Abstract
Water management that alters riverine ecosystem processes has strongly influenced deltas and the people who depend on them, but a full accounting of the trade-offs is still emerging. Using palaeoecological data, we document a surprising biogeochemical consequence of water management in the Colorado River basin. Complete allocation and consumptive use of the river's flow has altered the downstream estuarine ecosystem, including the abundance and composition of the mollusc community, an important component in estuarine carbon cycling. In particular, population declines in the endemic Colorado delta clam, Mulinia coloradoensis, from 50--125 individuals m−2 in the pre-dam era to three individuals m−2 today, have likely resulted in a reduction, on the order of 5900–15 000 t C yr−1 (4.1–10.6 mol C m−2 yr−1), in the net carbon emissions associated with molluscs. Although this reduction is large within the estuarine system, it is small in comparison with annual global carbon emissions. Nonetheless, this finding highlights the need for further research into the effects of dams, diversions and reservoirs on the biogeochemistry of deltas and estuaries worldwide, underscoring a present need for integrated water and carbon planning.
Keywords: carbon emission, carbon sequestration, estuary, geohistorical records, mollusc, water diversion
1. Introduction
Rivers worldwide have been profoundly modified to maximize the production of a subset of the services they provide, such as hydroelectric power and reliable water for irrigation and municipal use [1,2]. The upstream infrastructure and water diversions required to provide these services can, however, have a pronounced effect on downstream deltas and estuarine ecosystems. With human demands for freshwater remaining high or increasing, deltas and estuaries are poised to experience heightened stress from ocean acidification and sea-level rise as the climate changes. These compounded stressors may have wide reaching impacts, including perturbing carbon cycling in river systems [3,4]. Yet, our current understanding of the influence of large-scale water management on carbon sequestration and emission hinders the comprehensive evaluation of infrastructure or other water resource planning [5–7].
The need for research examining the carbon consequences of water management associated with the alterations of biophysical processes in rivers is particularly acute in deltas and their estuaries [3]. Carbon emissions from estuaries, which can range from 17 to 46 mol C m−2 yr−1 [8], are significant in regional carbon budgets [9,10] and cumulatively can amount to global emissions of 3.4–4.5 × 108 t C yr−1 [8,11]. Whereas overall emissions from estuaries are becoming well quantified [12], the individual components of the multifaceted estuarine carbon cycle (figure 1) merit further study, particularly molluscs.
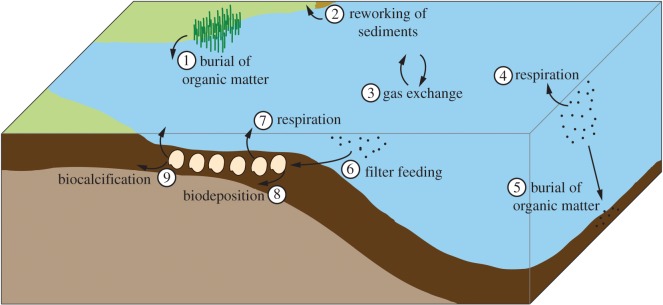
Figure 1.
A subset of the processes involved in estuarine carbon cycling. (1) Sequestration of carbon via vegetation (e.g. salt marshes, mangroves) growth, death and burial; (2) emission of carbon due to reworking of carbon-rich sediments; (3) constant gas exchange between ocean and atmosphere; (4) emission of carbon via respiration by microbes and zooplankton; (5) sequestration of carbon via burial of dead plankton; (6) filter feeding by bivalves; (7) carbon emission via bivalve respiration; (8) carbon sequestration via biodeposition and (9) carbon sequestration and emission via biocalcification.
Molluscs are a vital element of the tidal and subtidal benthic fauna of estuaries, collectively emitting 2–20 mol C yr−1 [13–15] and playing a large role in the pelagic–benthic cycling of nutrients (e.g. carbon, nitrogen, phosphorus [16–19]). In particular, bivalve molluscs (commonly, ‘clams’) can be found locally in densities of more than 1000 individuals m−2 [20,21] and can contribute significantly to estuarine carbon emissions via respiration and biogenic calcification (i.e. shell formation). Although carbon sequestration exceeds carbon emission during shell formation, the amount of carbon dioxide released via respiration typically exceeds the amount of carbon that is sequestered, resulting in net carbon emissions [13–15,22]. It is well documented that water management can affect molluscan populations [4,23,24]; however, to the best of our knowledge, the carbon implications of these effects have never been assessed.
The palaeoecological record on the Colorado River delta (CRD; figure 2) offers a unique means to conduct such an analysis. Prior to damming and diversions in the 1930s, natural annual flows ranged between 16 × 109 and 18 × 109 m3 at Lee's Ferry in northern Arizona [26], but the river now fails to reach the sea in most years (with the exception of the recent environmental pulse flow under the groundbreaking binational Minute 319 agreement [27]). The lack of Colorado River water has led to substantial declines in clam [23], shrimp [28] and fish [29] populations, in addition to reduction in estuarine, riparian and wetland vegetation [30]. Salt marshes, wetlands and riparian forests now cover a small fraction of their former area [31,32], and the once brackish estuary is now saltier than the sea [33,34].
