Abstract
Severe traumatic brain injury (TBI) often leads to deficits in physiological arousal and empathy, which are thought to be linked. This study examined whether injury-related brain volume loss in key limbic system structures is associated with these deficits. Twenty-four adults with TBI and 24 matched Controls underwent MRI scans to establish grey matter volumes in the amygdala, thalamus, and hippocampus. EEG and skin conductance levels were recorded to index basal physiological arousal. Self-report emotional empathy levels were also assessed. The TBI group had reduced brain volumes, topographic alpha differences, and lower emotional empathy compared to Controls. Regional brain volumes were differentially correlated to arousal and self-report empathy. Importantly, lower volume in pertinent brain structures correlated with lower empathy, for participants with and without TBI. Overall we provide new insights into empathic processes after TBI and their relationship to brain volume loss.
Keywords: Brain volume loss, Limbic system, Physiological arousal, Empathy, Severe traumatic brain injury
Highlights
-
•
EEG alpha power and SCL provide a stable measure of arousal disturbance following severe traumatic brain injury.
-
•
Diminished arousal was associated with reduced volume in the amygdala and thalamus.
-
•
Lower affective empathy was associated with reduced volume in the amygdala and hippocampus.
-
•
These relationships were found for participants with and without brain injury.
1. Introduction
Severe traumatic brain injury (TBI) results from acceleration-deceleration forces (often sustained in motor vehicle accidents, falls and assault) and leads to heterogeneous effects on the brain, with a preponderance of multifocal lesions in the lateral, anterior and ventral surfaces of the frontal and temporal lobes, and diffuse axonal damage (Bigler, 2007, Leunissen et al., 2014). Atrophy leading to volume loss generally begins in the months following injury and may continue for up to four years (Bigler, 2007). Consequently, the volume of these structures may provide a useful index of the extent of damage caused by TBI and serve as a predictor of clinical outcomes for TBI patients (Fearing et al., 2008).
TBI is often characterised by emotional changes, reduced behavioural regulation and impaired social function. In particular, around two thirds of patients with severe TBI self-report low empathy (de Sousa et al., 2010, Williams and Wood, 2010, Wood and Williams, 2008). Empathy is characterised by both cognitive and emotional components, which must function together in a relational and flexible manner in order for effective empathic responding to occur (Beadle et al., 2013, Driscoll et al., 2012). The cognitive component reflects our ability to represent and understand another person's mental state or perspective (Schnell et al., 2011). The emotional, or affective, component encompasses the ability to recognize and share another's emotional state, to distinguish between one's own and others' feelings, and regulate emotion effectively (Beadle et al., 2013, Driscoll et al., 2012). While cognitive and affective empathic processes interact, they appear to rely on at least partially distinct neural networks that can each be individually impaired by brain damage (Leigh et al., 2013). Affective empathy, in particular, is frequently associated with frontotemporal structures, particularly on the right hemisphere (Perry et al., 2001, Rankin et al., 2006).
Related to disorders of affective empathy is the finding that around two thirds of people with severe TBI experience deficits in arousal and emotional responsivity which are manifested both physiologically and behaviourally (de Sousa et al., 2012, Leunissen et al., 2014, Rushby et al., 2013a, Rushby et al., 2013b). A number of physiological measures have been used to examine affective arousal, particularly skin conductance level (SCL) and electroencephalographic (EEG) alpha activity, the latter being an index of neural activity during rest. Previous research has demonstrated an inverse relationship between lower SCL and higher alpha magnitude in normal adults, such that EEG systematically varies under low stimulation (e.g. when eyes are closed SCL is low and alpha is high) versus greater stimulation (e.g. when eyes are open SCL is high and alpha is suppressed) (Barry et al., 2005, Barry et al., 2007, Barry et al., 2008). Barry et al. (2007) argued that the inverse relationship between alpha and SCL reflects a stable measure of arousal in healthy populations. Rushby et al. (2013a) examined this relationship in participants with severe TBI. Relative to controls, the TBI group displayed lower amplitudes in both measures. Blunted alpha changes were largest in lateral-temporal regions, regions typically associated with TBI damage. A follow up study investigated levels of arousal during an emotion processing task, as well as whether disturbances in either process was related to atrophy of structures in the brain following TBI (Fisher et al., 2015). TBI participants showed this hypoarousal (i.e., blunted alpha change) in an emotional context, but only the control group showed a relationship with brain volume, such that larger left insula and right amygdala correlated positively with responsivity of alpha to task demands. Both structures uniquely contributed to variance in alpha. Although empathy was not examined in those papers, other research suggests that physiological hypoarousal may contribute to a reduced capacity to understand others' feelings, thoughts, and intentions in individuals with TBI (de Sousa et al., 2012, Leigh et al., 2013, Rushby et al., 2013b). The following study was designed to examine the inter-relationship between low empathy and arousal and loss of volume in relevant brain structures following TBI.
A useful framework for considering the neural processes underpinning affective empathy arises from the work of Phillips et al. (2003) who proposed two separate neural processing streams underlying emotion perception. The first is the ventral stream which mediates autonomic arousal and facilitates early rapid appraisal of emotional stimuli. This appraisal is relayed via the thalamus and the amygdala to the ventromedial prefrontal cortex, insula and ventral anterior cingulate. The second is the dorsal stream, which mediates slower effortful processing of these stimuli via the dorsal anterior cingulate gyrus, dorsolateral prefrontal cortex and hippocampus. This stream engages cognitive processes, such as memory, as well as the regulation of affective states. These streams are highly interactive and have reciprocal functional influences. Of these structures, the amygdala, thalamus and hippocampus are of particular relevance to the current study, as all are known to be highly vulnerable to TBI and are also implicated in empathy. Specifically, the amygdala and thalamus are likely to have a direct role in mediating arousal while the hippocampus is implicated in the cognitive regulation of empathic responses.
