS. What is the optimal treatment for obese patients with advanced ovarian carcinoma? Am J Obstet Gynecol. 2014;211:231, e231–239. doi: 10.1016/j.ajog.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 3.Eskander RN, Tewari KS. Epithelial cell-adhesion molecule-directed trifunctional antibody immunotherapy for symptom management of advanced ovarian cancer. Clin Pharmacol. 2013;5:55–61. doi: 10.2147/CPAA.S45885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med. 2010;31:60–74. doi: 10.1016/j.mam.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr. 2007;39:251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 9.Simonnet H, Alazard N, Pfeiffer K, Gallou C, Beroud C, Demont J, Bouvier R, Schagger H, Godinot C. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759–768. doi: 10.1093/carcin/23.5.759. [DOI] [PubMed] [Google Scholar]
- 10.Bresters TW, de Kok A, Veeger C. The pyruvate-dehydrogenase complex from Azotobacter vinelandii. 2. Regulation of the activity. Eur J Biochem. 1975;59:347–353. doi: 10.1111/j.1432-1033.1975.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel MS, Korotchkina LG. Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases. Exp Mol Med. 2001;33:191–197. doi: 10.1038/emm.2001.32. [DOI] [PubMed] [Google Scholar]
- 12.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 13.Brown GK, Otero LJ, LeGris M, Brown RM. Pyruvate dehydrogenase deficiency. J Med Genet. 1994;31:875–879. doi: 10.1136/jmg.31.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Kang HB, Shan C, Elf S, Lin R, Xie J, Gu TL, Aguiar M, Lonning S, Chung TW, Arellano M, Khoury HJ, Shin DM, Khuri FR, Boggon TJ, Kang S, Chen J. Tyr-301 phosphorylation inhibits pyruvate dehydrogenase by blocking substrate binding and promotes the Warburg effect. J Biol Chem. 2014;289:26533–26541. doi: 10.1074/jbc.M114.593970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Y, Li X, Yu D, Li X, Li Y, Long Y, Yuan Y, Ji Z, Zhang M, Wen JG, Nesland JM, Suo Z. Application of mitochondrial pyruvate carrier blocker UK5099 creates metabolic reprogram and greater stem-like properties in LnCap prostate cancer cells in vitro. Oncotarget. 2015;6:37758–37769. doi: 10.18632/oncotarget.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baak JP, Wisse-Brekelmans EC, Langley FA, Talerman A, Delemarre JF. Morphometric data to FIGO stage and histological type and grade for prognosis of ovarian tumours. J Clin Pathol. 1986;39:1340–1346. doi: 10.1136/jcp.39.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zivanovic O, Sima CS, Iasonos A, Hoskins WJ, Pingle PR, Leitao MM Jr, Sonoda Y, Abu-Rustum NR, Barakat RR, Chi DS. The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum. Gynecol Oncol. 2010;116:351–357. doi: 10.1016/j.ygyno.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rokita M, Stec R, Bodnar L, Charkiewicz R, Korniluk J, Smoter M, Cichowicz M, Chyczewski L, Niklinski J, Kozlowski W, Szczylik C. Overexpression of epidermal growth factor receptor as a prognostic factor in colorectal cancer on the basis of the Allred scoring system. Onco Targets Ther. 2013;6:967–976. doi: 10.2147/OTT.S42446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi A, Pervez S. Allred scoring for ER reporting and it’s impact in clearly distinguishing ER negative from ER positive breast cancers. J Pak Med Assoc. 2010;60:350–353. [PubMed] [Google Scholar]
- 24.Lim W, Jeong W, Song G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol Cell Endocrinol. 2015;422:172–81. doi: 10.1016/j.mce.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 27.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(Suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 28.Dupuy F, Tabaries S, Andrzejewski S, Dong Z, Blagih J, Annis MG, Omeroglu A, Gao D, Leung S, Amir E, Clemons M, Aguilar-Mahecha A, Basik M, Vincent EE, St-Pierre J, Jones RG, Siegel PM. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015;22:577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Roche TE, Baker JC, Yan X, Hiromasa Y, Gong X, Peng T, Dong J, Turkan A, Kasten SA. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog Nucleic Acid Res Mol Biol. 2001;70:33–75. doi: 10.1016/s0079-6603(01)70013-x. [DOI] [PubMed] [Google Scholar]
- 30.Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409–415. [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Quan M, Jiang W, Hu H, Jiao F, Li N, Jin Z, Wang L, Wang Y, Wang L. Suppressed expression of LDHB promotes pancreatic cancer progression via inducing glycolytic phenotype. Med Oncol. 2015;32:143. doi: 10.1007/s12032-015-0589-8. [DOI] [PubMed] [Google Scholar]
- 32.Lu X, Bennet B, Mu E, Rabinowitz J, Kang Y. Metabolomic changes accompanying transformation and acquisition of metastatic potential in a syngeneic mouse mammary tumor model. J Biol Chem. 2010;285:9317–9321. doi: 10.1074/jbc.C110.104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, Asara JM, Kalluri R. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1001–1015. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T, Bouzin C, Feron O, Michiels C, Gallez B, Sonveaux P. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–1907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


