Highlights
-
•
PRF in association with a new split crest augmentation technique was analyzed.
-
•
Ten patients five with the new technique and five by traditional one were treated.
-
•
All cases were successful and all implants achieved osteointegration.
-
•
Main advantages with this technique are soft tissues healing and bone regenerative properties.
Keywords: PRF, Split crest, Elderly patients, Bone regeneration
Abstract
Introduction
Some studies have demonstrated that platelet rich fibrin (PRF) is a healing biomaterial with a great potential for bone and soft tissue regeneration, without any inflammatory reactions and may be used alone or in combination with bone grafts, promoting hemostasis, bone growth, and maturation. PRF appears as a natural and satisfactory aid in bone regenerative surgery in elderly patients with favorable results and low risks.
Aim
This study wants to demonstrate how PRF in association with a new split crest augmentation technique can be a great aid in implant rehabilitation, especially in the elderly patients, when bone regeneration is required.
Materials and methods
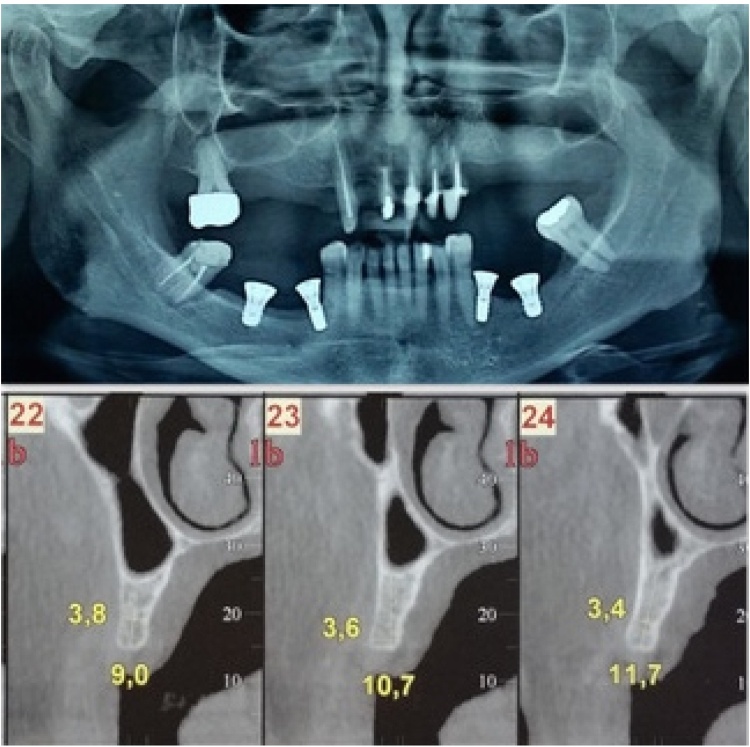
Ten patients were treated in this study, five following the flapless split crest new procedure and other five patients following traditional procedure without split crest as control. Five patients with an average age between 50 and 60 years were selected to be operated with a split crest flapless modified technique in order to optimize the regenerative conditions with a bone augmentation and implant insertion in one single stage procedure. For all the patients autologous PRF has been used to fill the split crest gap or simply as regenerative material. Orthopantomography, intraoral radiography and CT DentaScan/CT Cone beam were performed for every patient before the treatment and at follow-up time exeption made for CT.
Results
All cases were successful, there were no problems at surgery time, at post-operative and at osteointegration periods. All implants achieved osteointegration. These results were obtained by accurately managing immediate and late post operative period in all of the operated cases. Mean difference for height bone loss between the two groups of patients was 2.4 mm at T1 and 2.2 mm at T3.
Discussion
The rationale of this split crest flapless modified technique is to obtain a proper buccal cortex expansion preserving its vascular supply avoiding periosteal elevation for better cortical bone nourishing. Moreover, advantages are reported related to the use of PRF. The effectiveness of PRF is shown in promoting the healing of surgical wounds, it has, in fact, platelet growth factors that can improve the vascularisation of the surgical site, promoting neoangiogenesis. Furthermore, by simply changing the settings of the centrifuge, it is possible to obtain a normal gelling if it has to be used as regenerative and stimulating material, or more consistent substance to be used as a filler in the split crest gap.
