Introduction
Pyoderma gangrenosum (PG) is a rare, inflammatory, and necrotizing disease that belongs to the group of neutrophilic dermatosis.1, 2, 3 The pathogenesis of PG remains unknown. In the last 2 years, some researchers found a significant increase in interleukin (IL)-1β and in the IL-1β receptor in skin samples of PG patients.
Most PG cases respond to immunomodulators and immunosuppressants. However, there are no clear gold standards for PG treatment. Systemic corticosteroids and cyclosporine are considered first-line treatments. Azathioprine, mycophenolate mofetil, cyclophosphamide, and methotrexate are considered second-line therapies.4, 5, 6, 7, 8, 9, 10
Anti–tumor necrosis factor-α (TNF-α) biological agents show efficacy for a wide range of inflammatory conditions, including inflammatory bowel disease, rheumatoid arthritis, and psoriasis. Several studies report PG being successfully treated with infliximab (including 1 randomized, controlled trial11), etanercept, and adalimumab. Infliximab is a chimeric IgG monoclonal antibody that blocks the inflammatory cytokine TNF-α. Adalimumab is a fully human monoclonal antibody IgG1 against TNF-α. Etanercept is a human TNF receptor fusion protein that inhibits the binding of TNF-α to TNF receptors on cells' surfaces.
Because a significant contribution of IL-1 to the pathogenesis of PG has been confirmed, biologic agents that inhibit IL-1 represent a therapeutic option in PG.9, 10, 11, 12, 13, 14
Our patient had ulcerative PG for 24 years. Her disease was refractory to systemic corticosteroids, cyclosporine, infliximab, and adalimumab. After treatment with canakinumab, the lesions were completely healed in 7 months.
Clinical case
A 54-year-old female patient had a history of ulcerative colitis (UC) and ulcerative PG on her lower limbs that was diagnosed in 1991 and an 11-year treatment course with systemic corticosteroids and cyclosporine. Her PG became refractory to this treatment, so she was subsequently treated with infliximab, 5 mg/kg doses, in 3 infusions in weeks 0, 2, and 6. Pain immediately diminished, and the re-epithelialization of lesions was complete in 5 months. After 2 disease-free years, the patient relapsed with pyoderma gangrenosum associated with aggravated UC. She restarted treatment with infliximab, adding azathioprine (150 mg/d). Lesions re-epithelialized within 10 months.
In 2011, she relapsed with both pyoderma gangrenosum and UC. She restarted treatment with infliximab. UC was controlled, but the cutaneous lesions did not improve. We switched the therapy to adalimumab (80 mg the first week and then 40 mg/wk thereafter). After 4 months, lesions had worsened with pain, bleeding, and exposure of tendons (Figs 1, A and 2, A). Treatment was switched to canakinumab (150 mg once per month for 3 doses by subcutaneous injection). Two weeks after the first dose, the ulcers were filled with granulation tissue and were no longer painful (Fig 1, B). Two weeks after the third and last dose, the patient presented with complete re-epithelialization of the lesions in the right leg and a 70% reduction of the ulcers in the left leg (Figs 1, C and 2, B). After 7 months, re-epithelialization was complete.
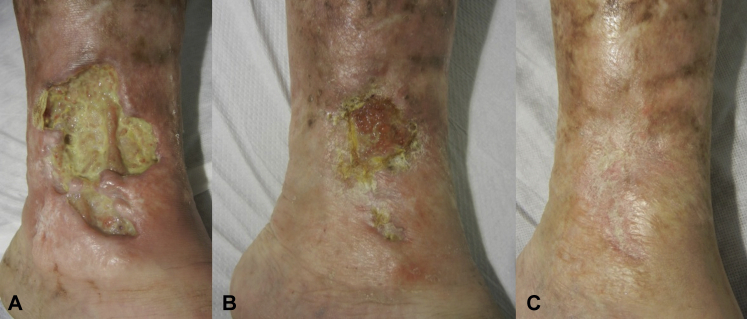
Fig 1.
