Abstract
Pluripotent stem cells (PSC) are promising resources for regeneration therapy, but teratoma formation is one of the critical problems for safe clinical application. After differentiation, the precise detection and subsequent elimination of undifferentiated PSC is essential for teratoma-free stem cell therapy, but a practical procedure is yet to be developed. CDy1, a PSC specific fluorescent probe, was investigated for the generation of reactive oxygen species (ROS) and demonstrated to induce selective death of PSC upon visible light irradiation. Importantly, the CDy1 and/or light irradiation did not negatively affect differentiated endothelial cells. The photodynamic treatment of PSC with CDy1 and visible light irradiation confirmed the inhibition of teratoma formation in mice, and suggests a promising new approach to safe PSC-based cell therapy.
Short abstract
Stain, shine, and kill: CDy1 selectively stains pluripotent stem cells and, upon shining visible light, kills only stem cells without affecting differentiated cells, opening the possibility of teratoma-free stem cell therapy.
Introduction
Human pluripotent stem cells (hPSC) have captured great interest as promising resources for regeneration therapy due to their pluripotency. Recently, hPSC-based clinical trials toward macular degeneration have been successfully performed in a number of research groups.1,2 Despite such advances, substantial challenges have remained for the wide clinical application of hPSC-based cell therapy. Among them, risk of teratoma formation arisen from the residual undifferentiated PSC should be resolved to secure the safety of the treatment.
Therefore, an efficient detection and consequent ablation of remaining PSC are holy grail research goals in the stem cell therapy field.3 To this end, chemical fluorescence probe4,5 and electrochemical biosensor6 have been reported for selective PSC detection. Also, ablation of PSC has been investigated using chemical,7,8 genetic,9,10 and antibody-based approaches.11,12 However, none of the known approaches can satisfy both selective detection and elimination of PSC simultaneously. Previously, we have demonstrated that mitochondrial ROS production by photoactivating gene expression was able to induce selective cell death of PSC.9 In parallel, we developed the first PSC selective fluorescent probe, CDy1, through a diversity oriented fluorescence library approach (DOFLA) utilizing a structurally diverse rosamine library combined with unbiased cell-based screening.13
Herein, we report that CDy1 not only selectively stains PSC but also efficiently kills them through its photodynamic property, without affecting other differentiated cells, opening up the possibility of a practical stem cell therapy (Figure 1A).
Figure 1.
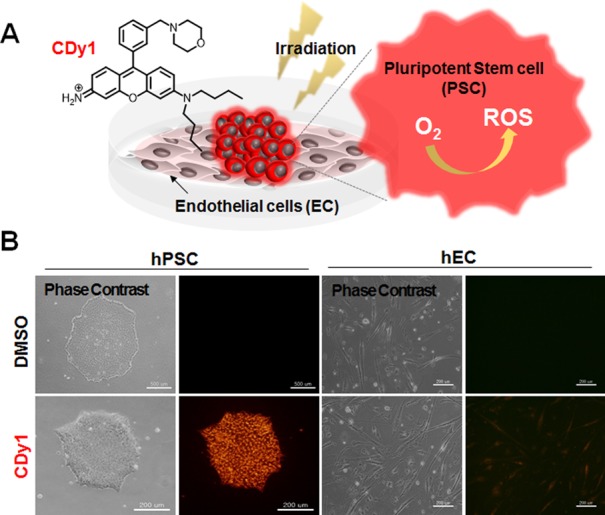
(A) Structure of CDy1 and concept of selective PSC death by photodynamic effect of CDy1. (B) Phase contrast and fluorescence microscopic images of hPSC (left) and hEC (right) in the absence (upper panel) and presence (lower panel) of 50 nM of CDy1. U-MWIG2 filter (ex 535 nm/580 nm) was used for fluorescence images.