The amygdala has traditionally been associated with the processing of emotionally salient stimuli and has been suggested to play a role in affective empathy (Fisher et al., 2015, Leigh et al., 2013, Rankin et al., 2006). Furthermore, since the amygdala mediates autonomic responses to emotional stimuli, damage to this structure is likely to be accompanied by abnormalities in arousal and responsivity to such stimuli (Fisher et al., 2015, Newsome et al., 2013). Amygdala volume correlates positively with both skin conductance levels (Williams et al., 2001) and alpha power (Fisher et al., 2015). The thalamus has been similarly implicated in the mediation of arousal. It has a strong influence on cortical EEG activity (Detari, 2000). Specific nuclei of the thalamus are thought to relay processed sensory information involving affective prosody and emotional facial expressions to the cortex (Leigh et al., 2013, Neumann et al., 2012). TBI is often associated with a significant reduction in thalamic volume, and since there are multiple projections from the thalamus to other structures, damage to the thalamus may globally affect a range of activities and account for a large proportion of the morbidity of TBI (Little et al., 2010, Neumann et al., 2012).
The hippocampus is rarely examined in empathy research. However, its role in declarative memory suggests that it is likely to play a role in the cognitive processes associated with affective empathy (Beadle et al., 2013, Kumaran and Maguire, 2005) such as self-reflection of past and present empathic abilities. Declarative memory supports the flexible expression of memories, which allows the application of prior learning to novel situations (Beadle et al., 2013). The hippocampus is essential in constructing, manipulating and updating information in order to respond appropriately to the task at hand (Rubin et al., 2014). Damage to this structure can significantly impair the ability to acquire and update new social information, (Beadle et al., 2013, Tate and Bigler, 2000). Hippocampal damage may also result in inflexible and maladaptive behaviour, when such behaviour places high demands on the generation, recombination and flexible use of information (Rubin et al., 2014).
In sum, atrophy of the amygdala and thalamus may be critical in determining the extent of deficits in both arousal and empathy in patients with TBI while the hippocampus may have a role limited to the cognitive appraisal of empathic abilities. Extending upon the results of our previous research (Fisher et al., 2015, Rushby et al., 2013a, Rushby et al., 2013b), the current study examined the degree of correspondence between measures of physiological arousal (SCL and alpha power) and self-reported emotional empathy, with atrophy in the amygdala and thalamus representing two primary ventral-stream structures. Examination of the hippocampus was included to assess correspondence between cognitive appraisal of empathy and atrophy in dorsal-stream structures. It was hypothesised that participants with TBI would self-report lower affective empathy levels than controls. It was further hypothesised that TBI participants would have significant atrophy in the bilateral amygdalae, hippocampi, and thalami, and deficits in resting arousal, reflected by lower SCL and elevated alpha power in temporal brain regions in comparison to healthy controls. Relationships between brain volume and arousal were expected for the ventral-stream structures (amygdalae and thalami volume), but not the dorsal-stream structure (hippocampi). Furthermore, self-reported affective empathy scores were predicted to correlate with brain volumes, specifically of the amygdalae, thalami and hippocampi.
2. Methods and materials
2.1. Participants
Participants included 24 adults (19 males) with a severe TBI of mean age 43.3 years (SD = 14.96) with 12.5 years of education, on average (SD = 2.99). Participants were recruited from the outpatient records of three metropolitan brain injury units in Sydney, who met the following inclusion criteria: they had sustained a severe brain injury resulting in altered consciousness of one day or greater, were discharged from hospital and living in the community, and had functional English.
The mean length of posttraumatic amnesia (PTA) was 59.63 days (SD = 47.72) and all participants were tested at least one year post-injury (M = 12.63 years, SD = 8.81). Cause of injury included motor car or bike accidents (n = 13, 54.8%), falls (n = 9, 37.5%), and assault (n = 2, 8.3%). Computerised tomography (CT) and magnetic resonance imaging (MRI) scans made for clinical purposes revealed that participants' injuries were left hemisphere-focused (n = 7, 29.2%), right-hemisphere-focused (n = 5, 20.8%), or bilateral (n = 4, 16.7%). For the remaining participants (n = 8, 33.3%), original (immediately post injury) scan readings were either unavailable (n = 2, 8.3%) or did not identify the injury site in a way that it could be characterised in terms of hemispheric focus (n = 6, 25.0%).
For the control group, 24 age- and gender-matched (p > 0.05) adults (20 males) without brain injuries were recruited from the general community via advertisements online and through local community groups (e.g., libraries, churches). This control group had a mean age of 42.4 years (SD = 14.9) and 14.7 years of education, on average (SD = 3.12). The TBI and control groups did not differ significantly with respect to gender distribution, age or education. Most participants (n = 33) included in the present study also participated in our previous study on arousal and EEG (Fisher et al., 2015).
All participants completed a screening questionnaire and were excluded if they had: uncorrected hearing or vision loss, a current diagnosed drug and/or alcohol addiction, active psychosis or were receiving treatment for a psychiatric condition, dementia or other neurodegenerative disease, aphasia, agnosia, or profound amnesia. All participants were requested not to ingest any caffeine for 2 h prior to collecting the physiological measures (skin conductance and EEG alpha power), as this has been shown to affect arousal (Barry et al., 2005, Barry et al., 2008). Ethics approval was obtained through the Human Research Ethics Advisory Panel (HREAP reference 103-049) at the University of New South Wales.
2.2. Procedure
This study comprised two parts, completed over one or two testing sessions. Upon commencing the (first) testing session participants were provided with an information sheet, provided written consent, and completed an MRI safety screening questionnaire along with the self-report questionnaires. For the MRI scan, participants followed standard scanning protocols and were instructed to lie with their eyes-open or closed as preferred.
Following their scan, participants were taken to the EEG laboratory where they were fitted with the SCL and EEG measurement equipment, seated approximately 60 cm from the computer screen, and instructed to keep their eyes fixated on the monitor in front of them. To ensure that participants were relaxed when recording commenced, they were asked to sit calmly for ten minutes and were provided with magazines during this time. Immediately prior to commencing recording, participants were asked to minimise their movement, and to remain as relaxed as possible. To establish resting physiological arousal, participants were instructed to close their eyes when they heard a tone and keep them closed until they heard a second tone (both 3 s duration 1000 Hz tones). During this time SCL and scalp-wide EEG alpha power were recorded simultaneously.
2.3. Materials
Two self-report questionnaires, described below, were used to assess affective empathy.
Balanced Emotional Empathy Scale (BEES) (Mehrabian, 2000). The 30-item BEES provides a measure of an individual's ability to empathise with another's emotional experiences (i.e., affective empathy), and has good reliability (α = 0.87) (Mehrabian, 2000). It has been used with a range of clinical populations, including TBI (de Sousa et al., 2010, de Sousa et al., 2012, Williams and Wood, 2010, Wood and Williams, 2008). Responses are rated on a nine-point Likert scale, ranging from + 4 (very strong agreement) to − 4 (very strong disagreement). Higher scores indicate greater levels of emotional empathy.
