Abstract

Efficient delivery of drugs to living cells is still a major challenge. Currently, most methods rely on the endocytotic pathway resulting in low delivery efficiency due to limited endosomal escape and/or degradation in lysosomes. Here, we report a new method for direct drug delivery into the cytosol of live cells in vitro and invivo utilizing targeted membrane fusion between liposomes and live cells. A pair of complementary coiled-coil lipopeptides was embedded in the lipid bilayer of liposomes and cell membranes respectively, resulting in targeted membrane fusion with concomitant release of liposome encapsulated cargo including fluorescent dyes and the cytotoxic drug doxorubicin. Using a wide spectrum of endocytosis inhibitors and endosome trackers, we demonstrate that the major site of cargo release is at the plasma membrane. This method thus allows for the quick and efficient delivery of drugs and is expected to have many invitro, ex vivo, and invivo applications.
Short abstract
Coiled-coil mediated membrane fusion between liposomes and cells provides a platform for the quick and efficient delivery of molecules inside live cells.
Introduction
The plasma membrane is the protecting interface between cells and their surrounding environment. Uptake of nutrients occurs through this interface using specialized mechanisms such as endocytosis.1 Nutrients, or drugs for that matter, are frequently internalized into small transport vesicles called endosomes, which are derived from the cell membrane. For many medicines to become an active drug, they have to enter the cell’s cytosol. However, the detrimental environment inside these endosomes can result in degradation of the drug. To date, intracellular delivery of macromolecules is still a major challenge in research and therapeutic applications.2,3 It is therefore highly desirable to develop new alternative delivery methods that circumvent the endocytosis pathway. So far, all attempts in drug delivery using particles as carriers have been unsuccessful in avoiding this pathway,4 hence current efforts to develop ways of enhancing endosomal escape.5
Cell penetrating peptides (CPPs) have been studied extensively to achieve efficient uptake into the cytosol. However, the current view is that CPPs conjugated to large molecular weight cargo (e.g., liposomes) predominantly are internalized via endocytosis.6−8 Moreover, the positive charge of CPPs such as the Tat peptide9 leads to unfavorable interaction with blood components. Other transfection techniques have been devised, such as viral vectors10 and physical methods.2,11,12 These methods have their own limitations, including safety issues or their reliance to electrical fields or high pressure.
Fusion of lipid membranes is a vital process in biological systems, facilitating the efficient transport of molecules across membranes.13−15In vivo membrane fusion shows a broad variety, from synaptic to viral and extracellular fusion, and was found to be a highly regulated process, specific in time and place, which is achieved by a complex interplay of different functional proteins.16 For example, in the process of neuronal exocytosis, docking of transport vesicles to the target plasma membrane is mediated by the coiled-coil formation of complementary SNARE protein subunits on the opposing membranes.17 This forces the opposing membranes into close proximity, resulting ultimately in lipid mixing followed by pore formation and concomitant content transfer.
As a bottom-up approach, several synthetic models systems have been developed to mimic membrane fusion events, but in general these simple systems do not always recapitulate the basic characteristics of native membrane fusion.18−22 Furthermore, all these approaches were limited to liposome–liposome fusion studies and have not shown to induce fusion events in live cells, thereby limiting their use for future drug delivery purposes.
Inspired by the SNARE protein complex, our laboratory has developed a fully artificial membrane fusion system composed of a complementary pair of lipidated coiled-coil peptides enabling targeted liposome-liposome fusion.23 This model system possesses all the key characteristics of targeted membrane fusion similar to SNARE mediated fusion including lipid and content mixing in the absence of leakage (Figure 1A–B).24,25 In our membrane fusion system, coiled-coil forming peptides “E3” [(EIAALEK)3] and “K3” [(KIAALKE)3] were conjugated to a cholesterol moiety via a polyethylene glycol (PEG) spacer, yielding lipopeptides CPE3 and CPK3. The cholesterol moiety allows for the immediate insertion of the lipidated peptides into any phospholipid membrane. We demonstrated that plain membranes could become fusogenic by the spontaneous insertion of CPE3 and CPK3 in the bilayer. A follow-up study showed that CPK3 modified cells and zebrafish embryos could be specifically labeled with the complementary fluorescently labeled E3 peptide,23 revealing that E3/K3 coiled-coil formation is also functional in an in vivo environment, thereby paving the way for targeted delivery using peptide modified liposomes.
Figure 1.
Schematic representation of (A) coiled-coil structure between peptides E and K (adapted from PDB 1UOI), (B) targeted liposome fusion mediated by coiled-coil formation between CPE4 modified liposomes and CPK4 modified liposomes, (C) CD spectra of CPE4 modified liposomes, and CPK4 modified liposomes and a equimolar mixture thereof. The total lipid concentrations were 0.5 mM with 1 mol % of lipidated peptide in PBS. (D) Lipid mixing and content mixing between CPE4-liposomes and CPK4-liposomes. Fluorescence traces showing lipid mixing between E and K decorated liposomes, as measured through an increase in NBD fluorescence. Total lipid concentrations were 0.1 mM with 1 mol % of lipididated peptide, in PBS; fluorescence graphs indicating content mixing between sulphorhodamine loaded (20 mM), CPE4 decorated liposomes and nonfluorescent, CPK4 decorated liposomes. Total lipid concentrations were 0.25 mM with 1 mol % lipidated peptide in PBS. (E) Scheme of fusion between cell and liposomes.
Here, we report a new drug delivery method based on targeted membrane fusion between liposomes and live cells. We demonstrate that a wide range of cell lines can be specifically modified with lipopeptide CPK4, and upon addition of CPE4 decorated liposomes membrane fusion occurs with concomitant efficient cytosolic delivery of a variety of compounds such as fluorescent dyes propidium iodide (PI), TOPRO3, and the cytotoxic drug doxorubicin (DOX). The mechanism of content uptake was studied using endocytosis inhibitors and endosome trackers in order to prove that the major site of cargo release into the cells is indeed at the plasma membrane due to liposome-cell fusion. Additionally, we show cytosolic dye (and drug) delivery in vivo using zebrafish embryos. Our method thus allows for quick and efficient delivery of drugs and bio(macromolecules) without cell damage and is expected to have many applications in vitro, ex vivo, and in vivo.