Conclusions
The main advantages in using the platelet-rich fibrin are healing and bone regenerative properties in combination with its complete resorption after surgery, thus avoiding a second surgery time, important factor in the elderly patients. Currently, it is a minimally invasive technique with low risks and satisfactory clinical results such preventing complications or implant failure particularly in elderly patients for age related conditions.
1. Introduction
Platelets’ regenerative potential was reported in the 70’s [1], when it was observed that they contain growth factors that are responsible for increase collagen production, cell mitosis, blood vessels growth, recruitment of other cells that migrate to the site of injury, and cell differentiation induction, among others [2].
Nowadays in oral surgery there are two kind of platelet concentrates for in vivo tissue engineering applications: platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). Platelet concentrates are a concentrated suspension of growth factors found in platelets, which act as bioactive surgical additives that are applied locally to induce wound healing [3].
PRF was first used specifically in oral surgery by Dohan et al. [4] and is currently considered as a new generation of platelet concentrate. It consists of a matrix of autologous fibrin [5] and has several advantages over PRP, including easier preparation and not requiring chemical manipulation of the blood, which makes it strictly an autologous preparation [6]. For these considerations, in our study we preferred to use PRF procedure instead of PRP.
PRF consists of an autologous leukocyte-platelet-rich fibrin matrix [7] composed of a tetra molecular structure, with cytokines, platelets, and stem cells within it [5], [8], which acts as a biodegradable scaffold [9] that favors the development of microvascularization and is able to guide epithelial cell migration to its surface [5], [10].
Some studies [11], [12] have demonstrated that PRF is a healing biomaterial with a great potential for bone and soft tissue regeneration, without inflammatory reactions and may be used alone or in combination with bone grafts, promoting hemostasis, bone growth, and maturation.
This autologous matrix demonstrated in the in vitro studies a great potential to increase cell attachment [13] and a stimulation to proliferate and differentiate osteoblasts [14].
In surgical procedures, PRF could serve as a resorbable membrane for guided bone regeneration (GBR) [15], preventing the migration of non-desirable cells into bone defect and providing a space that allows the immigration of osteogenic and angiogenic cells permitting the underlying blood clot to mineralize [16]; moreover, a normal PRF membrane has a rapid degradability (1–2 weeks) [17].
PRF membrane helps in wound healing, protecting the surgical site [18], [19] promoting soft tissue repair; when mixed with bone graft, it may act as a “biological connector”, which attracts stem cell, favors the migration of osteoprogenitor cells to the center of the graft, and provides a neo-angiogenesis [19].
In addition, PRF may act as a biologic adhesive to hold the particles together, facilitating the manipulation of the bone grafts [20].
In this study, all of the five patients of the first group (test) has been submitted to a new split crest flapless modified technique with the use of PRF. Platelet rich fibrin can be used both as a regenerative and stimulator material, both as a filler in bone defects; it depends on type of protocol preparation used. In this technique bone regeneration is similar to osteodistraction creating a regenerative chamber with bone walls covered by native periosteum not cut or elevated during osteotomies preserving full ability of feeding of the underlying bone and comparable to reconstructive procedures for treating periodontal intraosseous defects.
In this study, we want to demonstrate how PRF can be a great aid in implant rehabilitation, especially in the elderly patients, when bone regeneration is required.
2. Materials and methods
Ten patients with an age between 50 and 60 years were referred to the Medical Department of the University of Salerno for evaluation of a moderately reabsorbed edentulous ridge secondary to previous extraction. Five patients were treated with the split crest flapless new technique for implant insertion, while five other patients were treated with traditional technique with smaller implants to overcome alveolar crest thinness. The treatment plan included rehabilitation with an implant-supported restoration (Fig. 1, Fig. 2, Fig. 3). The patient’s past medical and social history were non-contributory, and they had good oral hygiene. All the patients had no controindications to implant placement.
