Key Points
Significant expansion only of native splenic macrophages that are F4/80+/Cd11blo occurs in both post-Epo and post–hemolysis-induced stress.
VCAM-1−/− mice, like Spi-C−/−, mice have significantly decreased macrophages but did not have a compromised E-stress response.
Abstract
Although the importance of native bone marrow and spleen macrophages in enhancing baseline and stress erythropoiesis has been emphasized over several decades, their kinetic and phenotypic changes during a variety of stress responses have been unclear. Furthermore, whether monocyte-derived recruited macrophages can functionally substitute for inadequate or functionally impaired native macrophages has been controversial and seem to be not only tissue- but also stress-type dependent. To provide further insight into these issues, we made detailed observations at baseline and post-erythroid stress (E-stress) in 2 mouse models with genetically depressed macrophage numbers and compared them to their controls. We documented that, irrespective of the stress-induced (hemolytic or post-erythropoietin [Epo]) treatment, only native CD11blo splenic macrophages expand dramatically post-stress in normal mice without significant changes in the monocyte-derived CD11bhi subset. The latter remained a minority and did not change post-stress in 2 genetic models lacking either Spi-C or VCAM-1 with impaired native macrophage proliferative expansion. Although CD11blo macrophages in these mice were one-fifth of normal at their peak response, surprisingly, their erythroid response was not compromised and was similar to controls. Thus, despite the prior emphasis on numerical macrophage reliance to provide functional rescue from E-stress, our data highlight the importance of previously described non–macrophage-dependent pathways activated under certain stress conditions to compensate for low macrophage numbers.
Introduction
The link between erythroid-cell responses and macrophage number and function has been extensively emphasized previously.1-7 This macrophage/erythroblast (Eb) relationship is exerted within the bone marrow (BM) or spleen in the context of cellular niches termed erythroid or erythroblastic islands (EIs). Although initial studies focused on the role of macrophages to provide iron for heme synthesis, and to phagocytize effete red blood cells and the expelled nuclei from Ebs, later studies provided several additional roles for macrophages, influencing proliferation and/or survival of erythroid cells. Guiding these functions were several adhesive signals between macrophages and erythroid cells, although the dominant or precise molecular pathways initiated in each case are unclear.2,8-12
Studies on the phenotypic characterization of central macrophages within EIs revealed that although there is no single molecule expression unique to the central macrophage, it can be characterized by a combination of molecules expressed on its surface. Among these are the pan-macrophage markers F4/80 and CD68, as well as CD169 and the adhesion molecule VCAM-1.13 Regarding the monocytic markers CD11b and Ly6G, there is currently a discrepancy in the literature. For example, CD169+ and Ly6G+ macrophages were identified as supporting BM erythropoiesis,14,15 but other studies have concluded that CD169+ macrophages or the EI macrophages “express low to intermediate CD11b, whereas Ly6G+ macrophages exhibit high CD11b expression.”13,16-18
Under conditions of erythroid stress (E-stress), when demands are imposed on enhancement of erythroid cell growth and maturation, a proper cellular crosstalk among components within the EIs and positive feedback pathways from their microenvironment become especially important. Thus, it is not surprising that various functional impairments described in either macrophages or Ebs are mainly manifested under stress. Molecules providing adhesive contact between macrophages and Ebs (alpha4 integrin/VCAM-1,8,19,20 ICAM4/alphav/alpha4 integrins,10,11,21,22 CD163,23 palladin,12 as well as extracellular matrix proteins, fibronectin,24,25 and laminin),26-28 exert regulatory roles mostly under stress by influencing growth, differentiation, and adhesion/migration of erythroid cells. In addition to direct contact, soluble factors secreted by either Ebs or macrophages influence stress erythropoiesis. These include the positive regulators growth arrest specific protein 6 (Gas6),29,30 vascular endothelial growth factor A (VEGF-A), and placental growth factor (PlGF)31 released by Ebs.
Beyond functional defects, the influence of macrophage numbers on the outcome of stress response has been studied by extraneous reductions of their numbers,13-15,32 or in certain genetic mouse models with low macrophage numbers.33-35 However, extraneous reductions by clodronate or diphtheria toxin-induced macrophage deletion may exert additional off-target effects on other tissue cells, as noted previously,36-38 making the data hard to interpret. On the other hand, in mice with genetic deficiencies in red pulp macrophages (RPMs), it has been concluded that monocyte-derived, CD11bhi cells, acquire post–E-stress a functionally competent macrophage phenotype to address functional demands.33 However, the above conclusions about the functional compensation of recruited CD11bhi macrophages are in conflict with other previously forwarded data, suggesting that in cases of non-genotoxic macrophage depletion, native tissue macrophages respond by enhanced in situ proliferation without any monocyte contribution to the functional tissue macrophage pool.39,40 Whether these controversial outcomes are dependent on different forms of stress is not clear.
In order to provide further insights on the contribution of specific macrophage subsets to the E-stress response, in the present study we assessed quantitative kinetic changes of macrophage subsets in response to hemolytic stress or post-erythropoietin (Epo) treatment in normal mice, and compared the data to the ones from 2 genetic mouse models with drastically reduced numbers of macrophages at baseline and post-stress. Despite the low number of macrophages in the latter mice carrying a putative unfavorable signature, no detrimental erythroid responses were noted. These unexpected data expand our current knowledge of players involved in E-stress response in mice.