Results and Discussion
Coiled-Coil Formation between CPE4 and CPK4
Previously, we reported docking of liposomes at cell membranes using peptides CPE3 and CPK3, but membrane fusion was not observed.23 In the present study we increased the number of heptad repeats in CPE and CPK to four thereby enhancing coiled-coil stability,26 expecting that this would favor liposome-cell fusion. Figure 1C shows that the cholesterol- and PEG-modified E4 and K4—hereafter called lipopeptides CPE4 and CPK4—when attached to liposomes, are capable of coiled-coil formation as evident from circular dichroism (CD) spectroscopy, in agreement with previous experiments using CPE3 and CPK3. Next, lipid mixing experiments were performed to investigate the fusogenicity of the CPE4/CPK4 pair in a liposome–liposome assay. In these experiments a fluorescence resonance energy transfer (FRET)-pair consisting of nitrobenzoxadiazole (NBD) and lissamine rhodamine (LR) fluorophore labeled lipids was incorporated into the membrane of CPK-decorated liposomes.21 Upon lipid mixing of the latter liposomes with CPE4-liposomes the distance between NBD and LR increased, resulting in increased NBD-fluorescence as shown in Figure 1D. Content mixing was quantified by incorporating a sulforhodamine B at a self-quenching concentration of 20 mM into CPE-decorated liposomes and mixing these with CPK-liposomes as described.23 The increase in sulforhodamine B fluorescence over time indicated that full fusion took place between CPE4 and CPK4-liposomes (Figure 1D). Control experiments verified that the increase in sulforhodamine B fluorescence was not caused by leakage during fusion (Supplementary Figure 1).
Coiled-Coil Formation Triggers Liposome-Cell Fusion
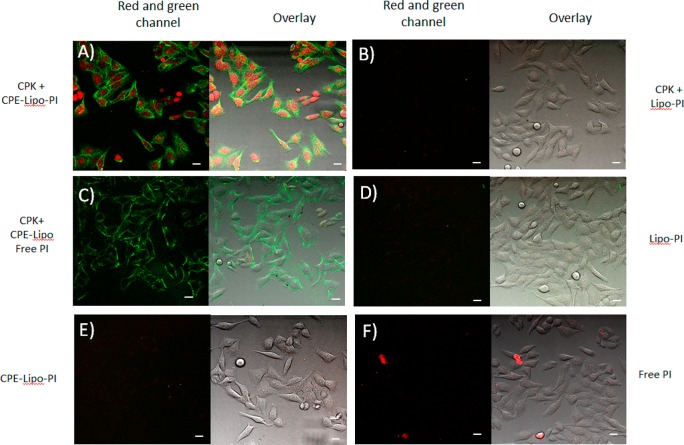
Next we investigated whether CPE4 and CPK4 could also mediate membrane fusion between liposomes and living cells. To this end, HeLa cells were preincubated with a micellar solution of CPK4 for 0.5–2 h before CPE4-decorated liposomes (lipid composition DOPC/DOPE/CH, 50:25:25 mol %) containing the nucleic acid stain propidium iodide (PI) or TOPRO3 in their aqueous interior were added as schematically shown in Figure 1E. In order to localize the lipid bilayer, these liposomes also contained 1 mol % of green-fluorescent NBD-DOPE lipids. As expected, confocal microscopy showed that cell membranes became labeled with the green NBD-dye on their outside in line with previous studies.23 Strikingly, the red dye was observed in the cytosol and nucleus, indicating that membrane fusion and content release had occurred (Figure 2A and Supplementary Figure 2A for TOPRO). Control experiments in which one of the two lipopeptides was omitted showed neither uptake of PI or TOPRO3 nor NBD-labeling of the cell plasma membrane (Figure 2B,C,E and Supplementary Figure 2). We note that when CPK-treated cells were incubated with empty CPE4-decorated liposomes in the presence of free dye only a weak fluorescent signal was observed inside cells (Figure 2C and Supplementary Figure 2C). This control experiment rules out the possibility that residual nonencapsulated dye in our liposome preparation entered the cell by transient membrane destabilization during fusion events. Finally cell incubation with free dyes also did not show any signal of the dye inside the cells (Figure 2F and Supplementary Figure 2F). Similar to CPE4 decorated liposomes, we also used CPK4 decorated liposomes containing PI and incubated these with CPE4 pretreated HeLa cells. However, the delivery of PI was less efficient. A reason might be the asymmetric nature of the fusion system. It was recently shown that peptide E does not interact with a membrane. In contrast, peptide K does interact with the membrane in a so-called snorkeling mode, and this peptide–membrane interaction is in equilibrium with either peptide K homocoiling or E/K coiled-coil formation.27,28 These studies suggest that peptide K-membrane interactions result in increased membrane curvature supporting membrane fusion. A cell membrane is more complex in composition and therefore less susceptible to undergo fusion as compared to the fusogenic liposomes (DOPC/DOPE/CH 2:1:1) used in this study. Our current thought is that peptide K needs to be on the cell membrane prior to a fusion event in order to activate the complex cell membrane by inducing membrane curvature.29 However, more studies are required to support this hypothesis.
Figure 2.
Delivery of PI by peptidated-liposomes is dependent on coiled-coil formation between CPK and CPE. Confocal microscopy images of Hela cells. Cells were preincubated with CPK (A, B, C) or medium (D, E, F) for 2 h, followed by treatment with CPE-decorated liposomes containing PI (A, E), liposomes containing PI (B, D), CPE-decorated liposomes plus free PI (C), or free PI (F). Green: NBD, red: PI. Scale bar is 25 μm. Overlay is red and green channel plus bright field image.