Results and Discussion
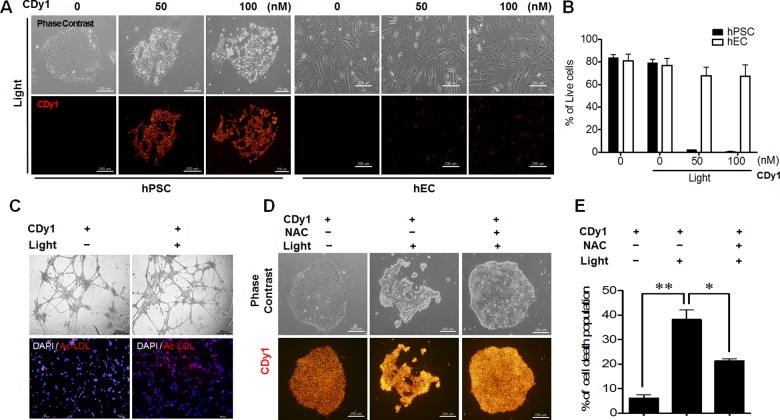
For this study, CDy1 (λex = 535, λem = 570) was first tested for selective staining of embryonic stem cells as representative PSC, while negative staining in the endothelial cells (EC) differentiated from the same PSC. The selective staining was confirmed both in human cells (hPSC vs hEC, Figure 1B and Figure S1A) and in mouse cells (mPSC vs mEC, Figure S1B,C) in the fluorescence imaging and flow cytometry analysis. The EC were carefully characterized as fully functional endothelial cells by expression of von Willebrand factor (vWF) and platelet EC adhesion molecule (PECAM), and also by acetylated low-density lipoprotein (Ac-LDL) uptake assay both in hEC and mEC (Figure S2).14 While the original report of CDy113 focused on the selective fluorescent labeling and safe isolation of PSC without affecting the biological function or viability of the cells, we carefully investigated the photodynamic property of CDy1. Interestingly, we found that a small amount of singlet oxygen can be generated by CDy1 upon irradiation of visible light with 1.1% of quantum yield (Figure S3) (with 76% of Rose Bengal as a reference).15 Although the singlet oxygen quantum yield of CDy1 was relatively low, the fact that CDy1 is localized in mitochondria,13 where cell death machinery is well developed in PSC,7,16,17 encouraged us to test CDy1 as a possible photodynamic agent for PSC. Given the fact that mitochondrial ROS production (e.g., oxidative stress) was a strong insult to induce cell death of PSC,9,18 we envisioned that the small amount of singlet oxygen generated by CDy1 selectively at the mitochondria may achieve PSC specific cell death without affecting other cells (Figure 1A). To this end, we irradiated CDy1 stained PSC with a green light (1.2 W/cm2, 532 nm, 1 min), which was previously proven to be safe to normal PSC and differentiated cells.9 Surprisingly, PSC underwent cell death in a dose dependent manner with CDy1 within 5 h after a single 1 min of exposure to green light, whereas CDy1 treated EC had no apparent alteration (Figure 2A). The phototoxic effects in CDy1-stained PSC were quantified by annexin V for apoptosis induction and 7-aminoactinomycin D (7-AAD) for membrane damage as double staining (Figure 2B). It is noteworthy that CDy1 itself did not induce noticeable morphological changes for both PSC and EC (Figure S4). For safe and efficient cell therapy, differentiated cells, such as EC, should remain fully functional after CDy1 treatment together with visual light exposure. In this regard, we examined whether light exposure following CDy1 staining affects the normal function of EC. The results showed that hEC remained fully functional in vitro regardless of light exposure as determined by in vitro tubule formation assay (Figure 2C, upper) and acetylated low-density lipoprotein (Ac-LDL) uptake assay (Figure 2C lower).19 With 100 nM CDy1 and 1 min of light exposure, not only hPSC but also mPSC were almost completely dead (Figure S5), while EC were not affected at all (Figure S6). The absolute amount of CDy1 difference in PSC and EC was measured by making cell extract, confirming good correlation with fluorescence imaging data (Figure S7).
Figure 2.
(A) CDy1-stained or nonstained hPSC (left) and hEC (right) exposed to 1.2 W/cm2 of green light (532 nm), 1 min (scale bar, 200 μm). CDy1 treated from zero to 100 nM concentration. (B) Live cell quantification by dual negative population of annexin V and 7-AAD after 5 h of 1 min irradiation. Mock are cells without CDy1 and irradiation. (C) Tubule formation (upper) and acetylated low-density lipoprotein (Ac-LDL, red) uptake assay of hEC 24 h after 1 min light exposure (scale bars, 100 μm). The results represent one of the experiments performed in triplicate. DAPI (blue) for nuclear counterstaining. (D) CDy1-stained hPSC were pretreated with 3 mM of NAC for 1 h prior to the light exposure. (E) Quantitative analysis data of cell death for images in panel D by annexin V staining.