Methods
Mice and treatments
Spi-C–deficient mice were described elsewhere.18,33 They were a generous gift from D. Shayakhmetov (Atlanta, GA). In our study, we used Spi-C+/− as controls for Spi-C−/− mice. VCAM-1Δ/Δ (Tie2cre+/VCAM-1f/f) mice41,42 in the present study were on a C57Bl/6 background, the same as our α4Δ/Δ mice (Tie2cre+/α4f/f)43 and control mice. Phenylhydrazine (PHZ)-induced stress: mice received a single PHZ injection (100 mg/kg, intraperitoneally) on day 1 and were euthanized later either on day 4 or on day 6. In some experiments, after the first PHZ injection, mice were allowed to recover for 3 weeks, received the second PHZ injection, and were euthanized on day 6. Epo-induced stress: mice received 3 injections of hu rEpo (100 U per mouse, intraperitoneally) on consecutive days and were euthanized on different days. Transplantation: wild-type (WT) recipients were lethally irradiated and received 5 × 106 of either WT (control) or VCAM-1Δ/Δ BM cells. After BM reconstitution (at 9 weeks), mice were challenged with PHZ. In some experiments, mice received a single injection of 100 μg control rat immunoglobulin G2b or CD24 murine monoclonal antibodies (mAbs) (clone M1/69), and were euthanized 5 days later. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Flow cytometry
Freshly isolated BM and spleen cells were immunostained at 4°C with combinations of mAbs. For macrophage evaluation, we used VCAM-1–Alexa 488 (clone 429; BioLegend, San Diego, CA), CD11b-phycoerythrin (PE) (BioLegend), and F4/80-allophycocyanin (APC) (eBioscience, San Diego, CA); in some experiments, CD169-PE (clone 3D6.112; BioLegend) was used in combination with VCAM-1, F4/80, and CD11b mAbs; to assess cycling macrophages, Ki-67–fluorescein isothiocyanate (FITC) (BD Biosciences, San Jose, CA) was used together with F4/80, CD11b, and VCAM-1 mAbs according to the manufacturer’s instructions. For erythroid evaluation, we used CD71-FITC (BD Biosciences), Ter119-PE (eBioscience), CD44-APC (BioLegend) or CD71-FITC (BD Biosciences), Ter119-PE (eBioscience), and CD117/cKit-APC (Southern Biotech, Birmingham, AL). Eb maturation profile was assessed as follows: pro-Ebs were Ter119loCD71hi, whereas the Ter119+ cells were subdivided into basophilic Ebs (Ter119+ CD44hi FSChi); polychromatic Ebs were Ter119+CD44medFSCmed; and orthochromatic Ebs were Ter119+CD44loFSClo. Fluorescence-activated cell sorter (FACS) analysis was done using a FACSCalibur system (BD Immunocytometry Systems, San Jose, CA) and FloJo software.
EI preparations
EI preparations were made from the BM and spleen, essentially as described.16,20 Cytospin preparations of EIs were stained with Hema3 (Thermo Fisher Scientific, Waltham, MA) or immunostained with biotinylated F4/80, or CD11b, or VCAM-1 antibodies, followed by Streptavidin-Alexa 488 (all from BD Biosciences). In some experiments, isolated EIs were incubated with 10 mM EDTA to strip Ebs from macrophages, and then stained with F4/80 and CD169 mAbs for FACS analysis.
Results
Quantitative and phenotypic changes in macrophages in response to E-stress
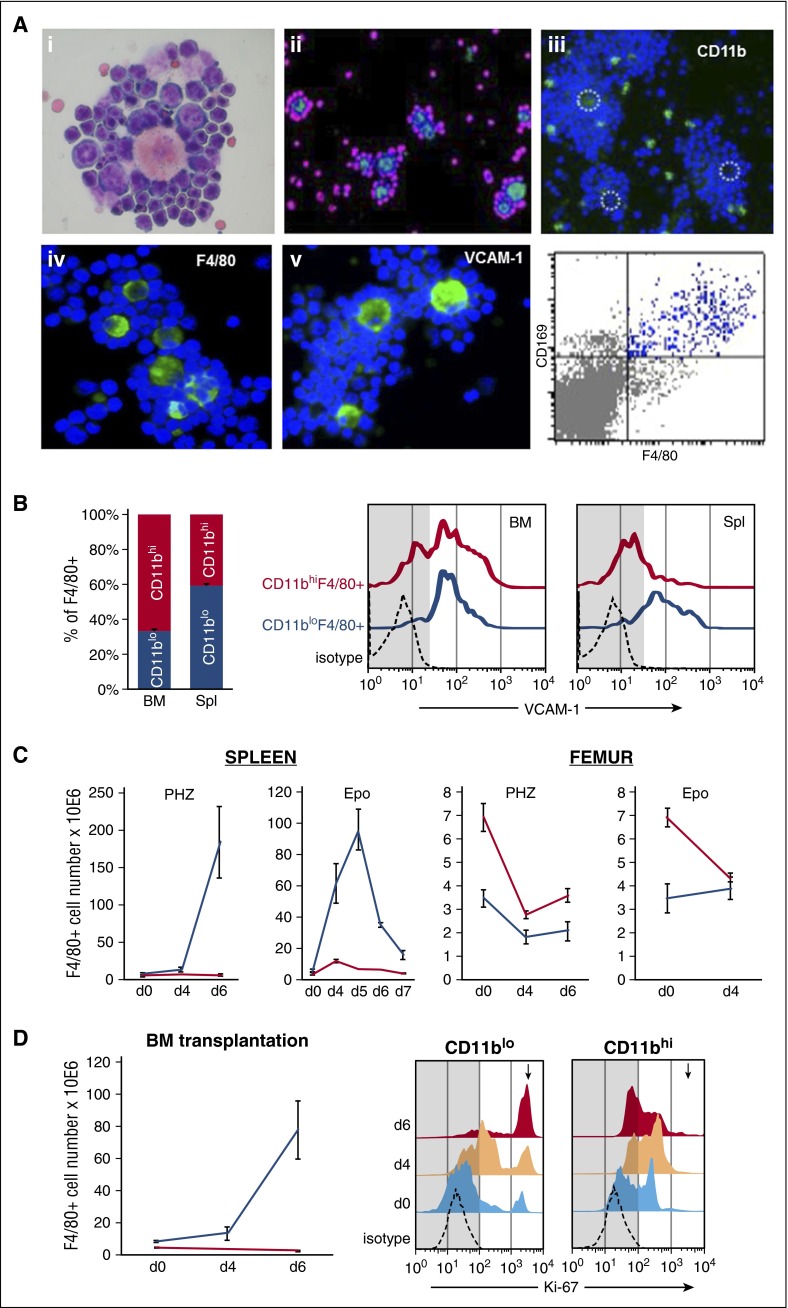
By isolating EIs from the BM or spleen after E-stress, we found that the phenotype of EI F4/80+ macrophages in normal mice is the one displaying low expression of CD11b and high expression of CD169 and VCAM-1 (Figure 1A), a conclusion consistent with several prior reports.13,16-18,33 In addition to the CD11blo macrophage populations that are engaged in EI formation, F4/80+/CD11bhi are also present, derived from circulating monocytes and represent a minority among total macrophages in the spleen, but are modestly higher in BM at baseline erythropoiesis (Figure 1B, left). Of note, VCAM-1 expression, a characteristic feature of EI macrophages, was expressed in the great majority of the CD11blo subset, both in the BM and spleen, whereas the monocyte-derived subset in the spleen was virtually negative for VCAM-1 expression (Figure 1B, right; and see supplemental Figures 1-2, available on the Blood Web site).
Figure 1.
