Abstract
Maternal obesity and high-fat diet (HFD) predisposes offspring to obesity and metabolic diseases. Due to uncoupling, brown adipose tissue (BAT) dissipates energy via heat generation, mitigating obesity and diabetes. The lactation stage is a manageable period for improving the health of offspring of obese mothers, but the impact of maternal HFD during lactation on offspring BAT function is unknown. To determine, female mice were fed either a control or HFD during lactation. At weaning, HFD offspring gained more body weight and had greater body fat mass compared to the control, and these differences maintained into adulthood, which correlated with glucose intolerance and insulin resistance in HFD offspring. Adaptive thermogenesis of BAT was impaired in HFD offspring at weaning. In adulthood, HFD offspring BAT had lower Ucp1 expression and thermogenic activity. Mechanistically, maternal HFD feeding during lactation elevated peripheral serotonin, which decreased the sensitivity of BAT to sympathetic β3-adrenergic signaling. Importantly, early postnatal metformin administration decreased serotonin concentration and ameliorated the impairment of offspring BAT due to maternal HFD. Our data suggest that attenuation of BAT thermogenic function may be a key mechanism linking maternal HFD during lactation to persisted metabolic disorder in the offspring.
There is concrete evidence supporting the association between maternal obesity and high energy intake with obesity and metabolic dysfunction in offspring1,2. Feeding the dam with high-fat diet (HFD) is the most common method to induce maternal obesity and over-nutrition in rodents3,4,5,6. Previous studies have demonstrated that lactation HFD predisposes offspring to obesity and metabolic disorder in rats5, and severely affects offspring metabolic profiles and neural development4. However, the mechanistic link between lactation diets and offspring metabolic dysfunction remains to be established.
There are two different adipose tissues, namely white adipose tissue (WAT) and brown adipose tissue (BAT)7,8,9, which have distinct characteristics and functions. The function of WAT is to store energy while BAT dissipates energy as heat10. Due to its high efficiency in oxidizing glucose and fatty acids and the recent discovery of its abundancy in adult human11,12,13,14, BAT is a critical organ against obesity and diabetes15,16,17. Consistently, activation of BAT by cold-exposure increased whole-body energy expenditure, glucose homeostasis and insulin sensitivity in humans18,19. Thermogenic activity of BAT can be activated or inhibited via the alteration of many factors including hormones and dietary bioactive compounds20,21,22. Serotonin (5-hydroxytryptamine, 5-HT)23 is a hormone elevated due to diet-induced obesity (DIO)24,25. Recently, ablation of serotonin genetically or pharmacologically was shown to prevent HFD-induced obesity through activation of BAT thermogenic function25,26, suggesting a possible mediatory role of serotonin in BAT function.
The fetal and neonatal stages are critical for BAT development, which is expected to have long-term impacts on offspring BAT function27. The objectives of the current study is to determine the impact of maternal HFD during lactation on the thermogenic function of offspring mice. We found that maternal HFD during lactation permanently attenuated BAT thermogenesis in offspring, which was partially mediated by enhanced serotonin signaling. Intervention of dams with metformin during lactation could protect offspring BAT function from impairment by maternal HFD.
Results
Maternal high-fat diet during lactation affected body composition and blood profiles of offspring at weaning and adulthood
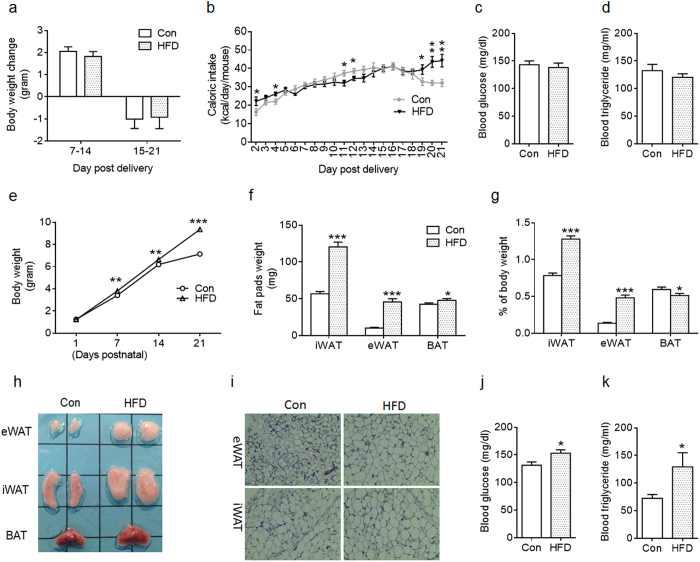
The dams were fed with Con or HFD throughout lactation, and no difference in body weight was observed (Fig. 1a). Con dams tended to have lower caloric intake during the beginning and the end of nursing period (Fig. 1b). There was no difference in blood glucose and triglyceride levels between Con and HFD dams at weaning (Fig. 1c,d). Milk triglyceride (Con vs HFD; 364 ± 37.3 vs 528 ± 67.1 mg/ml; P < 0.05) and insulin (Con vs HFD; 5 ± 0.9 vs 10 ± 1.5 ng/ml; P < 0.01) levels were elevated in HFD dams at weaning.
Figure 1. Characteristics of dams fed with Control (Con) and high-fat diet (HFD) during lactation and their pups at weaning.
(a,b) Body weight change (a) and caloric intake (b) of Con and HFD dams were monitored during lactation (n = 9 Con and n = 8 HFD). (c,d) Blood glucose (c) and triglyceride levels (d) of Con and HFD dams at weaning (n = 6 per group). (e) Offspring body weight were monitored each week during sucking (n = 42 per group). (f–h) Fat tissue weight (BAT: brown adipose tissue; iWAT: inguinal adipose tissue; eWAT: epididymal adipose tissue) (f), ratio of fat tissue to body weight (g) and image of fat pads at weaning (h) (n = 11 Con and n = 12 HFD). (i) Representative images of eWAT and iWAT sections stained with H&E. (j,k) Blood glucose (j) and triglyceride levels (k) of Con and HFD offspring at weaning (n = 8 per group). Data are presented as Means ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 as determined using a Student’s t-test.