To exclude the possibility that peptide-mediated liposomal dye delivery was a peculiarity of HeLa cells, the membrane fusion experiments were repeated with Chinese hamster ovary (CHO) and mouse fibroblast (NIH/3T3) cell lines. Again the appearance of TOPRO3 and PI was observed inside cells suggesting that the peptide-mediated delivery of the dye is cell type independent (Supplementary Figures 3 and 4). Importantly, we found that uveal melanoma cells (Mel270), which are generally hard to transfect,30,31 could also be modified with TOPRO3 using this method (Supplementary Figure 3D).
To address the potential toxicity of CPK4, CPE4, and liposomes toward CHO, NIH/3T3, and HeLa cells cell viability assays were carried out. These assays indicated that lipopeptides CPE4 and CPK4 and liposomes, with or without CPE4, at the concentrations used throughout this study are well tolerated by all cell lines (Supplementary Figure 5A). Higher concentrations of these lipopeptides, even up to 100 μM, did not significantly reduce cell viability when exposed for 2 h but only did so after 24 h of exposure (Supplementary Figure 5B,C).
Altogether, these results show that coiled-coil formation between CPK4 and CPE4 is critical for fusion and release of the dyes and that these compounds are not toxic for living cells at the concentrations used allowing to investigate potential applications and their uptake mechanism.
Delivery of Doxorubicin
Doxorubicin (DOX) is one of the mostly used drugs for cancer treatments in the clinic today but as a free drug has serious cardiotoxicity. DOX is a cell-permeable drug whose fluorescence is strongly enhanced upon binding to nucleic acids. Intercalation into DNA ultimately results in apoptosis.32 To test delivery of liposomal DOX, HeLa cells were preincubated with CPK4 and subsequently exposed to CPE4-decorated liposomes containing 5 μM DOX for 15 min. As can be seen in Figure 3a,b this resulted in strong nuclear (and cytosolic) fluorescence. Control experiments showed that DOX delivery is highly dependent on the presence of CPE4 and CPK4 (Supplementary Figure 6). To investigate cytotoxicity of liposomal delivered DOX, HeLa cells preincubated with CPK were exposed with increasing concentrations of DOX-loaded liposomes for 12 h. Cell viability was measured 24 h later. Figure 3c shows cell viability as a function of liposomal and free DOX. As expected, very low concentrations of free DOX (<1 μM) did not affect the viability of HeLa cells as passive crossing into cells is not efficient at this concentration. Importantly, in current treatments in the clinic the DOX concentration is up to 9 μM in the serum of patients. In contrast, liposomes loaded with 1 μM Dox did show a significant effect as the DOX uptake is significantly enhanced. Liposomally delivered DOX reduced cell viability at DOX concentrations as low as 0.1 nM with an IC50 of ∼0.01 μM, while free DOX did not affect cell viability at concentrations up to 1 μM (IC50 ∼ 5 μM). Control experiments in which either CPK4 or CPE4 was omitted showed 100-fold or higher IC50 values (Supplementary Figure 7). Thus, our peptide-mediated delivery of DOX can potentially reduce the dose of DOX needed for anticancer treatments thereby lowering the cardiotoxicity of DOX.33 The presented fusion mediated delivery approach is also promising for the delivery of other drugs or biomolecules like DNA or siRNA.
Figure 3.
Delivery of DOX into HeLa cells. (a) CPE/CPK mediated delivery of DOX into HeLa cells. Cells were treated with CPK for 1 h followed by incubation with 0.25 mM CPE-liposomes containing with DOX for 15 min. Images were taken after washing. (a) Bright field. (b) Fluorescence channel. The inset shows a magnified overlay image, revealing the presence of DOX in the nucleus. The concentration of DOX loaded into liposomes is 5 μM. Scale bar represents 25 μm. (c) Cytotoxicity of CPE/CPK delivered DOX and free DOX. HeLa cells were treated with CPK for 1 h and series of concentrations of CPE decorated liposomes containing DOX (blue line), or the same concentrations of free DOX (red line) for 12 h. After washing and incubation with medium for 24 h, cell viability was measured by a WST-1 assay.
Liposomes and Content Only Partially Colocalize with Endosomes
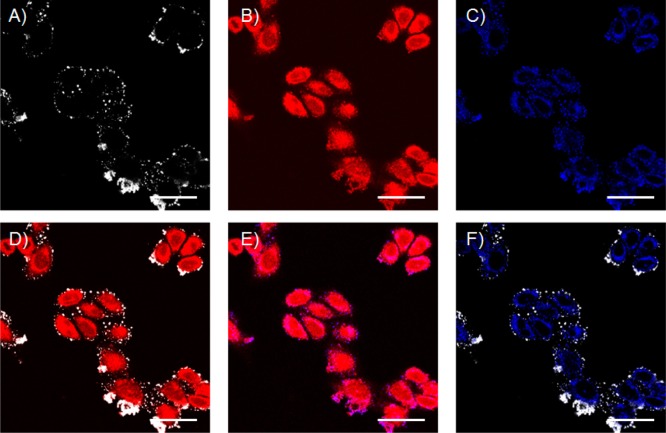
Endocytosis is the most common pathway for the uptake of small particles including liposomes by cells.4 To investigate whether endocytosis played a role in the liposomal delivery, the endosome tracker pHrodo, a fluorescently labeled dextran, was used in combination with TOPRO3 loaded liposomes. TOPRO3 was chosen as encapsulated dye for this experiment instead of PI because its emission (Ex/Em 642/661 nm) is expected not to interfere with emission of pHrodo (Ex/Em 560/585 nm) making investigation of colocalization of dyes easier. pHrodo and CPE-decorated liposomes containing 1 mol % NBD-DOPE and TOPRO3 were simultaneously added to CPK-modified HeLa cells. Confocal microscopy showed the presence of TOPRO3 in the cytosol and to a lesser extent in the nucleus (Figure 4B), while pHrodo was mainly observed as individual dots in the cytosol in agreement with its endosomal uptake (Figure 4C). Overlaying the fluorescent images of TOPRO3 and pHrodo revealed some overlap between TOPRO3 and endosomes (Figure 4E, pink dots), but the majority of TOPRO3 signal remains unmixed. Again, the signal from NBD-DOPE (Figure 4A, white dots) remained at the plasma membrane, although some overlap with pHrodo was observed at the plasma membrane (Figure 4F). This could be the result of both liposomes and endosome tracker binding at a common spot at the plasma membrane or could mean that some liposomes are initially taken up by endocytosis but then rapidly fuse with the endosomal membrane.