Interpersonal Reactivity Index (IRI) (Davis, 1980). The 28-item IRI comprises four seven-item subscales, which assess different processes underlying empathy: perspective-taking and fantasy items are thought to reflect one's ability to understand another person's thoughts or point-of-view, i.e., cognitive empathy; whereas empathic concern (ECS) and personal distress (PDS) are proposed to reflect one's ability to feel compassion or distress for, and thereby understand another's emotional state, i.e., affective empathy. Only the two affective empathy subscales are reported in this study. All responses are rated on a five-point Likert scale from 0 (does not describe me well) to 4 (describes me very well) and show acceptable to good reliability (α = 0.68–0.79) (Davis, 1980). While higher scores reflect greater levels of each corresponding construct, the PDS has been shown to be negatively correlated with the ECS (D'Orazio, 2004, Davis, 1980, Davis, 1983). Consequently the two subscales were reported separately.
2.4. Data acquisition and processing
2.4.1. Physiological data
Skin conductance level (SCL) was recorded via Ag/AgCl electrodes strapped to the distal volar surface of the index and fourth fingers of the non-dominant hand. SCLs were acquired continuously throughout the experiment using BioGraph Infiniti Software (Thought Technology Ltd., Quebec, Canada) at a sampling rate of 256 Hz, connected to a PC and triggered manually by the experimenter. SCL was calibrated before each session to detect activity in the range of 0–30 μS.
A PC-based digital signal-processing hardware and software package from Neuroscan was used for the acquisition of EEG data (Compumedics, Acquire Version 4.5). Continuous EEG was recorded from 64 scalp sites using the Neuroscan Quick-cap, sampled at 1000 Hz, referenced to the nose and grounded by the cap electrode. Vertical eye movement (vEOG) was monitored with tin cup electrodes placed 2 cm above and below the left eye. Horizontal eye movement (hEOG) was monitored with tin cup electrodes placed on the outer canthus of each eye. The maximum impedance was always < 5 kΩ for cap and EOG electrodes.
Continuous EEG data were segmented offline using Neuroscan Edit software (Compumedics 4.5). A 2 min segment was epoched into contiguous periods of 1000 ms, and the data were resampled to 1024 Hz. Epochs in which EEG amplitude exceeded ± 100 μV or contained ocular artefacts were rejected. For each subject, average power spectrum was calculated using Fast Fourier Transforms with a 10% Welch window. At each electrode, absolute power alpha (8–12 Hz) band was calculated. Mean SCL was calculated for the same 2 min period.
2.4.2. Brain volume data
Participants were scanned on a Phillips Achieva 3T TX scanner. Two sets of structural brain images were acquired using the following sequences with a standard 8-channel head coil: 3D high-resolution TFE T1 volume utilising an inversion pre-pulse at shot interval of 1800 ms (TR/TE/FL: 6.4/2.8/8 ms; flip angle: 8°; 1 mm isotropic voxel size, 200 coronal slices; field of view 256).
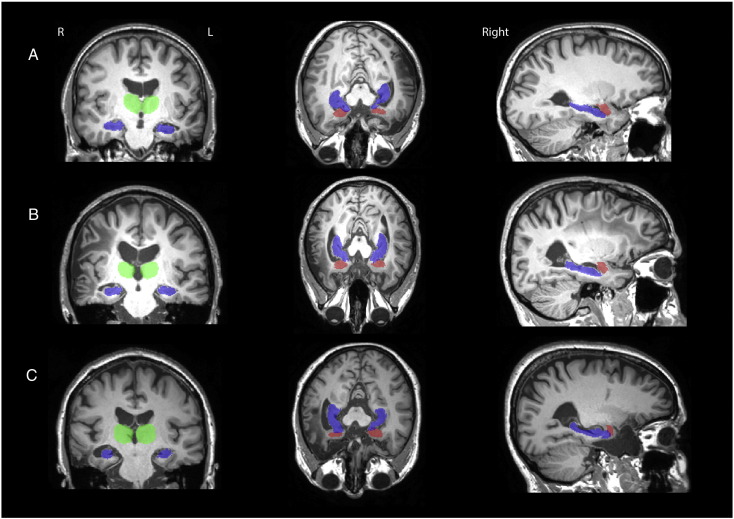
Prior to the analyses, the two T1 volumes were merged and averaged to increase the signal to noise ratio and the grey matter-white matter contrasts. Total brain tissue volume, normalised for subject head size, was estimated with SIENAX (Smith et al., 2002), part of FMRIBs Software Library V-4.1.9 (Smith et al., 2004). Automated segmentation of the amygdalae, thalami, and hippocampi were performed to obtain volume estimates of each structure in mm3 using FIRST from FSL (Kumaran and Maguire, 2005, Rubin et al., 2014). Analysis was then performed to observe local shape differences on a per-vertex basis between TBIs and Controls using FIRST. Manual assessment was performed after segmentation to ensure it was correct but no manual adjustments were necessary. In order to demonstrate the correspondence for the automated segmentation output, Fig. 1 presents the T1 images from three participants with TBI with their FIRST output overlaid in colour (green = thalamus, blue = hippocampus, red = amygdala).
Fig. 1.
T1 images from three participants with TBI with their FIRST output overlaid in colour (green = thalamus, blue = hippocampus, red = amygdala). Participant A: Male, 30 years, Acute left subdural haematoma, multiple left and parietal petechial haemorrhages and oedema, left frontal haematoma. Participant B: Male, 29, Bilateral subdural haematomas and fronto-temporal contusion. Participant C: Female, 59 years, Right temporal haematoma, right frontal contusion and fractured occipital bone.” (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. Statistical analyses
A series of independent sample t-tests analysed differences in self-reported empathy, brain volume (bilateral amygdalae, thalami, hippocampi) and SCL, as a function of group (TBI vs. Control).