Fig. 1.
Ortopantomography and CT Dentascan of one of patients selected for split crest flapless, a moderate resorbed edentulous ridge is shown.
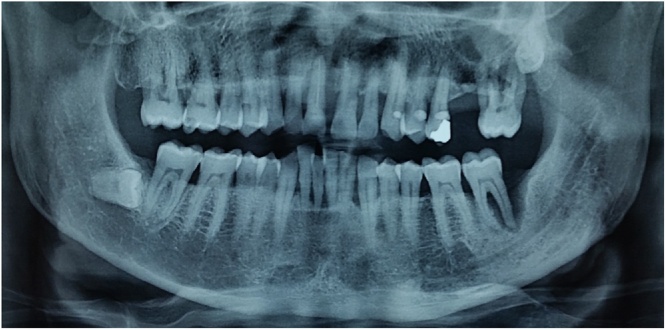
Fig. 2.
Ortopantomography of other patient selected for split crest flapless, there was the fracture of tooth in region 1.1.
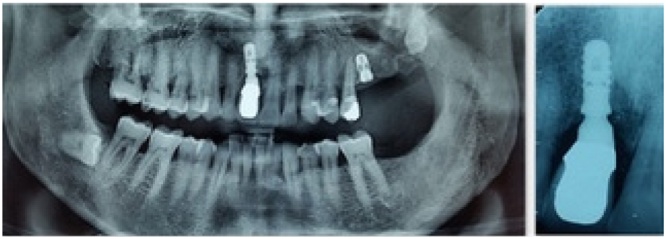
Fig. 3.
Ortopantomography after implant insertion. At time of insertion, a resorbed edentulous ridge was shown in region 1.1.
The surgeries were not performed in patients with systemic or psychological disorders that contraindicate oral surgery.
2.1. Surgical procedure
After administration of a local anesthesia, a palatally (maxilla) or lingually (mandibular) shifted incision was made to gain access to underlying bone ridge: periosteum elevation was performed only on the alveolar ridge up to the vestibular cortical wall. Split crest was performed making a thin milling, and then combining sharping osteotomy and than smooth osteotomes insertion for expansion. It continues with rotating drills of the implant kits in the basal bone preserving an adequate bone thickness for buccal and medial bone walls by splitted walls divarication during drilling up to 2.8 mm burs. To preserve cortical walls integrity further implant site preparation up to 3.5 mm. diameter was carried out by round osteotomes (Sommers) or ball burs. With this method there is a preservation of the vascular supply of the vestibular cortical wall living the periostium attached on the vestibular side, without any vestibular, mesial and distal osteotomies also achieving primary implant stability.
Particularly to obtain an optimal implant insertion, a two levels of implant site preparation was performed: 1) split crest flapless technique for the alveolar and small amount of basal bone and 2) additional and traditional drilling preparation by implant kit at the basal bone level (usually showing sufficient thickness).
In this way simultaneously were obtained optimal implant stability, adequate alveolar expansion, bone height-stability over time for adequate cortical walls thickness and optimal nourishment of the splitted vestibular walls by native periosteum with attached gingiva covering. Expansion with smooth chisel continued until appropriate site preparation for the selected implant was obtained, before performing the step two preparation with traditional implant kit drilling. Final expansion was maintained by the implants itself. The implants were positioned at the same time of surgery, bone level implants were preferred for the procedure.
The Vicryl polyglactin (91, 3/0) absorbable suture was used to close the flap.
To obtain a primary closure of the flap, essential for a proper healing of the osteotomy site, partial releasing incisions technique by scalpel at the mid-crest fibromucosa was performed for flap elongation. This aspect is particularly important because of the impossibility to apply a traditional membrane on the split crest osteotomy because of the flapless technique. In this way all the bone lack at the osteotomy site will be covered by fibromucosa after PRF apply at osteotomy rim for best healing.