The offspring of HFD dams gained more body weight at postnatal day 7 (PND7) compared to Con (Fig. 1e), and such difference maintained throughout lactation (Fig. 1e). At weaning, HFD offspring had greater inguinal white adipose tissue (iWAT) and epididymal white adipose tissue (eWAT) mass, and higher ratios of fat weight to the body weight compared to Con offspring (Fig. 1f–h). Maternal HFD offspring also had greater BAT mass (Fig. 1f,h), but a significantly lower ratio of BAT weight to the body weight compared to Con (Fig. 1g). Consistent with the greater iWAT and eWAT mass, white adipocytes appeared to undergo hypertrophy in HFD offspring (Fig. 1i). Higher blood glucose and triglyceride levels were detected in HFD offspring (Fig. 1j,k). Taken together, maternal HFD feeding during lactation accelerated body weight gain and changed blood profiles of offspring at weaning.
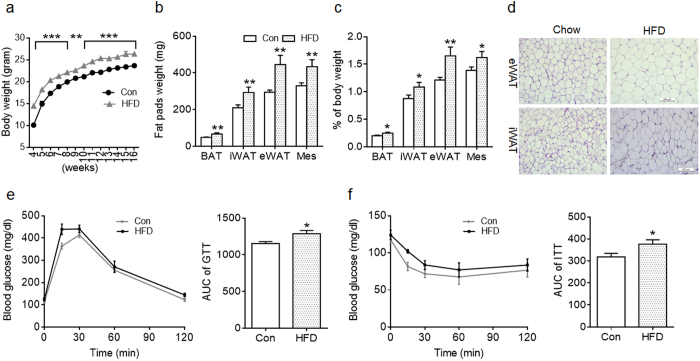
To analyze the long-term effect, offspring of Con and HFD dams were weaned on a chow diet up to 16 weeks of age when mice were euthanized. Offspring of HFD dams continued to have greater body weight compared to Con (Fig. 2a). Consistently, fat pad weight, and the ratios of individual fat weight to the body weight of HFD offspring were also higher (Fig. 2b,c), indicating adiposity in HFD offspring in adulthood. Adipocyte sizes of iWAT and eWAT of HFD offspring appeared to be larger than those of Con (Fig. 2d). Consistent with adiposity and large adipocytes, glucose tolerance and insulin sensitivity were impaired in HFD adult offspring (Fig. 2e,f). Together, maternal HFD during lactation has lasting effects on adiposity and metabolic disorders in offspring.
Figure 2. Maternal high-fat diet feeding during lactation reprograms body composition and metabolic syndrome in adult offspring.
(a) Body weight of offspring from control (Con) and high-fat diet (HFD) feeding dams (n = 8 per group). (b,c) Fat tissue weight (BAT: brown adipose tissue; iWAT: inguinal adipose tissue; eWAT: epididymal adipose tissue; Mes: mesenteric fat) (b) and ratio of fat tissue to body weight (c) in adult offspring at 16-week of age (n = 8 Con and n = 6 HFD); (d) Representative images of eWAT and iWAT sections stained with H&E. (e,f) Glucose tolerance test (GTT; at 14-week of age) (e) and insulin tolerance test (ITT; at 15-week of age)) (f) of adult offspring (n = 5 per group). AUC: area under curve. Data are presented as Means ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 as determined using a Student’s t-test.
Maternal HFD feeding during lactation affected Ucp1 expression in BAT of offspring at weaning
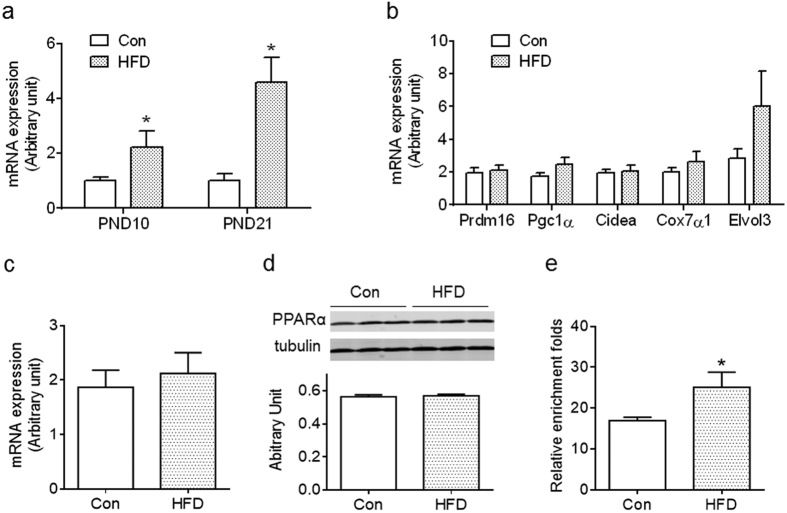
To examine the impact of maternal HFD on Ucp1 expression, BAT was collected at PND10 and PND21. Unexpectedly, offspring of HFD dams had higher Ucp1 expression than Con (Fig. 3a). Of interest, maternal HFD had no effect on the expression of other brown fat marker genes, Prdm16, Pgc1α, Cidea, Cox7α and Elvol3 (Fig. 3b). Because PPARα activates Ucp1 expression in BAT28,29, we analyzed mRNA and protein contents of PPARα and found no difference between Con and HFD offspring (Fig. 3c,d). We further examined PPARα binding to the Ucp1 promotor using ChIP-PCR and found that more PPARα bound to the Ucp1 promoter of HFD BAT compared to Con (Fig. 3e). Thus, maternal HFD feeding during lactation induced Ucp1 expression in BAT of offspring at weaning, likely mediated by recruiting PPARα to the Ucp1 promoter.
Figure 3. Maternal high-fat diet feeding during lactation elevates BAT Ucp1 expression in offspring preweaning.
(a) Ucp1 expression in offspring BAT of control (Con) and high-fat diet (HFD) dams at PND10 and PND21 (n = 4–6 per group). (b) mRNA expression of BAT-selective genes in BAT of Con and HFD offspring at weaning (n = 6 per group). (c,d) PPARα mRNA (c) and protein contents (d) in BAT of Con and HFD offspring at weaning (n = 6 per group). (e) Binding of PPARα to the Ucp1 promoter was elevated in HFD BAT as assayed by ChIP-PCR (n = 4 per group). Data are presented as Means ± S.E.M. *P < 0.05 as determined using a Student’s t-test.