Macrophage subsets in WT mice at steady state and during response to stress. (A) Morphology and expression of F4/80, CD11b, and VCAM-1 was analyzed in EI preparations isolated from spleens of PHZ-treated or Epo-treated mice on day 4; Hema3 stain: note the different maturation stages of Ebs that adhere to themselves and to the central macrophage engorged in damaged red cells after PHZ treatment (i); and DAPI-counterstained EIs (ii-v). Light green stain for F4/80 and magenta nuclear stain (assigned color) for surrounding Ebs (ii); light green CD11b+ cells (iii); dotted circles indicate central macrophages. Light green F4/80+ cells and DAPI-stained Ebs (iv); and green VCAM-1 positivity on central macrophages (v). CD169 expression on F4/80+ cells was analyzed by FACS after EI disaggregation (bottom right panel). (B) Percentage of F4/80+ cells in the BM and spleen that are CD11bhi or CD11blo at steady state (CD11bhi: red; CD11blo: blue). VCAM-1 expression in the 2 subsets (right panel). Note that only CD11blo express VCAM-1 in the spleen. (C) Quantitative kinetic changes in the 2 F4/80+ subsets after PHZ or Epo challenge. (D) Quantitative changes in the 2 F4/80+ subsets in transplanted mice (WT→WT) treated with PHZ 9 weeks after transplantation (left). Cycling status (Ki-67 antibodies; see “Methods”) of the 2 subsets before and after stress (right). Note differences only in the CD11blo subset. The high Ki-67 positivity in this subset changed from 9.63 ± 1.27% to 45.9 ± 6.15% and finally to 76.15 ± 1.25% on days 0 (light blue), 4 (peach) and 6 (red), respectively. No such population was present in CD11bhi subset (arrow). Number of mice: day 0, n = 6; PHZ-induced stress, day 4, n = 4; day 6, n = 6; and Epo-induced stress, n = 3. Images in (Ai,iv-v) were taken with a Leica DMLB camera (objective N PLAN 40×/0.65, eye piece HC PLAN 10×/22) and 20× objective for (Aii-iii). d, day; DAPI, 4,6 diamidino-2-phenylindole; Spl, spleen.
We next studied the dynamics of the above characterized macrophage subsets during E-stress triggered after acute PHZ-induced hemolysis. After PHZ injection, the number of both macrophage subsets in the BM dropped by 50% on day 4 and started to recover by day 6 (Figure 1C, right [femur]). In sharp contrast, the number of CD11blo macrophages in the spleen increased dramatically (32-fold) by day 6, whereas the CD11bhi subset remained unchanged and comprised only a very small fraction of total splenic macrophages (Figure 1C, left [spleen]; supplemental Figure 1). Consistency of macrophage responses was seen when two PHZ treatments were given 3 weeks apart (supplemental Figure 3). In our next model, stress erythropoiesis was induced by 3 daily Epo injections (100 U/mouse × 3). In this model, any putative heme-triggered changes in macrophages are absent. Again after Epo, as in after PHZ, we saw a dramatic increase in CD11blo macrophages in the spleen by day 4, 1 day after the last Epo injection (Figure 1C), although the magnitude of the response was somewhat lower compared with the PHZ-induced stress. In BM, the number of CD11bhi macrophages dropped, similarly to that seen after PHZ, likely reflecting their mobilization to peripheral blood (PB), whereas a modest increase in the number of CD11bhi macrophages in the spleen was seen after Epo compared with the baseline (Figure 1C, right).
The fact that we are dealing with selective proliferation of CD11blo native macrophages, especially after PHZ, was substantiated by showing that the proportion of Ki-67+ cycling CD11blo macrophages increased to ∼80% by day 6 (from 9% at day 0), whereas the proportion of Ki-67+ CD11bhi macrophages was almost unchanged from pretreatment values (Figure 1D, right).
Furthermore, to test whether the dynamic changes in macrophage subsets are the same or different after BM transplantation representing a genotoxic stress with possible elimination of native tissue macrophages, we carried out similar studies in normal lethally irradiated recipient mice transplanted with normal stem cells. As indicated by data presented in Figure 1D (left), PHZ stress induced 9 weeks posttransplant resulted in kinetic responses similar to the ones seen in nontransplanted mice, albeit of a lower magnitude. These data suggest that by 9 weeks posttransplantation, the donor-derived macrophage population in the spleen has acquired the phenotypic characteristics seen in nontransplanted mice (supplemental Figure 4).
In conclusion, native spleen macrophages phenotypically characterized by low expression of CD11b respond by a significant proliferation, both post-hemolytic stress by PHZ and post-Epo treatment, in contrast to CD11bhi macrophages, which do not show significant quantitative changes post–E-stress. Similar expansions of macrophage subsets were also observed in animals after BM transplantation.
Changes in macrophage population before and after E-stress in genetic mouse models with macrophage impairment
One of the models chosen for study is the Spi-C−/− mouse.33 Spi-C, a member of the Spi family of Ets transcriptional factors, is normally induced by heme through Bach1 degradation and is found to regulate the development of F4/80hi/CD11blo/VCAM-1+ macrophages (ie, the RPMs). Spi-C−/− mice show a severe reduction of F4/80hi/CD68+/CD169+/CD11blo macrophages in the spleen red pulp18 and in BM33 but not in other tissues. Importantly, Spi-C also regulates VCAM-1 expression (there is an Ets element within the VCAM-1 gene) and Spi-C−/− mice were reported to have33 virtually absent VCAM-1 levels in F4/80+/CD11blo cells. When E-stress response was induced in the Spi-C−/− mice, there was an influx of monocyte-derived CD11bhi macrophages in the spleen. Because the erythroid response in these mice was not very different than in controls,33 it was previously concluded but never convincingly documented in the same mice, that the recruited monocyte-derived population under heme stimulation (detrimental to native macrophages) morphed into a phenotypically and functionally competent macrophage population.33
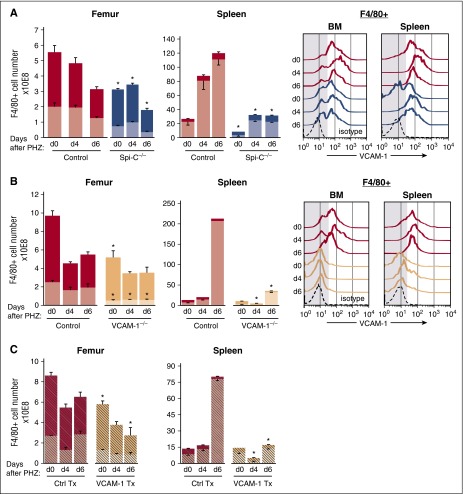
In our study, we confirmed the reduction of RPMs in spleens of Spi-C−/− mice (Figure 2A). In addition to decreased numbers, especially of the CD11blo subset (39% of control in BM and 14% of control in the spleen), the Spi-C−/− macrophages also had decreased VCAM-1 expression especially in the spleen (Figure 2A, right). Erythropoiesis was normal at steady state in these animals. To assess macrophage changes post–E-stress, we induced acute hemolysis in these mice by PHZ injection. As seen in Figure 2A, although a modest increase of CD11blo macrophages was noted in the spleen, the total number of these macrophages were only 19% ± 5 of control spleen values and in BM were 29% ± 6 of the corresponding control values at day 6. At the same time, the numbers of monocyte-derived CD11bhi macrophages remained at low levels and were similar in Spi-C−/− mice and their controls, both in the BM and spleen. Of interest, the level of VCAM-1 expression, although increased slightly post-stress, remained below the level expressed in controls (Figure 2A, right).