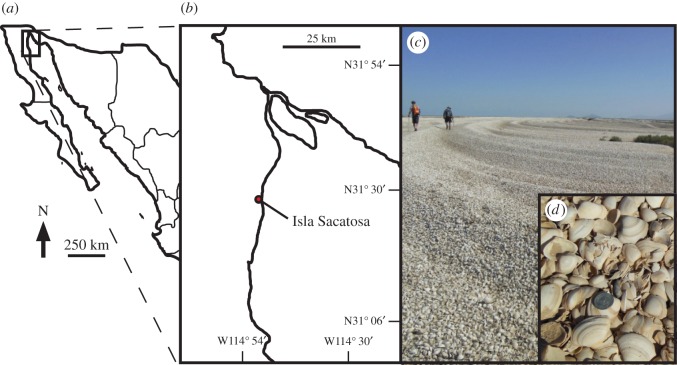
Figure 2.
Cheniers in the Colorado River delta. (a) Location of delta in Mexico. (b) Colorado River delta locality from Kowalewski et al. [23]. (c) A chenier in the Colorado River delta at a locality south of Isla Sacatosa. (d) Close-up of Mulinia coloradoensis; coin, 0.24 cm in diameter.
Very few environmental data were collected before the main period of dam construction (1900–1965) on the Colorado River. However, palaeoecological data from cheniers—beach ridges composed almost exclusively of molluscan remains [35,36]—permit estimates of the size and density of the pre-dam (prior to 1930) molluscan community, particularly the endemic Colorado delta clam, Mulinia coloradoensis [23] (figure 2). Cheniers of three ages are present, two that formed between 90–1500 and 2000–5000 years ago, and a third that has been actively forming over the past 90 years [25]. Dated shells from these cheniers indicate that M. coloradoensis accounted for as much as 95% of the individuals during the pre-dam era and occurred at densities that were likely well more than 50 individuals m−2 [23]. In contrast, current molluscan densities of 3–17 individuals m−2 [37] indicate the scope of the ecological change associated with river management [23,24,38]. Accordingly, we combined evidence of pre-dam conditions from cheniers with data from contemporary mollusc community surveys to investigate how reduced clam density following upstream river diversion altered carbon sequestration and emissions (in terms of CO2) in the estuary of the CRD.
2. Methods
2.1. Clam density
We established the estimates for the density of clams living on the CRD tidal flats during the pre-dam era and the present day based on the work of Kowalewski et al. [23] and Cintra-Buenrostro et al. [38]. Kowalewski et al. [23] estimated pre-dam clam density for the 90–1500 year old chenier by combining bulk samples from the molluscan assemblage, estimates of the total area of shell deposits and an average chenier thickness (table 1). They estimated an average density of 87 500 clams m−3, which translates to approximately 2.1 × 1012 total clams in the chenier. Amino acid racemization confirmed that 98% (n = 125) of the shells in the chenier dated from the period C.E. 950–1950. Based on estimates of the pre-dam tidal flat area (table 1) and an average ontogenetic age of three years, Kowalewski et al. [23] then calculated an average clam density of approximately 50 individuals m−2 during this period. This value likely represents a conservative estimate of clam density given that various taphonomic processes (e.g. shell dissolution, abrasion, fragmentation, etc.) remove shell material after death [25,44]. Based on the age distribution of dated shells and accounting for shell removal, Kowalewski et al. [23] proposed that the total number of clams alive during C.E. 950–1950 may have exceeded 5 × 1012, or a constant standing density of 125 individuals m−2 across the entire CRD tidal flat. This estimate remains highly conservative, however, because, for practical reasons, Kowalewski et al. [23] did not consider individuals smaller than the 12.5 mm mesh size that they used to sieve samples. Indeed, surveys of the living community suggest that large individuals (more than 12.5 mm) may compose the majority of biomass yet represent only 20% of individuals in the community [23]. Thus, we calculated changes in carbon based on pre-dam densities of 50 and 125 individuals m−2 in order to estimate a conservative range of values.
Table 1.
Parameter estimates and assumptions for carbon calculations. AFDM, ash-free dry mass.
| parameter | estimate | source |
|---|---|---|
| chenier area | 5.96 × 106 m2 | Kowalewski et al. [23] |
| chenier thickness | 4 m | Kowalewski et al. [23] |
| chenier volume | 2.4 × 107 m2 | Kowalewski et al. [23] |
| clams by volume in cheniers | 87 500 m−3 | Kowalewski et al. [23] |
| tidal flat area | 1.2 × 108 m2 | Kowalewski et al. [23] |
| pre-dam clam density | 50–125 ind m−2 | Kowalewski et al. [23] |
| modern clam density | 3–17 ind m−2 | Kowalewski et al. [23], Avila-Serrano et al. [37] |
| clam dry mass | 11.13 ± 8.3 g ind−1 | Measured (electronic supplementary material, table) |
| pre-dam salinity (north) | 22–32 psu | Cintra-Buenrostro et al. [39] |
| pre-dam salinity (south) | 30–38 psu | Cintra-Buenrostro et al. [39] |
| modern salinity | 35–42 psu | Dettman et al. [40] |
| dry tissue weight | 1.36 g ind−1 | Velasco & Navarro [41] |
| dry tissue (DT) to AFDM | 1 g DT = 0.81 g AFDM | Rumohr et al. [42] |
| AFDM gram to kilocalorie | 1 g AFDM = 5.492 kcal | Cummins & Wuycheck [43] |
| kilocalorie to grams carbon | 11.4 kcal = 1 g C | Chauvaud et al. [13] |
Surveys of the contemporary tidal flat molluscan community along seven transects throughout the CRD suggest an average density of approximately 17 individuals m−2 [23,37]. This estimate includes molluscs of all sizes; however, when only considering clams greater than 12.5 mm, density drops to three individuals m−2 [23]. We based present-day values on this latter estimate for consistency with the pre-dam data.