Figure 4.
Visualization of endosomes using an endosome tracker. CHO cells were treated with CPK for 2 h, followed by coincubation with pHrodo red dextran and CPE-decorated liposomes (0.25 mM total lipid concentration and 1 mol % CPE) loaded with TOPRO 3. (A) White channel showing DOPE-NBD liposomes. (B) Red channel (TOPRO3). (C) Blue channel (pHrodo). (D) Overlay of panels A and B. (E) Overlay of panels B and C. (F) Overlay of panels A and C. Scale bar is 25 μm.
These results suggest that the endosomal uptake pathway only plays a minor role in CPE4–CPK4 mediated liposomal uptake and that liposome-cell membrane fusion is the main route for cargo delivery. This is also illustrated by performing the same experiment at 4 °C, conditions under which active uptake by endocytosis is inhibited. Imaging of cells over a period of 3 h showed the increasing uptake of TOPRO3 (Figure 5A, upper panels). In contrast only a faint signal of endosome tracker pHrodo was observed after 3 h, indicating that endocytosis was severely limited at 4 °C (Figure 5A, lower panels). Quantification of the fluorescence intensity using software (ImageJ) showed that after 3 h the uptake of TOPRO3 reached ∼80% of the level obtained after 30 min at 37 °C (Figure 5B). The slower uptake is presumably caused by the reduced rate of liposome-cell fusion events at 4 °C. This is supported by the observation that liposome-liposome lipid mixing induced by CPE4/CPK4 is also significantly slower at 4 °C than at room temperature (Supplementary Figure 8).
Figure 5.
Investigation into the uptake mechanism. (A) Effect of low temperature incubation of HeLa cells on liposomal delivery of TOPRO3 and endosomal uptake of pHrodo. Cells were preincubated on ice with 5 μM CPK (2 h), followed by 15 min incubation with 0.25 mM CPE-decorated liposomes containing TOPRO3. After three washes confocal images were taken immediately (0 min) and after 60, 120, and 180 min. Top row: TOPRO3 (red), bottom row: pHrodo (blue). (B) Graphical representation of the percentage of TOPRO dye uptake by HeLa cells on ice. Fluorescence intensities were calculated by ImageJ and plotted as a percentage relative to the fluorescence of TOPRO3 delivery at 37 °C (100%). Scale bar is 25 μm. (C) Effect of endocytosis and macropinocytosis inhibitors on delivery of PI by liposomes to HeLa cells. Cells were incubated with medium (Ctrl+), or medium containing 0.25 μM wortmannin (Wor), 40 μM chlorpromazine (Chl), 200 μM genistein (Gen), 40 μM nocodazole (Noc) for 1 h, 0.01% w/v sodium azide (NaN3), followed by 2 h incubation with 5 μM CPK in the presence of inhibitors, and then treated for 15 min with CPE-liposomes containing PI. Final concentration of lipids (liposomes) was 0.25 mM. Cellular uptake was measured by flow cytometry. Positive control (100%): fluorescence of PI dye in the absence of inhibitors.
Endocytosis and Macropinocytosis Inhibitors Marginally Affect Delivery
As independent support for our conclusion that fusion at the plasma membrane is the major pathway for our liposome-based delivery system, several well-characterized inhibitors of endocytotic pathways were tested using flow cytometry measurements and confocal microscopy imaging. Wortmannin blocks PI3-kinase and inhibits macropinocytosis,34−37 chlorpromazine interferes with clathrin-dependent endocytosis,38−40 genistein inhibits tyrosine-phosphorylation of Cav 1 and caveola-dependent endocytosis.41−43 In addition, nocodazole, an inhibitor of microtubule formation, was used to investigate whether intracellular trafficking and internalization mechanisms are involved.36,37,40,44,45 Moreover, endocytosis of nanoparticles is an energy-dependent mechanism. Sodium azide was therefore used to deplete the energy needs for endocytosis and restrict metabolic activity.46,47
HeLa cells were first incubated for 1 h with each inhibitor at concentrations that have been reported by others to show optimal activity. After removal of the inhibitors, cells were treated with CPK and subsequently with CPE-decorated liposomes containing PI dye in the presence of freshly added inhibitors. FACS analysis showed that genistein and nocodazole had no adverse effect on the delivery of PI (Figure 5C), whereas in the presence of wortmannin, chlorpromazine and sodium azide PI uptake was reduced less than 20%. These results argue against a major role of endocytosis or pinocytosis in uptake of liposomal cargo and support that the dominant pathway for delivery is indeed targeted membrane fusion between liposomes with the plasma membrane of live cells.
Intracellular Delivery in Vivo
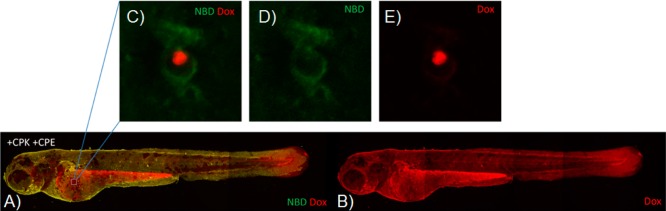
As a first step toward clinical application, we used zebrafish embryos to evaluate direct cytoplasmic delivery in vivo. We previously established coiled-coil mediated docking of liposomes onto the zebrafish embryonic skin.21 During embryonic stages, the zebrafish skin is composed of a layer of ridged, mucus-covered enveloping layer (EVL) cells. Through interspersed gaps in the EVL layer, cells within the underlying epidermal basal layer (EBL), including mucus-secreting cells and ionocytes, are exposed to the external environment.45 To test for in vivo delivery to skin epithelial cells, we exposed 48-h-old zebrafish embryos to CPK in embryo medium for 30 min. After washing, embryos were exposed to NBD-labeled, CPE-decorated liposomes containing DOX for 30 min. Consistent with previous results,21 we observed widespread liposome docking after 30 min of incubation, as evidenced by NBD and DOX colabeling. Importantly, we identified nuclear DOX labeling within a subset of skin epithelial cells (Figure 6) consistent with delivery into EBL-layer, but not EVL layer cells, which appeared to be inaccessible due to mucus covering or membrane ridging. Control experiments established that cytoplasmic delivery was specific to coiled-coil interaction (Supplementary Figure 9). We further confirmed intracellular delivery using liposomes loaded with PI, which becomes highly fluorescent only after interaction with cellular DNA or RNA (Supplementary Figures 10 and 11). Together, these results indicate the potential application of coiled-coil induced membrane fusion for direct cellular drug delivery in vivo.