Figure 2.
Total and CD11blo macrophage subsets are greatly reduced before and during E-stress response in Spi-C–deficient and VCAM-1–deficient mice. Numbers of CD11blo (lighter shades) and CD11bhi (darker shades) macrophages, as well as VCAM-1 expression in F4/80+ cells in the BM and spleen were determined at several points after PHZ challenge. (A) Spi-C–deficient mice (blue bars and lines, n = 3 for each time point). (B) VCAM-1 knockouts (peach bars and lines, n = 3 to 5 mice for each time point). (C) Normal irradiated recipients transplanted (Tx) with either normal donor cells (red hatched bars) or VCAM-1−/− donor cells (peach hatched bars, n = 5 for each time point). Red bars and lines represent the respective controls (n = 6 to 12 mice for each time point). Significant difference from control; *P < .05. Note that total F4/80+ cells are significantly reduced in mutant mice compared with their controls before and especially after PHZ challenge, mainly in the spleen. Far right panels in (A-B) indicate levels of VCAM-1 expression in total macrophages. Note that less VCAM-1 expression (lower MFI) is seen in Spi-C–deficient and virtually no expression in VCAM-1–deficient macrophages (low MFI VCAM-1 was seen in <10% of F4/80+ cells). Ctrl, control; d, day; MFI, mean fluorescence intensity.
Because VCAM-1 expression was not properly activated in heme-challenged Spi-C−/− macrophages (Figure 2A, right) and also because VCAM-1 was previously associated with impairment in E-stress response through the use of inhibitory antibodies,8,20 we also studied in detail the macrophage response of VCAM-1Δ/Δ mice following hemolytic stress (Figure 2B). We documented for the first time, that at steady state, CD11blo macrophages in the BM were one-fifth of control, whereas in the spleen they were about half of control; the CD11bhi subset was 50% of control in the BM and similar to controls in the spleen (Figure 2B). After PHZ challenge, CD11blo macrophages reached only 10% to 20% of the level seen in control spleen and 30% of the level seen in control femur. Whereas control macrophage CD11blo cells expanded ∼32 times, their VCAM-1Δ/Δ counterparts expanded only 10-fold, suggesting either impaired proliferative responses or excessive death post-stress.
Thus overall, the 2 mouse models used showed great similarities in their type and number of macrophage content, both at baseline and after stress, compared with their respective controls.
To test whether the lack of VCAM-1 expression in macrophages was intrinsically important for their low numbers, rather than microenvironmentally-induced by other nonmacrophage stromal cells that are VCAM-1Δ/Δ (ie, endothelial cells [ECs], stromal cells, or fibroblasts not expressing VCAM-1), we made observations in lethally irradiated normal mice transplanted with either VCAM-1Δ/Δ BM cells, or normal BM cells (Figure 2C). After donor reconstitution (9 weeks posttransplantation), the total macrophage populations (F4/80+, 98% donor-derived) were quantitatively lower than controls, especially the CD11blo populations in the BM. However, after hemolytic challenge, VCAM-1Δ/Δ donor-transplanted mice essentially did not expand at day 6 post-PHZ (the day of maximum expansion of CD11blo cells in the spleen), similarly to nontransplanted VCAM-1Δ/Δ animals and in sharp contrast to mice transplanted with +/+ donor cells, which had significant expansion of their total F4/80+ cells and total CD11blo cells. These data suggest that VCAM-1 expression in macrophages is likely intrinsically associated with their failure to expand post-hemolytic stress.
Erythroid responses post-stress in Spi-C−/− and VCAM-1Δ/Δ mice
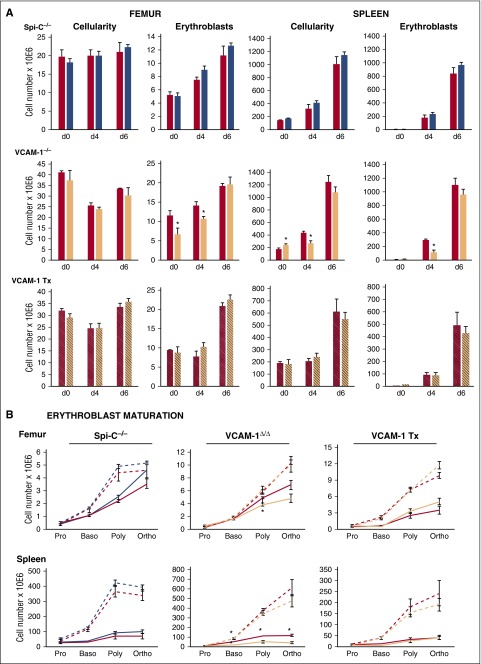
Because significant increases in tissue macrophages post-PHZ were seen in normal mice, such changes may be intuitively interpreted as necessary for erythroid response. Therefore, we evaluated in detail erythroid responses in normal mice and compared them to those with drastically reduced macrophages, the Spi-C−/− and VCAM-1Δ/Δ mice. Evaluation included spleen weight, spleen cellularity, total nucleated erythroid cells (TER119+) and their maturation profile, EI preparations from spleens of treated animals, and PB analysis (hematocrit [Hct], red blood cell count [supplemental Figure 5], and white blood cell count). Briefly, spleen responses in terms of splenic weight, cellularity, and Eb content in Spi-C−/− mice were similar to controls (Figure 3A). BM parameters were also similar to controls. In addition to total Eb numbers, their maturation profile, as assessed by staining with CD71, TER119, and CD44 antibodies, was also similar to control mice (Figure 3B). EI preparations were qualitatively similar to those of control mice (supplemental Figure 6A). Hct levels at day 6 post-PHZ were 44.4 ± 2.4% in control mice and 45.6 ± 1.9% in Spi-C−/− mice (n = 5 per group; supplemental Figure 5). In VCAM-1Δ/Δ mice, Hcts were 40.5 ± 0.7% vs 41.1 ± 2.9% in the respective controls (supplemental Figure 5). Nevertheless, certain differences from controls were seen in VCAM-1Δ/Δ mice in terms of Eb content, maturation profile (Figure 3), and EI preparations (supplemental Figure 6A-B). Despite these differences, by day 6, VCAM-1Δ/Δ mice caught up to the control levels, an outcome that is in agreement with recent data in which VCAM-1 was deleted only in macrophages.44 In contrast to the primary VCAM-1Δ/Δ mice, normal mice reconstituted with VCAM-1Δ/Δ donor cells fared somewhat better in their erythroid responses, because the transient changes seen in nontransplanted mice were not readily apparent (Figure 3). It is tempting to attribute the differences seen to a putative positive influence by the largely normal microenvironment in the spleen and BM in transplanted mice.