2.2. Carbon storage and emission
Using these pre-dam and present-day estimates of clam density, we then calculated annual carbon sequestration and production from biogenic calcification and carbon emission due to respiration. Annual carbon sequestration was calculated based on standing clam density and one-third average shell dry mass (assuming constant growth over a clam's three-year lifespan for ease of calculation; table 1), with mass corrected to account for the approximately 5% of compounds other than calcium carbonate in clam shells [45].
We determined average shell dry mass (11.13 ± 8.3 g ind−1) by taking the mass of 100 specimens of M. coloradoensis (electronic supplementary material, table S1), which composed as much as 95% of the molluscan community in the pre-dam era [24,25]. The M. coloradoensis specimens were randomly selected from a bulk sample [46] that was sieved with a 12.5 mm screen to ensure comparability with the data reported by Kowalewski et al. [23]. We estimated grams of carbon per square metre by adjusting for the atomic weight of calcium carbonate:
We then multiplied g C m−2 by the total area of the tidal flat to provide values of annual average sequestration (table 1).
We calculated the production of carbon due to biogenic calcification according to the ratio of released (CO2) to precipitated (CaCO3) carbon (ψ [47]). Carbon emission to the atmosphere during biogenic calcification is buffered by water chemistry, with the exchange rate influenced by temperature and salinity. Higher temperatures and greater salinities reduce ψ, decreasing the emission of CO2 to the atmosphere relative to the precipitation of carbonate (i.e. fresh, cold water leads to the least precipitation). Seawater temperature data are available for the present day and vary seasonally from 5 to 40°C [48,49], but data from the pre-dam era are limited to a handful of data points at 18–20°C, collected by the US Fish Commission steamer Albatross in March 1889 [50]. Owing to this limitation on temperature data, we used the univariate ψ equation for salinity, which does not include temperature (i.e. assumes constant temperature)
where ψ is the ratio of released : precipitated carbon, and S is salinity in practical salinity units (psu), and pCO2 = 350 µatm1 [13,15,22,47].
Salinity in the CRD has increased following the construction of dams throughout the basin [24,33]. Today, salinities range from 35 to 42 psu [40], though periodic flow releases (at significantly lower volumes than occurred historically) have occasionally reduced salinities to 29–36 psu [40,51]. We therefore assumed a value of 38.5 psu for the present. Isotopic data from the shells of M. coloradoensis suggest individuals near the mouth of the river grew under salinities of 22–33 psu, whereas individuals further south experienced salinities of 30–38 psu [39]. We used the mean value from the isotopic data, 30 psu, which is in the range reported during more recent flows, to calculate changes in carbon emissions.
Finally, carbon emitted due to respiration was calculated using the relationship established by Schwinghamer et al. [52]:
where R and P are respiration and biomass production (kcal m−2 yr−1), respectively. We applied the conversion factor of 5.492 kcal = 1 g ash-free dry mass (AFDM) to estimate P [43] and estimated carbon emission from R as 11.4 kcal = 1 g C [13]. Live M. coloradoensis were not available to determine AFDM; however, dry tissue weights of 1.36 g individual–1 have been reported in the literature for the closely related and morphologically similar Mulinia edulis [41] and can be converted to AFDM-equivalent using the conversion factor of 1 g dry tissue weight = 0.81 g AFDM [42]. Thus, we used a value of 1.1 g AFDM in our calculations.
3. Results
Both sequestration, via biogenic calcification, and emission, via respiration and calcification, have fallen sharply with the alteration of the CRD estuarine ecosystem. Estimated sequestration from current clam populations is 0.1 mol C m−2 yr−1, whereas pre-dam abundances imply sequestration of 1.8–4.4 mol C m−2 yr−1 (table 2, at low and high densities, respectively). Atmospheric emissions due to calcification have also declined by roughly an order of magnitude from 1.3 to 3.1 mol C m−2 yr−1 (at low and high pre-dam densities, respectively) to 0.07 mol C m−2 yr−1. Similarly, historic emissions from respiration ranging from 4.9 to 12.2 mol C m−2 yr−1 (at low and high densities, respectively) have declined to 0.3 mol C m−2 yr−1. As a consequence, net carbon emissions have decreased from a range of 4.4–10.9 to 0.26 mol C m−2 yr−1. These values correspond to a cumulative annual reduction in tidal flat carbon emissions ranging from 5.9 × 103 to 15.0 × 103 t.
Table 2.
Estimated carbon sequestration and emission for the pre-dam and modern eras. Pre-dam low (50 ind m−1) and high (125 ind m−1) refer to the number of individuals per square metre inferred from chenier deposits [23]. Emissions via calcification were estimated at salinities of 30 psu and 38.5 psu for the pre-dam and modern eras, respectively. Emissions from respiration were estimated based on an ash-free dry mass of 1.1 g ind−1.