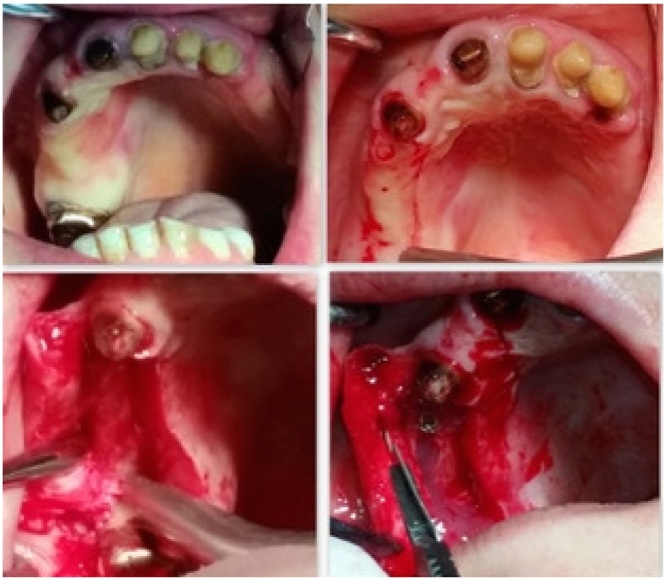
For all of the five patients of the first group autologous platelet rich fibrin (PRF) has been used to fill the osteotomy gap or simply as regenerative material. It depends on type of protocol preparation used. PRF protocol requires only centrifuged blood without any addition of anticoagulant and bovine thrombin. Then, a blood sample is taken without anticoagulant in 10-mL tubes in a glass or glass-coated plastic tube, then immediately centrifuged at 2700 rpm for 12 min. This protocol was used in 2 of 5 cases, in the other 3 cases, the protocol was changed (3000 rpm for 13 min) to obtain a more consistent substance to use as a filler in the split crest gap (Fig. 4).
Fig. 4.
Split crest flapless technique. After implant insertion, membranes of PRF were used to assure a better soft tissue healing, making the wound healing faster. The PRF was used in a regenerative chamber of native bone fed by periosteum to test the osteoinductive and osteoconductive power.
The five other patients treated with the traditional technique as control group were selected with a thin alveolar crest in order to allow smaller implant insertion without bone augmentation at a deeper bone position.
Orthopantomography, intraoral radiographs and CT DentaScan/CT Cone beam were performed before surgery for each patient in order to have a preliminary radiological investigation and to give a general overview of the jaw bone and relevant anatomic landmarks in a bidimensional and tridimensional reconstructed planes. Intraoral and face photographs were taken for aesthetic and functional evaluation of the patients status. For all the patients a beta-lactam antibiotic (Amoxicillin) was given orally 2gr. one hour before the surgery. The post-operative therapy required good oral hygiene, rinsing with mouthwash containing 0.2% chlorhexidine solution twice a day and an evening application of the same product in gel form, as well as the administration of a non-steroidal anti-inflammatory aid (Ketoprofen 80 mg) for three consecutive days.
Follow up was carried out postoperatively at 30 days (clinical), 3 months and 6 months (ortopantomography and intraoral X-ray).
This study was performed following the principles of the Declaration of Helsinki regarding research on humans; signature of a written informed consent form from all patients was requested. Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
3. Results
All cases ended well, there were no problems at surgery time, post-operative and in the period of osteointegration. All implants achieved osteointegration. In all implants a good degree of primary stability was achieved at the surgery time.
All patients underwent an uneventful implant surgery. All implants were placed according to the manufacturer’s instructions and achieving primary stability (≥35 Ncm). No intraoperative surgical complications were recorded.
Although postoperative complications were generally modest. The clinical healing was optimal in the short term, no dehiscence was reported.
