Abstract
Transarterial chemoembolization (TACE) is the principal therapy for unresectable hepatocellular carcinoma (HCC). However, its efficacy is currently limited owing to tumor progression or treatment failure. It has been shown that aspirin reduces the incidence of multiple malignant tumors including HCC and plays a synergistic role with chemotherapy in the treatment of colon cancer. Therefore, we aimed to investigate the adjuvant effect of aspirin on patients with unresectable HCC who underwent TACE therapy. A retrospective matched-pairs analysis was performed to evaluate the efficacy of aspirin in combination with TACE therapy. A total of 120 patients with HCC, including 60 patients treated with aspirin for treatment of cardiovascular disease, transient ischemic attack, and arthritis, and 60 paired matching HCC patients without aspirin treatment in the same period, were enrolled. Compared with non-aspirin users, patients treated with aspirin showed improved OS (P = 0.050). Specifically, patients treated with a full dose of aspirin showed prolonged OS (P = 0.027), which was an independent factor associated with OS in multivariate analysis (hazard ratio 0.498, 95% confidence interval 0.280-0.888, P = 0.018). Aspirin in combination with TACE might improve OS in patients with unresectable HCC. Thus, the impact of aspirin on patients with HCC warrants further investigation prospectively.
Keywords: Hepatocellular carcinoma (HCC), aspirin, overall survival (OS), transarterial chemoembolization (TACE)
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer mortality [1]. While surgical resection and local ablation remain the standard options for treating HCC, long-term survival prognosis remains unsatisfactory because of a high incidence of recurrence. Less than 30% of patients detected with HCC are initially eligible for curative therapy, owing to advanced stage or poor liver function at diagnosis.
Transarterial chemoembolization (TACE) is the standard treatment method for unresectable HCC [2]. Currently, TACE has shown to improve the median survival of patients from 16 to 20 months for intermediate-stage, and up to 8 months for advanced-stage HCC [3]. However, not all patients respond well to this procedure. TACE failure or refractoriness worsens disease progression, even in patients who show a good response to the procedure [4]. Various strategies to improve the outcome of TACE have included combination regimes; however, their efficacy is still limited [5-7]. Therefore, there is a need for effective and accessible adjuvant therapies that improve the outcome of patients with HCC who are undergoing TACE.
Aspirin is currently used extensively as a cardioprotective, antithrombotic, and anti-inflammatory agent [8]. Daily aspirin intake has demonstrated noticeable chemopreventive and chemotherapeutic benefits in colorectal carcinoma. Cyclooxygenase (COX)-dependent and -independent mechanisms have been suggested to explain this effect [8]. Taking into account that HCC is a chronic inflammation- related carcinogenesis characterized by high levels of COX-2, it is tempting to speculate that aspirin might be a potential therapeutic agent for HCC.
At present, an increasing number of preclinical studies have revealed the possible preventive and therapeutic benefits of aspirin for treatment of HCC [9]. Moreover, it has been reported that aspirin may reverse apoptosis resistance in HCC cell lines [10,11]. The antiplatelet function of aspirin is well known, and, to some extent, accounts for its chemotherapeutic effect, considering that platelet-secreted factors such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) play important roles in cancer initiation and progression [12]. Thus far, only a few clinical studies using aspirin have been performed in patients with HCC. Although Sahasrabuddhe et al. initially provided a large-scale, population-based evidence for reduced risk of liver cancer incidence and mortality associated with aspirin administration [13], the role of aspirin as an adjuvant therapy in patients with HCC remains to be defined.
Therefore, we performed a retrospective study and evaluated the association between aspirin intake and overall survival among patients with unresectable HCC who underwent comprehensive treatment based on TACE.
Methods
Study population
The retrospective study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University, and it conformed to the principles of the Declaration of Helsinkin- Ethical principles for Medical Research Involving Human subjects.
We performed a retrospective analysis using a database in patients diagnosed with HCC at the Department of Hepatic Oncology, Liver Cancer Institute, Zhongshan Hospital, Fudan University. Patients were diagnosed by histological examination or using noninvasive methods following the American Association for the Study of the Liver (AASLD) criteria [14].
Patients who had received the initial TACE during the period from January 1, 2008 to December 31, 2013 at the Department of Hepatic Oncology and patients who were administered acetylsalicylic acid (aspirin) for more than three months during the observation period (from one year before the diagnosis of HCC till the date of death/the latest follow-up, according to the medical records and the prescription records of the outpatients in the database, were included in the study. Patients with multiple types of primary cancers and those with a history of other types of cancer were excluded. Finally, 64 patients who were administered aspirin in addition to routine comprehensive TACE treatment were identified and enrolled in this study.