| Δcarbon (low, high) |
|||||
|---|---|---|---|---|---|
| pre-dam low (mol C m−2 yr−1) | pre-dam high (mol C m−2 yr−1) | modern (mol C m−2 yr−1) | mol C m−2 yr−1 | t C yr−1 | |
| sequestration via calcification | 1.8 | 4.4 | 0.11 | 1.7, 4.3 | 2400, 6200 |
| emission via calcification | 1.3 | 3.1 | 0.068 | 1.2, 3.0 | 1700, 4400 |
| emission via respiration | 4.9 | 12.2 | 0.3 | 4.6, 11.9 | 6600, 17 000 |
| net emission | 4.4 | 10.9 | 0.26 | 4.1, 10.6 | 5900, 15 200 |
4. Discussion
The reduction in carbon emissions, by 4.1–10.6 mol C m−2 yr−1, due to the decline of M. coloradoensis populations in the CRD likely corresponds to a large proportional decline in carbon emissions from the estuary as a whole. Borges et al. [8] estimated for estuaries from low latitudes (0–30°) and high latitudes (30–60°) that carbon emissions were 17 and 46 mol m−2 yr–1, respectively. The CRD is located at approximately 31°N, suggesting that the carbon reductions calculated here represent a reduction of roughly 9–23% for the entire estuary. The estimates from Borges et al. [8] may, however, overestimate estuarine emissions owing to the abundance (69%; n = 16) of high pCO2 European river systems used to make the estimates [12,53,54]. In contrast, emissions from several ‘high latitude’ estuaries in the United States were reported to be considerably lower: 15–36 mol C m−2 yr−1 [12,55]. Thus, the reductions reported here might correspond to a decline of up to 70% for annual estuary carbon emissions. This considerable change for the estuary has the potential to significantly alter the CRD ecosystem [16,56] and influence economically important local mariculture [57]. Despite these implications, the conveyance, storage and emission of carbon by rivers—under natural or human-altered conditions—has only recently [6,7,53] factored prominently into assessments of the trade-offs that accompany decisions to store water in reservoirs, to divert it for agricultural and municipal use, or to use it for hydroelectric power generation [58–61]. Even so, these decisions certainly imply different outcomes for the carbon footprint associated with the managed river network [7].
Our calculations were constrained in part by the uncertainties inherent to using palaeoecological data; however, we most likely underestimated the difference in carbon emissions before and after extensive river diversion. The largest uncertainty in our analyses was the estimate of clam density prior to extensive water diversions. Many processes transport (e.g. wave action, currents) and degrade (e.g. fragmentation, dissolution2) the remains of organisms after death [25], and the shells preserved in cheniers provide a conservative, lower bound on original density. Additional uncertainty was due to the absence of precise estimates for M. coloradoensis AFDM. Despite being congeneric, slight differences in shell morphology between M. coloradoensis and M. edulis may have introduced minor errors into our calculations. Notwithstanding these considerations, our estimates for pre-dam carbon emission and sequestration are comparable to values reported for other calcifying organisms such as clams [13,15], corals [62], barnacles [63] and brittle stars [64] (table 3). These studies support the validity of our parameter estimates, and strengthen the conclusion that carbon emissions from the molluscan community in the estuary of the CRD have dropped precipitously following the complete appropriation of the river's flow.
Table 3.
Carbon sequestration and emission for other calcifying systems.
| species | sequestration via calcification (mol C m−2 yr−1) | emission via calcification (mol C m−2 yr−1) | emission via respiration (mol C m−2 yr−1) | source |
|---|---|---|---|---|
| Potamocorbula amurensis | 2.2 | 1.5 | 3.1 | Chauvaud et al. [13] |
| Ruditapes philippinarum | 8.2 | 5.6 | 22.7 | Mistri & Munari [15] |
| Mytilus galloprovincialis | 136.6 | 86.8 | 187.8 | Munari et al. [22] |
| barnacles | 4.8–18.0 | 3.4–12.7 | 3.9–14.1 | Golléty et al. [63] |
| brittle stars | 6.8 | 4.8 | – | Migne et al. [64] |
| corals | 15.0 | 12.0 | – | Ware et al. [62] |
Whereas the reduction in carbon emissions is likely a significant portion of the pre-dam era estuary emissions, the mass is small relative to overall carbon emissions resulting from water management in the southwestern USA. For instance, the United States Bureau of Reclamation uses a 24.3% share of power from the coal-fired Navajo Generating Station to lift Colorado River water to Phoenix and Tucson through the Central Arizona Project, emitting 1.1 × 106 t (approx. 9.2 × 1010 mol) of carbon annually (http://ghgdata.epa.gov/ghgp/main.do#). Similarly, Shrestha et al. [6] estimated that 1.4 × 105 t (approx. 1.2 × 1010 mol) of carbon are emitted annually as a consequence of conveyance of Colorado River water to the Las Vegas valley. By comparison, the reduced carbon emissions at the delta resulting from diverted flow are vastly outweighed by the carbon emissions required to divert that flow.
The estuary emissions reduction may not be significant compared with other carbon emissions related to water management in the Colorado River system; however, extrapolating to a global scale, the mass of reduced carbon emissions becomes much larger. An overview of the world's largest river systems revealed that 172 out of 292 have been diverted by dams and water management [1]. Assuming the conditions in the Colorado River system are representative of the average large river system, then global reductions in carbon emissions associated with molluscan populations are on the magnitude of 1.0 × 106–2.6 × 106 t C yr−1, using the low and high estimates reported here. Estimates such as these are often prone to a large degree of uncertainty (±50%) given the tenuous nature of the assumptions behind them [10,65]. Keeping this in mind, the hypothetical reduction in global carbon emissions is at most on the scale of a large power plant (i.e. Navajo Generating Station).