These results were obtained by accurately managing immediate and late post operative period in all of the operated cases.
Particular attention was paid to oral hygiene, and to inappropriate early clinical loading at immediate and late post operative time.
The main characteristics and results achieved of the 10 patients belonging to the study are presented in Table 1, Table 2.
Table 1.
Selected elderly patients (split crest flapless technique) with implants distribution, type of PRF used, bone height loss at T0(pre-operative time), T1(immediate post-operative time), T2(3 months after surgery), T3(6 months after surgery).
| Age | Implants placed | Implant length, mm | Torque, N.cm |
PRF type | Bone height loss (T0,T1,T2,T3), mm | |
|---|---|---|---|---|---|---|
| Patient 1 | 53 | 1 | 11 | 35 | More consistent | T0:0; T1:0; T2:0; T3:1 |
| Patient 2 | 59 | 4 | ≥ 8; ≤ 13 |
40 | Normal gel | T0:0; T1:0; T2:0; T3:1 |
| Patient 3 | 60 | 2 | 11 | 35 | More consistent | T0:0; T1:0; T2:1; T3:2 |
| Patient 4 | 57 | 1 | 8.5 | 35 | More consistent | T0:0; T1:0; T2:0; T3:1 |
| Patient 5 | 55 | 2 | ≥ 8; ≤ 11 |
40 | Normal gel | T0:0; T1:0; T2:0; T3:1 |
Table 2.
Control patients with traditional technique with implants distribution, bone height loss at T0(pre-operative time), T1(immediate post-operative time), T2(3 months after surgery), T3(6 months after surgery).
| Age | Implants placed | Implant length, mm | Torque, N.cm |
Bone height loss (T0,T1,T2,T3), mm | |
|---|---|---|---|---|---|
| Patient 1 | 57 | 2 | 11 | 40 | T0:0; T1:2; T2:3; T3:3 |
| Patient 2 | 60 | 1 | 8 | 35 | T0:0; T1:3; T2:4; T3:4 |
| Patient 3 | 52 | 4 | ≥11; ≤13 |
35 | T0:0; T1:2; T2:2; T3:3 |
| Patient 4 | 55 | 2 | 11 | 40 | T0:0; T1:3; T2:3; T3:4 |
| Patient 5 | 57 | 2 | ≥ 8; ≤ 11 |
35 | T0:0; T1:2; T2:3; T3:3 |
Comparing results shown in Table 1, Table 2 for height decrease at T0, T1, T2 and T3 between the new and traditional technique it is evident less bone height loss for the new split crest flapless technique. Bone height was measured from lower border of the mandible and from the nose or sinus floor of the maxillae up to the alveolar bridge at the pre-operative time and up to the most coronal level of bone to implant contact at the post-operative time.
At T1 mean height loss was of 0 mm and of 1.2 mm at T3 for group 1 (new flapless technique) while for group 2 patients (traditional implant insertion technique) mean height bone loss was 2.4 mm at T1 and of 3.4 at T3.
Mean difference for height bone loss between the two groups of patients was 2.4 mm at T1 and 2.2 mm at T3. In this way bone height loss was calculated also referring to pre-operative time: heavier bone height loss in control group can be explained considering deeper implant insertion necessity in thin alveolar crest without expansion by smaller implant selection with related worsening of the final aesthetic result.
4. Discussion
Use of PRF in oral and maxillofacial surgery has been implicated in different procedures such as socket preservation, sinus lift and bone augmentation, root coverage procedures, and healing in donor site with good results [6], [7].
Some advantages are reported in the literature related to the use of PRF, such as the following:
its preparation is a simplified and efficient technique, with centrifugation in a single step, free and openly accessible for all clinicians [21], [22]; it is obtained by autologous blood sample [10]; minimized blood manipulation [23]; it does not require the addition of external thrombin because polymerization is a completely natural process, without any risk of suffering from an immunological reaction [4], [23].
It has a natural fibrin framework with growth factors within that may keep their activity for a relatively longer period and stimulate tissue regeneration effectively [13].