Next, to study the mechanism of the cell death by photoactivated CDy1, a well-known antioxidant, N-acetyl cysteine (NAC), was pretreated prior to light exposure to the PSC in an attempt to block ROS-dependent cell death. The cell death was monitored by morphological analysis and flow cytometry analysis, demonstrating that NAC effectively rescued the cell death of CDy1-stained hPSC, determined by cellular morphology (Figure 2D) and annexin V analysis (Figure 2E). The direct measurement of ROS formation also confirmed the selective induction of ROS only in CDy1-stained PSC upon irradiation (Figure S8A). In a similar format, superoxide dismutase (SOD), a key redox enzyme, was tested for the expression level difference, but no obvious change was observed (Figure S8B). ROS reacts with unsaturated fatty acid to form reactive 4-hydroxynonenal (4-HNE), which can further form protein adducts. As CDy1 is a relatively hydrophobic dye, it may react with mitochondrial unsaturated lipid forming 4-HNE. When tested, a clear increase of 4-HNE–protein adducts was observed only in CDy1 light treated mPSC (Figure S9). The immunoblotting assay showed that NAC treatment in hPSC significantly reduced formation of active caspase 3 and Parp-1 cleavage, a typical event of apoptotic cell death (Figure S10). Therefore, it seems that ROS production itself, rather than redox status, is directly correlated to the cytotoxicity of photoactivated CDy1.
For further mechanistic understanding, we incorporated two MitoTracker dyes (orange and red, Figure S11A), which have the same chemical scaffold of rosamine with CDy1, for the phototoxic effect. The MitoTracker dyes stain PSC at a similar level as CDy1, but showed only mild cell deaths in PSC (Figure S11B,C). It is noteworthy that MitoTracker dyes also stain EC unlike CDy1, but did not show apparent phototoxicity. These data suggest that the CDy1’s selective PSC toxicity is, while partially due to mitochondrial localization, also due to its cell type specific staining property.
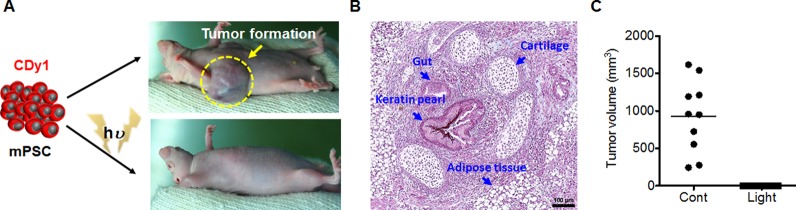
For practical teratoma-free cell therapy, it would be critical to confirm that ablation of PSC by the photodynamic effect of CDy1 is sufficient to inhibit teratoma formation in vivo. It is noteworthy that teratoma was developed after transplantation of cells differentiated from mPSC in a mouse model, even though there was no sign of existing leftover mPSC20 or even after cell surface marker antibody-based purification through cell sorting.21 In contrast, a number of mouse-model studies with hPSC indicated much lower efficiency of teratoma formation.14,22 For comparison, as few as 500 mPSC23 is enough, but at least 10,000 hPSC24 are required for teratoma formation in a mouse model. These results clearly imply that teratoma model of hPSC with a mouse would not be suitable for assessing teratoma risk. Thereby, we performed a mouse teratoma forming test with mPSC transplantation after photodynamic treatment with CDy1. CDy1-stained mPSC, with and without exposure to light, were subcutaneously injected into the mice, and teratoma formation was monitored. After 21 days, 100% (ten out of ten) mice developed teratomas (Figure 3A, right upper) with various sizes (Figure 3C and Figure S12), when injected with CDy1-stained mPSC, but without light exposure. According to typical three germ layer analysis (gut for endoderm, cartilage and adipose tissue for mesoderm, and keratin pearl for ectoderm), the teratoma formation was confirmed histologically (Figure 3B). These data support our previous results that CDy1 itself is nontoxic and harmless for the properties of the PSC.4,13 In contrast, none of the 10 mice showed any sign of teratoma formation, when injected with CDy1-stained mPSC after light exposure (Figure 3A lower). It is a clear contrast between two experiment sets with just 1 min of light exposure to small amount of CDy1 treatment (100 nM), albeit the low efficiency of singlet oxygen generation efficiency of CDy1. Once the small amount of ROS is enough to kill PSC, then it would also be beneficial to other non stem cells guaranteeing the lower side effect, which would be the basis of cell selectivity for the photodynamic effect of CDy1.
Figure 3.
(A) Introduction of CDy1-stained mPSC with or without light exposure through subcutaneous injection; images of teratoma developed from CDy1-stained mPSC with (0/10: zero out of ten mice) and without (10/10: ten out of ten mice) light exposure are shown. (B) Image of typical three germ layers from the teratoma stained with hematoxylin and eosin containing gut for endoderm, adipose tissue and cartilage for mesoderm, and keratin pearl for ectoderm (scale bar; 100 μm). (C) Size distribution of 10 teratomas measured in CDy1 treated mice with and without light exposed.
