Introduction
Rosai-Dorfman disease, also called sinus histiocytosis with massive lymphadenopathy, is a rare histiocytic condition of unknown etiology first described in 1969.1 Primary cutaneous manifestation without systemic involvement of Rosai-Dorfman disease is even more uncommon.2 Here we present a case of primary cutaneous manifestation of Rosai-Dorfman disease with excellent response to oral thalidomide.
Case report
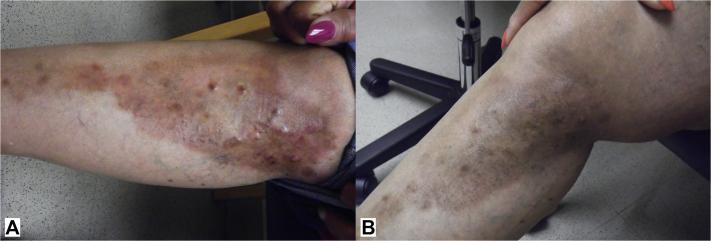
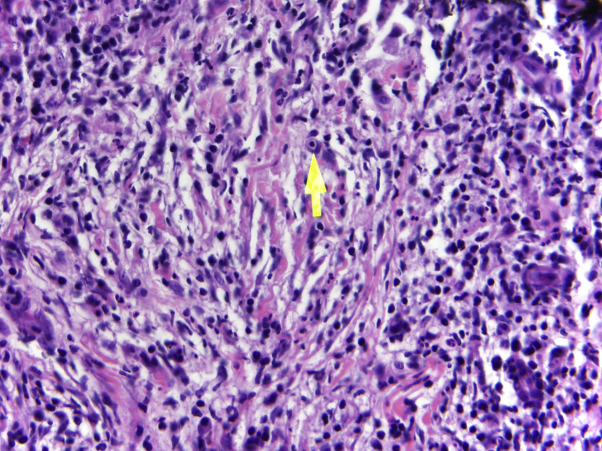
A 49-year-old African-American woman initially presented with a 6-year history of an eruption on the lower extremities. She did not report systemic symptoms in association with this eruption. On physical examination, the patient had scattered violaceous-to-pink firm nodules overlying indurated plaques within hyperpigmented patches on the medial right lower leg (Fig 1, A) and medial left thigh. The patient did not have any lymphadenopathy. An excisional biopsy from the right leg found histiocytic proliferation with emperipolesis (Fig 2). Immunohistochemical staining of the histiocytes showed positive stain for S100, CD68, CD20, CD3, and CD30 and negative stain for CD1a and langerin. These findings were most consistent with a non-Langerhans cell histiocytosis, specifically Rosai-Dorfman disease.
Fig 1.
A, Indurated plaques within atrophic hyperpigmented patches on the medial right lower leg before initiation of thalidomide. B, Medial right lower leg after 5 months of treatment with thalidomide
Fig 2.
Histiocyte engulfing lymphocyte (emperipolesis), highlighted by arrow. (Original magnification: ×40.)
The patient was initially treated with intralesional triamcinolone (20 mg/mL) to the nodules on legs and fluocinonide 0.5% cream to all the affected areas. Because of poor response, she was referred to the radiation oncology department and subsequently treated with 20 fractionated radiation doses, 40 Gy total, with minimal improvement. She was subsequently treated with methotrexate (15 mg weekly) but did not respond. Thalidomide, 50 mg nightly, was started and increased to 100 mg nightly. The methotrexate was slowly tapered and discontinued. After 5 months of treatment, the nodules on the patient's thigh and lower leg showed a noticeable improvement in erythema with decrease in size (Fig 1, B). The patient was taking thalidomide, 100 mg nightly, with no relapse of the disease process at her 9-month follow-up visit. The patient did report more fatigue with the increased dose of thalidomide.
Discussion
Rosai-Dorfman disease is a rare, generally benign and self-limited condition that primarily presents as painless, cervical lymphadenopathy accompanied by fever, neutrophilia, polyclonal hypergammaglobulinemia, and elevated erythrocyte sedimentation rate (ESR).3 Cutaneous Rosai-Dorfman disease is characterized by nonspecific macules, papules, plaques, or nodules of variable color. Because of the nonspecific skin findings, biopsy is critical to diagnosis, which reveals a hallmark characteristic of emperipolesis or histiocytes with lymphocytes within their cytoplasm. The histiocytes stain positive for S-100 and CD68 and negative for CD1a and langerin.4 Because cutaneous Rosai-Dorfman disease usually follows a benign course, treatment is generally not indicated unless there is extensive involvement, if the condition persists, or if the patient is significantly affected by disease.