Matched control patients were enrolled based on the criteria of age, gender, date of HCC diagnosis, Child-Pugh score, following treatment after the initial TACE, tumor size, tumor number, vascular invasion, and metastasis. Further, given that certain diseases for which aspirin was prescribed might influence life expectancy, these diseases were considered for this paired-matching study. If more than one patient fulfilled these criteria, the initial date of HCC diagnosis was used for optimal matching.
Dose of patients treated with aspirin
Patients who were administered at least 100 mg/day of aspirin continuously for more than 3 months were considered “full dose users” and patients who received aspirin for more than 3 months but intermittently were defined as “non-full dose users”.
TACE procedure
TACE was performed according to the standard protocols of the institution [2]. Briefly, angiographies were performed to identify the tumor-feeding artery. The desired tumor-feeding artery was catheterized using a 5-Fr RH catheter (Cook Inc., Bloomington, IN, USA), and microcatheters (2.7-Fr; PROGREAT, Terumo Co., Tokyo, Japan) were used if required. Following perfusion of chemotherapeutic agents such as 5-fluorouracil 1.0 g and oxaplatin 150 mg, 5-20 mL of lipiodol was slowly infused. For some patients with large tumors and hypervascularity, further embolization with gelatin sponge particles (1-2 mm) was performed.
The general condition of the patients, and the liver function and blood test levels were taken into account while preparing the individual dosage regimen. Patients with confirmed residual tumor or tumor progression received repeated TACE with an interval of six weeks.
Follow-up
All patients underwent routine follow-up, as described previously [2]. Briefly, serum alpha-fetoprotein, liver and renal function, blood routine, and contrast-enhanced tomography/magnetic resonance imaging (CT/MRI) were evaluated one month after TACE. When complete response (as identified with no arterial phase enhancement of CT/MRI) was achieved, a follow-up with CT/MRI was conducted at an interval of 8 or 12 weeks. All patients were followed up until May 31, 2015.
The primary endpoint was the overall survival (OS). Survival time was defined as the time from the initial TACE to the last follow-up or death. Patients who were alive at the last follow-up were considered as censored data.
Statistical analysis
Baseline characteristics were reported as numbers with percentages for categorical variables and medians for continuous variables. Paired t test and Chi-square test (McNemar-Bowker test) were used to test for significant differences between the two groups of patients. Survival curves were constructed using the Kaplan-Meier method and compared using a log-rank test. Cox proportional hazards model was used for multivariate analysis. All statistics were two-tailed, and P < 0.05 was considered statistically significant. All data were analyzed using the SPSS v.19.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 64 patients who were administered aspirin in addition to routine comprehensive TACE treatment were identified. Among these, four patients were unmatchable, finally leaving 60 patients and 60 control subjects for analysis.
Among aspirin users, a total of 45 patients were prescribed aspirin for treatment of cardiovascular disease, including acute coronary syndrome (ACS), ischemic stroke, and transient ischemic attack (TIA), five patients were prescribed aspirin for arthritis, and ten patients received aspirin for primary prevention of ACS. In total, 46 (76.6%) patients were full dose aspirin users. The characteristics of patients with aspirin intake and control subjects are shown in Table 1. No statistically significant differences in baseline characteristics were observed between the groups.
Table 1.
