Abstract
Background
The klotho (Klt)-fibroblast growth factor-23 (FGF-23)-vitamin D axis is the main component of calcium (Ca) and phosphorus (P) metabolisms; on the contrary, it is also secreted from the choroid plexus (CP).
Purpose
This study is aimed at evaluating serum soluble Klt (sKlt), FGF-23, and 25-(OH)-vitamin D levels in multiple sclerosis (MS) patients.
Methods
Thirty-two relapsing-remitting MS patients (11 males and 21 females; mean age 38.3 years) and 31 age-sex matched healthy controls (12 males and 19 females; median age 38.5 years) were included in this study. All patients were diagnosed with MS according to the criteria of McDonald.
Results
Serum sKlt, FGF-23, and P levels were significantly higher in MS patients compared to the control group (p < 0.01, p < 0.01, and p = 0.02, respectively). Serum 25-(OH)-vitamin D and Ca levels were significantly lower in MS patients (p < 0.01 and p = 0.04, respectively).
Conclusion
Klt, which is secreted from CP, could be a response to the inflammatory condition in MS. Elevated FGF-23 levels suppress 1α-hydroxylase and upregulates 24α-hydroxylase, which results in a decrease in 1,25-(OH)2D3 levels. Thus, the neuroprotective and immunomodulatory effects of vitamin D might not be seen in MS patients.
Key Words: Multiple sclerosis, Vitamin D, Klotho, Fibroblast growth factor-23, Choroid plexus, Demyelinization, Oligodendrocyte
Introduction
Multiple sclerosis (MS) is the most common disease of the central nervous system (CNS) that results from inflammatory demyelinating events. It is the leading cause of disabilities in young adults. Although it does not have a limiting effect on survival, it is associated with the potential of development of disability over years, thus having significant socioeconomic outcomes [1,2,3].
MS is characterized by a loss of oligodendrocytes (with partial protection of axons), astroglial scars, and multiple demyelinization areas. Despite certain characteristic descriptive clinical features, MS has considerably variable clinical progression and atypical forms. Research-oriented examinations are usually required to confirm the diagnosis and to eliminate other diseases. The advances in disease monitoring and treatment are promising with respect to decelerating the progression of disabilities. Current understanding of the fundamental nature of MS is limited, and future goals are directed towards better disease management and repair of injured CNS tissue [1,4].
In 1997, Kuro-o et al. [5] discovered a gene that was associated with ageing, and named it after Klotho (Klt), one of Zeus's daughters who was responsible for fate. Since then, Klt has attracted significant attention, and various studies have been carried out. Klt is a transmembrane molecule that is predominantly expressed in the kidneys and brain. It is also separated from the cell surface and forms bioactive soluble Klt (sKlt) in body fluids [6]. In Klt-knockout mice, the onset of increased atherosclerosis, infertility, osteoporosis, skin atrophy, alopecia, and age-related diseases such as dementia occurred earlier, compared to the wild-type mice [5]. Klt, which is considered as the youth protein, has antioxidant, anti-inflammatory, tumor suppressive features; in addition, it can also suppress insulin, IGF, and Wnt signaling [7,8]. Another important feature of Klt is that it plays a role in calcium (Ca)-phosphorus (P) homeostasis, together with fibroblast growth factor-23 (FGF-23) [9,10]. While FGF-23 is secreted from the bones, Klt is secreted from kidneys. Klt is required to bind FGF-23 to the FGF receptor, and the activation of intracellular signal molecules [6]. Basically, if we think of the FGF receptor as a bridge, Klt functions as its piers, and FGF-23 binds to the Klt-FGF receptor complex. The normal physiological role of FGF-23 is to decrease the expression of Type II Na-P co-transporter and increase P secretion [11]. In addition, FGF-23 decreases 1,25-(OH)2D3 levels by suppressing 1α-hydroxylase activity, which in turn leads to the suppression of P absorption from the intestines [12,13].