Figure 3.
Erythroid responses to PHZ challenge in Spi-C–deficient and VCAM-1–deficient mice. (A) Cellularity and total number of Ebs in the femur (left panels) and spleen (right panels) of respective controls for each group of mice (red bars), Spi-C–deficient mice (blue bars, upper panels), VCAM-1–deficient mice (peach bars, middle panels), and control mice irradiated and transplanted with either control donor cells (red striped bars) or VCAM-1–deficient donor cells (peach striped bars, lower panel). (B) Erythroid maturation profiles in femur and spleen of Spi-C–deficient mice (blue lines, left panels) VCAM-1–deficient mice (peach lines, middle panels) and control mice irradiated and transplanted (Tx) with either control donor cells or VCAM-1–deficient donor cells (peach lines, right panels) and their respective controls (red lines) at day 4 (solid lines) and at day 6 post-PHZ (broken blue, peach, and red lines, respectively). Blue bars and lines, n = 3 for each time point; and peach bars and lines, n = 3 to 5 mice for each time point; red bars and lines, n = 6 to 12 mice for each time point. Significant difference over controls; *P < .05 d, day; Baso, basophilic Eb; Poly, polychromatophilic Eb; Ortho, orthochromatic Eb; Pro, proerythroblast.
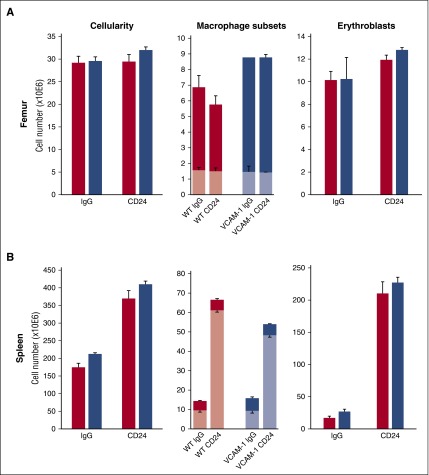
Because macrophages and dendritic cells (DCs) appear to have a common origin,45,46 and a certain class of DCs (CD8α+, cDC1) (ie, with high CD24 expression but negative for CD169 and located adjacent to RPMs) appear to exert a macrophage-independent influence on E-stress response,47 we tested whether this pathway was functional in VCAM-1Δ/Δ mice. Thus, we treated our VCAM-1Δ/Δ mice with aCD24 antibodies to engage a DC response. As seen in Figure 4, both control and VCAM-1Δ/Δ mice responded to this treatment by eliciting similar increases in CD11blo macrophages in the spleen and similar increments in Eb populations. It is of interest that following this treatment, macrophage expansion in VCAM-1Δ/Δ mice was not significantly different from that of controls, in stark contrast to PHZ response (Figure 1). These data highlight the fact that differences in both macrophage and erythroid population responses depend on the stimulus applied.
Figure 4.
Dynamics of macrophage subsets CD11blo (lighter shades) and CD11bhi (darker shades) in response to aCD24 antibody treatment. WT (red bars) and VCAM-1−/− blue bars) mice were treated with aCD24 antibody or control immunoglobulin G (see “Methods”). Cellularity (left panels), macrophage (middle panels), and Eb (right panels) responses were followed in the BM (A) and spleen (B). Note the major changes after treatment in the spleen only. These data are consistent with the fact that the spleen and not the BM is the major source of SCF produced after CD24 engagement.47 SCF, stem cell factor.
Overall, certain phenotypic features of the VCAM-1Δ/Δ mice at baseline and their responses post-stress are reminiscent of the ones seen in integrin α4Δ/Δ mice.37,48 Interaction of α4 on Ebs with its ligand VCAM-1 on macrophages was previously considered important in EI stabilization through the use of inhibitory antibodies for either α4 or VCAM-1.8,20 At baseline, VCAM-1Δ/Δ mice, like α4Δ/Δ mice, are also not anemic and show increased release of progenitor cells in the PB.42,49 After E-stress, both types of mice exhibited a delayed response to stress. However, of interest, only VCAM-1Δ/Δ mice at baseline have significantly decreased total F4/80+ cells in the spleen, especially the ones that are CD11blo (Figure 2B). These data reinforce the participation of α4/VCAM-1 interaction partners in the context of EIs for the enhancement of terminal erythroid maturation and for firm retention of Ebs, in conjunction with other cooperating adhesive connections.
Discussion
The association of differentiating Ebs with macrophages within a defined complex, the EI, has been emphasized on multiple occasions since its inception (some 50 years ago1), as a necessary component of developing erythroid cells. Reduction in the number of macrophages by external treatments (ie, clodronate liposomes or by genetic manipulations) has been deemed as highly detrimental to developing erythroid cells, especially in response to stress.13,14,32 Whether the existing population of native tissue macrophages at baseline was adequate for stress response, or whether their numbers need to increase also has not been conclusively determined, because quantitative changes in the population of macrophages were rarely assessed kinetically post-stress. Our data in normal mice suggest that both post-hemolytic stress and post-Epo treatment in the population of native spleen macrophages, characteristically the ones with low expression of CD11b (native macrophages), is dramatically expanded post-stress (Figure 1C). Although the exact proliferative stimulus for the expansion of CD11blo macrophages post–E-stress is not clear (increase in macrophage colony-stimulating factor, reactive oxygen species, bone morphogenetic protein 4, or release of inflammatory cytokines), this outcome may be intuitively viewed as necessary for optimal erythroid response. The fact is that the number of monocyte-derived CD11bhi macrophages did not change. As documented here, this is in agreement with previously expressed views covering macrophage tissue responses to non-genotoxic stress.39,40 However, an alternative view has been expressed suggesting that CD11bhi macrophages represent a recruited “rescue squad” capable of transforming and supplanting the low numbers of native, CD11blo tissue macrophages6,33,50,51 following the same type of non-genotoxic stress. Beyond these inconsistencies regarding splenic responses, there are also conflicting data about the influence of CD11bhi macrophages on BM erythropoiesis post-cytokine treatments. A negative role in BM erythropoiesis was found after reduction of their numbers post-treatment with granulocyte colony stimulating factor14 or with Fms-like tyrosine kinase (ie, Flt3),15 although a reduction in CD11b+ macrophages also seen in earlier studies after Epo treatment or post-hypoxia led to the opposite conclusion.52 Whether the CD11bhi macrophages are the ones participating in EI formation in BM14 in contrast to spleen,13,16,18 also varies in different reports.