The topographic distribution of alpha power was examined with a mixed-measures MANOVA, with a between factor of group (CTL vs. TBI). In line with previous studies nine electrodes were chosen for the analysis: left frontal (F3), midline frontal (Fz), right frontal (F4), left central (C3), midline central (Cz), right central (C4), left posterior (P3), midline posterior (Pz), and right posterior (P4). The sagittal regions: frontal (F: F3, Fz, F4), central (C: C3, Cz, C4), and parietal (P: P3, Pz, P4), served as one within-subjects factor, and the lateral regions: left-hemisphere (L: F3, C3, P3), right-hemisphere (R: F4, C4, P4), and midline (M: Fz, Cz, Pz), served as the other. Planned contrasts compared frontal (F) and parietal (P) sites, and central (C) sites to the fronto-parietal (F/P) mean along the sagittal plane. Similarly in the lateral plane, the left (L) and right (R) hemispheres were compared, as were the midline (M) sites to the hemispheric mean (L/R). As all these contrasts were planned, and there were no more of them than the degrees of freedom for effect, no Bonferroni-type adjustment to α is required (Tabachnick and Fidell, 2012). For all analyses p < 0.05 was treated as statistically significant. All F tests were reported with (1, 46) degrees of freedom.
Pearson's partial correlations tested bivariate relationships between brain volume (bilateral amygdalae, hippocampi, and thalami), SCL, alpha power; and each of the empathy measures for the whole sample, and separately for each group (TBI, Control). Correlations between age and years and education with the brain volume and arousal measures were also initially assessed, neither correlated with any of the variables examined. p-Values for correlations were corrected for false discovery rate. This procedure avoids the inflated rate of false negatives arising from Bonferroni adjustments while still controlling for false positives (Benjamini and Hochberg, 1995, Glickman et al., 2014). Significant correlations found in the whole sample were tested for between group differences with the Fisher r-to-z transformation.
3. Results
3.1. Group differences on self-report empathic measures
The TBI group reported significantly lower emotional empathy on the BEES compared to Controls, but no differences were shown for the Empathic Concern (ECS) or Personal Distress (PD) subscales (see Table 1 for the associated statistics). The BEES was positively correlated with the ECS (p < 0.001) but not the PD subscale (p = 0.08), and a non-significant negative correlation was found between the ECS and PDS.
Table 1.
Summary of between group differences for SCL, brain structure volume, and self-report empathy measures.
| Variable | Mean difference (TBI vs. Controls) | t (1,46) | p |
|---|---|---|---|
| L Amygdala | − 134.38 | − 1.26 | ns |
| R Amygdala | − 260.63 | − 2.45 | < 0.05 |
| L Hippocampus | − 415.62 | − 2.15 | < 0.05 |
| R Hippocampus | − 672.31 | − 2.95 | < 0.01 |
| L Thalamus | − 914.87 | − 2.20 | < 0.05 |
| R Thalamus | − 1128.75 | − 2.72 | < 0.01 |
| SCL | − 2.69 | − 3.51 | < 0.01 |
| BEES | − 15.58 | − 1.94 | < 0.05a |
| IRI PDS | 1.08 | 0.69 | ns |
| IRI ECS | − 0.71 | − 0.54 | ns |
Notes. SCL = skin conductance level; L = left; R = right; BEES = Balanced Emotional Empathy Scale; IRI = Interpersonal Reactivity Index; PDS = Personal Distress Subscale; ECS = Empathic Concern Subscale.
Significant for 1-tail t-test.
3.2. Group differences in brain volume
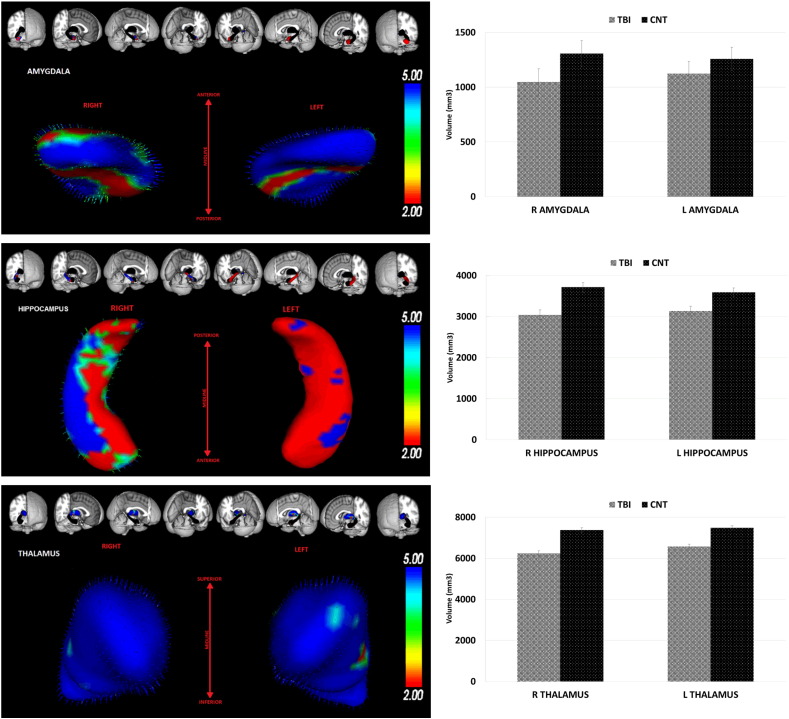
As can be seen in Table 1, compared to Controls, the TBI participants showed significant volume reductions in most of the brain structures of interest, with the exception of the left amygdala (p = 0.12). Fig. 2 illustrates 3D reconstructions of the bilateral amygdalae, hippocampi, and thalami showing volume reductions in the TBI group relative to Controls. As can be seen, atrophy in the TBI group was substantial with reductions of 11.3 and 11.9% in left-hemisphere structures (left thalamus and left hippocampus, respectively) and reductions ranging from 9.3% (right hippocampus) to 17.8% (right amygdala) in the right-hemisphere.
Fig. 2.
The left panel illustrates 3D reconstruction of the bilateral Amgydale, Hippocampi, and Thalami showing volume reductions in the TBI group relative to Controls. Surface colouring is the multivariate F statistic (based on Pillai's trace) corrected for multiple comparisons. Significant differences between groups are illustrated as increasing in significance from red to blue. Vectors show the direction of shape change from controls to TBI. The right panels show the mean grey matter volume differences (± standard errors, SEM) between TBI and Control participants. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Group differences in SCL
An independent samples t-test revealed a significantly lower SCL for the TBI group compared to controls; the corresponding statistics are presented in Table 1.