Several studies have demonstrated the importance of vitamin D in the progression and prevalence of MS. A majority of these studies have shown that vitamin D intake attenuates the progression of MS, and vitamin D has a key role in MS pathogenesis [14,15]. In addition, a lower risk of MS in individuals who are exposed to more sunlight during childhood and early adolescence has supported this theory [16]. On the contrary, FGF-23 and Klt have significant effects, in addition to sunlight, diet, and other factors in vitamin D metabolism [17]. Therefore, the aim of this study was to analyze serum sKlt, FGF-23, and 25-(OH)-vitamin D levels in MS patients, and to interpret the interactions between these molecules. This study is the first of its kind to investigate vitamin D levels together with FGF-23 and sKlt in MS patients.
Methods
The following study included 32 patients who were admitted to the Antalya Education and Research Hospital with stable relapsing-remitting MS. The mean age of this group was 38.3 (21-60) years, and the study group comprised 21 females and 11 males. In accordance with McDonald's [18] criteria, all participants had clinically definite MS. The Kurtzke's Expanded Disability Status Scale (EDSS) [19] was used to measure the severity and clinical disability, and the date of neurological diagnosis was determined as the starting point from which to determine the duration of the disease. The group was also assessed using the progression index (PI; defined as the ratio between EDSS/MS duration).
Thirty-one healthy volunteers, free of any indication or family history for neurological diseases, were selected as the control group. The volunteers had a mean age of 38 (23-57) years and consisted of 19 females and 12 males. All participants belonged to the same ethnic group and had comparable socioeconomic status, and they matched in age, gender, and geographic origins with the study group.
The study group was enrolled between September and November. The neurologists examined, evaluated, and diagnosed all patients. Patients with probable MS or clinically isolated syndrome or any other acute or chronic inflammatory disease, anaemia, malignancy, cerebrovascular disease, kidney disease, pregnancy, or patients using any antioxidant drugs that could influence the results, were not included in the study. Each participant underwent a full physical and nephrological examination. Data collection included age, gender, medical history, alcohol consumption, and smoking habits. The study was approved by the local Ethical Committee upon completion of a general questionnaire and signed consent from each participant before the onset of the study.
Samples
Blood samples were obtained after an overnight fasting. Five millilitres of blood from each patient and control were drawn into a BD Vacutainer (Becton-Dickinson, USA) SSTTM II Advance tube. Serum tubes were coated with micronised silica particles which activate clotting. Samples were checked for haemolysis or other interfering substances. Serum was then separated from the cells by centrifugation at 1,500 g for 10 min. Serum creatinine was measured freshly. Remaining serum portions were stored at −80°C and used to analyze Klt and FGF-23.
Analytical Methods
Measurement of Serum Calcidiol
25-(OH)-vitamin D assays (DiaSorin, Stillwater, Minn., USA) were performed using the direct competitive chemiluminescence immunoassay method. The LIAISON assay was linear up to 125 ng/ml total 25-(OH)-vitamin D in unaltered samples. The limit of detection was 3.5 ng/ml and the coefficient of variation ranged between 4.8 and 11.1% 25-(OH)-D for this assay.
Measurement of Serum Parathyroid Hormone
Serum intact parathyroid hormone (PTH) levels were determined using commercially available assay kits (Beckman Coulter) and an autoanalyzer (Access DxI800; Beckman Coulter Diagnostics, USA). The intact PTH assay is a 2-site immunoenzymatic (‘sandwich’) assay. Intact PTH assay was linear up to 3,500 pg/ml. The limit of detection was 1 pg/ml and the coefficient of variation ranged between 3.5 and 6.4% for this assay.
Measurement of Serum Klt and FGF-23
Klt and FGF-23 levels were measured using a commercially available ELISA kit (YH Biosearch, Shanghai, China) (%CV: <10 for both parameters, Klt assay range 0.05-20 ng/ml, FGF-23 assay range 5-1,500 pg/ml). The assays employed the quantitative sandwich enzyme immunoassay technique. To avoid variation within an assay, measurements were performed in duplicate and simultaneously using the same ELISA kit.
Rutin Parameters
Serum blood urea nitrogen, creatinine, Ca, and P levels were determined using commercially available assay kits (Beckman Coulter) and an autoanalyzer (Beckman AU5800; Beckman Coulter Diagnostics, USA).