To provide further insight into these controversial issues, we made detailed observations in 2 genetic models, Spi-C−/− and VCAM-1Δ/Δ, with distinct macrophage impairment. We documented that the native spleen macrophages were dramatically diminished at steady state, as documented for Spi-C−/−33 mice and presented for the first time for our VCAM-1Δ/Δ animals. The total number of F4/80+ cells in both types of animals was <20% of that in control animals (Figure 2). Post-hemolytic stress, although the number of RPMs, or native spleen macrophages, was expanded from their low numbers in both types of animals (Figure 2) in contrast to the CD11bhi macrophages, which did not expand, the total macrophage population remained ∼80% below the number achieved in E-stressed controls (Figure 2). An explanation for the low number of RPMs in Spi-C−/− macrophages at homeostasis has been previously advanced,33 however, the reason why VCAM-1Δ/Δ macrophages are low is not clear. Activation of VCAM-1 in both ECs and in macrophages by increased levels of glucocorticoids was found to be part of the E-stress response.53,54 Further, VCAM-1 appears to be a target for hypoxia-inducible factor 1-α in ECs playing an essential role in supporting erythropoiesis,55 because it impairs erythroid maturation. The increased reactive oxygen species occurring post-stress could also affect VCAM-1 activation in macrophages, as it occurs in ECs. Therefore, the inability to activate VCAM-1 in macrophages may have curtailed their proliferative response. Whether macrophages alone or in cooperation with other stromal cells (ECs, fibroblasts, etc) respond functionally by increasing their VCAM-1 activation and their numbers post-stress has been addressed in our transplantation experiments (Figure 2C). Because VCAM-1Δ/Δ macrophages did not expand within normal host splenic stroma, phenocopying the nontransplanted phenotype, the data support a dominant effect of VCAM-1 expression in macrophages as intrinsically associated with their proliferation.
Despite the low number of native RPMs in both types of animals, we were surprised to see that CD11bhi macrophages could not supplant the void of native macrophages, especially post-stress, as suggested in recent commentaries.6,50,51 Because proliferation was only documented in CD11blo macrophages (Figure 1D), it is possible that the presence of CD11b or the absence of VCAM-1 limits their activation and/or proliferation post-stress (Figure 1), suggesting the need to suppress their CD11b and/or enhance their VCAM-1 expression before their proliferation. In this context, it is of note that in adipose tissue macrophages, the presence of CD11b limits their proliferation and their alternative activation.56
A further and unexpected surprise was the fact that the erythroid response in Spi-C−/− and in VCAM-1Δ/Δ mice was similar to control mice despite the very low number of total F4/80+ cells (both CD11blo and CD11bhi). The adequate response of Spi-C−/− to hemolytic challenge was previously attributed to recruited macrophages (although neither quantitative kinetic data for macrophage numbers nor erythroid cell numbers were provided to support this claim).33 It was argued that heme challenge led to “local differentiation of monocytes into RPMs, thus replenishing the resident population lost by heme toxicity”4 by adapting a phenotype that is by definition heme-vulnerable? Because we found that the CD11bhi macrophages did not significantly expand post-stress in control and knockout animals, our data challenge this claim. In this context, it is also important to note that the kinetic responses we described after PHZ challenge were similar to the ones seen after just Epo treatment, away from any heme-triggered changes. Similar erythroid response data were also documented in VCAM-1Δ/Δ mice (Figure 3B). In these mice, despite their low macrophage numbers, the erythroid proliferative response was similar to controls by all criteria (Hct, spleen size, spleen cellularity, and total erythroid cells in the spleen and BM) and with only some modest transient differences from their controls (Figure 3B, day 4 responses).
Several possibilities can be envisioned that come into play to enhance the E-stress response in these 2 animal models: in addition to macrophages, and independently from the contribution of macrophages, DCs (having a shared origin with macrophages but divergent molecular signaling) expressing CD24+/CD8a+ have been found to be important for the amplification of erythroid expansion post-stress, especially for the initiation of stress response.47 CD24−/− mice had a delayed response to stress (post-hemolysis or Epo, or cisplatin challenge), despite the presence of normal macrophages and normal erythroid cells. According to this scenario, an active response is initiated through the presence of CD24+ cells and is further amplified through positive feedback from erythroid cells, and direct or indirect effects by ECs and macrophages. It needs to be emphasized that this scenario may not require increased numbers of macrophages, as long as no inhibitory signals are emanated by them. Because the stress response of both Spi-C−/− and VCAM-1Δ/Δ mice was adequate, we have to surmise that no negative signals were derived by the above cells in response to stimuli elicited by the E-stress modality. Indeed, we have documented that the CD24 pathway of response is actively engaged in VCAM-1Δ/Δ animals, because when they were treated with the activating CD24 antibody, they responded similar to controls (Figure 4). It is important to note however, that the overall erythroid response was over threefold higher. Therefore, possibly either sustained high Epo levels post-PHZ or heme-mediated stress, triggers additional pathways enhancing the erythroid response. The recently reported effect of heme on CD83 expression by DCs57 dampening pro-inflammatory responses is intriguing, and may support the above speculation.
Another possibility is that a positive feedback by developing erythoid cells themselves kicks in once a response is initiated. Multiple pathways have been previously described, but their quantitative contribution was only studied in impaired pathways. In response to Epo, Ebs release Gas6 (important for cell survival) by boosting Epo receptor signaling and by enhancing adhesion to fibronectin through very-late-activation antigen 4 activation.24,25 Data in Gas6−/− mice convincingly show that Gas6 regulates the response to acute anemia in both the spleen and BM.29 Apart from the erythroid direct effects, Gas6 exerts a paracrine effect by dampening the release of erythroid-inhibitory factors by macrophages. Further, erythroid cells, in addition to Gas6, secrete two angiogenic factors, VEGF-A and PlGF.29-31 These factors promote in a paracrine fashion interactions with macrophages or with ECs carrying their receptors. (Neuropilin 1 is expressed by BM stromal cells and immature hematopoietic cells express neuropilin 1 ligands, such as VEGF and PlGF1 and 2.) In addition to paracrine effects exercised by VEGF and PlGF, EphB4+ erythroid progenitors can interact, especially under hypoxic conditions, with Ephrin-B2 on BM stromal cells58 and early erythroid cells express GDF11 supporting their survival.59 These collective data indicate that during E-stress, Epo receptor signaling sets up in motion a signaling network response, which includes unique stress-related positive feedback by erythroid cells, and which is also aided by paracrine positive and/or by inhibiting negative responses from stromal cells. Finally, the engagement of additional pathways enhancing the erythroid response may be stress dependent. Furthermore, it is possible that the height of proliferative response for macrophages and erythroid cells may be independently controlled and also stress-type dependent.