There are several treatments described in the literature for cutaneous Rosai-Dorfman disease, including methotrexate, chemotherapy, surgery, and steroids.4, 5, 6, 7, 8, 9 Li et al5 described successful resolution of cutaneous Rosai-Dorfman disease with low-dose thalidomide of 100 mg. Our patient did not respond to intralesional triamcinolone, topical corticosteroids, radiation therapy, and methotrexate over 3 years but responded well to thalidomide. We conducted a literature review of the successful and failed cases of thalidomide for treatment of cutaneous Rosai-Dorfman disease. Data gathered for 7 patients from 5 publications are presented in Table I.
Table I.
Successful and failed treatments of Rosai-Dorfman disease with thalidomide in the literature
| Patient | Age (y)/sex | Presentation | Site | Additional history | Duration before diagnosis | Treatment prethalidomide | Dosage/duration of thalidomide treatment | Response to thalidomide/follow-up |
|---|---|---|---|---|---|---|---|---|
| 15 | 43/M | Papules and crusting | Face, chest, and upper extremities | Negative | 12 mo |
|
50 mg/d for 2 wk, 100 mg/d for 8 mo | Near resolution of nodules in face and limbs with lightening of erythematous papules 2 y: no recurrence of lesions |
| 26 | 45/F | Erythematous papules and plaques | Bilateral cheeks, periorbital, neck, inner thighs | Polyclonal hypergammaglobulinemia, elevated ESR | 5 mo |
|
300 mg/d | Gradual regression 5 mo: all lesions resolved except scattered axillary lesions |
| 310 | 41/F | Erythematous papules and plaques | Face, neck, periorbital, limbs, upper trunk | Uveitis, IgG hyperglobulinemia, mild anemia, elevated ESR | 2 mo | N/A | 300 mg/d, tapering to 150 mg/d | 22 mo: partial response |
| 410 | 60/M | Erythematous plaques/nodules with satellite papules | Back | Negative | 4-5 mo | N/A | 300 mg/d | 4 mo: clearing of old lesions |
| 511 | 72/F | Erythematous papules | Right breast | Negative | 8 mo |
|
50 mg/d for 1 mo, 100 mg/d for 5 mo | Minimal response Response to methotrexate 0.25 mg/kg within 1 mo, sustained partial response with methotrexate at 6 mo |
| 612 | 41/F | Erythematous papules, plaques, and nodules | Right cheek from inner canthus to nasal ala | Negative | 10 mo |
|
50 mg/d for 3 mo | Significant improvement No follow up information |
| 713 | 70/M | Erythematous nodules | Right chest | Negative | 1 y |
|
300 mg/d for 8 mo | No improvement on thalidomide Surgical excision of nodes; no recurrence after 1 y |
The review consisted of 3 men and 4 women. The ages ranged from 41 to 72 years, with an average age for presentation of 53.1 years. The average duration of disease before diagnosis was 7.6 months. Five of the 7 cases had a negative or unavailable medical history; 1 patient had mild anemia, bilateral uveitis, elevated ESR, and IgG hypergammaglobulinemia,10 whereas another had elevated ESR and polyclonal hypergammaglobulinemia.6 Five patients did not respond to multiple treatments before starting thalidomide. Five of the 7 cases reported improvement with thalidomide, whereas 2 cases had minimal response. Of the cases that reported response to thalidomide, only 2 cases used low-dose thalidomide.5, 12
It is not precisely known how thalidomide works, but the drug is used for several cutaneous conditions because of its immune-modulating and anti-inflammatory effects. Thalidomide decreases the CD4:CD8 ratio in patients with erythema nodosum leprosum.14 Thalidomide also affects chemotaxis of polymorphonuclear leukocytes and phagocytosis through cytokine alterations and inhibits tumor necrosis factor-α.14 It has antitumor properties and in vitro effects of converting a T-helper cell type 1 to a T-helper cell type 2 response.14 Thalidomide is effective in treating several dermatologic conditions, including chronic graft-versus-host disease, cutaneous lupus erythematosus, recurrent aphthous stomatitis, and Behçet's disease.14 Moreover, thalidomide is documented as a treatment for histiocytic disorders. There are cases of purely cutaneous, low-risk Langerhans cell histiocytosis effectively treated with thalidomide.15, 16 Thalidomide was also found to be effective in treating vulvar Langerhans cell histiocytosis.17
Side effects reported from thalidomide use include peripheral neuropathy, pulmonary toxicity, neutropenia, somnolence, and constipation.14, 16 Our patient did not note peripheral neurologic symptoms, but she noted a decreased sense of taste 5 months after starting therapy as well as fatigue.