Statistical Analysis
Statistical Package for Social Sciences (SPSS) was performed using 13.0 package program. Normally distributed data for the comparison of groups was carried out using Student's t test, and for non-normal distributions the Mann-Whitney U test was used. Fisher's exact test was used for non-parametric data. For correlation analysis; Pearson correlation test for data with normal distribution was used. We used the Spearman test for non-normal distributions. p < 0.05 was considered statistically significant.
Results
All MS patients belonged to the stable relapsing remitting subtype. The mean disease duration was 6.6 ± 5.5 years (minimum 1 year; maximum 20 years). The mean EDSS was 1.7 (range 0.5-5.5), and the mean PI was 0.35 (range 0.04-1.16). Only 2 patients did not receive medical treatment. There were no significant differences in age, gender, smoking habits, and body mass index between patients and controls. Demographic and clinical features are summarized in table 1.
Table 1.
Demographic and clinical data of patients with MS and controls
| Parameters | Patients (n = 32) | Controls (n = 31) | p value |
|---|---|---|---|
| Age, years, mean ± SD | 38.3±9.9 | 38.5±7.5 | 0.14 |
| Female, n | 21 | 19 | 0.79 |
| BMI, kg/m2 | 27±4.3 | 26±5.2 | 0.32 |
| Smoking, n (%) | 10 (31) | 11 (35) | 0.79 |
| Disease duration, years, mean ± SD | 6.6±5.5 | – | |
| Disease course and activity | All RRMS | – | |
| EDSS level, mean (range) | 1.7 (0.5–5.5) | – | |
| PI | 0.35 (0.04–1.16) | – | |
| Treatment | |||
| Interferon | 21 | – | |
| Glatiramer acetate | 9 | – | |
| No treatment | 2 | – |
RRMS = Relapsing-remitting MS.
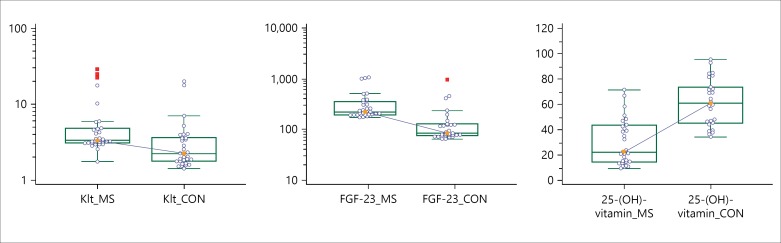
Serum sKL, FGF-23, and P levels were significantly higher in MS patients compared to the control group (p < 0.01, p < 0.01 and p = 0.02, respectively; fig. 1). On the contrary, serum 25-(OH)-vitamin D and Ca levels were significantly lower in MS patients (p < 0.01 and p = 0.04, respectively). Serum PTH levels were lower in MS patients, but this difference was not statistically significant (p = 0.09; table 2).
Fig. 1.
The levels of sKlt, FGF-23 and 25-(OH)-vitamin D in MS patients and controls. Serum sKlt and FGF-23 levels were significantly higher in patients with MS than in controls, whereas 25-(OH)-vitamin D levels were significantly lower in MS patients.
Table 2.
Biochemical markers
| Parameters | Patients (n = 32) | Controls (n = 31) | p value |
|---|---|---|---|
| BUN, mmol/l, mean (range) | 3.7 (3.2–5) | 4.5 (3.6–5.3) | 0.10 |
| Creatinine, µmol/l, mean (range) | 78.6 (77–85.7) | 75.1 (70.7–84.8) | 0.22 |
| Ca, mmol/l, mean ± SD | 2.29±0.11 | 2.35±0.10 | 0.04 |
| P, mmol/l, mean (range) | 1.09 (1.03–1.19) | 1.03 (0.90–1.16) | 0.02 |
| PTH, ng/l, mean (range) | 45.3 (37–61) | 52 (41.5–67) | 0.09 |
| 25-(OH)-vitamin D, ng/ml, mean (range) | 22 (14.2–43.2) | 60 (45.1–73.5) | <0.01 |
| sKlt, ng/ml, mean (range) | 3.31 (3.1–4.8) | 2.25 (1.8–3.6) | <0.01 |
| FGF-23, pg/ml, mean (range) | 222 (197–360) | 85 (76–127) | <0.01 |
BUN = Blood urea nitrogen.