It can be used solely or in combination with bone grafts, depending on the purpose [8], [21]; increases the healing rate of the grafted bone [8], [23]; it is an economical and quick option compared with recombinant growth factors when used in conjunction with bone grafts [24]
When used as a membrane, it avoids a donor site surgical procedure and results in a reduction in patient discomfort during the early wound-healing period [25].
PRF may present some disadvantages as follows:
the final amount available is low because it is autologous blood [8]; the success of the PRF protocol depends directly on the handling, mainly, related to blood collection time and its transference for the centrifuge [4]; need of using a glass-coated tube to achieve clot polymerization [22]; possible refusal of treatment by the puncture required for blood collection at surgery time [21].
This procedure only needs a minimal experience of clinician for PRF manipulation [7], [21].
With this flapless technique with lingual or palatal mucosa incision, buccal alveolar cortex are preserved shifting incision and periosteal elevation on palatal or lingual sides where cortical wall are thicker and more resistant to reabsorption. Furthermore, this type of technique reduces the possibility of vestibular bone fenestration, avoiding periimplantitis and medicolegal consequences, especially in elderly patients where surgical problems could happen more often for the considerable anatomical difficulties [26]. Comparing the two groups of patients operated for this study, we can state that with this new technique it is possible to preserve alveolar bone height at implant insertion while achieving transversal alveolar expansion by proper bone regenerative conditions.
In literature, in addition to the split crest, other innovative and minimally invasive techniques for the expansion of the jaws are described, that are able to improve the aesthetics of the face [27], [28], [29], [30].
Recent literature suggests it is essential to leave unchanged the vascularity of the site, as evidenced by Schwartz-Arad [31]. To allow proper osseointegration with good healing of the surgical sites and to assure primary closure of the surgical site, releasing incisions by scalpel at the mid crest collapsed fibromucosa were performed obtaining sufficient flap elongation for primary closure and osteotomy site coverage with adherent (firm) fibromucosa.
To promote bone healing at the osteotomy sites PRF (Platelet Rich Fibrin) can be used. PRF seemed to assure a better soft tissue healing, making the wound healing faster [8]. Even in case of PRF membrane exposure, there is no risk of membrane infection or bone loss, as PRF can assure also a second intention healing of the soft tissues. Moreover, several clinical works have demonstrated the effectiveness of PRF in promoting the healing of surgical wounds; the PRF has, in fact, platelet growth factors that can improve the vascularisation of the surgical site, promoting neoangiogenesis [4].
The PRF if used in a regenerative chamber of native bone fed by periosteum, it may obtain regeneration, while not having osteoinductive properties. The osteoconductive power, over the acceleration healing process already demonstrated in the literature, has recently been proved in the article of Schwarz-Arad [31].
5. Conclusions
The biggest advantage in using the platelet-rich fibrin is for its complete resorption, thus avoiding a second surgical time, absolutely a crucial factor in the elderly patients.
Moreover, by simply changing the settings of the centrifuge, it is possible to obtain a normal gelling if it is to be used as regenerative and stimulating material, or a more consistent substance to use as a filler in the split crest bone gap.
Currently, it seems to be a minimally invasive technique with low risks and satisfactory clinical results such preventing complications or implant failure particularly in elderly patients for age related conditions.
Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal on request.
Author contributions
All authors contributed significantly to the present research and reviewed the entire manuscript.
AC: Partecipated substantially in conception and execution of the study and in the analysis and interpretation of data; also partecipated substantially in the drafting and editing of the manuscript.
GP: Partecipated substantially in execution of the study and in the analysis and interpretation of data; also partecipated substantially in the drafting and editing of the manuscript.
AB: Partecipated substantially in the analysis and interpretation of data; also partecipated substantially in the drafting and editing of the manuscript.
MG: Partecipated substantially in the analysis and interpretation of data; also partecipated substantially in the drafting and editing of the manuscript.