Baseline characteristics of patients
| Characteristics | Aspirin users (n = 60) | Non-aspirin users (n = 60) | P-value |
|---|---|---|---|
| Age (y) | 67.2 ± 15.5 | 66.0 ± 15.6 | 0.56 |
| Gender (Male/Female) | 48/12 | 48/12 | 1 |
| Underlying liver disease | |||
| TB (≤ 3 mg) | 54 (90%) | 57 (95%) | 0.491 |
| GGT (≤ 75 µ/L) | 34 (56.7%) | 27 (45%) | 0.273 |
| PLT (≤ 100 × 109/L) | 17 (28.3%) | 20 (33.3%) | 0.693 |
| Child-Pugh A | 59 (98.3%) | 59 (98.3%) | 1 |
| HBV infection | 56 (93.3%) | 54 (90%) | 0.764 |
| Anti-HCV positive | 2 (3.3%) | 3 (5.0%) | 1 |
| Cirrhosis | 49 (81.7%) | 48 (80%) | 1 |
| Anti-HBV treatment | 9 (15%) | 15 (25%) | 0.238 |
| Tumor characteristics at initial TACE | |||
| AFP (ng/mL) | 2533.6 ± 10993.6 | 3943.4 ± 12681.3 | 0.43 |
| Tumor size (cm) | 4.5 ± 3.3 | 4.8 ± 3.0 | 0.063 |
| Tumor number (solitary) | 45 (75%) | 47 (78.3%) | 0.662 |
| Vascular invasion | 9 (15%) | 9 (15%) | 1 |
| Metastasis | 0 | 0 | 1 |
| Combined treatment | |||
| Hepatic resection | 12 (19.7%) | 10 (16.4%) | 0.687 |
| TACE number | 2.3 ± 2.3 | 2.5 ± 2.2 | 0.616 |
| RFA | 19 (31.7%) | 21 (35%) | 0.150 |
Abbreviations: TB, total bilirubin; GGT, gamma-glutamyl transferase; PLT, platelet; AFP, alpha-fetoprotein; RFA, radiofrequency ablation.
Overall survival (OS)
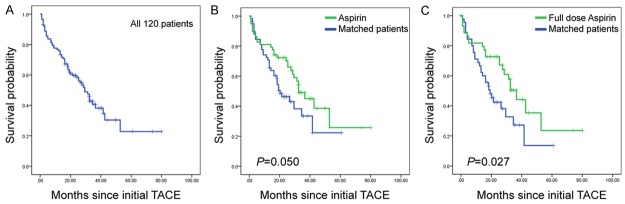
The median overall follow-up duration was 25.4 (range, 4.0-82.2) months. As showed in Figure 1A and 1B, patients from the “aspirin users” group showed improved OS (P = 0.050). The median OS was 32.5 (95% confidence interval [CI]; range, 23.6-41.4) months in aspirin users, and the 1-, 2-, 3-, and 4-year cumulative survival rates were 81%, 72%, 47%, and 38%, respectively. The median OS in “non-aspirin users” was 20.3 (95% CI; range, 12.2-28.3) months, and the 1-, 2-, 3-, and 4-year cumulative survival rates were 73%, 45%, 34%, and 23%, respectively. We then performed further characterization of the aspirin use. The full dose aspirin users had a median OS of 36.5 months, whereas the median OS of the matched control subjects was 19.2 months; the difference was statistically significant (P = 0.027, Figure 1C).
Figure 1.
Kaplan-Meier curves illustrating overall survival. A: Overall survival curve of all 120 patients. B: Overall survival curves of aspirin users and their matched pairs. C: Overall survival curves of full dose aspirin users and their matched pairs.
For the 46 full dose aspirin users and their matched pairs, further multivariable analysis showed that full aspirin use (hazard ratio [HR] 0.498, 95% CI 0.280-0.888, P = 0.018) was independently associated with OS after adjusting for age at initial TACE, gender, total bilirubin and gamma-glutamyl transferase (GGT) levels, tumor size and number, tumor vascular invasion, and after HCC treatment (Table 2). However, this was not the case for aspirin users, including both full dose users and non-full dose users, and their matched pairs (HR 0.604, 95% CI 0.354-1.033, P = 0.066). Further, GGT levels, tumor size, and tumor vascular invasion were crucial factors for the OS of patients with HCC (Tables 2 and 3).
Table 2.
Univariate and multivariable Cox proportional hazards regression: risk of mortality (46 full dose aspirin users vs. 46 matched subjects)
| Characteristics | Univariate P | Multivariate P | HR (95% CI) |
|---|---|---|---|
| Full dose aspirin usage | 0.027* | 0.018* | 0.498 (0.280-0.888) |
| Age (≤ 65 y/> 65 y) | 0.917 | 0.245 | 1.419 (0.787-2.559) |
| Gender (male/female) | 0.464 | 0.084 | 0.545 (0.274-1.084) |
| Underlying liver disease | |||
| TB (≤ 3 mg/> 3 mg) | 0.023* | 0.071 | 2.356 (0.930-5.974) |
| GGT (≤ 75 µL/> 75 µL) | 0.011* | 0.008* | 2.303 (1.249-4.246) |
| PLT (≤ 100 × 109/L)/> 100 × 109/L) | 0.156 | ||
| Anti-HBV treatment | 0.156 | ||
| Cirrhosis | 0.782 | ||
| Tumor characteristics at initial TACE | |||
| Tumor size (≤ 5 cm/> 5 cm) | 0.001* | 0.031* | 2.087 (1.070-4.068) |
| Tumor number (solitary/multiple) | 0.899 | 0.529 | 0.784 (0.367-1.674) |
| Vascular invasion (without/with) | 0.044* | 0.049* | 2.539 (1.003-6.426) |
| Combined treatment | |||
| Hepatic resection (with/without) | 0.183 | 0.191 | 0.543 (0.217-1.356) |
| RFA (with/without) | 0.089 | 0.881 | 0.953 (0.504-1.801) |
Abbreviations: TB, total bilirubin; GGT, gamma-glutamyl transferase; PLT, platelet; AFP, alpha-fetoprotein; RFA, radiofrequency ablation.