Considering a cut-off value of 30 ng/ml for 25-(OH)-vitamin D in MS patients [20], 17 patients had sufficient serum 25-(OH)-vitamin D levels, whereas 15 patients had sufficient 25-(OH)-vitamin D levels.
With the exception of a weak negative correlation between sKlt and EDSS (r = −0.328, p = 0.06), we did not find any significant correlation between other parameters (FGF-23, PTH, EDSS, PI, Ca, P, and disease duration between the groups). We found a strong positive correlation between sKlt and FGF-23 in MS patients (r = 0.773, p < 0.01). Similarly, a strong positive correlation was also found between sKlt and FGF-23 in the control group (r = 0.797, p < 0.01). In addition, we found positive correlations between sKlt and 25-(OH)-vitamin D and FGF-23 and 25-(OH)-vitamin D in the control group (r = 0.495, p = 0.01; r = 0.403, p = 0.04, respectively; table 3). We did not find any significant correlation between sKlt, FGF-23, and 25-(OH)-vitamin D, and age, gender, EDSS, PI, and other parameters.
Table 3.
Correlation analysis
| r value | p value | |
|---|---|---|
| For MS patients | ||
| sKlt and FGF-23 | 0.773 | <0.01 |
| sKlt and EDSS | –0.328 | 0.06 |
| For controls | ||
| sKlt and FGF-23 | 0.797 | <0.01 |
| sKlt and 25-(OH)-vitamin D | 0.495 | 0.01 |
| FGF-23 and 25-(OH)-vitamin D | 0.403 | 0.04 |
Discussion
In this study, we analyzed sKlt, FGF-23, and 25-(OH)-vitamin D levels in MS patients. Our results showed that sKlt and FGF-23 levels were significantly higher in MS patients compared to the control group. On the contrary, 25-(OH)-vitamin D levels were significantly lower in MS patients compared to the control group. In both groups, there was a strong positive correlation between sKlt and FGF-23. In the control group, we found a positive correlation between sKlt and 25-(OH)-vitamin D, and FGF-23 and 25-(OH)-vitamin D; on the contrary, such a correlation was not found in MS patients.
As mentioned previously, Klt is mainly expressed in 2 locations in the human body: the kidneys and brain. Within the brain, Klt is particularly expressed to a greater extent in the choroid plexus (CP) [6]. Considering the fact that none of the MS patients had previously had any kidney pathology, we could conclude that CNS is the source of sKlt in MS patients. Accordingly, we can evaluate the results in 4 main headings.
CP and Klt
CP is a major component of the blood-brain barrier (BBB), and also contributes to the production of cerebrospinal fluid (CSF) via epithelial cells. Interestingly, Sathyanesan et al. [21] have demonstrated that gene expression in CP is more similar to the kidneys, compared to other regions of the brain, and various receptors in kidney, Klt, and carrier proteins have high similarities with the proteins in CP. Given the fact that it maintains the ionic equilibrium of the CSF, Spector and Johanson [22] described CP as the ‘kidney of the brain’ in 1989.
Under normal conditions, the BBB is an effective barrier for the CNS against blood cells. In case of bacterial and viral infections, or autoimmune diseases such as MS, the leukocyte count in CNS is elevated - the entry site of these leukocytes to CNS is presumably CP [23,24,25] - and Klt secretion from CP could mediate this process. The Wnt signaling pathway is responsible for the epithelial polarity in CP, and regulation of endothelial tight junction in the BBB [26,27]. Liu et al. [8] have shown that Klt binds to several members of the Wnt family and inhibits them. Accordingly, Klt secretion from CP might inhibit Wnt signaling, leading to the disruption of the BBB integrity and eventually entry of leukocytes into CNS.