3.4. Group differences in alpha power
The topographic distribution of power in the alpha band for the TBI and Control groups, and their difference (TBI-CTL) can be seen in Fig. 3. In both groups, alpha power was elevated in both the parietal (F < P: F = 41.74, p < 0.001, ηp2 = 0.47) and fronto-parietal (C < F/P: F = 15.78, p < 0.001, ηp2 = 0.25) regions. Power was further elevated in the midline compared to the hemisphere sites (M > L/R: F = 4.17, p = 0.047, ηp2 = 0.08). As can be seen in Fig. 3 (lower panel) an interaction between region and group indicates that, relative to controls, the TBI group had lower power in the midline region compared to the hemispheres regions, which showed an elevation (Control > TBI × M > L/R: F = 10.70, p = 0.002, ηp2 = 0.19).
Fig. 3.
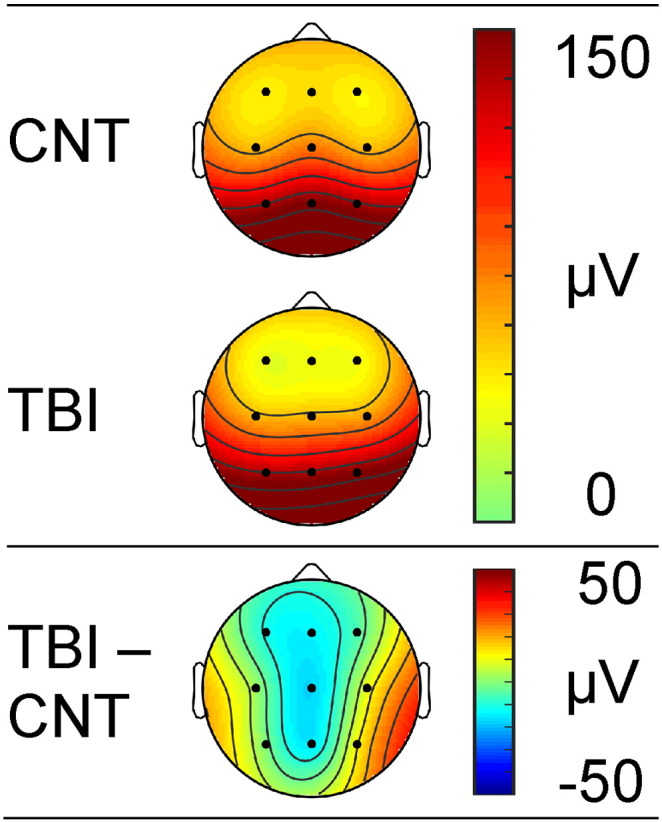
Topographic distribution of mean alpha power for control participants (top), TBI participants (centre), the difference between groups (bottom).
3.5. Correlations between brain volume and arousal
A summary of significant correlations is shown in Table 2. The differences between the correlation coefficients found for each group are also included. A positive z-score indicates that the correlation coefficient was larger for the Control compared to the TBI group, and a negative score reflects a larger coefficient for the TBI group.
Table 2.
Summary of significant correlations across the whole sample, and the between groups z-scores.
| Structure | SCL |
aAlpha |
Alpha (C3) |
|||
|---|---|---|---|---|---|---|
| r | z | r | z | r | z | |
| L Amygdala | 0.24b | − 0.15 | − 0.25c | − 1.31 | ||
| R Amygdala | 0.25c | − 0.03 | − 0.29c | − 0.70 | ||
| L Thalamus | 0.29c | − 0.14 | − 0.27c | − 0.40 | ||
| R Thalamus | 0.39d | 0.06 | − 0.29c | − 0.07 | ||
| L Hippocampus | − 0.31c | 0.85 | − 0.32d | 0.43 | ||
| R Hippocampus | − 0.36d | − 1.06 | − 0.39c | − 1.19 | ||
Reflects mean at central electrodes (C3, Cz, C4).
= 0.054.
< 0.05.
< 0.01.
Across the whole sample significant positive correlations were found between the right amygdala and bilateral thalami volumes with SCL, and significant inverse relationships were found for each brain structure examined with alpha power. For bilateral amygdalae and thalami, correlations with alpha power were confined to the left central electrode (C3). The hippocampi also showed negative correlations across central brain regions (C3, Cz, C4). While the majority of coefficients were larger for the TBI group, none differed significantly to controls.
3.6. Correlations between brain volume and empathy
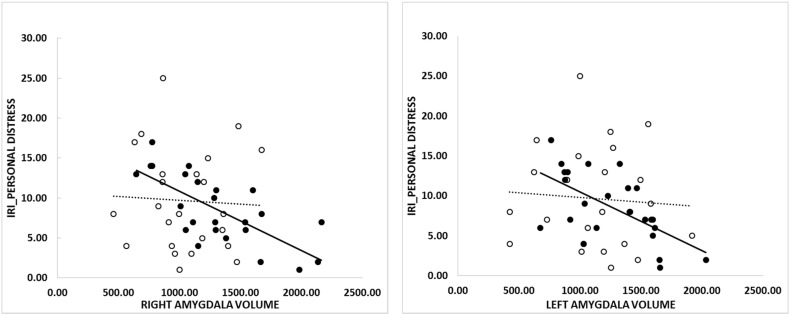
Whole group significant correlations and between group z-scores, between brain volume and the empathy measures are shown in Table 3. Left and right amygdala were inversely correlated with the PDS, while the left amygdala and right hippocampus were positively correlated with ECS. Additionally the right hippocampus was also positively correlated with the BEES. While the majority of z-scores were larger for TBI participants, the only significant differences to emerge were between the bilateral amygdalae and the PDS. As can be seen in Fig. 4, a strong correlation between measures was found for control participants, but no relationship was evident in participants with TBI.
Table 3.
Summary of significant correlations across the whole sample, and the between groups z-scores.
| BEES |
ECS |
PDS |
||||
|---|---|---|---|---|---|---|
| r | z | r | z | r | z | |
| L Amygdala | 0.26a | − 0.52 | − 0.29a | 2.1a | ||
| R Amygdala | − 0.36b | 2.8b | ||||
| L Thalamus | ||||||
| R Thalamus | ||||||
| L Hippocampus | ||||||
| R Hippocampus | 0.27a | 1.36 | 0.29a | − 0.69 | ||
< 0.05.