Maternal HFD feeding during lactation impaired thermogenic activity of offspring BAT at weaning and adulthood
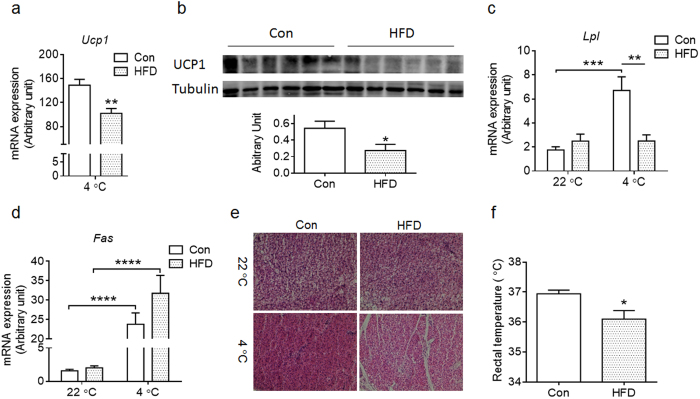
Given the unexpectedly higher Ucp1 expression in HFD BAT at weaning, we further examined the response of Ucp1 expression to cold exposure. Though the BAT of HFD offspring had higher Ucp1 expression at room temperature (Fig. 3a), the Ucp1 mRNA and protein contents were not elevated by cold exposure, in contrast to the Con BAT, where cold exposure dramatically increase Ucp1 expression (Fig. 4a,b). These data show the impairment of thermogenic adaption of HFD offspring to cold stimulus.
Figure 4. Maternal high-fat diet feeding during lactation impairs BAT thermogenic capacity of offspring at weaning under cold stimulus.
Control (Con) and high fat diet (HFD) offspring at PND21 were subjected to a cold stimulus for 6 h at 4 °C. (a,b) Ucp1 mRNA (a) and protein (b) levels in offspring BAT after cold exposure. (c,d) Lpl (c) and Fas (d) expression in BAT of offspring at room temperature (22 °C) and after cold exposure (4 °C) (n = 6 per group). (e) Histological changes of interscapular BAT from offspring of Con and HFD in room temperature and under cold exposure. (f) Rectal temperature of Con and HFD offspring at weaning after cold exposure (4 °C for 6 h) (n = 7 Con and n = 6 HFD). Data are presented as Means ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 as determined using a Student’s t-test or, where appropriate, two-way ANOVA and Tukey’ multiple comparison.
Lipoprotein lipase (LPL) is activated by β-adrenergic signaling, which releases free fatty acids for BAT absorption and oxidation, while fatty acid synthase (FAS) catalyzes fatty acid synthesis. Both of these enzymes regulate the availability of free fatty acids, which provide fuels for thermogenesis in BAT30. We found that Lpl expression in BAT was induced by cold exposure in Con offspring, but not in HFD BAT (Fig. 4c). Fas expression in BAT was similarly induced both in Con and HFD BAT by cold exposure (Fig. 4d). In addition, no obvious difference in histological structures was found between Con and HFD BAT at room temperature (Fig. 4e), but under cold stimulus, Con BAT became denser, with less fat droplets compared to that of room temperature, suggesting effective oxidation of fatty acids for thermogenesis in Con BAT, in contrast to the HFD BAT where no obvious reduction in lipid droplets was detected (Fig. 4e)3. Accordingly, HFD offspring had lower rectal temperature under cold exposure when compared to Con offspring (Fig. 4f). Because the major physiological function of BAT is to maintain body thermostasis, the lower body temperature of HFD offspring under cold exposure confirmed the impairment in BAT thermogenic function. All these results demonstrated that the adaptive thermogenesis in BAT was impaired in HFD offspring at weaning.
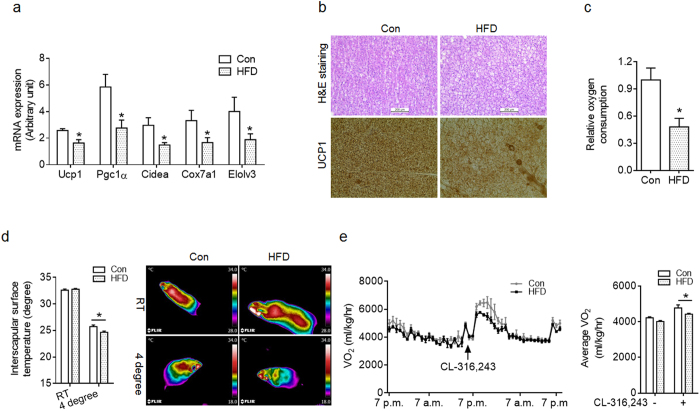
Next, we examined the long-term effect of maternal HFD on thermogenic function in offspring BAT. Samples were collected from mice at 16-week of age. We then determined the mRNA expression of brown fat-selected genes. The HFD BAT had lower Ucp1, Pgc1α, Cidea, Cox7α1 and Elolv3 expression compared to Con (Fig. 5a). There was noticeable histological difference in BAT between HFD and Con offspring (Fig. 5b). The HFD BAT had bigger brown adipocytes with larger fat droplets (Fig. 5b), which was in agreement with higher BAT mass in HFD adult offspring (Fig. 2b,c). Furthermore, less UCP1 was detected in HFD compared to Con BAT (Fig. 5b). To determine BAT function, interscapular BAT was isolated from mice under room temperature and immediately analyzed oxygen consumption ex vivo. Minced HFD BAT had less oxygen consumption compared to Con indicating impairment of BAT function (Fig. 5c). BAT function was further examined by measurement of interscapular surface temperature. There was no difference in interscapular surface temperature in room temperature, but HFD offspring was lower than Con offspring under cold-stimulus (Fig. 5d). HFD offspring had a slight reduction in basal oxygen consumption compared to Con offspring (Fig. 5e). When injected with CL-316, 243, a β3-specific agonist targeting BAT, the oxygen consumption was higher in Con than in HFD offspring (Fig. 5e), suggesting that lactation HFD impaired BAT function. There was no difference in daily food intake between Con and HFD offspring (Con: HFD; 3.4 ± 0.11 versus 3.6 ± 0.14 g; n = 8), suggesting that the adiposity and metabolic dysfunctions in HFD offspring was not due to change in caloric intake. Taken together, maternal HFD feeding during lactation attenuated BAT thermogenesis in adult offspring.
Figure 5. Maternal high-fat diet has long-term negative effects on BAT function of adult offspring at 4 months of age.