Neurons, Oligodendrocytes, and Klt
Despite the ongoing studies on Klt, our understanding of the function of this biomolecule is not fully clear, especially within the CNS. In Klt knock-out mice, an increased total neurodegeneration in CNS, including memory loss, altered cholinergic function, decreased dopaminergic and hippocampal neurons, decreased Purkinje cells, insufficient axonal transport, decreased synaptic protein expression, increased apoptotic markers, and elevated oxidative stress are seen compared to the wild-type mice [28]. While Klt is mostly expressed in CP and Purkinje cells in CNS, Clinton et al. [28] showed that Klt is extensively expressed in rats, including the white and grey matter in the brain. In this study, the authors have shown that Klt is co-localized with neuronal and oligodendrocyte markers but not with microglial and astrocyte markers. Chen et al. [29] showed another important function of Klt in CNS. In CNS, Klt leads to the differentiation and maturation of oligodendrocyte progenitor cells to mature oligodendrocytes, but does not increase the proliferation of these cells. In conclusion, peripheral leukocytes are required to repair the chronic myelin damage in MS, and oligodendrocytes are required for myelin production. Neurons could increase the Klt synthesis, and lead to the differentiation of oligodendrocyte progenitor cells to mature oligodendrocytes. In addition, they could also decrease the specificity of BBB, and result in the entry of leukocytes into the CNS. In short, Klt secretion from the CNS could be a response to the inflammatory condition in MS.
FGF-23 and MS
FGF-23 is a hormone that is released from osteoblasts, which regulates Ca and P metabolisms [6]. Currently, it is not known whether FGF-23 is secreted from the brain; however, in CNS, the effects of its sister FGF-2 [30,31] on astrocytes, oligodendrocytes, and BBB have been shown. Similar to Klt, FGF-23 secretion from neurons or CP could also lead to the disruption of BBB integrity and alter the phosphate metabolism in CSF. On the contrary, Klt secretion from CP could increase the FGF-23 expression in osteoblasts.
Irrespective of its origin, elevated FGF-23 levels in MS could disrupt the P equilibrium in MS patients, especially in CNS. P reabsorption from CSF could disrupt the function of cellular structures (such as proteins and nucleotides); alternatively, P absorption in CSF could lead to excess P accumulation, resulting in cytotoxicity. Apart from its effects on the P metabolism, overexpression of FGF-23 - in our study, FGF-23 levels were approximately 2.5-fold higher in MS patients compared to the control group and could lead to comorbid diseases, such as cardiovascular diseases in MS patients.
Klt-FGF-23-Vitamin D Axis in MS
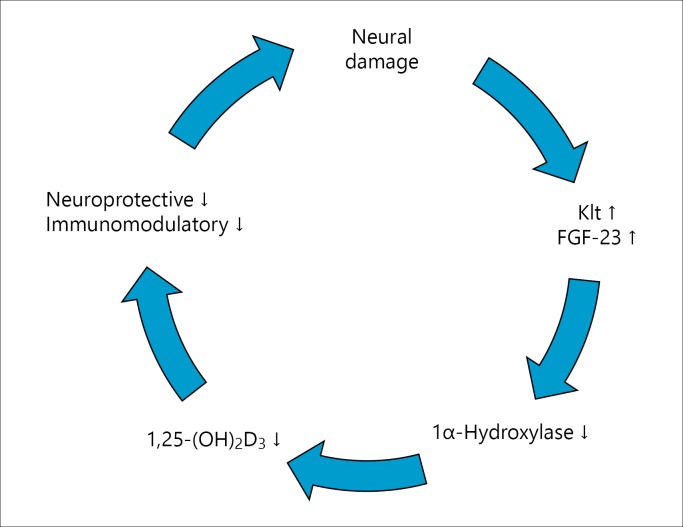
The FGF-23/Klt axis has a key role in the regulation of P metabolism. This system ensures the regulation of PTH and vitamin D synthesis [13]. Different studies have shown that low vitamin D levels are associated with MS [14,15,16]. Indeed, previous studies have shown that vitamin D has immunomodulatory effects on experimental autoimmune encephalitis, an experimental model of MS; inhibits the IL-12/IFN-γ axis and Th17 cell differentiation; and blocks the entry of inflammatory cells to CNS [32,33]. Previous studies have shown that vitamin D could pass through