Figure 6.
In vivo delivery of DOX using CPK and CPE. 2 dpf zebrafish were treated with CPK for 30 min, followed by 30 min incubation with CPE-decorated liposomes (0.25 mM total lipid concentration and 1 mol % CPE) loaded with DOX. (A, B) Whole-embryo imaging showing widespread DOX delivery in living zebrafish embryos (control experiments in Supplementary Figure S9). (C–E) Single zebrafish skin epithelial cell (from the indicated site of the embryo in (A, B) displaying membrane associated DOPE-NBD labeling (NBD) and predominantly nuclear DOX labeling.
Conclusions
Numerous methods exist to deliver drugs and (bio)macromolecules to living cells. Depending on the nature of these molecules they can be delivered into cells via electroporation, microinjection, calcium phosphate coprecipitation, nanoparticles, or viral particles. However, many of these methods are either not suitable for in vitro use or cannot be safely applied in in vivo applications, or are inefficient due to endosomal entrapment and degradation. The membrane fusion system described here involves the targeted fusion of liposomes with the plasma membrane of live cells. As a result, endosomal pathways are almost completely circumvented, and therefore this efficient drug delivery method is suited for labile (bio)molecules. In addition, the lipopeptides and modified liposomes have a low toxicity at the used concentration—in contrast to CPP-based delivery approaches or PEG-induced liposome fusion.48 We anticipate that this membrane fusion strategy will spark new in vitro, ex vivo research in the field of chemical biology and possibly in long term in vivo applications, enabling new basic and applied research studies for gene therapy. Moreover any compound that can be encapsulated in liposomes like hydrophilic low molecular weight drugs49 or DNA/siRNA50,51 could be considered as well as many hydrophilic drugs are unable to enter cells effectively and are known to be degraded in a lysosomal environment thereby lowering their therapeutic efficacy.52 Here, fusion mediated delivery could result in less degradation of sensitive molecules and might therefore find use as a new transfection agent in in vitro cell studies. Also lipid bilayer-coated nanoparticles53−57 might be delivered more efficiently when coiled-coil mediated membrane fusion is applied thereby increasing the scope of molecules and nanoparticles/nanomedicines that can be delivered into cells. Future in vivo application of this technique requires cells to be premodified with one of the two peptides and is currently not cell-type specific due to the cholesterol-anchor; several applications are still conceivable. These include topical administration of drugs to treat, e.g., pulmonary disease or combat respiratory infections like influenza. On the other hand, delivery of liposomally encapsulated mRNA or DNA coding for the tumor suppressor p53 will only affect tumor cells and leave healthy cells unharmed.58 Similarly, liposomal delivery of miRNA or siRNA to upregulate tumor suppressors or downregulate oncogenes could selectively kill only tumor cells.59
Finally, a certain degree of selectivity can be achieved using a light-induced membrane fusion system that was recently developed in our laboratory. This system makes use of photoinduced deshielding of a PEGylated CPE and thus allows potentially for spatiotemporal control of liposomal drug delivery in vivo.60
Materials and Methods
Fmoc-protected amino acids were purchased from Novabiochem, and Biosolve Sieber Amide resin was purchased from Chem-Impex International and Agilent Technologies. DOPE, DOPC, DOPE-NBD, and DOPE-LR were purchased from Avanti Polar Lipids. Cholesterol, propidium iodide (#BCBM1455V), and sulphorhodamine were obtained from Sigma-Aldrich. Topro3-Iodide (#1301286) and pHrodo Red dextran 10,000MW were purchased from Life Technologies. Eight wells slide Lab-tek was purchased from Thermo Scientific, USA. DMEM medium was obtained from Gibco, life technologies. N3-PEG4-COOH61 and 3-azido-5-cholestene62 were synthesized following literature procedures.
Lipopeptide Synthesis and Purification
The peptide components of CPK4 and CPE4, i.e., E4 (EIAALEK)4 and K4 (KIAALKE)4, were synthesized on an automatic CEM peptide synthesizer on a 250 μmol scale using Fmoc chemistry and standard solid-phase peptide synthesis protocols as previously described.23 After Fmoc deprotection N3-(ethylene glycol)4-COOH was coupled to the peptide on the resin. After azide reduction cholesteryl-4-amino-4-oxobutanoic acid was coupled to the PEG4 linker to yield the CPE4 and CPK4 peptides as described. The final products were purified by HPLC using a C4 column, and their identity was confirmed by LC-MS.
Liposome Preparation and Characterization
Lipids were dissolved in CHCl3 in the molar ratio DOPC, DOPE, cholesterol, and DOPE-NBD of 49.5:24.75:24.75:1 [total lipid concentration] = 1 mM. Peptide stock solutions of 50 μM were prepared in CHCl3/CH3OH (1:1 v/v). Liposomes were prepared by mixing the appropriate amount of lipids and CPE4 in a 20 mL glass vial and evaporating the solvents over air pressure to form lipid films. Traces of solvent were removed under high vacuum for 3–4 h at 25 °C. Each sample was then hydrated with 15 mM PI (Sigma Aldrich #BCBM1455V) or 0.25 mM Topro3 (Life Technologies, #1301286, after removing DMSO by freeze-drying) or FITC-dextran (35 mg/mL) in PBS buffer and sonicated for 2–3 min in a sonication bath at 55 °C. Nonencapsulated dyes or FITC-dextran were removed via Sephadex G25 or G50 size-exclusion PD-10 Columns (GE-Healthcare, USA). Liposomes were characterized by dynamic light scattering (DLS) at 25 °C to determine the average diameter (80–100 nm in general). The final concentration of lipids and CPE4 in each sample before cell treatments was 250 μM and 2.5 μM, respectively.