Evolution of the ulcer in inner left lower leg. A, Before treatment. B, Two weeks after the last doses of canakinumab. C, 6 months after the beginning of treatment.
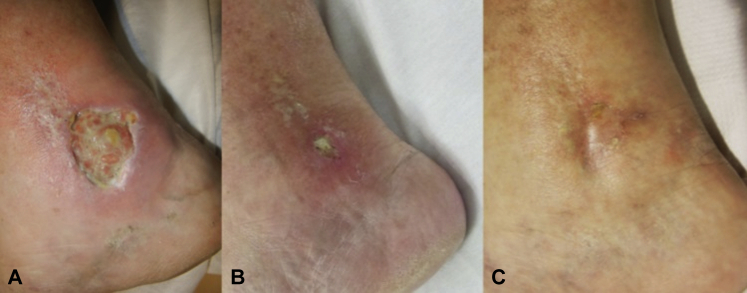
Fig 2.
Evolution of the ulcer in inner right lower leg. A, Before treatment. B, Two weeks after the last doses of canakinumab. C, None months after the beginning of treatment.
Comments
The pathogenesis of PG remains unknown. Dinarello et al9 classified PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne) and PASH (pyoderma gangrenosum, acne, and suppurative hidradenitis) syndromes as autoinflammatory disorders in which a genetic predisposition favors an increase in inflammatory cytokines that belong to the innate immune system (such as IL-1β).9 In these diseases, inflammasomes activate caspase-1 and cleave the pro–IL-1β into its active form. IL-1β activates endothelial cells, leading to an increased expression of adhesion molecules that stimulate the recruitment of neutrophils. This cytokine also increases the average life of the neutrophils.9
In 2013, 2 articles commented on the successful use of anti IL-1β monoclonal antibodies in a patient with PAPA-like syndrome and in a patient who had PG associated with hidradenitis suppurativa; both diseases were refractory to systemic corticosteroids.10, 12
Marzano et al15 measured the levels of inflammatory cytokines in the histopathology of 16 PG patients and compared them with the histopathology of healthy patients. In PG patients, they observed an increase in TNF-α and IL-17 and their receptors and a significant increase in IL-1β.15
Kolios et al14 quantified cytokine mRNA levels by performing real-time quantitative polymerase chain reaction on the skin samples of 7 PG patients and compared them with healthy skin and dermatitis samples. They found a significant increase in IL-1β and in the IL-1β receptor in PG patients. They also observed an increase in IL-1α, IL-6, IL-8, and IL-32. They showed no significant increase in TNF-α, IFN-γ, IL-36, or IL-12.14
Research advances in the role of IL-1β in pyoderma gangrenosum's pathogenesis published in international journals in the last 3 years support the use of the biologic agent anti IL-1β in PG that is resistant to systemic corticosteroids or immunomodulators.
Anakinra was the first biologic anti–IL-1 available on the market. It is a recombinant nonglycosylated protein of the IL-1 receptor antagonist. It blocks the biologic activity of IL-1α and IL-1β by competitively inhibiting the binding of IL-1 to their receptor. It can be administered subcutaneously or intravenously. A daily dose is recommended. Because of its daily use, anakinra could be a problem in patients who have positive pathergy. Rilonacept is a dimeric fusion protein that joins IL-1β and IL-α, thereby obstructing them. Rilonacept is used on a weekly basis.9, 13
Canakinumab is a fully humanized monoclonal IgG1 anti–IL-1β antibody that binds to human IL-1β and neutralizes its activity by selectively blocking its interaction with IL-1 receptors. It is given subcutaneously every 1 or 2 months.9, 13 Canakinumab may be the best treatment for PG that is refractory to classic therapy. This judgment is based on physiopathology and on the risk of pathergy.