In conclusion, our study highlights additional insights to erythroid cell response by emphasizing that: (1) in the presence of a small number of macrophages, amounting to one-fifth of normal, an optimal E-stress response is possible presumably aided by autocrine feedback contributions by erythroid cells themselves and paracrine effects by other than macrophage (stromal) cells; (2) F4/80+CD11bhi, the monocyte-derived subset in the BM and spleen does not numerically supplant the low number of CD11blo RPMs in Spi-C−/− (and VCAM-1Δ/Δ), in contrast to some previous suggestions33; (3) the presence of VCAM-1 is positively associated, whereas CD11b expression is negatively associated with macrophage proliferative expansion; and (4) whether macrophage-independent pathways are engaged in the E-stress response may depend on the type and severity of E-stress.
Acknowledgments
The authors thank Nelson Di Paolo for providing supplemental Figure 2, and Betty Nakamoto for editing the figures and manuscript preparation.
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant DK094702.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.U. generated data and edited the paper; S.R.P. carried out all mouse procedures; and T.P. designed the experiments, supervised data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thalia Papayannopoulou, University of Washington, Box 357710, Health Sciences Building, Room K243, Seattle, WA 98195; e-mail: thalp@uw.edu.
References
- 1.Bessis M. Erythroblastic island, functional unity of bone marrow. Rev Hematol. 1958;13(1):8–11. [PubMed] [Google Scholar]
- 2.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manwani D, Bieker JJ. The erythroblastic island. Curr Top Dev Biol. 2008;82:23–53. doi: 10.1016/S0070-2153(07)00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korolnek T, Hamza I. Macrophages and iron trafficking at the birth and death of red cells. Blood. 2015;125(19):2893–2897. doi: 10.1182/blood-2014-12-567776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giger KM, Kalfa TA. Phylogenetic and ontogenetic view of erythroblastic islands. BioMed Research International. 2015;2015:873628. doi: 10.1155/2015/873628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koury MJ. Tracking erythroid progenitor cells in times of need and times of plenty. Exp Hematol. 2016;44(8):653–663. doi: 10.1016/j.exphem.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen RN, Perkins AC, Levesque JP. Macrophages and regulation of erythropoiesis. Curr Opin Hematol. 2015;22(3):212–219. doi: 10.1097/MOH.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 8.Sadahira Y, Yoshino T, Monobe Y. Very late activation antigen 4-vascular cell adhesion molecule 1 interaction is involved in the formation of erythroblastic islands. J Exp Med. 1995;181(1):411–415. doi: 10.1084/jem.181.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92(8):2940–2950. [PubMed] [Google Scholar]
- 10.Spring FA, Parsons SF, Ortlepp S, et al. Intercellular adhesion molecule-4 binds alpha(4)beta(1) and alpha(V)-family integrins through novel integrin-binding mechanisms. Blood. 2001;98(2):458–466. doi: 10.1182/blood.v98.2.458. [DOI] [PubMed] [Google Scholar]
- 11.Mankelow TJ, Spring FA, Parsons SF, et al. Identification of critical amino-acid residues on the erythroid intercellular adhesion molecule-4 (ICAM-4) mediating adhesion to alpha V integrins. Blood. 2004;103(4):1503–1508. doi: 10.1182/blood-2003-08-2792. [DOI] [PubMed] [Google Scholar]
- 12.Liu XS, Li XH, Wang Y, et al. Disruption of palladin leads to defects in definitive erythropoiesis by interfering with erythroblastic island formation in mouse fetal liver. Blood. 2007;110(3):870–876. doi: 10.1182/blood-2007-01-068528. [DOI] [PubMed] [Google Scholar]
- 13.Chow A, Huggins M, Ahmed J, et al. CD169⁺ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen RN, Forristal CE, Raggatt LJ, et al. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80(+)VCAM1(+)CD169(+)ER-HR3(+)Ly6G(+) erythroid island macrophages in the mouse. Exp Hematol. 2014;42(7):547–561.e4. doi: 10.1016/j.exphem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen RN, Nowlan B, Brunck ME, Barbier V, Winkler IG, Levesque JP. Fms-like tyrosine kinase 3 (Flt3) ligand depletes erythroid island macrophages and blocks medullar erythropoiesis in the mouse. Exp Hematol. 2016;44(3):207–212. e4. doi: 10.1016/j.exphem.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Crocker PR, Gordon S. Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med. 1985;162(3):993–1014. doi: 10.1084/jem.162.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldar M, Murphy KM. Origin, development, and homeostasis of tissue-resident macrophages. Immunol Rev. 2014;262(1):25–35. doi: 10.1111/imr.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohyama M, Ise W, Edelson BT, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457(7227):318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadahira Y, Yasuda T, Yoshino T, et al. Impaired splenic erythropoiesis in phlebotomized mice injected with CL2MDP-liposome: an experimental model for studying the role of stromal macrophages in erythropoiesis. J Leukoc Biol. 2000;68(4):464–470. [PubMed] [Google Scholar]
- 20.Toda S, Segawa K, Nagata S. MerTK-mediated engulfment of pyrenocytes by central macrophages in erythroblastic islands. Blood. 2014;123(25):3963–3971. doi: 10.1182/blood-2014-01-547976. [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Lo A, Short SA, et al. Targeted gene deletion demonstrates that the cell adhesion molecule ICAM-4 is critical for erythroblastic island formation. Blood. 2006;108(6):2064–2071. doi: 10.1182/blood-2006-03-006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, Spring FA, Parsons SF, et al. Novel secreted isoform of adhesion molecule ICAM-4: potential regulator of membrane-associated ICAM-4 interactions. Blood. 2003;101(5):1790–1797. doi: 10.1182/blood-2002-08-2529. [DOI] [PubMed] [Google Scholar]
- 23.Fabriek BO, Polfliet MM, Vloet RP, et al. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. 2007;109(12):5223–5229. doi: 10.1182/blood-2006-08-036467. [DOI] [PubMed] [Google Scholar]
- 24.Patel VP, Lodish HF. A fibronectin matrix is required for differentiation of murine erythroleukemia cells into reticulocytes. J Cell Biol. 1987;105(6 pt 2):3105–3118. doi: 10.1083/jcb.105.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vuillet-Gaugler MH, Breton-Gorius J, Vainchenker W, et al. Loss of attachment to fibronectin with terminal human erythroid differentiation. Blood. 1990;75(4):865–873. [PubMed] [Google Scholar]