BBB directly and CNS cells express vitamin D receptors [34]. Interestingly, Shirazi et al. [35] found that 1,25-(OH)2D3 increases the number of neural stem cells, and converts them to mature neurons and oligodendrocytes. Unfortunately, it might not be possible to see all of these neuroprotective and immunomodulatory effects of vitamin D in MS patients. It is known that FGF-23 inhibits 1α-hydroxylase and decreases 1,25-(OH)2D3 levels. In addition, FGF-23 upregulates 24α-hydroxylase and eliminates 1,25-(OH)2D3[18]. In this study, we found that FGF-23 levels were approximately 2.5-fold higher in MS patients; however, despite the correlation between 25-(OH)-vitamin D levels and FGF-23 and Klt in the control group, we did not find such a correlation in MS patients. When we compared MS patients and the control group, we found significant differences in Ca and P levels. Taken together, we conclude that the Klt/FGF-23/vitamin D axis is altered in MS. Moreover, low vitamin D levels in MS patients are not the primary cause of MS but rather a result of elevated FGF-23 levels. Neuronal destruction in MS and Klt and FGF-23 secretion from these neurons and CP, disrupt the peripheral Klt/FGF-23 axis. Moreover, elevated FGF-23 levels suppress 1,25-(OH)2D3, which in turn exacerbates inflammation and creates a vicious cycle (fig. 2).
Fig. 2.
A vicious cycle of KL/FGF-23/vitamin D axis in MS patients.
Taken together, we found that serum sKlt and FGF-23 levels were higher in MS patients compared to the control group; on the contrary, 25-(OH)-vitamin D levels were lower in MS patients. While it is possible that this outcome could represent the main pathogenesis mechanism underlying MS, it could also represent a response to the chronic inflammation in MS. A single bioactive molecule is not responsible for a single function, and complex diseases such as MS might not have a single cause. However, we believe that the Klt/FGF-23/vitamin D axis has a significant role in the pathogenesis and progression of MS. The evaluation of the Klt/FGF-23/vitamin D axis in other subtypes of MS and evaluation of MRI, electrophysiological findings, CSF oligoclonal band, CSF Klt, and FGF-23 levels are important for the diagnosis and treatment of this disease. Klt and FGF-23 could serve as important markers for evaluating BBB in MS and other neurological diseases. Our study has certain limitations, such as low sample size, involving a single subtype of MS, and not analyzing 1,25-(OH)2D3 levels. Yet, this is the first step and the first study of its kind to investigate the Klt/FGF23/vitamin D axis in MS.
Authorship Contributions
H.Y. Ellidag: research concept and design, data analysis and interpretation, laboratory analysis; N. Yilmaz: critical revision of the article; F. Kurtulus: neurological examination and selection of patients; O. Aydın: final revision of article; E. Eren: data analysis and interpretation; A. Inci: nephrological examination and selection of patients; S. Dolu: nephrological examination and selection of patients; F.D.A. Ince: data analysis; Ö. Giray: language revision; A. Yaman: neurological examination and selection of patients.
Disclosure Statement
There is no potential conflict of interest. This study received no funding or sponsorship of any form. This manuscript complies with International Committee of Medical Journal Editor's guidelines.