(a) mRNA expression of genes enriched in the BAT of control (Con) and high-fat diet (HFD) adult offspring (n = 7 each group). (b) Representative images of offspring BAT of Con and HFD feeding dams stained with H&E (top) and immunohistochemically with UCP1 antibody (bottom). (c) Oxygen consumption of isolated BAT from adult offspring under room temperature (n = 4 per group). (d) Dorsal interscapular surface temperature of Con and HFD offspring under room temperature and 4 °C for 6 h (n = 4–5 each group). Right (d), representative thermal images of mice under room temperature and 4 °C. (e) Oxygen consumption of Con and HFD adult offspring in basal status and injected with CL316,243 (n = 4 per group). Data are presented as Means ± S.E.M. *P < 0.05 as determined using a Student’s t-test or, where appropriate, two-way ANOVA and Tukey’ multiple comparison.
Maternal HFD during lactation affected BAT thermogenesis through inhibition of β3-adrenergic signaling
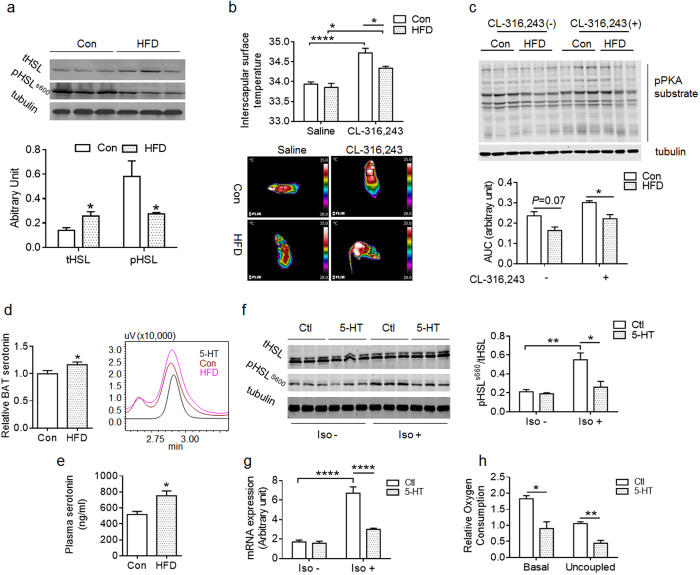
To understand the underlying molecular mechanisms contributing to the impairment of BAT activity in HFD offspring, β3-adrenergic signaling pathway was examined in offspring at one-month of age. As a key effector of β3-adrenergic signaling, hormone-sensitive lipase (HSL) activity is essential for fatty acid oxidation in BAT. Total HSL was higher, while HSL phosphorylation was lower in BAT HFD offspring compared to Con, indicating the inhibition of β3-adrenergic signaling in the BAT of HFD offspring (Fig. 6a). There was no difference in basal interscapular surface temperature between Con and HFD mice (Fig. 6b), while the temperature of Con mice was higher than that of HFD offspring under CL-316, 243-stimulation (Fig. 6b). In agreement, the phosphorylation of basal PKA substrates was slightly lower in BAT of HFD compared to Con (P = 0.07), while the HFD had significantly lower PKA substrate phosphorylation under the treatment by β3-adrenoceptor agonist CL-316, 243 (Fig. 6c). These data indicated that the HFD BAT was less sensitive to β3-adrenergic agonist stimulation.
Figure 6. Inhibition of β3-adrenergic signaling in BAT of HFD offspring due to elevated serotonin.
(a) Total and phosphorylated HSL (S660) in BAT of Con and HFD offspring at one-month of age (n = 6 per group, 3 shown). (b) Dorsal interscapular surface temperature of one-month old Con and HFD offspring at 20 min following an injection with saline or CL-316,243 (n = 4 per group; bottom: representative thermal images). The circle indicated that the area used for dorsal interscapular surface temperature measurement. (c) Protein kinase A (PKA) substrate phosphorylation in BAT of Con and HFD offspring at one-month age at 20 min following an injection with saline or CL-316,243 (n = 4 per group; upper: representative western blot). (d) Relative serotonin level in interscapular BAT of Con and HFD offspring at weaning (Normalized to Con; n = 4 per group; right (d), representative serotonin level determined by HPLC). (e) Serotonin level in plasma of adult offspring of Con and HFD dam (n = 5 per group). (f) HSL (S600) phosphorylation in control and isoproterenol-stimulated (Iso) brown adipocytes (n = 3 per group; (f) left, representative western blot). (g) Ucp1 expression in control and isoproterenol-stimulated (Iso) brown adipocytes pretreated with serotonin. (h) Basal and uncoupled respiration rate of brown adipocytes pretreated with vehicle or serotonin as performed in (f). Serotonin = 5-HT (5-hydroxytryptamine). *P < 0.05; **P < 0.01 and ****P < 0.0001 as determined using a Student’s t-test or, where appropriate, two-way ANOVA and Tukey’ multiple comparison.
Next, we explored the underlying mechanisms leading to inhibition of β3-adrenergic signaling in HFD BAT. Because peripheral serotonin inhibits thermogenesis in BAT26, we measured serotonin contents in offspring. The HFD BAT had higher serotonin content at weaning (Fig. 6d) and also higher circulatory serotonin than that of Con in adulthood (Fig. 6e). To test whether elevated serotonin inhibited β3-adrenergic signaling in HFD BAT, we pretreated brown adipocytes with serotonin in vitro, and found that serotonin had no effect on basal HSL phosphorylation (Fig. 6f). But under the stimulation of isoproterenol, a β-adrenergic receptor agonist, serotonin inhibited HSL phosphorylation (Fig. 6f). Accordingly, there was no difference of basal Ucp1 expression in brown adipocytes with/without serotonin treatment, but serotonin inhibited Ucp1 expression under the stimulation of isoproterenol (Fig. 6g). The basal and uncoupling cellular respiration of brown adipocyte was reduced due to serotonin treatment (Fig. 6h). These data showed that elevated peripheral serotonin inhibited β3-adrenergic signaling in BAT of maternal HFD offspring.