< 0.01.
Fig. 4.
Correlations between left and right amygdala and the personal distress subscale of the IRI. Solid circles and solid trend-line reflect control participants, open circles and dashed trend-line reflect participants with TBI.
4. Discussion
The primary aim of the current study was to examine the degree of correspondence between measures of atrophy in pertinent brain regions in individuals with severe traumatic brain injury and physiological arousal and self-reported empathy. Our first and second hypotheses were confirmed. Compared with controls, participants with TBI self-reported lower empathy, had significant amygdala (right only), thalamic and hippocampal loss and showed blunted resting arousal, reflected in lower SCL and alpha power. Like our previous research (Fisher et al., 2015, Rushby et al., 2013a, Rushby et al., 2013b) focal alpha magnitude differences were found, such that people with TBI displayed reduced alpha power (hypoarousal) in midline brain regions, and elevated power (hyperarousal) in outer hemisphere regions.
We predicted that a relationship would emerge between bilateral amygdalae and thalami volumes with the arousal variables, but no such relationship would be found for the hippocampus. This hypothesis was partially supported, such that a positive relationship emerged between amygdalae and thalami volumes with SCL, and a negative relationship was found between these structures and alpha power, which was localised to the left hemisphere central electrode (C3). These effects indicate that volume loss is associated with lower SCL and higher alpha power. Barry and colleagues have previously demonstrated that EEG systematically varies under low stimulation (e.g. when eyes are closed SCL is low and alpha is high) versus greater stimulation (e.g. when eyes are open SCL is high and alpha is suppressed), and that the degree of change is functionally relevant (Barry et al., 2005, Barry et al., 2007, Barry et al., 2008). The functional significance of this inverse relationship was apparent in our previous study (Fisher et al., 2015), which showed that elevated alpha power in the hemisphere regions was associated with reduced alpha suppression when viewing emotional facial expressions in TBI participants. The magnitude of alpha suppression correlated positively with right amygdala volume (and left insula) in that study, but only for control participants. This suggests that hyper-arousal in the hemispheres leads to reduced alpha responsivity to task demands, and these effects were independently mediated by amygdala volume. In the current study, significant relationships were found for the whole sample, and though correlation coefficients were larger in TBI participants, none differed significantly to the control group. Further research examining the relationship between physiological resting states and physiological/behavioural responsivity to task demands, may further delineate the mechanisms that underlie arousal dysregulation in people following TBI.
In contrast to expectations an inverse correlation between alpha power and the bilateral hippocampi was also found, and this was evident for both left and right central electrode sites, as well as the mean over central regions, but no association was found between hippocampal volumes and SCL. Although the key mechanisms of EEG generation are not fully understood, primary EEG oscillations appear to be dependent on interactions between the cortex and the thalamus, along with inhibitory contributions from the hippocampus. These interactions are reflected in rapid brain changes between slow wave (delta, theta) and fast wave (alpha, beta) activity, in response to sensory input (Larson et al., 1998, Steriade, 2006). The correlations found in the current study may reflect the importance of hippocampal volumes in supporting alpha responsivity, but the lack of association with SCL suggests that, as hypothesised, volume of this structure is not as directly involved in the regulation of arousal.
Although our group with TBI did report lower affective empathy than the control group, this was limited to the BEES. This reduction in self-reported empathy is likely to be valid. Numerous studies have shown consistency in the prevalence of self-reported empathy deficits across groups of people with severe TBI. Furthermore, the ability of individuals with even severe TBI to accurately reflect upon emotional changes has been established empirically (Kinsella et al., 1988). Despite differences on the BEES the group with TBI did not differ from controls for the IRI affective empathy subscales. This pattern may suggest that the BEES is more sensitive to the presence of TBI than is the IRI. Certainly, the BEES has been found to discriminate people with severe TBI from matched controls in several studies apart from this one (de Sousa et al., 2010, de Sousa et al., 2011, Williams and Wood, 2010, Wood and Williams, 2008). Fewer studies have examined the IRI and, of those, at least one found no difference between people with severe TBI and controls on the IRI (Muller et al., 2010) while another found only marginal differences between groups on the IRI compared to the BEES (de Sousa et al., 2010). Despite this, the BEES was positively correlated with the IRI empathic-concern subscale (ECS). The personal distress subscale, PDS was correlated to neither the BEES nor the ECS. It has been argued that some items on the PDS (e.g. “In emergency situations, I feel apprehensive and ill-at-ease”) suggest it is less sensitive to empathy than it is to emotional regulation (Baron-Cohen and Wheelwright, 2004) and this may be a reason for its apparent lack of association with the other measures.
We predicted that volume of all three structures (amygdala, thalamus and hippocampus) would correlate with affective empathy measures, albeit for different reasons. Again partial support was found. The amygdalae volume, a structure that is known to be critical in affective and autonomic responses, correlated with affective empathy as measured by the IRI and this relationship was positive for the ECS and negative for the PDS. Additionally, the hippocampus, a structure hypothesised to be critical in the cognitive appraisal of empathy (such as reflecting on one's empathic abilities) was also correlated. Specifically, hippocampal volume (right) was positively correlated with both the BEES and the ECS. This finding of a right lateralised contribution of the hippocampus is interesting in that it accords with results conducted in people with different dementia diagnoses, in which atrophy of the right temporal pole, right inferior frontal gyrus and right caudate nucleus (although interestingly not the amygdala) were all correlated with low scores on the ECS subscale of the IRI (Rankin et al., 2006). Similar patterns of right temporal involvement have been reported in other cases of frontotemporal dementia (Perry et al., 2001). The positive relationships between empathy and volume in the current study were found for both participants with TBI and the healthy control group. These findings suggest that lower volume in pertinent brain structures is associated with lower empathy, and this outcome is irrespective of a known brain injury.
While the majority of correlations indicated that increased empathy was associated with larger volume in specific structures, an inverse relationship was found for the PDS. In this case, lower scores were associated with increasing amygdalae volume bilaterally. Previous research has suggested that high scores for the PDS are associated with social dysfunction (Davis, 1983, Eisenberg and Fabes, 1990). The current research indicates that higher personal distress is related to smaller volume, but only in healthy adults, i.e. as can be seen in Fig. 4 larger amygdale volume was associated with lower PDS score for the controls, but almost no effect was found for the TBI participants. Given the high rates of comorbidity in anxiety and depression in adults with severe TBI, especially in the chronic stages (Alway et al., 2016), it may well be that the personal distress/emotion regulation subscale of the IRI is tapping into a variety of psychological adjustment issues in the TBI group that overlay individual differences in emotion regulation and/or empathy as measured by the PDS.