References
- 1.Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2 suppl):S15–S20. [PubMed] [Google Scholar]
- 2.Ellidag HY, Eren E, Erdogan N, et al. Comparison of neurophysiological and MRI findings of patients with multiple sclerosis using oligoclonal band technique. Ann Neurosci. 2013;20:149–154. doi: 10.5214/ans.0972.7531.200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellidag HY, Kurtulus F, Yaman A, et al. Serum iron metabolism markers including hepcidin in multiple sclerosis patients. Neurochem J. 2014;8:226–230. [Google Scholar]
- 4.Aydin O, Ellidag HY, Eren E, et al. Ischemia modified albumin is an indicator of oxidative stress in multiple sclerosis. Biochem Med (Zagreb) 2014;24:383–389. doi: 10.11613/BM.2014.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 6.Nitta K, Nagano N, Tsuchiya K. Fibroblast growth factor 23/klotho axis in chronic kidney disease. Nephron Clin Pract. 2014;128:1–10. doi: 10.1159/000365787. [DOI] [PubMed] [Google Scholar]
- 7.Abraham CR, Chen C, Cuny GD, et al. Small-molecule klotho enhancers as novel treatment of neurodegeneration. Future Med Chem. 2012;4:1671–1679. doi: 10.4155/fmc.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 9.Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113–122. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawires HK, Essam RM, Morgan MF, Mahmoud RA. Serum klotho: relation to fibroblast growth factor-23 and other regulators of phosphate metabolism in children with chronic kidney disease. Nephron. 2015;129:293–299. doi: 10.1159/000377633. [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Urakawa I, Yamazaki Y, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 12.Bai X, Miao D, Li J, et al. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 13.Donate-Correa J, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. FGF23/klotho axis: phosphorus, mineral metabolism and beyond. Cytokine Growth Factor Rev. 2012;23:37–46. doi: 10.1016/j.cytogfr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Smolders J, Menheere P, Thewissen M, et al. Regulatory T cell function correlates with serum 25-hydroxyvitamin D, but not with 1,25-dihydroxyvitamin D, parathyroid hormone and calcium levels in patients with relapsing remitting multiple sclerosis. J Steroid Biochem Mol Biol. 2010;121:243–246. doi: 10.1016/j.jsbmb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Harandi AA, Harandi AA, Pakdaman H, Sahraian MA. Vitamin D and multiple sclerosis. Iran J Neurol. 2014;13:1–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HL, Wu J. Role of vitamin D in immune responses and autoimmune diseases, with emphasis on its role in multiple sclerosis. Neurosci Bull. 2010;26:445–454. doi: 10.1007/s12264-010-0731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glade MJ. Vitamin D: health panacea or false prophet? Nutrition. 2013;29:37–41. doi: 10.1016/j.nut.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Gadi R, Spertus JA, et al. Prevalence of vitamin D deficiency in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1636–1638. doi: 10.1016/j.amjcard.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sathyanesan M, Girgenti MJ, Banasr M, et al. A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl Psychiatry. 2012;2:e139. doi: 10.1038/tp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector R, Johanson CE. The mammalian choroid plexus. Sci Am. 1989;261:68–74. doi: 10.1038/scientificamerican1189-68. [DOI] [PubMed] [Google Scholar]
- 23.Kooij G, De Vries HE. The best basic science paper in multiple sclerosis in 2014: important role for the choroid plexus in the central nervous system entry of leukocytes. Mult Scler. 2015;21:372–373. doi: 10.1177/1352458515573095. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119:75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- 26.Liebner S, Corada M, Bangsow T, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development. Int J Dev Biol. 2011;55:467–476. doi: 10.1387/ijdb.103224sl. [DOI] [PubMed] [Google Scholar]
- 28.Clinton SM, Glover ME, Maltare A, et al. Expression of klotho mRNA and protein in rat brain parenchyma from early postnatal development into adulthood. Brain Res. 2013;1527:1–14. doi: 10.1016/j.brainres.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CD, Li H, Liang J, et al. The anti-aging and tumor suppressor protein klotho enhances differentiation of a human oligodendrocytic hybrid cell line. J Mol Neurosci. 2015;55:76–90. doi: 10.1007/s12031-014-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuss B, Dono R, Unsicker K. Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood-brain barrier permeability: evidence from mouse mutants. J Neurosci. 2003;23:6404–6412. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Bao L, Yang L, et al. Roles of intracellular fibroblast growth factors in neural development and functions. Sci China Life Sci. 2012;55:1038–1044. doi: 10.1007/s11427-012-4412-x. [DOI] [PubMed] [Google Scholar]
- 32.Muthian G, Raikwar HP, Rajasingh J, et al. 1,25 dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83:1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Jin J, Tang Y, et al. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182:3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 34.DeLuca GC, Kimball SM, Kolasinski J, et al. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39:458–484. doi: 10.1111/nan.12020. [DOI] [PubMed] [Google Scholar]
- 35.Shirazi HA, Rasouli J, Ciric B, et al. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol. 2015;98:240–245. doi: 10.1016/j.yexmp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]