This is our first experience with canakinumab. Successful resolution showed that it might be an effective and well-tolerated treatment for patients with PG whose disease is refractory to classic therapy and supports recent discoveries on the prime role of IL-1β in this dermatosis. There are only a few reports on PG treated with canakinumab and, at present, there is not enough evidence to draw a firm conclusion.
Footnotes
Funding sources: None.
Conflicts of interest: Prof. Galimberti is a senior researcher at Novartis, Eli Lilly, and AbbVie. The rest of the authors have no conflicts to declare.
References
- 1.Wolff K., Stingl G., Feedberg I.M. 6th ed. Vol. 2. Médica Panamericana; Mexico City, Mexico: 2005. Pyoderma gangrenosum; pp. 1088–1096. (Dermatología en Medicina General de Fitzpatrick). [Google Scholar]
- 2.Brunsting L.A., Goeckerman W.H., O'sLeary P.A. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol Syphilol. 1930;22:655–680. [Google Scholar]
- 3.Bossi G., Offidani A. Síndromes cutáneos paraneoplásicos. In: Giannetti A., Galimberti R.L., editors. Tratado de dermatología. Piccin Nuova Libreria; Rome, Italy: 2012. pp. 23–94. [Google Scholar]
- 4.Sakiyama M., Kobayashi T., Nagata Y. Bullous pyoderma gangrenosum: a case report and review of the published work. J Dermatol. 2012;39:1010–1015. doi: 10.1111/j.1346-8138.2012.01676.x. [DOI] [PubMed] [Google Scholar]
- 5.Reichrath J., Bens G., Bonowitz A., Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273–283. doi: 10.1016/j.jaad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Binuss A.M., Qureshi A., Li V.W., Witerfield L.S. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244–1250. doi: 10.1111/j.1365-2133.2011.10565.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruocco E., Sangiuliano S., Gravina A.G., Miranda A., Nicoletti G. Pyoderma Gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008–1017. doi: 10.1111/j.1468-3083.2009.03199.x. [DOI] [PubMed] [Google Scholar]
- 8.Malieni D., Torre A.C., Baztan M.C., Anselmi C., Galimberti R. Pioderma gangrenoso asociado a colitis ulcerosa tratado con infliximab. Dermatología Argentina. 2009;15(3):191–195. [Google Scholar]
- 9.Dinarello C.A., Simon A., Van der Meer J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geusau A., Mothes-Luksch N., Nahavandi H. Identification of a Homozygous PSTPIP1 Mutation in a Patient With a PAPA-Like Syndrome Responding to Canakinumab Treatment. JAMA Dermatol. 2013;149(2):209–215. doi: 10.1001/2013.jamadermatol.717. [DOI] [PubMed] [Google Scholar]
- 11.Brooklyn T.N., Dunnill M.G., Shetty A. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double blind, placebo controlled trial. Gut. 2006;55:505–509. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaeger T., Andres C., Grosber M. Pyoderma gangrenosum and concomitant hidradenitis suppurativa-rapid response to canakinumab (anti-IL-1b) Eur J Dermatol. 2013;23(3):408–410. doi: 10.1684/ejd.2013.2018. [DOI] [PubMed] [Google Scholar]
- 13.Ljung T., Staun M., Grove O. Pyoderma gangrenosum associated with Crohn disease: effect of TNF alfa; blockade with infliximab. Scand J Gastroenterol. 2002;37:1108–1110. doi: 10.1080/003655202320378338. [DOI] [PubMed] [Google Scholar]
- 14.Kolios A.G.A., Maul J.T., Meier B. Canakinumab for steroid-refractory pyoderma gangrenosum. Br J Dertmatol. 2015;173:1216–1223. doi: 10.1111/bjd.14037. [DOI] [PubMed] [Google Scholar]
- 15.Marzano A.V., Fanoni D., Antiga E. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet's syndrome. Clin Exp Immunol. 2014;178(1):48–56. doi: 10.1111/cei.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]