Although modest in comparison with the present-day emissions resulting from river management, the change that we document nonetheless illustrates the need to advance and refine the science to support better accounting of the carbon budgets associated with rivers and water management systems [6,7,66]. Carbon emission from clams is one of many components that contribute to a river's total carbon footprint (figure 1). The complexity of carbon cycling in rivers and estuaries reflects the diverse organisms that inhabit the interwoven components of these systems and understanding these connections will be critical to well-informed planning and policymaking under an uncertain future. For instance, as climate change increases temperatures and the frequency, duration and severity of drought in the southwestern USA [67,68], integrated management of water, energy and ecosystem services is essential. The unintended reduction in net carbon emissions following the decline of mollusc populations in the CRD further demonstrates the need to seek solutions to pressing global challenges that maximize ecosystem services while maintaining ecosystem function as new social priorities emerge or new scientific insight is gained [69–71].
Supplementary Material
Acknowledgements
We thank John Warren Huntley, Xinping Hu and two additional anonymous reviewers whose comments greatly improved the manuscript.
Footnotes
350 µatm was used by Frankignoulle et al. [47] to establish the ψ equation and is maintained here. Frankignoulle et al. [47] reported an increase in ψ as pCO2 increases, resulting in positive feedback. Consequently, ψ is higher today than it was in the past, but 350 µatm remains an appropriate estimate for the entire post-dam construction era (1930–present). In the pre-dam era, ψ was likely lower; however, a decrease to 290 µatm (an estimate for the pre-industrial era [47]) results in only a small change (0.04) in ψ. Thus, given the small difference and because the pre-dam era also encompassed higher pCO2 during the industrial era, 350 µatm was also used for pre-dam era calculations.
The process of dissolution releases carbon dioxide and contributes to carbon cycling. Because cheniers are subaerial accumulations, however, they are not affected by the same set of destructive processes as assemblages in other marine environments [36]. Thus, shell dissolution via seawater was likely limited. The dissolution that did occur on the tidal flat would have effectively reduced the amount of carbon sequestered in clam shells, resulting in greater net emissions proportionate to total clam abundance today and in the pre-dam era.
Ethics
This study was based on data available in the literature and, as such, there are no ethical concerns.
Permission to carry out fieldwork
No fieldwork was conducted for the completion of this study.
Data accessibility
All data used in this study are reported within. See electronic supplementary material for the raw data on shell mass.
Authors' contributions
J.S. conceived the study, collected data, carried out analyses, interpreted data and drafted the manuscript. D.A. conceived the study, interpreted data and helped draft the manuscript. K.F. A.F. and G.D. interpreted data and contributed to manuscript revisions. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
No funding was obtained for the completion of this study.
References
- 1.Nilsson C. 2005. Fragmentation and flow regulation of the world's large river systems. Science 308, 405–408. (doi:10.1126/science.1107887) [DOI] [PubMed] [Google Scholar]
- 2.Vörösmarty CJ, et al. 2010. Global threats to human water security and river biodiversity. Nature 467, 555–561. (doi:10.1038/nature09440) [DOI] [PubMed] [Google Scholar]
- 3.Bauer JE, Cai W-J, Raymond PA, Bianchi TS, Hopkinson CS, Regnier PAG. 2013. The changing carbon cycle of the coastal ocean. Nature 504, 61–70. (doi:10.1038/nature12857) [DOI] [PubMed] [Google Scholar]
- 4.Cloern JE, et al. 2015. Human activities and climate variability drive fast-paced change across the world's estuarine-coastal ecosystems. Glob. Change Biol. 22, 513–529. (doi:10.1111/gcb.13059) [DOI] [PubMed] [Google Scholar]
- 5.Strutt J, Wilson S, Shorney-Darby H, Byers A. 2008. Assessing the carbon footprint of water production. J. Am. Water Works Assoc. 100, 80–91. [Google Scholar]
- 6.Shrestha E, Ahmad S, Johnson W, Batista JR. 2011. The carbon footprint associated with water management policy options in the Las Vegas Valley, Nevada. J. Nev. Water Resour. Assoc. 6, 2–9. [Google Scholar]
- 7.Shrestha E, Ahmad S, Johnson W, Shrestha P, Batista JR. 2011. Carbon footprint of water conveyance versus desalination as alternatives to expand water supply. Desalination 280, 33–43. (doi:10.1016/j.desal.2011.06.062) [Google Scholar]
- 8.Borges AV, Delille B, Frankignoulle M. 2005. Budgeting sinks and sources of CO2 in the coastal ocean: diversity of ecosystems counts. Geophys. Res. Lett. 32, L14601 (doi:10.1029/2005GL023053) [Google Scholar]
- 9.Frankignoulle M, Abril G, Borges AV, Bourge I, Canon C, Delille BE, Libert E, Théate J-M. 1998. Carbon dioxide emission from European estuaries. Science 282, 434–436. (doi:10.1126/science.282.5388.434) [DOI] [PubMed] [Google Scholar]
- 10.Borges AV, Schiettecatte L-S, Abril G, Delille B, Gazeau F. 2006. Carbon dioxide in European coastal waters. Estuar. Coast. Shelf Sci. 70, 375–387. (doi:10.1016/j.ecss.2006.05.046) [Google Scholar]