Our hypotheses were partly determined by a dual stream model of emotion perception (Phillips et al., 2003). In partial agreement with expectations, volume of the ventral stream structures examined (amygdala and thalamus) were directly associated with the regulation of arousal, whereas hippocampus volume only reflected alpha magnitude, further supporting its role in the “regulatory” dorsal stream. In regards to relationships with self-reported empathy however, a role was shown for amygdala and hippocampus volumes, but not for the thalamus. It is possible that this lack of association reflects a specific role for the thalamus in empathic processes that is less readily captured in empathy self-report scales compared to functions mediated by the other structures examined. For example, while thalamic volume was associated with SCL (arousal) in this study, it also plays a central role in the on-line processing of incoming emotional sensory stimuli. Such processing may not be directly relevant to self-reported empathy, which taps into more general empathic experience.
It is important to emphasise that the dual streams are highly interactive and have reciprocal functional influences. Evidence of this reciprocity was found here, such that both amygdala and hippocampus volume were associated with affective empathy scores. Independence was also shown, such that only the amygdala showed an additional relationship with emotional-regulation. While the current findings are generally consistent with the interaction between autonomic arousal, empathy and dorsal and ventral structures as reviewed by Phillips et al. (2003), they do not preclude a role for the involvement of other structures of the limbic system (Carr et al., 2003). The current study focused on three key limbic structures that had a hypothesised role in both empathy and arousal. Other brain regions, including noted above (right temporal pole, right inferior frontal gyrus and right caudate nucleus, Rankin et al., 2006), have also been shown to mediate self-reported empathy. Damage to these structures are also likely amongst the TBI group examined here, and future examination of these pathways may untangle some of the anomalous findings noted in the current study. The current study was also limited by the MRI scan sequence. We collected two T1 sequences, primarily in order to increase the signal to noise ratio and the grey matter-white matter contrasts. However, focal lesions/abnormalities have the potential to distort automated segmentation. Future studies might consider collecting more detailed sequences such as susceptibility-weighted imaging (SWI) or fluid-attenuated inversion recovery (FLAIR), as these have been shown to be more sensitive in detecting abnormalities and larger size of lesions compared with other conventional sequences (Wilde et al., 2015).
In conclusion, the present study has provided new insights into empathic processes after TBI and their relationship to brain volume loss. Arousal and associated responsivity to emotional situations is an integral facet of empathy and are thought to be mediated by ventral structures such as the amygdalae and thalami. Other structures such as the hippocampi play a role in cognitive processing of affective experience. Our participants with TBI had reduced volume in bilateral hippocampi and thalamus as well as the right amygdala. Moreover, direct relationships between brain volume and arousal measures were found in the adults with TBI as well as normal healthy adults, suggesting a robust relationship. Lower self-reported affective empathy was also related to lower (left) amygdala and (right) hippocampal volume across groups, only deviating for the PDS scale, which appears not to reflect affective empathy per se. This pattern conforms to our expectation that an interactive system entailing both dorsal and medial structures supports different facets of empathic processing. Further it suggests that TBI can interfere with these systems in variable ways resulting in complex disorders of empathic processes.
Acknowledgements
We are grateful to the Ryde, Westmead and Liverpool Brain Injury Units in Sydney, Australia, for their assistance with recruitment. We are indebted to the people with traumatic brain injuries and their families, as well as our volunteers from the general community, who gave willingly of their time to make this research possible.
References
- Alway Y., Gould K.R., Johnston L., McKenzie D., Ponsford J. A prospective examination of Axis I psychiatric disorders in the first 5 years following moderate to severe traumatic brain injury. Psychol. Med. 2016;46:1331–1341. doi: 10.1017/S0033291715002986. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Barry R.J., Rushby J.A., Wallace M.J., Clarke A.R., Johnstone S.J., Ilinka Z. Caffeine effects on resting-state arousal. Clin. Neurophysiol. 2005;116:2693–2700. doi: 10.1016/j.clinph.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Barry R.J., Clarke A.R., Johnstone S.J., Magee C.A., Rushby J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Barry R.J., Clarke A.R., Johnstone S.J., Rushby J.A. Timing of caffeine's impact on autonomic and central nervous system measures: clarification of arousal effects. Biol. Psychol. 2008;77:304–346. doi: 10.1016/j.biopsycho.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Beadle J.N., Tranel D., Cohen N.J., Duff M.C. Empathy in hippocampal amnesia. Front. Psychol. 2013;4:1–12. doi: 10.3389/fpsyg.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
- Bigler E.D. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychol. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M.C., Maxzziotta J.C., Lenzi G.L. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. PNAS USA. 2003;100:5487–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. A multidimensional approach to individual differences in empathy. JSAS Catalog Psychol. 1980;10:85. [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113–126. [Google Scholar]
- de Sousa A., McDonald S., Rushby J., Li S., Dimoska A., James C. Why don't you feel how I feel? Insight into the absence of empathy after severe traumatic brain injury. Neuropsychologia. 2010;48:3585–3595. doi: 10.1016/j.neuropsychologia.2010.08.008. [DOI] [PubMed] [Google Scholar]
- de Sousa A., McDonald S., Rushby J., Li S., Dimoska A., James C. Understanding deficits in empathy after traumatic brain injury: the role of affective responsivity. Cortex. 2011;47:526–535. doi: 10.1016/j.cortex.2010.02.004. [DOI] [PubMed] [Google Scholar]