P < 0.05.
Table 3.
Univariate and multivariable Cox proportional hazards regression: risk of mortality (60 aspirin users vs. 60 matched)
| Characteristics | Univariate P | Multivariate P | HR (95% CI) |
|---|---|---|---|
| Aspirin usage | 0.050* | 0.066 | 0.604 (0.354-1.033) |
| Age (≤ 65 y/> 65 y) | 0.957 | 0.387 | 1.301 (0.717-2.363) |
| Gender (male/female) | 0.203 | 0.193 | 0.637 (0.323-1.257) |
| Underlying liver disease | |||
| TB (≤ 3 mg/> 3 mg) | 0.014* | 0.091 | 2.253 (0.879-5.771) |
| GGT (≤ 75 µL/> 75 µL) | 0.000* | 0.006* | 2.327 (1.268-4.272) |
| PLT (≤ 100 × 109/L /> 100 × 109/L) | 0.880 | ||
| Anti-HBV treatment | 0.246 | ||
| Cirrhosis | 0.918 | ||
| Tumor characteristics at initial TACE | |||
| Tumor size (≤ 5 cm/> 5 cm) | 0.000* | 0.094 | 1.757 (0.908-3.402) |
| Tumor number (solitary/multiple) | 0.993 | 0.579 | 0.805 (0.373-1.733) |
| Vascular invasion (without/with) | 0.194 | 0.071 | 2.393 (0.929-6.161) |
| Combined treatment | |||
| Hepatic resection (with/without) | 0.093 | 0.210 | 0.547 (0.214-1.403) |
| RFA (with/without) | 0.103 | 0.767 | 0.908 (0.479-1.720) |
Abbreviations: TB, total bilirubin; GGT, gamma-glutamyl transferase; PLT, platelet; AFP, alpha-fetoprotein; RFA, radiofrequency ablation.
P < 0.05.
During the follow-up, 63 deaths were observed, which included 29 aspirin users and 34 matched control subjects. Of the 63 deaths, 76.2% were related to progression of HCC. Six patients in the aspirin group and seven patients in the non-aspirin group died because of upper gastrointestinal bleeding. Two patients in the aspirin group and five patients in the non-aspirin group died of cerebral/myocardial infarction. No significant difference was observed in the causes of death between the groups (P = 0.803).
Discussion
In this retrospective study, it was observed that adjuvant aspirin therapy in patients with unresectable HCC who underwent TACE treatment may improve OS without increasing the chance of fatal bleeding. To our knowledge, this is the first study evaluating the combined effects of aspirin and TACE therapy in palliative treatment of patients with HCC.
Several studies have supported a possible chemotherapeutic role of aspirin in colorectal carcinoma. However, the underlying mechanism by which aspirin acts as an anticancer agent remains elusive.
The antiplatelet function of aspirin, to some extent, accounts for its anticancer effect. It is well known that the apparent anti-colorectal cancer effect of aspirin is detectable at low-dose intake (75 mg daily), and much higher doses (1,200 mg daily) are not more effective [15]. In fact, aspirin exhibits a very short half-life in serum and exerts a relatively selective inhibition of COX-1 in platelets. Thus, long-lasting suppression of platelet activation might be the main mechanism of action of low-dose aspirin, acting upstream of platelet-driven COX-2 expression in other cells in the tumor site [16]. Direct evidence using antiplatelet therapy has shown to prevent HCC and improve median survival [12]. This might also help to explain the reduced risk of cancer metastases in cardiovascular trials associated with low-dose aspirin [17], and the prolonged survival of patients with unresectable HCC observed in our study.