MA: Partecipated substantially in the analysis and interpretation of data; also partecipated substantially in the drafting and editing of the manuscript.
Contributor Information
Antonio Cortese, Email: ancortese@unisa.it.
Giuseppe Pantaleo, Email: giuseppepantaleo88@gmail.com.
Antonio Borri, Email: antonio-borri@alice.it.
Mario Caggiano, Email: dr.mariocaggiano@gmail.com.
Massimo Amato, Email: mamato@unisa.it.
References
- 1.Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc. Natl. Acad. Sci. U. S. A. 1974;71:1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiran N.K., Mukunda K.S., Tilak Raj T.N. Platelet concentrates: a promising innovation in dentistry. J. Dent. Sci. Res. 2011;2:50–61. [Google Scholar]
- 3.Borie E., Olivì D.G., Orsi I.A., Garlet K., Weber B., Beltrán V., Fuentes R. Platelet-rich fibrin application in dentistry: a literature review. Int. J. Clin. Exp. Med. 2015;15:7922–7929. [PMC free article] [PubMed] [Google Scholar]
- 4.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Passaretti F., Tia M., D’Esposito V., De Pascale M., Del Corso M., Sepulveres R., Liguoro D., Valentino R., Beguinot F., Formisano P., Sammartino G. Growth-promoting action and growth factor release by different platelet derivatives. Platelets. 2014;25:252–256. doi: 10.3109/09537104.2013.809060. [DOI] [PubMed] [Google Scholar]
- 7.Gupta V., Bains B.K., Singh G.P., Mathur A., Bains R. Regenerative potential of platelet rich fibrin in dentistry: literature review. Asian. J. Oral. Health. Allied. Sci. 2011;1:22–28. [Google Scholar]
- 8.Choukroun J., Diss A., Simonpieri A., Girard M.O., Schoeffler C., Dohan S.L., Dohan A.J., Mouhyi J., Dohan D.M. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Li Q., Pan S., Dangaria S.J., Gopinathan G., Kolokythas A., Chu S., Geng Y., Zhou Y., Luan X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed. Res. Int. 2013;2013:638043. doi: 10.1155/2013/638043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:e51–e55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Saluja H., Dehane V., Mahindra U. Platelet-Rich fibrin: a second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann. Maxillofac. Surg. 2011;1:53–57. doi: 10.4103/2231-0746.83158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim T.H., Kim S.H., Sándor G.K., Kim Y.D. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch. Oral Biol. 2014;59:550–558. doi: 10.1016/j.archoralbio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Wu C.L., Lee S.S., Tsai C.H., Lu K.H., Zhao J.H., Chang Y.C. Platelet-rich fibrin increases cell attachment: proliferation and collagen-related protein expression of human osteoblasts. Aust. Dent. J. 2012;57:207–212. doi: 10.1111/j.1834-7819.2012.01686.x. [DOI] [PubMed] [Google Scholar]
- 14.Dohan Ehrenfest D.M., Diss A., Odin G., Doglioli P., Hippolyte M.P., Charrier J.B. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108:341–352. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y.C., Zhao J.H. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust. Dent. J. 2011;56:365–371. doi: 10.1111/j.1834-7819.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 16.Lo Giudice G., Iannello G., Terranova A., Lo Giudice R., Pantaleo G., Cicciù M. Transcrestal sinus lift procedure approaching atrophic maxillary ridge. A 60 months clinical and radiological follow-up evaluation. Int. J. Dent. 2015:261652. doi: 10.1155/2015/261652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawase T., Kamiya M., Kobayashi M., Tanaka T., Okuda K., Wolff L.F., Yoshie H. The heat-compression technique for the conversion of platelet-rich fibrin preparation to a barrier membrane with a reduced rate of biodegradation. J. Biomed. Mater Res. B Appl. Biomater. 2015;103:825–831. doi: 10.1002/jbm.b.33262. [DOI] [PubMed] [Google Scholar]