Doxorubicin (DOX) was entrapped as follows. The lipid film was hydrated with citrate buffer (pH 3.5) and sonicated in a sonication bath at 50 °C for 30 min. The citrate buffer was replaced by PBS (pH 7.4) through Sephadex G-25 filtration, leaving the inside of liposomes acidic. Doxorubicin powder (Sigma Aldrich #44538) was added into liposomal dispersion at a drug-to-lipid molar ratio of 1:3 and subsequently rotated at 4 °C overnight. Untrapped free DOX was separated from liposomes by size exclusion chromatography using a Sephadex G-25 column. The entrapment efficiency was determined using UV–vis spectrophotometry (see Supporting Information). Liposomes obtained were ∼120 nm in diameter with a PDI of <0.2.
A CPK4 stock solution (50 μM) was prepared in CHCl3/CH3OH (1:1). For a typical cell treatment the appropriate amount of CPK stock solution was taken, and the organic solvent was evaporated under air stream. After that it was hydrated by DMEM (± FCS, w/o phenol red) and sonicated at 55 °C for 1–2 min.
Cellular Uptake Assay and Confocal Microscopy Measurements
All incubations were done in complete medium without phenol red. Cells were grown in an 8-well slide at a density of 2.5 × 104 cells per well and incubated at 37 °C in 7% CO2 atmosphere. After 21 h, medium was removed and a CPK4 solution (5 μM) in medium was added and incubated for 0.5–2 h at 37 °C in 7% CO2. After removal of CPK4, cells were washed with medium and incubated with CPE4-decorated liposomes (250 μM) containing NBD, PI, TOPRO3. After 15 min incubation, cells were washed three times with medium, and fluorescent images were acquired on Leica TCS SP8 confocal laser scanning microscope. Leica application suite advanced fluorescence software (LAS AF, Leica Microsystems B.V., Rijswijk, The Netherlands) and ImageJ (developed by the National Institutes of Health) were used for image analysis and liposome colocalization studies. Wavelength settings for pHrodo Red dextran were Ex/Em: 560/585 nm (Ex laser: 488 nm), for Topro3 Ex/Em: 641/662 nm (Ex laser: 633 nm), for propidium iodide Ex/Em: 535/617 nm (Ex laser: 543 nm), for NBD-DOPE Ex/Em: 455/530 nm (Ex laser: 488 nm) and for DOX Ex/Em: 490/590 nm (Ex laser: 543 nm).
When performing cellular uptake assays on ice, an 8-well slide was placed on ice for 1 h, before adding CPK4. After 2 h on ice, CPK4 was removed and after washing CPE4-decorated liposomes loaded with TOPRO3 and endosome tracker were added simultaneously. After 15 min incubation on ice, cells were washed three times with ice-cold medium and imaged immediately (time point 0 h). After 1, 2, and 3 h the slide was transferred to the microscope and images were recorded. In between measurements the cells were kept on ice.
Cell Viability Assay
Cells were seeded in a 96 well-plate at a concentration of 1 × 104 cells per well and incubated for 24 h prior to the WST-1 assay. The medium was removed, and cells were incubated with 100 μL of CPK4 (5 μM) solution in medium (w/o phenol red) for 2 h. After 2 h CPK4 was removed by washing three times with medium, and the cells were incubated with liposomes containing 1 mol % CPE4 decorated liposomes for 15 min. In parallel cells were incubated with liposomes in the absence of lipopeptides. After these treatments, fresh medium was added to each well, and the plate was incubated at 37 °C for 24 h prior to the WST assay. After 24 h, medium was removed and 200 μL of cell proliferation reagent WST-1 (Serva, #140330 and PMS-Ome Santa Cruz Biotechnology, #D3013) in DMEM (w/o phenol red) was added to each well, and the plate was incubated for 3 h at 37 °C. After 3 h the absorbance at 450 nm was measured at room temperature using a Tecan infinite M1000 and a 96-well plate, which was shaken for 60 s prior to measurement (2 mm linearly, 654 rpm). The values for metabolic activity (cell survival) were normalized with respect to control (no liposomes), which was set at 100% cell survival.
For the DOX cell viability assay, Hela cells were incubated with CPK4 for 2 h and then treated with series of diluted CPE decorated liposomes loaded with DOX (stock lipid concentration was 1 mM, containing 1 mol % of CPE; DOX concentration was 0.25 mM), final concentration of DOX in liposomes were from 100 μM to 0.1 nM (100 μM, 50 μM, 25 μM, 10 μM, 5 μM, 2.5 μM, 1 μM, 0.1 μM, 0.05 μM, 0.01 μM, 1 nM, 0.5 nM, 0.1 nM). In parallel cells were incubated with liposomes in the absence of lipopeptides. After 12 h, all the medium was removed from the wells, and cells were incubated in fresh medium for 24 h prior to the WST assay.
Flow Cytometry Measurements
HeLa cells and NIH/3T3 cells were seeded in a 24-well plate at a density of 1 × 105 cells per well and incubated at 37 °C. After 21 h medium was removed and cells were incubated with 500 μL of nocodazole (40 μM), wortmannin (0.25 μM), chlorpromazine (40 μM), genistein (200 μM), or sodium azide 0.01% w/v in medium. After 1 h preincubation, inhibitors were removed, and the cells were treated with 500 μL of CPK4 5 (μM) for 2 h followed by addition of 500 μL of CPE4-liposomes containing PI (250 μM) in the presence of fresh inhibitors. After 15 min liposomes and inhibitors were removed and washing steps were performed. The cells were incubated at 37 °C for 1 h. Finally the cells were detached using PBS/EDTA for 15 min, centrifuged, and resuspended in fresh medium at a concentration of 200,000 cells/mL medium. The mean fluorescence intensity of the cells was measured by flow cytometry using a Beckman Coulter Quanta SC machine.