It has also been suggested that the anti-colorectal cancer effect of aspirin might be, at least partly, via inhibition of COX-2 activity [18]. It has been reported that aspirin may increase the expression pattern as well, indicating a mesenchymal-to-epithelial transition in tumor cells through inhibition of COX-2, thus leading to improvement of OS in vivo [19]. Unlike “housekeeping” COX-1, “inducible” COX-2 is less sensitive to aspirin. Thus, higher doses, shorter intervals, and continuous intake of aspirin are necessary to inhibit COX-2. A possible explanation for our findings is the fact that exposure to a full dose of aspirin improved the OS of patients with HCC.
The anticancer effect of aspirin could be attributed to its ability to enhance the sensitivity of cancer cells to anticancer therapy, thus improving OS. Several in vitro studies have shown that 5-fluorouracil (5-Fu), a commonly used drug in TACE, in combination with aspirin, increases the sensitivity of initially 5-Fu-resistant cancer cells [20]. Moreover, aspirin improves the anti-HCC effect of sorafenib [19]. Further, aspirin-pretreated cells have shown increased sensitivity towards radiation-induced apoptosis [21]. Jacobs et al. reported prolonged survival of a patient with prostate cancer who received aspirin in conjunction with radiation therapy [22].
Despite the chemotherapeutic role of aspirin in cancer, clinicians do not prescribe aspirin for HCC at present, probably because of its adverse effects including the risk of gastrointestinal bleeding. However, it has been suggested that low-dose aspirin (75-100 mg) reduces the number of major bleeding complications; adverse effects are observed at a dose of 500-1000 mg. However, some individuals might show increased susceptibility to bleeding owing to advanced age, a history of malignant bleeding, and concomitant treatment with other non-steroidal anti-inflammatory drugs or anticoagulant agents [23]. Concomitantly, in our study, where the majority of aspirin prescriptions contained a dosage of 100 mg, we detected only one patient in the “aspirin users” group who discontinued aspirin treatment because of gastrointestinal bleeding. Further, the ratio of deaths due to gastrointestinal bleeding in both groups was similar. Additionally, in the matched-pairs “non-aspirin users” group, more patients died because of the presence and/or recurrence of a serious cardiovascular event. Thus, low-dose aspirin regimen may be continued unless foreseeable bleeding risks are present in patients who are taking aspirin for other diseases, prior to the diagnosis of HCC.
Although low-dose aspirin intake might currently be safe for patients with cancer, efforts to reduce the risk of bleeding are warranted. Previously, proton pump inhibitors were used to reduce the risk of developing aspirin-associated ulcers. However, no randomized control trial (RCT) for this therapy was completed using bleeding as the primary endpoint [23]. Recently, Kodela et al. showed that NOSH-aspirin, a hybrid entity capable of releasing two gasotransmitters (nitric oxide and hydrogen sulfide) showed improved chemoprevention and gastrointestinal safety than that observed when using aspirin alone [24].
The retrospective nature of our study represents its main limitation. Therefore, selection bias cannot be ruled out. Patients were prescribed aspirin for prevention of other diseases such as ACS, and not specifically as therapy for HCC. Although a 1:1 matched-pairs analysis was performed and basic diseases, patient age, and other characteristics were carefully considered, the influence of these diseases on OS cannot be excluded. Patients with well-preserved liver function and adequate bone marrow function showed better tolerance to the side effects of aspirin. Further, observational bias such as failure of aspirin administration or bleeding evidence in our hospital records cannot be ignored.
In summary, this is the first study to propose a role of aspirin as a combined approach with TACE therapy in patients with unresectable HCC. Given the retrospective nature and the relatively small sample size of our study, further prospective studies and RCTs are encouraged to validate the adjuvant role of aspirin in the treatment of HCC.