- 11.Borges AV. 2005. Do we have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries 28, 3–27. (doi:10.1007/BF02732750) [Google Scholar]
- 12.Cai W-J. 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annu. Rev. Mar. Sci. 3, 123–145. (doi:10.1146/annurev-marine-120709-142723) [DOI] [PubMed] [Google Scholar]
- 13.Chauvaud L, Thompson J, Cloern J, Thouzeau G. 2003. Clams as CO2 generators: the Potamocorbula amurensis example in San Francisco Bay. Limnol. Oceanogr. 48, 2086–2092. (doi:10.4319/lo.2003.48.6.2086) [Google Scholar]
- 14.Martin S, Thouzeau G, Richard M, Chauvaud L, Jean F, Clavier J. 2007. Benthic community respiration in areas impacted by the invasive mollusk Crepidula fornicata. Mar. Ecol. Prog. Ser. 347, 51–60. (doi:10.3354/meps07000) [Google Scholar]
- 15.Mistri M, Munari C. 2012. Clam farming generates CO2: a study case in the Marinetta lagoon (Italy). Mar. Pollut. Bull. 64, 2261–2264. (doi:10.1016/j.marpolbul.2012.07.010) [DOI] [PubMed] [Google Scholar]
- 16.Newell R. 2004. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: a review. J. Shellfish Res. 23, 51–61. [Google Scholar]
- 17.Nizzoli D, Bartoli M, Viaroli P. 2006. Nitrogen and phosphorous budgets during a farming cycle of the Manila clam Ruditapes philippinarum: an in situ experiment. Aquaculture 261, 98–108. (doi:10.1016/j.aquaculture.2006.06.042) [Google Scholar]
- 18.Petersen JK, Hansen JW, Laursen MB, Clausen P, Carstensen J, Conley DJ. 2008. Regime shift in a coastal marine ecosystem. Ecol. Appl. 18, 497–510. (doi:10.1890/07-0752.1) [DOI] [PubMed] [Google Scholar]
- 19.Filgueira R, et al. 2015. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar. Ecol. Prog. Ser. 518, 281–287. (doi:10.3354/meps11048) [Google Scholar]
- 20.Virnstein RW. 1977. The importance of predation by crabs and fishes on benthic infauna in Chesapeake Bay. Ecology 58, 1200–1217. (doi:10.2307/1935076) [Google Scholar]
- 21.Sikora WB, Sikora JP.1982. Ecological characterization of the benthic community of Lake Pontchartrain, Louisiana. DTIC Document. See http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA126648. (accessed 6 May 2015).
- 22.Munari C, Rossetti E, Mistri M. 2013. Shell formation in cultivated bivalves cannot be part of carbon trading systems: a study case with Mytilus galloprovincialis. Mar. Environ. Res. 92, 264–267. (doi:10.1016/j.marenvres.2013.10.006) [DOI] [PubMed] [Google Scholar]
- 23.Kowalewski M, Serrano GEA, Flessa KW, Goodfriend GA. 2000. Dead delta's former productivity: two trillion shells at the mouth of the Colorado River. Geology 28, 1059–1062. (doi:10.1130/0091-7613(2000)28<1059:DDFPTT>2.0.CO;2) [Google Scholar]
- 24.Rodriguez CA, Flessa KW, Téllez-Duarte MA, Dettman DL, Ávila-Serrano GA. 2001. Macrofaunal and isotopic estimates of the former extent of the Colorado River estuary, upper Gulf of California, México. J. Arid Environ. 49, 183–193. (doi:10.1006/jare.2001.0845) [Google Scholar]
- 25.Kowalewski M, Flessa KW, Aggen JA. 1994. Taphofacies analysis of recent shelly cheniers (beach ridges), Northeastern Baja California, Mexico. Facies 31, 209–242. (doi:10.1007/BF02536940) [Google Scholar]
- 26.Meko DM, Woodhouse CA, Baisan CA, Knight T, Lukas JJ, Hughes MK, Salzer MW. 2007. Medieval drought in the upper Colorado River Basin. Geophys. Res. Lett. 34, L10705 (doi:10.1029/2007GL029988) [Google Scholar]
- 27.Flessa KW, Glenn EP, Hinojosa-Huerta O, de la Parra-Renteria CA, Ramirez-Hernandez J, Schmidt JC, Zamora-Arroyo FA. 2013. Flooding the Colorado River Delta: a landscape-scale experiment. Eos Trans. Am. Geophys. Union 94, 485–486. (doi:10.1002/2013EO500001) [Google Scholar]
- 28.Galindo-Bect MS, Glenn EP, Page HM, Fitzsimmons K, Galindo-Bect LA, Hernandez-Ayon JM, Petty RL, Garcia-Hernandez J, Moore D. 2000. Penaeid shrimp landings in the upper Gulf of California in relation to Colorado River freshwater discharge. Fish. Bull. 98, 222–225. [Google Scholar]
- 29.Rowell K, Flessa KW, Dettman DL, Román M. 2005. The importance of Colorado River flow to nursery habitats of the Gulf corvina (Cynoscion othonopterus). Can. J. Fish Aquat. Sci. 62, 2874–2885. (doi:10.1139/f05-193) [Google Scholar]
- 30.Glenn EP, Flessa KW, Pitt J. 2013. Restoration potential of the aquatic ecosystems of the Colorado River Delta, Mexico: introduction to special issue on ‘Wetlands of the Colorado River Delta’. Ecol. Eng. 59, 1–6. (doi:10.1016/j.ecoleng.2013.04.057) [Google Scholar]
- 31.Zamora-Arroyo F, et al. 2005. Conservation priorities in the Colorado River Delta, Mexico and the United States. Tucson, AZ: Sonoran Institute. [Google Scholar]
- 32.Zamora-Arrroyo F, Flessa KW. 2009. Nature's fair share: finding and allocating water for the Colorado River Delta. In Conservation of shared environments: learning from the United States and Mexico (eds Lopez-Hoffman L, McGovern D, Varady R & Flessa KW), pp. 23–28. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 33.Carbajal N, Souza A, Durazo R. 1997. A numerical study of the ex-ROFI of the Colorado River. J. Mar. Syst. 12, 17–33. (doi:10.1016/S0924-7963(96)00086-3) [Google Scholar]
- 34.Carriquiry JD, Sánchez A. 1999. Sedimentation in the Colorado River delta and Upper Gulf of California after nearly a century of discharge loss. Mar. Geol. 158, 125–145. (doi:10.1016/S0025-3227(98)00189-3) [Google Scholar]
- 35.Thompson RW. 1968. Tidal flat sedimentation on the Colorado River delta, northwestern Gulf of California. Memoir. Boulder, CO: Geological Society of America.