Zebrafish Embryo Assay
Zebrafish (Danio rerio) were handled in compliance with the local animal welfare regulations and maintained according to standard protocols (http://ZFIN.org). Embryos were treated with 0.16 mM 1-phenyl-2-thiourea from 24 h post fertilization (hpf) to prevent pigment formation. At 48 hpf, embryos were exposed in groups of 10 in 12-well plates to 5 μM CPK at 31 °C for 30 min; untreated embryos were used as controls. Next, embryos were washed and treated for 30 min with liposomes containing CPE, NBD-PE and DOX or PI (15 μM). Liposomes without CPE, or liposomes without PI or DOX, or with free PI or DOX added to the medium, were used as controls. After 3× washing in embryo medium, embryos were anesthetized in 0.02% tricaine methanesulfonate, mounted in 0.4% agarose, and imaged by confocal microscopy.
Acknowledgments
We thank Gerda Lamers and Kirsten Martens for their help with confocal microscopy and Hans de Dulk for the assistance with NIH/3T3 cell line stock. This work was funded primarily through a CSC scholarship (to J.Y.), the STW VENI fellowship (No. 12520), which is financed by The Netherlands Organization for Scientific Research (NWO) (to J.B.), the European Research Council via an ERC starting grant (Contract 240391) and an ERC Proof of Concept grant (to A.B and A.K.), and the NWO and FOM via a VICI grant (No. 724.014.001) and a programme grant (to A.K.).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.6b00172.
Full details of the methods (PDF)
Author Contributions
# J.Y. and A.B. contributed equally to this manuscript.
Author Contributions
A.K. designed the study and supervised the work. J.Y., A.B., and J.B. performed biological studies and synthesized compounds. G.D. aided in peptide synthesis. J.Y., A.B., J.B., R.C.L.O., and A.K. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Conner S. D.; Schmid S. L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Sharei A.; Zoldan J.; Adamo A.; Sim W. Y.; Cho N.; Jackson E.; Mao S.; Schneider S.; Han M. J.; Lytton-Jean A.; Basto P. A.; Jhunjhunwala S.; Lee J.; Heller D. A.; Kang J. W.; Hartoularos G. C.; Kim K. S.; Anderson D. G.; Langer R.; Jensen K. F. A vector-free microfluidic platform for intracellular delivery. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 2082–2087. 10.1073/pnas.1218705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup A.; Ai A.; Liu X.; Hamar P.; Trifonova R.; Charisse K.; Manoharan M.; Kirchhausen T.; Lieberman J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015, 33, 870–876. 10.1038/nbt.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E.; Madler L.; Velegol D.; Xia T.; Hoek E. M. V.; Somasundaran P.; Klaessig F.; Castranova V.; Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Vasir J. K.; Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Delivery Rev. 2007, 59, 718–728. 10.1016/j.addr.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock R. The uptake of arginine-rich cell-penetrating peptides: putting the puzzle together. Bioconjugate Chem. 2014, 25, 863–868. 10.1021/bc500017t. [DOI] [PubMed] [Google Scholar]
- Tunnemann G.; Martin R. M.; Haupt S.; Patsch C.; Edenhofer F.; Cardoso M. C. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. FASEB J. 2006, 20, 1775–1784. 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- Karagiannis E. D.; Urbanska A. M.; Sahay G.; Pelet J. M.; Jhunjhunwala S.; Langer R.; Anderson D. G. Rational design of a biomimetic cell penetrating peptide library. ACS Nano 2013, 7, 8616–8626. 10.1021/nn4027382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin V. P.; Rammohan R.; Weissig V.; Levchenko T. S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 8786–8791. 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S.; Herzog R. W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010, 17, 295–304. 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. J. Gene therapy progress and prospects: Electroporation and other physical methods. Gene Ther. 2004, 11, 1363–1369. 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- Boukany P. E.; Morss A.; Liao W. C.; Henslee B.; Jung H. C.; Zhang X. L.; Yu B.; Wang X. M.; Wu Y.; Li L.; Gao K. L.; Hu X.; Zhao X.; Hemminger O.; Lu W.; Lafyatis G. P.; Lee L. J. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat. Nanotechnol. 2011, 6, 747–754. 10.1038/nnano.2011.164. [DOI] [PubMed] [Google Scholar]
- Chen Y. A.; Scheller R. H. Snare-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2001, 2, 98–106. 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V.; Kozlov M. M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 675–683. 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P.; Bacaj T.; Yang X. F.; Pang Z. P. P.; Sudhof T. C. Lipid-anchored SNAREs lacking transmembrane regions fully support membrane fusion during neurotransmitter release. Neuron 2013, 80, 470–483. 10.1016/j.neuron.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R.; Lang T.; Südhof T. C. Membrane fusion. Cell 2003, 112, 519–533. 10.1016/S0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jahn R.; Scheller R. H. SNAREs - engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Sudhof T. C. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.; Bong D. Controlled fusion of synthetic lipid membrane vesicles. Acc. Chem. Res. 2013, 46, 2988–2997. 10.1021/ar400065m. [DOI] [PubMed] [Google Scholar]
- Pahler G.; Panse C.; Diederichsen U.; Janshoff A. Coiled-coil formation on lipid bilayers-implications for docking and fusion efficiency. Biophys. J. 2012, 103, 2295–2303. 10.1016/j.bpj.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.; Guha S.; Diederichsen U. SNARE protein analog-mediated membrane fusion. J. Pept. Sci. 2015, 21, 621–629. 10.1002/psc.2773. [DOI] [PubMed] [Google Scholar]
- Voskuhl J.; Ravoo B. J. Molecular recognition of bilayer vesicles. Chem. Soc. Rev. 2009, 38, 495–505. 10.1039/B803782P. [DOI] [PubMed] [Google Scholar]