Acknowledgements
This study was supported by National Clinical Key Special Subject of China and Nation Natural Science Foundation of China (81172275 and 81272565).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Li JH, Xie XY, Zhang L, Le F, Ge NL, Li LX, Gan YH, Chen Y, Zhang JB, Xue TC, Chen RX, Xia JL, Zhang BH, Ye SL, Wang YH, Ren ZG. Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol. 2015;21:3970–7. doi: 10.3748/wjg.v21.i13.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanoff HK, Chang Y, Stavas JM, Sturmer T, Lund J. Effectiveness of Initial Transarterial Chemoembolization for Hepatocellular Carcinoma Among Medicare Beneficiaries. J Natl Compr Canc Netw. 2015;13:1102–10. doi: 10.6004/jnccn.2015.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T Liver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 5.Erhardt A, Kolligs F, Dollinger M, Schott E, Wege H, Bitzer M, Bitzer M, Gog C, Lammert F, Schuchmann M, Walter C, Blondin D, Ohmann C, Häussinger D. TACE plus sorafenib for the treatment of hepatocellular carcinoma: results of the multicenter, phase II SOCRATES trial. Cancer Chemother Pharmacol. 2014;74:947–54. doi: 10.1007/s00280-014-2568-8. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Lu L, Tak WY, Yu X, Lee JH, Lin SM, Wu C, Tanwandee T, Shao G, Walters IB, Dela Cruz C, Poulart V, Wang JH. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60:1697–707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Ng J, Christos PJ, Goldenberg AS, Sparano J, Sung MW, Hochster HS, Muggia FM. Chronic thalidomide and chemoembolization for hepatocellular carcinoma. Oncologist. 2014;19:1229–30. doi: 10.1634/theoncologist.2014-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasche B, Wang M, Pennison M, Jimenez H. Prevention and treatment of cancer with aspirin: where do we stand? Semin Oncol. 2014;41:397–401. doi: 10.1053/j.seminoncol.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrat F. Statin and aspirin for prevention of hepatocellular carcinoma: what are the levels of evidence? Clin Res Hepatol Gastroenterol. 2014;38:9–11. doi: 10.1016/j.clinre.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Zhang S, Fang H, Yan B, Zhao Y, Feng L, Ma X, Ye X. Aspirin overcomes Navitoclax-resistance in hepatocellular carcinoma cells through suppression of Mcl-1. Biochem Biophys Res Commun. 2013;434:809–14. doi: 10.1016/j.bbrc.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Dong ZR, Guo ZY, Wang CH, Tang ZY, Qu SF, Chen ZT, Li XW, Zhi XT. Aspirin enhances IFN-alpha-induced growth inhibition and apoptosis of hepatocellular carcinoma via JAK1/STAT1 pathway. Cancer Gene Ther. 2013;20:366–74. doi: 10.1038/cgt.2013.29. [DOI] [PubMed] [Google Scholar]
- 12.Sitia G, Iannacone M, Guidotti LG. Anti-platelet therapy in the prevention of hepatitis B virus-associated hepatocellular carcinoma. J Hepatol. 2013;59:1135–8. doi: 10.1016/j.jhep.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, Hollenbeck AR, Freedman ND, McGlynn KA. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:1808–14. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 16.Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Munch G, John CM, Suess B, Sgambato A, Steinhilber D, Patrignani P. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84:25–40. doi: 10.1124/mol.113.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 18.Alfonso L, Ai G, Spitale RC, Bhat GJ. Molecular targets of aspirin and cancer prevention. Br J Cancer. 2014;111:61–7. doi: 10.1038/bjc.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, Sun HC, Zhang W, Chai ZT, Zhu XD, Kong LQ, Wang WQ, Zhang KZ, Zhang YY, Zhang QB, Ao JY, Li JQ, Wang L, Wu WZ, Tang ZY. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PLoS One. 2013;8:e65023. doi: 10.1371/journal.pone.0065023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman M, Selvarajan K, Hasan MR, Chan AP, Jin C, Kim J, Chan SK, Le ND, Kim YB, Tai IT. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia. 2012;14:624–33. doi: 10.1593/neo.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KY, Seol JY, Jeon GA, Nam MJ. The combined treatment of aspirin and radiation induces apoptosis by the regulation of bcl-2 and caspase-3 in human cervical cancer cell. Cancer Lett. 2003;189:157–66. doi: 10.1016/s0304-3835(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs CD, Chun SG, Yan J, Xie XJ, Pistenmaa DA, Hannan R, Lotan Y, Roehrborn CG, Choe KS, Kim DW. Aspirin improves outcome in high risk prostate cancer patients treated with radiation therapy. Cancer Biol Ther. 2014;15:699–706. doi: 10.4161/cbt.28554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrono C. The Multifaceted Clinical Readouts of Platelet Inhibition by Low-Dose Aspirin. J Am Coll Cardiol. 2015;66:74–85. doi: 10.1016/j.jacc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Kodela R, Chattopadhyay M, Velazquez-Martinez CA, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem Pharmacol. 2015;98:564–72. doi: 10.1016/j.bcp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]