- 36.Lluch-Cota SE, et al. 2007. The Gulf of California: review of ecosystem status and sustainability challenges. Prog. Oceanogr. 73, 1–26. (doi:10.1016/j.pocean.2007.01.013) [Google Scholar]
- 37.Avila-Serrano GE, Flessa KW, Téllez-Duarte MA, Cintra-Buenrostro CE. 2006. Distribution of the shelly intertidal macrofauna of the Colorado River delta, northern Gulf of California, Mexico. Cienc. Mar. 2, 649–661. (doi:10.7773/cm.v32i4.1163) [Google Scholar]
- 38.Cintra-Buenrostro CE, Flessa KW, Avila-Serrano GE. 2005. Who cares about a vanishing clam? Trophic importance of Mulinia coloradoensis inferred from predatory damage. PALAIOS 20, 296–302. (doi:10.2110/palo.2004.p04-21) [Google Scholar]
- 39.Cintra-Buenrostro CE, Flessa KW, Dettman DL. 2012. Restoration flows for the Colorado River estuary, México: estimates from oxygen isotopes in the bivalve mollusk Mulinia coloradoensis (Mactridae: Bivalvia). Wetl. Ecol. Manage. 20, 313–327. (doi:10.1007/s11273-012-9255-5) [Google Scholar]
- 40.Dettman DL, Flessa KW, Roopnarine PD, Schöne BR, Goodwin DH. 2004. The use of oxygen isotope variation in shells of estuarine mollusks as a quantitative record of seasonal and annual Colorado river discharge. Geochim. Cosmochim. Acta 68, 1253–1263. (doi:10.1016/j.gca.2003.09.008) [Google Scholar]
- 41.Velasco L, Navarro J. 2003. Energetic balance of infaunal (Mulinia edulis King, 1831) and epifaunal (Mytilus chilensis Hupé, 1854) bivalves in response to wide variations in concentration and quality of seston. J. Exp. Mar. Biol. Ecol. 296, 79–92. (doi:10.1016/S0022-0981(03)00316-2) [Google Scholar]
- 42.Rumohr H, Bery T, Ankar S. 1987. A compilation of biometric conversion factors for benthic invertebrates of the Baltic Sea. Uppsala, Sweden: Baltic Marine Biologists. [Google Scholar]
- 43.Cummins KW, Wuycheck JC. 1971. Caloric equivalents for investigations in ecological energetics. Komitee fur Limnologische Methoden. Stuttgart, Germany: E. Schweizernart. [Google Scholar]
- 44.Kowalewski M, Goodfriend GA, Flessa KW. 1998. High-resolution estimates of temporal mixing within shell beds: the evils and virtues of time-averaging. Paleobiology 24, 287–304. [Google Scholar]
- 45.Goulletquer P, Wolowicz M. 1989. The shell of Cardium edule, Cardium glaucum and Ruditapes philippinarum: organic content, composition and energy value as determined by different methods. J. Mar. Biol. Assoc. UK 69, 563–572. (doi:10.1017/S0025315400030976) [Google Scholar]
- 46.Kowalewski M. 2002. The fossil record of predation: an overview of analytical methods. Paleontol. Soc. Pap. 8, 3–42. [Google Scholar]
- 47.Frankignoulle M, Canon C, Gattuso J-P. 1994. Marine calcification as a source of carbon dioxide: positive feedback of increasing atmospheric CO2. Limnol. Oceanogr. 39, 458–462. (doi:10.4319/lo.1994.39.2.0458) [Google Scholar]
- 48.Goodwin DH, Flessa KW, Schone BR, Dettman DL. 2001. Cross-calibration of daily growth increments, stable isotope variation, and temperature in the Gulf of California bivalve mollusk Chione cortezi: implications for paleoenvironmental analysis. PALAIOS 16, 387–398. (doi:10.1669/0883-1351(2001)016<0387:CCODGI>2.0.CO;2) [Google Scholar]
- 49.Ramirez-Leon MR. 2015. Nutrient input from the Colorado River to the northern Gulf of California is not required to maintain a productive pelagic ecosystem. Cienc. Mar. 41, 169–188. (doi:10.7773/cm.v41i2.2483) [Google Scholar]
- 50.Townsend CH. 1901. Dredging and other records of the United States Fish Commission Steamer Albatross with bibliography relative to the work of the vessel. Washington, DC: Government Printing Office. [Google Scholar]
- 51.Lavın MF, Sánchez S. 1999. On how the Colorado River affected the hydrography of the Upper Gulf of California. Cont. Shelf Res. 9, 1545–1560. (doi:10.1016/S0278-4343(99)00030-8) [Google Scholar]
- 52.Schwinghamer P, Hargrave B, Peer D, Hawkins CM. 1986. Partitioning of production and respiration among size groups of organisms in an intertidal benthic community. Mar. Ecol. Prog. Ser. 31, 131–142. (doi:10.3354/meps031131) [Google Scholar]