- de Sousa A., McDonald S., Rushby J. Changes in emotional empathy, affective responsivity and behaviour following severe traumatic brain injury. J. Clin. Exp. Neuropsychol. 2012;34:606–623. doi: 10.1080/13803395.2012.667067. [DOI] [PubMed] [Google Scholar]
- Detari L. Tonic and phasic influences of basal forebrain unit activity on the cortical EEG. Beh Brain Res. 2000;115:159–170. doi: 10.1016/s0166-4328(00)00256-4. [DOI] [PubMed] [Google Scholar]
- D'Orazio D.M. The journal's publication of research that incorrectly employs Davis' interpersonal reactivity index. Sex. Abus. 2004;16:173–174. doi: 10.1177/107906320401600207. [DOI] [PubMed] [Google Scholar]
- Driscoll D.M., Dal Monte O., Solomon J., Krueger F., Grafman J. Empathic deficits in combat veterans with traumatic brain injury: a voxel-based lesion-symptom mapping study. Cog Beh Neurol. 2012;25:160–166. doi: 10.1097/WNN.0b013e318280cf4e. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A. Empathy: conceptualization, measurement, and relation to prosocial behavior. Motiv. Emot. 1990;14:131–149. [Google Scholar]
- Fearing M.A., Bigler E.D., Wilde E.A., Johnson J.L., Hunter J.V., Li X., Hanten G., Levin H.S. Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. J. Child Neurol. 2008;23:729–737. doi: 10.1177/0883073808314159. [DOI] [PubMed] [Google Scholar]
- Fisher A.C., Rushby J.A., McDonald S., Parks N., Piguet O. Neurophysiological correlates of dysregulated emotional arousal in severe traumatic brain injury. Clin. Neurophysiol. 2015;126:314–324. doi: 10.1016/j.clinph.2014.05.033. [DOI] [PubMed] [Google Scholar]
- Glickman M.E., Rao S.R., Schyltz M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Kinsella G., Moran C., Ford B., Ponsford J. Emotional disorder and its assessment within the severe head injured population. Psychol. Med. 1988;18:57–63. doi: 10.1017/s0033291700001884. [DOI] [PubMed] [Google Scholar]
- Kumaran D., Maguire E.A. The human hippocampus: cognitive maps or relational memory? J. Neurosci. 2005;25:7254–7259. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C.L., Davidson R.J., Abercrombie H.C., Ward R.T., Schaefer S.M., Jackson D.C., Holden J.E., Perlman S.B. Relations between PET-derived measures of thalamic glucose metabolism and EEG alpha power. Psychophysiol. 1998;35:162–169. [PubMed] [Google Scholar]
- Leigh R., Oishi K., Hsu J., Lindquist M., Gottesman R.F., Jarso S., Crainiceanu C., Mori S., Hillis A.E. Acute lesions that impair affective empathy. Brain. 2013;136:2539–2549. doi: 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunissen I., Coxon J.P., Caeyenberghs K., Michiels K., Sunaert S., Swinnen S.P. Subcortical volume analysis in traumatic brain injury: the importance of the fronto-striato-thalamic circuit in task switching. Cortex. 2014;51:67–81. doi: 10.1016/j.cortex.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Little D.M., Kraus M.F., Joseph J., Geary E.K., Susmaras T., Zhou X.J., Pliskin N., Gorelick P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian A. 2000. Manual for the Balanced Emotional Empathy Scale (BEES) (Available From Albert Mehrabian, 1130 Alta Mesa Road, Monterey, CA 93940) [Google Scholar]
- Muller F., Simion A., Reviriego E., Galera C., Mazaux J.M., Barat M., Joseph P.A. Exploring theory of mind after severe traumatic brain injury. Cortex. 2010;46:1088–1099. doi: 10.1016/j.cortex.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Neumann D., Zupan B., Babbage D.R., Radnovich A.J., Tomita M., Hammond F., Willer B. Affect recognition, empathy, and dysosmia after traumatic brain injury. Arch. Phys. Med. Rehabil. 2012;93:1414–1420. doi: 10.1016/j.apmr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Newsome M.R., Scheibel R.S., Mayer A.R., Chu Z.D., Wilde E.A., Hanten G., Steinberg J.L., Lin X., Merkley T.L., Hunter J.V., Vasquez A.C., Cook L., Lu H., Vinton K., Levin H.S. How functional connectivity between emotion regulation structures can be disrupted: preliminary evidence from adolescents with moderate to severe traumatic brain injury. J. Int. Neuropsychol. Soc. 2013;19:911–924. doi: 10.1017/S1355617713000817. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Rosen H.R., Kramer J.H., Beer J.S., Levenson R.L., Miller B.L. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–160. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M.L., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. Structual anatomy of empathy in frontolobar disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R.D., Watson P.D., Duff M.C., Cohen N.J. The role of the hippocampus in flexible cognition and social behaviour. Front. Hum. Neurosci. 2014;8:1–15. doi: 10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushby J.A., McDonald S., Randall R., de Sousa A., Trimmer E., Fisher A. Impaired emotional contagion following severe traumatic brain injury. Int. J. Psychophysiol. 2013;89:466–474. doi: 10.1016/j.ijpsycho.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Rushby J.A., Fisher A.C., McDonald S., Murphy A., Finnigan S. Autonomic and neural correlates of dysregulated arousal in severe traumatic brain injury. Int. J. Psychophysiol. 2013;89:460–465. doi: 10.1016/j.ijpsycho.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Schnell K., Bluschke S., Konradt B., Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. NeuroImage. 2011;54:1743–1754. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A., De Stefano N. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister, De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neurosci. 2006:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S. 6th ed. Allyn and Bacon; New York: 2012. Using Multivariate Statistics. [Google Scholar]
- Tate D.F., Bigler E.D. Fornix and hippocampal atrophy in traumatic brain injury. Learn. Mem. 2000;7:442–446. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Bouix S., Tate D., Lin A.P., Newsome M.R., Taylor B.A., Stone J.R., Montier J., Gandy S.E., Biekman B., Shenton M.E., York Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: state of the art and potential benefits. Brain Imaging Behav. 2015;9:367–402. doi: 10.1007/s11682-015-9444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C., Wood R.L. Alexithymia and emotional empathy following traumatic brain injury. J. Clin. Exp. Neuropsychol. 2010;32:259–267. doi: 10.1080/13803390902976940. [DOI] [PubMed] [Google Scholar]
- Williams L.M., Phillips M.L., Brammer M.J., Skerrett D., Lagopoulos J., Rennie C., Gordon. E. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance responses. NeuroImage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Wood R.L., Williams C. Inability to empathize following traumatic brain injury. J. Int. Neuropsychol. Soc. 2008;14:289–296. doi: 10.1017/S1355617708080326. [DOI] [PubMed] [Google Scholar]