- 26.Gu Y, Sorokin L, Durbeej M, Hjalt T, Jönsson JI, Ekblom M. Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood. 1999;93(8):2533–2542. [PubMed] [Google Scholar]
- 27.Parsons SF, Lee G, Spring FA, et al. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind alpha5 chain-containing human laminin with high affinity. Blood. 2001;97(1):312–320. doi: 10.1182/blood.v97.1.312. [DOI] [PubMed] [Google Scholar]
- 28.Mankelow TJ, Burton N, Stefansdottir FO, et al. The Laminin 511/521-binding site on the Lutheran blood group glycoprotein is located at the flexible junction of Ig domains 2 and 3. Blood. 2007;110(9):3398–3406. doi: 10.1182/blood-2007-06-094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angelillo-Scherrer A, Burnier L, Lambrechts D, et al. Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest. 2008;118(2):583–596. doi: 10.1172/JCI30375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87(5):869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 31.Tordjman R, Delaire S, Plouët J, et al. Erythroblasts are a source of angiogenic factors. Blood. 2001;97(7):1968–1974. doi: 10.1182/blood.v97.7.1968. [DOI] [PubMed] [Google Scholar]
- 32.Ramos P, Casu C, Gardenghi S, et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat Med. 2013;19(4):437–445. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haldar M, Kohyama M, So AY, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156(6):1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116(26):6054–6062. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao YA, Kusy S, Luong R, Wong RJ, Stevenson DK, Contag CH. Heme oxygenase-1 deletion affects stress erythropoiesis. PLoS One. 2011;6(5):e20634. doi: 10.1371/journal.pone.0020634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijffels JF, de Rover Z, Beelen RH, Kraal G, van Rooijen N. Macrophage subpopulations in the mouse spleen renewed by local proliferation. Immunobiology. 1994;191(1):52–64. doi: 10.1016/s0171-2985(11)80267-6. [DOI] [PubMed] [Google Scholar]
- 37.Ulyanova T, Jiang Y, Padilla S, Nakamoto B, Papayannopoulou T. Combinatorial and distinct roles of α₅ and α₄ integrins in stress erythropoiesis in mice. Blood. 2011;117(3):975–985. doi: 10.1182/blood-2010-05-283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Xu X, Feng X, Murphy PM. The macrophage-depleting agent clodronate promotes durable hematopoietic chimerism and donor-specific skin allograft tolerance in mice. Sci Rep. 2016;6:22143. doi: 10.1038/srep22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulyanova T, Scott LM, Priestley GV, et al. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106(1):86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulyanova T, Priestley GV, Nakamoto B, Jiang Y, Papayannopoulou T. VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Exp Hematol. 2007;35(4):565–571. doi: 10.1016/j.exphem.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ α4(f/f) mice are hematopoietic cell autonomous. Blood. 2007;109(1):109–111. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Q, Frenette PS. Macrophage erythroblast attacher (MAEA), but not VCAM1, is required for the bone marrow erythroblastic niche [abstract]. Blood. 2015;126(23) Abstract 2128. [Google Scholar]
- 45.Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208(2):227–234. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim TS, Hanak M, Trampont PC, Braciale TJ. Stress-associated erythropoiesis initiation is regulated by type 1 conventional dendritic cells. J Clin Invest. 2015;125(10):3965–3980. doi: 10.1172/JCI81919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulyanova T, Jiang Y, Padilla SM, Papayannopoulou T. Erythroid cells generated in the absence of specific β1-integrin heterodimers accumulate reactive oxygen species at homeostasis and are unable to mount effective antioxidant defenses. Haematologica. 2013;98(11):1769–1777. doi: 10.3324/haematol.2013.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulyanova T, Padilla SM, Papayannopoulou T. Stage-specific functional roles of integrins in murine erythropoiesis. Exp Hematol. 2014;42(5):404–409.e4. doi: 10.1016/j.exphem.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koury MJ. Heme-regulated differentiation of monocytes to macrophages reveals interconnections of hemolysis, iron metabolism, and macrophage differentiation. The Hematologist, ASH News and Reports. 2014;11(6):11. [Google Scholar]
- 51.Mildner A, Yona S, Jung S. A close encounter of the third kind: monocyte-derived cells. Adv Immunol. 2013;120:69–103. doi: 10.1016/B978-0-12-417028-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 52.Wang CQ, Udupa KB, Xiao H, Lipschitz DA. Evidence suggesting a negative regulatory role for macrophages in murine erythropoiesis in vivo. Exp Hematol. 1994;22(4):370–376. [PubMed] [Google Scholar]
- 53.Lacal PM, Petrillo MG, Ruffini F, et al. Glucocorticoid-induced tumor necrosis factor receptor family-related ligand triggering upregulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 and promotes leukocyte adhesion. J Pharmacol Exp Ther. 2013;347(1):164–172. doi: 10.1124/jpet.113.207605. [DOI] [PubMed] [Google Scholar]
- 54.Falchi M, Varricchio L, Martelli F, et al. Dexamethasone targeted directly to macrophages induces macrophage niches that promote erythroid expansion. Haematologica. 2015;100(2):178–187. doi: 10.3324/haematol.2014.114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita T, Ohneda O, Sakiyama A, Iwata F, Ohneda K, Fujii-Kuriyama Y. The microenvironment for erythropoiesis is regulated by HIF-2alpha through VCAM-1 in endothelial cells. Blood. 2008;112(4):1482–1492. doi: 10.1182/blood-2007-11-122648. [DOI] [PubMed] [Google Scholar]
- 56.Zheng C, Yang Q, Xu C, et al. CD11b regulates obesity-induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages. Proc Natl Acad Sci USA. 2015;112(52):E7239–E7248. doi: 10.1073/pnas.1500396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godefroy E, Liu Y, Shi P, et al. Altered heme-mediated modulation of dendritic cell function in sickle cell alloimmunization [published online ahead of print May 26, 2016]. Haematologica. 2016 doi: 10.3324/haematol.2016.147181. doi: pii: haematol.2016.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suenobu S, Takakura N, Inada T, et al. A role of EphB4 receptor and its ligand, ephrin-B2, in erythropoiesis. Biochem Biophys Res Commun. 2002;293(3):1124–1131. doi: 10.1016/S0006-291X(02)00330-3. [DOI] [PubMed] [Google Scholar]
- 59.Dussiot M, Maciel TT, Fricot A, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nat Med. 2014;20(4):398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]