Metformin intervention improved BAT thermogenic activity in HFD offspring
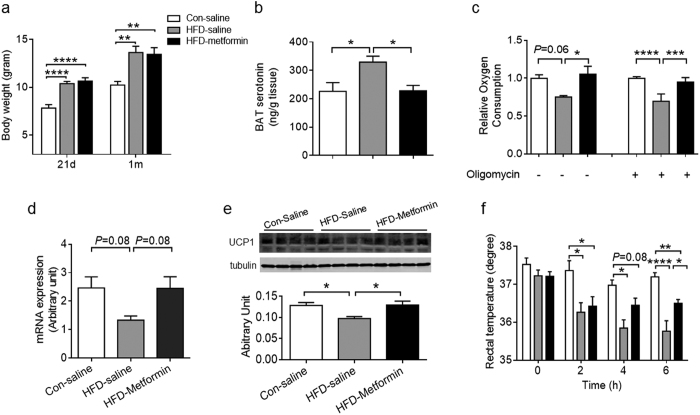
Metformin is the first-line drug for treating diabetes. To study whether early postnatal intervention could protect the offspring BAT from the impairment due to maternal HFD feeding, the offspring of Con and HFD were administered with saline or metformin during lactation. Metformin treatment did not affect the body weight gain resulting from maternal HFD feeding (Fig. 7a). However, metformin inhibited the elevation of serotonin in HFD BAT (Fig. 7b). Importantly, metformin enhanced basal and uncoupled oxygen consumption of BAT, showing the improvement of BAT thermogenic activity (Fig. 7c). Although metformin treatment had no effect on Ucp1 mRNA expression in BAT (HFD-saline versus HFD-metformin, P = 0.08) (Fig. 7d), the UCP1 protein was higher in HFD-metformin compared to HFD-saline under cold-stimulation (Fig. 7e). There was no difference in the rectal temperature among three groups at the beginning of cold exposure (0 h). But at the end of cold exposure (6 h), rectal temperature of HFD-saline was lower than that of Con-Saline mice, while HFD-metformin mice had higher rectal temperature compared to HFD-saline (Fig. 7f). Taken together, early postnatal metformin intervention protected the BAT function of HFD offspring.
Figure 7. Early postnatal metformin treatment ameliorates thermogenic impairment in BAT due to maternal HFD feeding during lactation.
(a) Body weight of Con-saline, HFD-saline and HFD-metformin at PND21 and one-month of age (Con-saline: Con offspring injected with saline; HFD-saline: HFD offspring injected with saline; HFD-metformin: HFD offspring injected with metformin from PND2-PND21, then weaned on chow diet at PND21. n = 7–11 each group). (b) BAT serotonin content of Con-saline, HFD-saline and HFD-metformin mice at one month of age (n = 6 each group). (c) Coupled and uncoupled respiration rate of isolated BAT from Con-saline, HFD-saline and HFD-metformin mice at one-month of age (n = 4 per group). (d,e) UCP1 mRNA (d) and protein contents (e) in BAT of Con-saline, HFD-saline and HFD-metformin at one-month of age under cold-stimulus for 6 h (n = 6 per group). (f) Rectal temperature changes of Con-saline, HFD-saline and HFD-metformin mice at one-month of age during 6 h under cold exposure (n = 6–8 per group). Data are presented as Means ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 and ***P < 0.0001 were determined by One-way ANOVA with Tukey’s post doc test.
Discussion
Previous studies showed that neonates at the lactation stage is highly sensitive to reprogramming effects of maternal diets in rodents4,5. In this study, we examined whether and how the maternal HFD feeding during lactation affects thermogenic activity in BAT of mice offspring. We found that lactation HFD impaired BAT thermogenic function of offspring which was associated with persistent adiposity and metabolic syndrome. We further demonstrated that the impairment of BAT thermogenesis was partially due to the attenuation of cellular β3-adregernic signaling, which was linked to the elevation of peripheral serotonin by maternal HFD. More importantly, early postnatal metformin intervention ameliorated the impairment of BAT function in HFD offspring.
In this study, we observed that HFD offspring at weaning and in adults had excessive body weight gain, due to higher fat accumulation. Furthermore, HFD offspring developed glucose intolerance and insulin insensitivity in adulthood. However, there were no differences in body weight and blood profiles between Con and HFD dams during lactation, suggesting that the metabolic dysfunction in HFD offspring was not due to maternal metabolic disorder, but a specific alteration of maternal diet composition and then the changes of milk compositions4. This is quite different from maternal DIO model, where dams were fed HFD up to several weeks before mating and throughout the whole gestation and lactation stages, which led to maternal adiposity, alterations of blood profiles and development of glucose intolerance in dams31,32,33,34. In our study, HFD offspring continued to have greater body weight associated with reduction in oxygen consumption and unaffected caloric intake, suggesting that the adiposity of HFD adult offspring was primarily derived from reduced energy expenditure, likely associated with impaired BAT function.
To test whether impairment of BAT function in HFD offspring is one of the primary reasons leading to reduced energy expenditure, we first examined the Ucp1 expression in BAT. Surprisingly, Ucp1 expression was elevated in HFD BAT at PND10 and weaning, with no changes in the expression of other BAT markers. Despite higher Ucp1 expression, the transcription levels of other BAT marker gene Prdm16, Pgc1α, Cidea, Cox7a1 and Elovl3 were unchanged in BAT of HFD offspring at weaning, suggesting there were defects in BAT functions. It was further confirmed by the fact that adaptive thermogenesis was attenuated in HFD BAT. The elevation of Ucp1 expression was consistent with an earlier report where postnatal over-nutrition due to small litter size increases BAT Ucp1 expression in rats3. HFD offspring gained more weight at weaning, which could be due to excessive energy intake from milk4 and also attenuated BAT adaptive thermogenesis in HFD offspring.
PPARα is a critical transcription factor regulating Ucp1 expression35, and we found that there was more PPARα bound to the Ucp1 promoter, providing an explanation for the elevated Ucp1 expression in HFD BAT at weaning. HFD feeding induces the Ucp1 expression in brown adipose tissue in rodents36. The higher fatty acids in milk of HFD dam increases fatty acid supply to pre-weaning offspring4, which might activate PPARs to increase Ucp1 expression28,37.
