Abstract
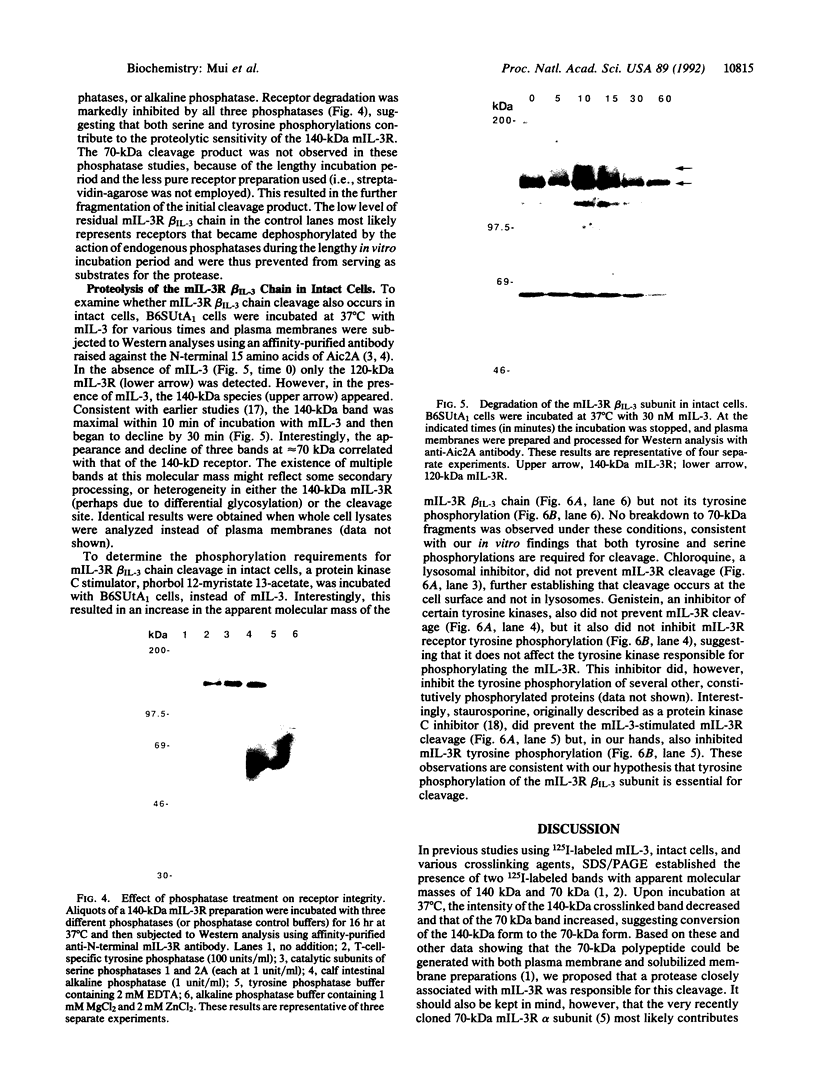
The murine interleukin 3 receptor (mIL-3R) is a heterodimer consisting of a 70-kDa alpha subunit and one of two alternative 120-kDa beta subunits termed beta IL-3 and beta c. beta IL-3 (originally called Aic2A) is capable of binding mIL-3 by itself, whereas beta c (Aic2B) does not bind any ligand on its own but increases the affinity of mIL-3, murine granulocyte/macrophage-colony-stimulating factor, and mIL-5 for their respective alpha subunits. Interestingly, although the mIL-3R does not possess tyrosine kinase activity, its beta IL-3 subunit does become tyrosine phosphorylated upon binding mIL-3. To further investigate the properties of this subunit, we have purified it from the cell line B6SUtA1, which expresses a high level of mIL-3R. Intriguingly, studies comparing the stability of the 140-kDa, tyrosine-phosphorylated form of this subunit with its 120-kDa, non-tyrosine-phosphorylated form reveal that the former is far less stable and is rapidly degraded to a 70-kDa fragment. Mixing experiments demonstrate that the differential stability of the two forms is due to an intrinsic difference in protease susceptibility. Phosphatase studies indicate that the higher protease susceptibility of the tyrosine-phosphorylated beta IL-3 is due to the presence of both phosphotyrosine and phosphoserine residues. Western analyses using an anti-N-terminal mIL-3R beta IL-3 chain antibody reveal that this proteolytic cleavage also occurs rapidly in intact cells following stimulation with mIL-3 and occurs at the cell surface, since it takes place within minutes at 37 degrees C, is observed with purified plasma membranes, and is not inhibited by chloroquine. This degradative step may play an important role in the mechanism of action of mIL-3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damen J., Mui A. L., Hughes P., Humphries K., Krystal G. Erythropoietin-induced tyrosine phosphorylations in a high erythropoietin receptor-expressing lymphoid cell line. Blood. 1992 Oct 15;80(8):1923–1932. [PubMed] [Google Scholar]
- Downing J. R., Roussel M. F., Sherr C. J. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989 Jul;9(7):2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Stevens D., May W. S., Ihle J. N. Interleukin 3 binds to a 140-kDa phosphotyrosine-containing cell surface protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7982–7986. doi: 10.1073/pnas.85.21.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G., Alai M., Cutler R. L., Dickeson H., Mui A. L., Wognum A. W. Hematopoietic growth factor receptors. Hematol Pathol. 1991;5(4):141–162. [PubMed] [Google Scholar]
- Mills G. B., May C., McGill M., Fung M., Baker M., Sutherland R., Greene W. C. Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated. J Biol Chem. 1990 Feb 25;265(6):3561–3567. [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Mui A. L., Kay R. J., Humphries R. K., Krystal G. Purification of the murine interleukin 3 receptor. J Biol Chem. 1992 Aug 15;267(23):16523–16530. [PubMed] [Google Scholar]
- Murthy S. C., Mui A. L., Krystal G. Characterization of the interleukin 3 receptor. Exp Hematol. 1990 Jan;18(1):11–17. [PubMed] [Google Scholar]
- Murthy S. C., Sorensen P. H., Mui A. L., Krystal G. Interleukin-3 down-regulates its own receptor. Blood. 1989 Apr;73(5):1180–1187. [PubMed] [Google Scholar]
- Sorensen P. H., Mui A. L., Murthy S. C., Krystal G. Interleukin-3, GM-CSF, and TPA induce distinct phosphorylation events in an interleukin 3-dependent multipotential cell line. Blood. 1989 Feb;73(2):406–418. [PubMed] [Google Scholar]
- Sorensen P., Mui A. L., Krystal G. Interleukin-3 stimulates the tyrosine phosphorylation of the 140-kilodalton interleukin-3 receptor. J Biol Chem. 1989 Nov 15;264(32):19253–19258. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]