- 18.Del Corso M., Toffler M., Dohan Ehrenfest D.M. Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post-avulsion sites: an overview of Choukroun’s PRF. J. Implant. Adv. Clin. Dent. 2010;1:27–35. [Google Scholar]
- 19.Toffler M., Toscano N., Holtzclaw D., Corso M.D., Dohan Ehrenfest M.D. Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. J. Implant Clin. Adv. Dent. 2009;1:21–30. [Google Scholar]
- 20.Cortese A., Pantaleo G., Amato M., Claudio P.P. Chin Wing osteotomy for bilateral goldenhar syndrome treated by chin wing mentoplasty: aesthetic, functional, and histological considerations. J. Craniofac. Surg. 2015;26:1628–1630. doi: 10.1097/SCS.0000000000001859. [DOI] [PubMed] [Google Scholar]
- 21.Simonpieri A., Del Corso M., Vervelle A., Jimbo R., Inchingolo F., Sammartino G., Dohan Ehrenfes D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012;13:1231–1256. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 22.Dohan D.M., Del Corso M., Charrier J.B. Cytotoxicity analyses of Choukroun’s platelet-rich fibrin (PRF) on a wide range of human cells: the answer to a commercial controversy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007;103:587–593. [Google Scholar]
- 23.Kang Y.H., Jeon S.H., Park J.Y., Chung J.H., Choung Y.H., Choung H.W., Kim E.S., Choung P.H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A. 2011;17:349–359. doi: 10.1089/ten.TEA.2010.0327. [DOI] [PubMed] [Google Scholar]
- 24.Cortese A., Pantaleo G., Ferrara I., Vatrella A., Cozzolino I., Di Crescenzo V., Amato M. Bone and soft tissue non-hodgkin lymphoma of the maxillofacial area: report of two cases, literature review and new therapeutic strategies. Int. J. Surg. 2014;12:S23–8. doi: 10.1016/j.ijsu.2014.08.388. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic S., Aleksic Z., Klokkevold P., Lekovic V., Dimitrijevic B., Kenney E.B., Camargo P. Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int. J. Periodontics Restor. Dent. 2012;32:e41–50. [PubMed] [Google Scholar]
- 26.Di Lorenzo P., Niola M., Buccelli C., Re D., Cortese A., Pantaleo G., Amato M. Professional responsibility in dentistry: analysis of inter-departmental case study. Dent. Cadmos. 2015;83:324–340. [Google Scholar]
- 27.Cortese A., Savastano G., Amato M., Cantone A., Boschetti C., Claudio P.P. New palatal distraction device by both bone-borne and tooth-borne force application in a paramedian bone anchorage site: surgical and occlusal considerations on clinical cases. J. Craniofac. Surg. 2014;25:589–595. doi: 10.1097/SCS.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 28.Cortese A., Savastano M., Savastano G., Claudio P.P. One-step transversal palatal distraction and maxillary repositioning: technical considerations, advantages, and long-term stability. J. Craniofac. Surg. 2011;22:1714–1719. doi: 10.1097/SCS.0b013e31822e6417. [DOI] [PubMed] [Google Scholar]
- 29.Cortese A., Savastano M., Cantone A., Claudio P.P. A new palatal distractor device for bodily movement of maxillary bones by rigid self-locking miniplates and screws system. J. Craniofac. Surg. 2013;24:1341–1346. doi: 10.1097/SCS.0b013e31828041a7. [DOI] [PubMed] [Google Scholar]
- 30.Cortese A., Pantaleo G., Amato M., Claudio P.P. Ridge expansion by flapless split crest and immediate implant placement: evolution of the technique. J. Craniofac. Surg. 2016;27(March (6)):e123–8. doi: 10.1097/SCS.0000000000002367. [DOI] [PubMed] [Google Scholar]
- 31.D. Schwartz-Arad, L. Levin, L. Sigal, Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation 14 (2005) 131–138. [DOI] [PubMed]