The thermogenic function of BAT is tightly regulated by cellular β-adrenoceptor signaling pathway14. Cold stimulus activates BAT thermogenesis via the β3-adrenergic signaling, which involves PKA activation and HSL phosphorylation14,38. To further examine BAT function, we subjected offspring at weaning to cold temperature20,39, which stimulated Ucp1 expression in Con BAT, but absent in HFD BAT, showing the cold-stimulated β3-adrenergic signaling was impaired in HFD BAT. Consistently, Lpl expression also was lower in HFD offspring than Con, whereas Lpl is essential for triglyceride uptake into BAT under cold condition40. Furthermore, there was obvious histological difference between room temperature and cold-stimulated BAT of Con offspring, which were absent in BAT of HFD offspring, reinforcing the notion of attenuated cold-stimulated thermogenic signaling in HFD BAT. In agreement with above data, at weaning, the rectal temperature of HFD offspring was lower compared to Con offspring under cold-stimulation. In adult, HFD BAT had lower expression of BAT marker genes, Ucp1, Pgc1α, Cidea, Cox7a1 and Elovl3 compared to Con BAT. Consistently, BAT of HFD offspring had reduced oxygen consumption and impaired thermogenic capacity under cold stimulus. Thus the attenuation of BAT function is a linking mediator of the long-term effect of maternal HFD on adiposity and metabolic disorder in offspring.
Serotonin is separately secreted by the central nervous system and peripheral tissue41, which regulates whole-body energy balance42,43. Obesity is known to elevate peripheral serotonin level24,25,26. Both genetically and pharmacologically knockdown peripheral serotonin protects the mice from HFD induced obesity via elevating β3-adrenergic pathway and BAT thermogenesis26. We found that maternal HFD offspring had higher peripheral serotonin level than Con at weaning and in adulthood. Consistently, in vitro treatment of brown adipocytes with serotonin inhibited β3-adrenergic intracellular signaling, which was associated with lower Ucp1 expression and reduced oxygen consumption. These data showed that the elevated peripheral serotonin due to maternal HFD contributes to the attenuation β3-adrenergic signaling in HFD BAT.
Lastly, we tested metformin administration during lactation on BAT function of HFD offspring, and observed astonishing recovery of HFD BAT function. Supportively, the serotonin concentration was elevated in HFD BAT, which was recovered due to metformin treatment. Because metformin is known to enhance lipid oxidation44, these data suggest that serotonin secretion is coupled with lipid metabolism in adipose tissue25. Of note, further studies are needed to explore the inhibitory effect of metformin on peripheral serotonin level in maternal HFD offspring, in addition to other likely mechanisms for the recovery actions of metformin on BAT thermogenic function of maternal HFD offspring.
In summary, to our best knowledge, for the first time, we observed that adaptive thermogenesis in HFD BAT was impaired at weaning, despite elevated Ucp1 expression in BAT before cold exposure. Importantly, such BAT impairment due to HFD during lactation has lasting effects on BAT function in offspring, partially explaining the developmental programming of maternal HFD on offspring obesity and metabolic dysfunction. Lastly, the lactation stage represents an amiable time window for intervention to ensure offspring metabolic health.
Methods
Mice
Animal studies were conducted in AAALAC-approved facilities and according to protocols approved by the Institutional Animal Use and Care Committee (IAUCC) of Washington State University. To obtain offspring, 10 to12 week-old female C57/BL mice were mated. Success in mating was confirmed by the presence of vaginal plug and mice were continually maintained on chow diet until delivery. On the day of birth, litter sizes were balanced to 6 pups and then dams were randomly assigned to either control diet (Con; 10% energy from fat, D12450, Research Diets, New Brunswick, NJ) or high-fat diet (HFD; 60% energy from fat, D12492, Research Diets) during lactation. Animals were maintained under 12 h-light/12 h-dark cycles and with ad libitum access to water. The pups were weaned on chow diet on postnatal day 21 (PND21). For metformin treatment, newborn pups were administered daily with 200 mg/kg body weight metformin (Sigma, St. Louis, MO) dissolved in sterilized saline or saline only from PND2 to PND2144. To avoid confounding sexual effect, only male offspring were used in this study.
Cell culture and treatment
Stromal vascular cells were isolated from BAT of one month old mice as previously described22. The brown adipogenesis was induced using an induction medium containing 15% fetal bovine serum (FBS), 5 μg/ml insulin, 1 μM dexamethasone, 0.5 mM isobutyl-1-methylxanthine, 125 nM indomethacin and 1 nM T3 for 2 days and subsequently maintained in a differentiation medium containing 5 μg/ml insulin and 1 nM T3.
Brown adipocytes were treated with serotonin as previously described26. Briefly, cells were treated with serotonin for 30 min (100 μM; Sigma) followed by isoproterenol (1 nM; Sigma) for 30 min or 4 h, respectively, and collected for western blotting or gene expression analyses. Coupled and uncoupled respiration rates of brown adipocytes were measured by addition of vehicle (1:1000) or 1 μM oligomycin (Sigma) dissolved with DMSO45, respectively. The O2 level was analyzed using an Orion Dissolved Oxygen platform (Thermo Scientific, Waltham, MA) as previously described22.
Insulin and triglyceride assays
Blood triglyceride level was analyzed with triglyceride colorimetric assay kit from Cayman (Cat. 10010303; Ann Arbor, MI). Insulin level was analyzed with Mouse Ultrasensitive Insulin ELISA Kit (Cat. 80-INSMSU-E10; ALPCO Diagnostics, Salem, NH).
Serotonin assay
BAT serotonin was extracted from frozen tissue samples using HOCL4. Homogenized samples were centrifuged at 12,000 × g for 5 min, and supernatants were carefully collected and neutralized using KOH. Neutralized supernatants were centrifuged at 12,000 × g for 10 min to remove any remaining precipitates. To isolate serotonin from serum, the same volume of serum of Con and HFD offspring was used. Proteins were precipitated by addition of cold acetonitrile, and precipitated proteins were removed by centrifugation as previously described24. Serotonin level was analyzed using HPLC as previously described46.
Glucose and insulin tolerance test (GTT and ITT)
Mice were subjected to GTT (at 14-week of age) and ITT (at 15-week of age) as previously described32. Briefly, mice were fasted for 6 h before testing and, then, administered (i.p.) with 2 g/kg D-glucose or 0.75 U/kg insulin for GTT and ITT, respectively. Blood samples were collected from the tail veil at 0, 15, 30, 60 and 120 min post injection and glucose concentrations were measured by glucose oxidase method using a glucose meter (Bayer Contour, Tarrytown, NY, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNAs were extracted using TRIzol (Life technologies, Grand Island, NY) and cleaned with Zymo Research RNA clean & concentrator kit (Zymo research, Irvine, CA). cDNA was synthesized using an iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to manufacturer’s instructions. qRT-PCR reactions were performed with SYBR green master mix (Bio-Rad, Hercules, CA) on a Bio-Rad real-time PCR unit. 18S rRNA was used as a reference gene. All primers are listed in Table 1.