Taken together, we discovered that CDy1 produces strong enough ROS to induce selective PSC death by simple visible light irradiation, without affecting other differentiated cells. Most importantly, a single 1 min exposure of CDy1-stained PSC to visible light completely eliminated teratoma formation in mice. Dual functions of CDy1 not only as an imaging probe but also as a photodynamic agent for PSC will be extremely advantageous for biochemical studies and also for clinically applicable teratoma free stem cell therapy.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grants 2014R1A2A2A01005970 and 2011-0030043 and the Korea Health Industry Development Institute (KHIDI) Grant HI14C3365 and funded by the Korea government and A*STAR (Agency for Science, Technology and Research, Singapore) Biomedical Research Council and the Joint Council Office (JCO) Development Programme, A*STAR (1334k00083).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.6b00099.
Materials, methods, and additional data (PDF)
The authors declare the following competing financial interest(s): Young-Tae Chang and Nam-Young Kang are inventors for CDy1 patent, and CDy1 is licensed to Activemotif for commercialization.
Supplementary Material
References
- Schwartz S. D.; Regillo C. D.; Lam B. L.; Eliott D.; Rosenfeld P. J.; Gregori N. Z.; Hubschman J. P.; Davis J. L.; Heilwell G.; Spirn M.; Maguire J.; Gay R.; Bateman J.; Ostrick R. M.; Morris D.; Vincent M.; Anglade E.; Del Priore L. V.; Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Song W. K.; Park K. M.; Kim H. J.; Lee J. H.; Choi J.; Chong S. Y.; Shim S. H.; Del Pri-ore L. V.; Lanza R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U.; Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- Kang N. Y.; Yun S. W.; Ha H. H.; Park S. J.; Chang Y. T. Embryonic and induced pluripotent stem cell staining and sorting with the live-cell fluorescence imaging probe CDy1. Nat. Protoc. 2011, 6, 1044–1052. 10.1038/nprot.2011.350. [DOI] [PubMed] [Google Scholar]
- Hirata N.; Nakagawa M.; Fujibayashi Y.; Yamauchi K.; Murata A.; Minami I.; Tomioka M.; Kondo T.; Kuo T. F.; Endo H.; Inoue H.; Sato S.; Ando S.; Kawazoe Y.; Aiba K.; Nagata K.; Kawase E.; Chang Y. T.; Suemori H.; Eto K.; Nakauchi H.; Yamanaka S.; Na-katsuji N.; Ueda K.; Uesugi M. A Chemical Probe that Labels Human Pluripotent Stem Cells. Cell Rep. 2014, 6, 1165–1174. 10.1016/j.celrep.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Yea C. H.; Jeong H. C.; Moon S. H.; Lee M. O.; Kim K. J.; Choi J. W.; Cha H. J. In situ label-free quantification of human pluripotent stem cells with electrochemical potential. Biomaterials 2016, 75, 250–259. 10.1016/j.biomaterials.2015.10.038. [DOI] [PubMed] [Google Scholar]
- Lee M. O.; Moon S. H.; Jeong H. C.; Yi J. Y.; Lee T. H.; Shim S. H.; Rhee Y. H.; Lee S. H.; Oh S. J.; Lee M. Y.; Han M. J.; Cho Y. S.; Chung H. M.; Kim K. S.; Cha H. J. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E3281–E3290. 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U.; Gan Q. F.; Golan-Lev T.; Arora P.; Yanuka O.; Oren Y. S.; Leikin-Frenkel A.; Graf R.; Garippa M.; Boehringer M.; Gro-mo G.; Benvenisty N. Selective Elimination of Human Pluripotent Stem Cells by an Oleate Synthesis Inhibitor Discovered in a High-Throughput Screen. Cell Stem Cell 2013, 12, 167–179. 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Cho S. J.; Kim S. Y.; Jeong H. C.; Cheong H.; Kim D.; Park S. J.; Choi J. J.; Kim H.; Chung H. M.; Moon S. H.; Cha H. J. Repair of Ischemic Injury by Pluripotent Stem Cell Based Cell Therapy without Teratoma through Selective Photosensitivity. Stem Cell Rep. 2015, 5, 1067–1680. 10.1016/j.stemcr.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu S.; Hoyos V.; Del Bufalo F.; Brenner M. K. An Inducible Caspase-9 Suicide Gene to Improve the Safety of Therapy Using Human Induced Pluripotent Stem Cells. Mol. Ther. 2015, 23, 1475–1485. 10.1038/mt.