- 53.Chen C-T, Huang T-H, Chen Y-C, Bai Y, He X, Kang Y. 2013. Air–sea exchanges of CO2 in the world's coastal seas. Biogeosciences 10, 6509–6544. (doi:10.5194/bg-10-6509-2013) [Google Scholar]
- 54.Laruelle GG, Dürr HH, Slomp CP, Borges AV. 2010. Evaluation of sinks and sources of CO2 in the global coastal ocean using a spatially-explicit typology of estuaries and continental shelves: global CO2 fluxes in coastal waters. Geophys. Res. Lett. 37, L15607 (doi:10.1029/2010GL043691) [Google Scholar]
- 55.Jiang L-Q, Cai W-J, Wang Y. 2008. A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries. Limnol. Oceanogr. 53, 2603–2615. (doi:10.4319/lo.2008.53.6.2603) [Google Scholar]
- 56.Kristensen E, Delefosse M, Quintana CO, Flindt MR, Valdemarsen T. 2014. Influence of benthic macrofauna community shifts on ecosystem functioning in shallow estuaries. Front. Mar. Sci. 1, 41 (doi:10.3389/fmars.2014.00041) [Google Scholar]
- 57.Jiang ZJ, Fang JG, Han TT, Mao YZ, Li JQ, Du MR. 2014. The role of Gracilaria lemaneiformis in eliminating the dissolved inorganic carbon released from calcification and respiration process of Chlamys farreri. J. Appl. Phycol. 26, 545–550. (doi:10.1007/s10811-013-0110-8) [Google Scholar]
- 58.Barros N, Cole JJ, Tranvik LJ, Prairie YT, Bastviken D, Huszar VLM, del Giorgio P, Roland F. 2011. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nat. Geosci. 4, 593–596. (doi:10.1038/ngeo1211) [Google Scholar]
- 59.Brown PH, Tullos D, Tilt B, Magee D, Wolf AT. 2009. Modeling the costs and benefits of dam construction from a multidisciplinary perspective. J. Environ. Manage. 90(Suppl 3), S303–S311. (doi:10.1016/j.jenvman.2008.07.025) [DOI] [PubMed] [Google Scholar]
- 60.Kareiva PM. 2012. Dam choices: analyses for multiple needs. Proc. Natl Acad. Sci. USA 109, 5553–5554. (doi:10.1073/pnas.1203263109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auerbach DA, Deisenroth DB, McShane RR, McCluney KE, LeRoy Poff N. 2014. Beyond the concrete: accounting for ecosystem services from free-flowing rivers. Ecosyst. Serv. 10, 1–5. (doi:10.1016/j.ecoser.2014.07.005) [Google Scholar]
- 62.Ware JR, Smith SV, Reaka-Kudla ML. 1992. Coral reefs: sources or sinks of atmospheric CO2. Coral Reefs 11, 127–130. (doi:10.1007/BF00255465) [Google Scholar]
- 63.Golléty C, Gentil F, Davoult D. 2008. Secondary production, calcification and CO2 fluxes in the cirripedes Chthamalus montagui and Elminius modestus. Oecologia 155, 133–142. (doi:10.1007/s00442-007-0895-8) [DOI] [PubMed] [Google Scholar]
- 64.Migne A, Davoult D, Gattuso J-P. 1998. Calcium carbonate production of a dense population of the brittle star Ophiothris fragilis (Echniodermata: Ophiuriudea): role in the carbon cycle of a temperature coastal ecosystem. Mar. Ecol. Prog. Ser. 173, 305–308. (doi:10.3354/meps173305) [Google Scholar]
- 65.Woodwell G, Rich P, Hall C. 1973. Carbon in estuaries. Brookhaven Symp. Biol. 30, 221–240. [PubMed] [Google Scholar]
- 66.Frijns J. 2012. Towards a common carbon footprint assessment methodology for the water sector. Water Environ. J. 26, 63–69. (doi:10.1111/j.1747-6593.2011.00264.x) [Google Scholar]
- 67.US Department of Interior Bureau of Reclamation Secure Water Act Section 9503(c) - Reclamation, Climate Change, and Water. 2011.
- 68.Dawadi S, Ahmad S. 2012. Changing climatic conditions in the Colorado River Basin: implications for water resources management. J. Hydrol. 430–431, 127–141. (doi:10.1016/j.jhydrol.2012.02.010) [Google Scholar]
- 69.Doyle MW. 2012. America's rivers and the American experiment. J. Am. Water Resour. Assoc. 48, 820–837. (doi:10.1111/j.1752-1688.2012.00652.x) [Google Scholar]
- 70.Auerbach DA. 2013. Discussion: ‘America's rivers and the American experiment’ by Martin W. Doyle. J. Am. Water Resour. Assoc. 49, 973–974. (doi:10.1111/jawr.12045) [Google Scholar]
- 71.Doyle MW. 2013. Reply to discussion. J. Am. Water Resour. Assoc. 49, 975–976. (doi:10.1111/jawr.12061) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are reported within. See electronic supplementary material for the raw data on shell mass.