Table 1. Sequences of primer used for real-time PCR and ChIP-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT |
| Cox7α | CAGCGTCATGGTCAGTCTGT | AGAAAACCGTGTGGCAGAGA |
| Elovl3 | TCCGCGTTCTCATGTAGGTCT | GGACCTGATCCAACCCTATGA |
| Fas | TCTGCGATGAAGAGCATGGTTTAGAG | GTTTGTATTGCTGGTTGCTGTGCAC |
| Lpl | TCATCAACTGGATGGAGGAGGAGTT | AAGTTAGGCCCAGCTGGATCCA |
| Pgc1a | CCATACACAACCGCAGTCGC | GTGGGAGGAGTTAGGCCTGC |
| Prdm16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG |
| Pparα | GGATGTCACACAATGCAATTCGC | GTTGAAGTTCTTCAGGTAGGCTTCGT |
| Ucp1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG |
| Ucp1 ChIP | AAGAGAAGAACACGGACACTAGGTAAGT | TGGGAAGTGAAAGAAAGTTTGCC |
Western blotting
Analyses were conducted as previously described32. Antibodies including phospho-HSL (#4126), phospho-PKA substrate (#9624), and β-tubulin (#3873) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against UCP1 (SC-6529), HSL (SC-25843) and PPARα (SC-9000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). IRDye 800CW goat anti-rabbit (Cat. 926-32211) and IRDye 680 goat anti-mouse (Cat. 926-68070) secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE). The target proteins were detected by using an Odyssey infrared imaging system (Li-cor Biosciences, Lincoln, NE) and bands were quantified using Software Image Studio Lite (LI-COR Biosciences, Version 4).
Chromatin immunoprecipitation (ChIP)-PCR assay
Briefly, tissue samples were homogenized in liquid nitrogen, fixed in 1% formaldehyde solution, and then sonicated. The supernatant was precleaned with ChIP-grade protein G (Cell Signaling Technology) and then incubated with antibody against PPARα (SC-9000; Santa Cruz, CA), or normal rabbit IgG (#2729; Cell Signaling Technology, Danvers, MA). The antibody-chromatin complex was precipitated with protein G and further treated with RNaseA and proteinase K. DNA was purified with ChIP DNA clean & concentrator kit (Zymo Research, Irvine, CA) and used as templates for PCR using the primers listed in Table 1. Target enrichment was calculated after normalization to input31.
Hematoxylin & Eosin (H&E) and Immunohistochemical staining
Fat tissues were fixed and sectioned into 5 μm thickness. H&E and immunohistochemical staining were conducted as previously described46. The ABC kit (Vector Laboratory Inc., Burlingame, CA) was used for antigen detection.
Tissue oxygen (O2) consumption measurement
Tissue O2 consumption was determined as previously reported47. Approximately 10 mg of BAT tissue was minced with a fine scissors in a buffer comprised of 2% BSA, 1.1 mM sodium pyruvate, and 25 mM glucose in PBS. O2 consumption was measured using an Orion Dissolved Oxygen platform (Thermo Scientific, Waltham, MA) for approximately 20 min. O2 consumption was normalized to the BAT tissue weight.
Oxygen consumption in vivo
Oxygen consumption of mice was measured in a Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, OH, USA). Measurements were taken every 30 seconds for 24 h (12 h-light/12 h-dark cycle). Mice could access ad libitum to water and one pellet of diet to avoid fasting stress.
Rectal temperature measurement
To monitor the rectal temperature, an animal temperature controller with a rectal probe for mice was used (Physitemp, Clifton, NJ). Temperature was measured every 2 h during the cold exposure.
Surface interscapular BAT temperature
Surface interscapular BAT temperature was measured with an infrared camera (E6, FLiR Systems) as previously described48. Briefly, mice were i.p. injected with sterilized saline or 0.033 nmol/g of β3-adrenergic agonist CL-316, 243 (Santa Cruz Biotech). The images were acquired 20 min post injection. Then, mice were immediately euthanized for BAT collection for biochemical analysis.
Statistical analysis
Data were presented as mean ± S.E.M. Results were analyzed using Student’s t-test or ANOVA where appropriate, with GraphPad Prism software (GraphPad Prism, version 6.0). P ≤ 0.05 was considered as significant difference.
Additional Information
How to cite this article: Liang, X. et al. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 6, 34345; doi: 10.1038/srep34345 (2016).
Acknowledgments
No potential conflicts of interest relevant to this article were reported. The work was funded by NIH R01HD067449 and R21AG049976. Authors thank Dr. Shuming Zhang for assistance in measurement of serotonin by HPLC, and Mrs. Jeanene Deavila for management of mice. M.D. is the guarantor who takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Footnotes
Author Contributions X.L., Q.Y., L.Z., J.W.M. and M.D. researched data and wrote the manuscript. M.-J.Z. and B.D.R. contributed to experimental design and discussion and reviewed and edited the manuscript. M.D. designed the experiments. All authors reviewed the manuscript.