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.; Lee A. S.; Volkmer J. P.; Sahoo D.; Nag D.; Mosley A. R.; Inlay M. A.; Ardehali R.; Chavez S. L.; Pera R. R.; Behr B.; Wu J. C.; Weissman I. L.; Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat. Biotechnol. 2011, 29, 829–834. 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo A. B.; Tan H. L.; Ang S. N.; Fong W. J.; Chin A.; Lo J.; Zheng L.; Hentze H.; Philp R. J.; Oh S. K.; Yap M. Selection Against Undifferentiated Human Embryonic Stem Cells by a Cytotoxic Antibody Recognizing Podocalyxin-Like Protein-1. Stem Cells 2008, 26, 1454–1463. 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- Im C. N.; Kang N. Y.; Ha H. H.; Bi X.; Lee J. J.; Park S. J.; Lee S. Y.; Vendrell M.; Kim Y. K.; Lee J. S.; Li J.; Ahn Y. H.; Feng B.; Ng H. H.; Yun S. W.; Chang Y. T. A Fluorescent Rosamine Compound Selectively Stains Pluripotent Stem Cells. Angew. Chem., Int. Ed. 2010, 49, 7497–7500. 10.1002/anie.201002463. [DOI] [PubMed] [Google Scholar]
- Cho S. W.; Moon S. H.; Lee S. H.; Kang S. W.; Kim J.; Lim J. M.; Kim H. S.; Kim B. S.; Chung H. M. Improvement of Postnatal Neovascularization by Human Embryonic Stem Cell–Derived Endothelial-Like Cell Transplantation in a Mouse Model of Hindlimb Ischemia. Circulation 2007, 116, 2409–2419. 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- Boso G.; Ke D.; Korzh B.; Bouilloux J.; Lange N.; Zbinden H. Time-resolved singlet-oxygen luminescence detection with an efficient and practical semiconductor single-photon detector. Biomed. Opt. Express 2016, 7, 211–224. 10.1364/BOE.7.000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. C.; Guan X.; Ryan J. A.; Rivera A. G.; Mock C.; Agarwal V.; Letai A.; Lerou P. H.; Lahav G. High Mitochondrial Priming Sensitizes hESCs to DNA-Damage-Induced Apoptosis. Cell Stem Cell 2013, 13, 483–491. 10.1016/j.stem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru R.; Ga-ma V.; Fagan B. M.; Bower J. J.; Swahari V.; Pevny L. H.; Deshmukh M. Human Embryonic Stem Cells Have Constitutively Active Bax at the Golgi and Are Primed to Undergo Rapid Apoptosis. Mol. Cell 2012, 46, 573–583. 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M. K.; Song E. K.; Guo Y.; Ou X.; Mantel C.; Broxmeyer H. E. SIRT1 Regulates Apoptosis and Nanog Expression in Mouse Embryonic Stem Cells by Controlling p53 Subcellular Localization. Cell Stem Cell 2008, 2, 241–251. 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. O.; Song S. H.; Jung S.; Hur S.; Asahara T.; Kim H.; Kwon S. M.; Cha H. J. Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler., Thromb., Vasc. Biol. 2012, 32, 343–352. 10.1161/ATVBAHA.111.237651. [DOI] [PubMed] [Google Scholar]
- Fujikawa T.; Oh S. H.; Pi L.; Hatch H. M.; Shupe T.; Petersen B. E. Teratoma Formation Leads to Failure of Treatment for Type I Diabetes Using Embryonic Stem Cell-Derived Insulin-Producing Cells. Am. J. Pathol. 2005, 166, 1781–1791. 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold S.; Klein H.; Semkova I.; Addicks K.; Schraermeyer U. Neurally Selected Embryonic Stem Cells Induce Tumor Formation after Long-Term Survival following Engraftment into the Subretinal Space. Invest. Ophthalmol. Visual Sci. 2004, 45, 4251–4255. 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- Zhang S. C.; Wernig M.; Duncan I. D.; Brustle O.; Thomson J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133. 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Erdo F.; Buhrle C.; Blunk J.; Hoehn M.; Xia Y.; Fleischmann B.; Focking M.; Kustermann E.; Ko-lossov E.; Hescheler J.; Hossmann K. A.; Trapp T. J. Host-Dependent Tumorigenesis of Embryonic Stem Cell Transplantation in Experimental Stroke. J. Cereb. Blood Flow Metab. 2003, 23, 780–785. 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- Lee A. S.; Tang C.; Cao F.; Xie X.; Van der Bogt K.; Hwang A.; Connolly A. J.; Robbins R. C.; Wu J. C. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 2009, 8, 2608–2612. 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.