Cutaneous Rosai-Dorfman disease is a rare condition that does not have a consensus treatment, although several treatment options are available. Thalidomide, both low and high doses, was found to be an effective treatment in some cases. However, further investigation is needed to understand why thalidomide works in some patients and not in others as well as the ideal dosage for pediatric and adult patients and optimal duration for thalidomide treatment. These questions would be best addressed in a prospective, multicenter clinical trial for cutaneous Rosai-Dorfman disease.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Rosai J., Dorfman R.F. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63–70. [PubMed] [Google Scholar]
- 2.Chu P., LeBoit P.E. Histologic features of cutaneous sinus histiocytosis (Rosai-Dorfman disease): study of cases both with and without systemic involvement. J Cutan Pathol. 1992;19(3):201–206. doi: 10.1111/j.1600-0560.1992.tb01659.x. [DOI] [PubMed] [Google Scholar]
- 3.Thawerani H., Sanchez R.L., Rosai J. The cutaneous manifestations of sinus histiocytosis with massive lymphadenopathy. Arch Dermatol. 1978;114:191–197. [PubMed] [Google Scholar]
- 4.Gebhardt C., Averbeck M., Paasch U. A case of cutaneous Rosai-Dorfman disease refractory to imatinib therapy. Arch Dermatol. 2009;145(5):571–574. doi: 10.1001/archdermatol.2008.597. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Hong Y., An Q. Successful treatment of Rosai-Dorfman Disease with Low-Dose Oral Thalidomide. JAMA Dermatol. 2013;149(8):992–993. doi: 10.1001/jamadermatol.2013.4399. [DOI] [PubMed] [Google Scholar]
- 6.Tjiu J.W., Hsiao C.H., Tsai T.F. Cutaneous Rosai-Dorfman disease: remission with thalidomide treatment. Br J Dermatol. 2003;148(5):1060–1061. doi: 10.1046/j.1365-2133.2003.05311.x. [DOI] [PubMed] [Google Scholar]
- 7.Satter E.K., Graham B.S., Steger J.W. Response of cutaneous Rosai-Dorfman disease to topical and intralesional steroids. Br J Dermatol. 2003;149(3):672–674. doi: 10.1046/j.1365-2133.2003.05499.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang H.S., Son S.J., Cho K.H., Lee J.H. Therapeutic challenge of dapsone in the treatment of purely cutaneous Rosai-Dorfman disease. Clin Exp Dermatol. 2011;36(4):420–422. doi: 10.1111/j.1365-2230.2010.03966.x. [DOI] [PubMed] [Google Scholar]
- 9.Sun N.Z., Galvin J., Cooper K.D. Cutaneous Rosai-Dorfman Disease Successfully Treated With Low-Dose Methotrexate. JAMA Dermatol. 2014;150(7):787–788. doi: 10.1001/jamadermatol.2013.8679. [DOI] [PubMed] [Google Scholar]
- 10.Lu C.I., Kuo T.T., Wong W.R., Hong H.S. Clinical and histopathologic spectrum of cutaneous Rosai-Dorfman disease in Taiwan. J Am Acad Dermatol. 2004;51(6):931–939. doi: 10.1016/j.jaad.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Nadal M., Kervarrec T., Machet M.C., Petrella T., Machet L. Cutaneous Rosai-Dorfman Disease Located on the Breast: Rapid Effectiveness of Methotrexate After Failure of Topical Corticosteroids, Acitretin and Thalidomide. Acta Derm Venerol. 2015;95(6):758–759. doi: 10.2340/00015555-2057. [DOI] [PubMed] [Google Scholar]
- 12.Wang F., Zhou H., Luo D.Q., Han J.D., Chen M.K. Dermatoscopic findings in cutaneous Rosai-Dorfman disease and response to low-dose thalidomide. J Dtsch Dermatol Ges. 2014;12(4):350–352. doi: 10.1111/ddg.12256. [DOI] [PubMed] [Google Scholar]
- 13.Du J., Ding X., Cai L., Sun P., Zhou C., Zhang J. Rosai-Dorfman disease: three difficult-to-diagnose cases. Eur J Dermatol. 2010;20(5):650–651. doi: 10.1684/ejd.2010.1026. [DOI] [PubMed] [Google Scholar]
- 14.Faver I.R., Guerra S.G., Su W.P., el-Azhary R. Thalidomide for dermatology: a review of clinical uses and adverse effects. Int J Dermatol. 2005;44(1):61–67. doi: 10.1111/j.1365-4632.2004.02445.x. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniyan R., Ramachandran R., Rajangam G., Donaparthi N. Purely cutaneous Langerhans cell histiocytosis presenting as an ulcer on the chin in an elderly man successfully treated with thalidomide. Indian Dermatol Online J. 2015;6(6):407–409. doi: 10.4103/2229-5178.169743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClain K.L., Kozinetz C.A. A phase II trial using thalidomide for Langerhans cell histiocytosis. Pediatr Blood Cancer. 2007;48(1):44–49. doi: 10.1002/pbc.20578. [DOI] [PubMed] [Google Scholar]
- 17.Santillan A., Montero A.J., Kavanagh J.J., Liu J., Ramirez P.T. Vulvar Langerhans cell histiocytosis: a case report and review of the literature. Gynecol Oncol. 2003;91(1):241–246. doi: 10.1016/s0090-8258(03)00370-6. [DOI] [PubMed] [Google Scholar]