References
- Khan I. Y. et al. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. American journal of physiology. Regulatory, integrative and comparative physiology 288, R127–133, doi: 10.1152/ajpregu.00354.2004 (2005). [DOI] [PubMed] [Google Scholar]
- Oben J. A. et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. Journal of hepatology 52, 913–920, doi: 10.1016/j.jhep.2009.12.042 (2010). [DOI] [PubMed] [Google Scholar]
- Xiao X. Q. et al. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology 148, 4150–4159, doi: 10.1210/en.2007-0373 (2007). [DOI] [PubMed] [Google Scholar]
- Vogt M. C. et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156, 495–509, doi: 10.1016/j.cell.2014.01.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B. et al. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 61, 2833–2841, doi: 10.2337/db11-0957 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida D. L. et al. Early overfeed-induced obesity leads to brown adipose tissue hypoactivity in rats. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 32, 1621–1630, doi: 10.1159/000356598 (2013). [DOI] [PubMed] [Google Scholar]
- Cypess A. M. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine 19, 635–639, doi: 10.1038/nm.3112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell M. E. et al. Evidence for two types of brown adipose tissue in humans. Nature medicine 19, 631–634, doi: 10.1038/nm.3017 (2013). [DOI] [PubMed] [Google Scholar]
- Cypess A. M. et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine 360, 1509–1517, doi: 10.1056/NEJMoa0810780 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M. & Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine 19, 1252–1263, doi: 10.1038/nm.3361 (2013). [DOI] [PubMed] [Google Scholar]
- Stanford K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of clinical investigation 123, 215–223, doi: 10.1172/JCI62308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen K. A. et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine 360, 1518–1525, doi: 10.1056/NEJMoa0808949 (2009). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell research 23, 851–854, doi: 10.1038/cr.2013.64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S. & Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual review of physiology 76, 225–249, doi: 10.1146/annurev-physiol-021113-170252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L. & Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of clinical investigation 125, 478–486, doi: 10.1172/JCI78362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099, doi: 10.2337/db14-0746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks H. & Symonds M. E. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes 62, 1783–1790, doi: 10.2337/db12-1430 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W. D. et al. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine 360, 1500–1508, doi: 10.1056/NEJMoa0808718 (2009). [DOI] [PubMed] [Google Scholar]
- Saito M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531, doi: 10.2337/db09-0530 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K. L. & Tseng Y. H. Brown fat fuel utilization and thermogenesis. Trends in endocrinology and metabolism: TEM 25, 168–177, doi: 10.1016/j.tem.2013.12.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd C. & Obici S. Brown fat fuel use and regulation of energy homeostasis. Current opinion in clinical nutrition and metabolic care 17, 368–372, doi: 10.1097/MCO.0000000000000063 (2014). [DOI] [PubMed] [Google Scholar]
- Wang S. et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. International Journal of Obesity 39, 967–976, doi: 10.1038/ijo.2015.23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport M. M., Green A. A. & Page I. H. Serum vasoconstrictor, serotonin; isolation and characterization. The Journal of biological chemistry 176, 1243–1251 (1948). [PubMed] [Google Scholar]
- Kim H. J. et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. Journal of proteome research 10, 722–731, doi: 10.1021/pr100892r (2011). [DOI] [PubMed] [Google Scholar]
- Oh C. M. et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nature communications 6, 6794, doi: 10.1038/ncomms7794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. D. et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nature medicine, doi: 10.1038/nm.3766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q. et al. AMPK/α-ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell metabolism, http://dx.doi.org/10.1016/j.cmet.2016.08.010 (2016). [DOI] [PMC free article] [PubMed]
- Cannon B. & Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews 84, 277–359, doi: 10.1152/physrev.00015.2003 (2004). [DOI] [PubMed] [Google Scholar]
- Barbera M. J. et al. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. The Journal of biological chemistry 276, 1486–1493, doi: 10.1074/jbc.M006246200 (2001). [DOI] [PubMed] [Google Scholar]
- Cannon B. & Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews 84, 277–359 (2004). [DOI] [PubMed] [Google Scholar]
- Yang Q. Y. et al. Maternal obesity induces epigenetic modifications to facilitate zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 62, 3727–3735, doi: 10.2337/db13-0433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. et al. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. The Journal of physiology 594, 4453–4466, doi: 10.1113/JP272123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfiglio D. C. et al. Obesity impairs lactation performance in mice by inducing prolactin resistance. Scientific reports 6, 22421, doi: 10.1038/srep22421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson A. M. et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–392, doi: 10.1161/HYPERTENSIONAHA.107.101477 (2008). [DOI] [PubMed] [Google Scholar]
- Mottillo E. P., Bloch A. E., Leff T. & Granneman J. G. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) alpha and delta in brown adipocytes to match fatty acid oxidation with supply. The Journal of biological chemistry 287, 25038–25048, doi: 10.1074/jbc.M112.374041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme T. & Klingenspor M. Uncoupling protein 1 expression and high-fat diets. American journal of physiology. Regulatory, integrative and comparative physiology 300, R1–8, doi: 10.1152/ajpregu.00411.2010 (2011). [DOI] [PubMed] [Google Scholar]
- Petzke K. J., Friedrich M., Metges C. C. & Klaus S. Long-term dietary high protein intake up-regulates tissue specific gene expression of uncoupling proteins 1 and 2 in rats. European journal of nutrition 44, 414–421, doi: 10.1007/s00394-004-0545-4 (2005). [DOI] [PubMed] [Google Scholar]
- Kooijman S. et al. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proceedings of the National Academy of Sciences of the United States of America 112, 6748–6753, doi: 10.1073/pnas.1504239112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A. M. et al. Cold-induced changes in gene expression in brown adipose tissue, white adipose tissue and liver. PloS one 8, e68933, doi: 10.1371/journal.pone.0068933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A. et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine 17, 200–205, doi: 10.1038/nm.2297 (2011). [DOI] [PubMed] [Google Scholar]
- Berger M., Gray J. A. & Roth B. L. The expanded biology of serotonin. Annual review of medicine 60, 355–366, doi: 10.1146/annurev.med.60.042307.110802 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. D. & Heisler L. K. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert reviews in molecular medicine 9, 1–24, doi: 10.1017/S1462399407000245 (2007). [DOI] [PubMed] [Google Scholar]
- Tecott L. H. Serotonin and the orchestration of energy balance. Cell metabolism 6, 352–361, doi: 10.1016/j.cmet.2007.09.012 (2007). [DOI] [PubMed] [Google Scholar]
- Geerling J. J. et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes 63, 880–891, doi: 10.2337/db13-0194 (2014). [DOI] [PubMed] [Google Scholar]
- Rajakumari S. et al. EBF2 determines and maintains brown adipocyte identity. Cell metabolism 17, 562–574, doi: 10.1016/j.cmet.2013.01.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. et al. Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK. Diabetes 65, 188–200, doi: 10.2337/db15-0647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M. J. et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell metabolism 19, 593–604, doi: 10.1016/j.cmet.2014.03.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. D., Mottillo E. P., Farncombe T. H., Morrison K. M. & Steinberg G. R. A standardized infrared imaging technique that specifically detects UCP1-mediated thermogenesis in vivo. Molecular metabolism 3, 490–494, doi: 10.1016/j.molmet.2014.04.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]