- Zope H. R.; Versluis F.; Ordas A.; Voskuhl J.; Spaink H. P.; Kros A. In vitro and in vivo supramolecular modification of biomembranes using a lipidated coiled-coil motif. Angew. Chem., Int. Ed. 2013, 52, 14247–14251. 10.1002/anie.201306033. [DOI] [PubMed] [Google Scholar]
- Robson Marsden H.; Elbers N. A.; Bomans P. H. H.; Sommerdijk N. A. J. M.; Kros A. A reduced SNARE model for membrane fusion. Angew. Chem., Int. Ed. 2009, 48, 2330–2333. 10.1002/anie.200804493. [DOI] [PubMed] [Google Scholar]
- Robson Marsden H.; Korobko A. V.; Zheng T. T.; Voskuhl J.; Kros A. Controlled liposome fusion mediated by SNARE protein mimics. Biomater. Sci. 2013, 1, 1046–1054. 10.1039/c3bm60040h. [DOI] [PubMed] [Google Scholar]
- Zheng T.; Voskuhl J.; Versluis F.; Zope H. R.; Tomatsu I.; Marsden H. R.; Kros A. Controlling the rate of coiled coil driven membrane fusion. Chem. Commun. (Cambridge, U. K.) 2013, 49, 3649–3651. 10.1039/c3cc38926j. [DOI] [PubMed] [Google Scholar]
- Rabe M.; Zope H. R.; Kros A. Interplay between lipid interaction and homo-coiling of membrane-tethered coiled-coil peptides. Langmuir 2015, 31, 9953–9964. 10.1021/acs.langmuir.5b02094. [DOI] [PubMed] [Google Scholar]
- Rabe M.; Schwieger C.; Zope H. R.; Versluis F.; Kros A. Membrane interactions of fusogenic coiled-coil peptides: implications for lipopeptide mediated vesicle fusion. Langmuir 2014, 30, 7724–7735. 10.1021/la500987c. [DOI] [PubMed] [Google Scholar]
- McMahon H. T.; Kozlov M. M.; Martens S. Membrane Curvature in Synaptic Vesicle Fusion and Beyond. Cell 2010, 140, 601–605. 10.1016/j.cell.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Micka B.; Trojaneck B.; Niemitz S.; Lefterova P.; Kruopis S.; Huhn D.; Wittig B.; Schadendorf D.; Schmidt-Wolf I. G. Comparison of non-viral transfection methods in melanoma cell primary cultures. Cytokine+ 2000, 12, 828–833. 10.1006/cyto.1999.0621. [DOI] [PubMed] [Google Scholar]
- Nowak J.; Cohen E. P.; Graf L. H. Jr. Cytotoxic activity toward mouse melanoma following immunization of mice with transfected cells expressing a human melanoma-associated antigen. Cancer Immunol. Immunother. 1991, 33, 91–96. 10.1007/BF01742535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang W. J.; Xiong Y.; Wang Y.; Tong Q.; Yang G. S. Sirt1 attenuates camptothecin-induced apoptosis through caspase-3 pathway in porcine preadipocytes. Exp. Cell Res. 2013, 319, 670–683. 10.1016/j.yexcr.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Singal P. K.; Iliskovic N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- Arcaro A.; Wymann M. P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993, 296, 297–301. 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N.; Johnson M. T.; Swanson J. A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996, 135, 1249–1260. 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C. K.; Jones S. A.; Chen C.; Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic (Oxford, U. K.) 2007, 8, 389–401. 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola S.; Estrada L. C.; Digman M. A.; Pozzi D.; Cardarelli F.; Gratton E.; Caracciolo G. Intracellular trafficking of cationic liposome-DNA complexes in living cells. Soft Matter 2012, 8, 7919–7927. 10.1039/c2sm25532d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H.; Rothberg K. G.; Anderson R. G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117. 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff K.; Sanders N. N.; Vandenbroucke R.; De Smedt S. C.; Wagner E.; Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol. Ther. 2006, 14, 745–753. 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huth U. S.; Schubert R.; Peschka-Suss R. Investigating the uptake and intracellular fate of pH-sensitive liposomes by flow cytometry and spectral bio-imaging. J. Controlled Release 2006, 110, 490–504. 10.1016/j.jconrel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Gabrielson N. P.; Pack D. W. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J. Controlled Release 2009, 136, 54–61. 10.1016/j.jconrel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Akiyama T.; Ishida J.; Nakagawa S.; Ogawara H.; Watanabe S.; Itoh N.; Shibuya M.; Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [PubMed] [Google Scholar]
- Orlandi P. A.; Fishman P. H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998, 141, 905–915. 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J.; Oberle V.; Zuhorn I. S.; Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. 10.1042/bj20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves C.; Mennesson E.; Fuchs R.; Gorvel J. P.; Midoux P.; Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol. Ther. 2004, 10, 373–385. 10.1016/j.ymthe.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Simoes S.; Slepushkin V.; Duzgunes N.; Pedroso de Lima M. C. On the mechanisms of internalization and intracellular delivery mediated by pH-sensitive liposomes. Biochim. Biophys. Acta, Biomembr. 2001, 1515, 23–37. 10.1016/S0005-2736(01)00389-3. [DOI] [PubMed] [Google Scholar]
- Gao H.; Yang Z.; Zhang S.; Cao S.; Shen S.; Pang Z.; Jiang X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci. Rep. 2013, 3, 2534. 10.1038/srep02534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F.; Magnusson K. E.; Wojcieszyn J.; Hou Y.; Derzko Z.; Jacobson K. Use of lectins and polyethylene glycol for fusion of glycolipid-containing liposomes with eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 1981, 78, 1685–1689. 10.1073/pnas.78.3.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora N. L.; Bahreman A.; Valkenier H.; Li H. Y.; Sharp T. H.; Sheppard D. N.; Davis A. P.; Kros A. Targeted anion transporter delivery by coiled-coil driven membrane fusion. Chem. Sci. 2016, 7, 1768–1772. 10.1039/C5SC04282H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Blenke E. E.; van den Dikkenberg J.; van Kolck B.; Kros A.; Mastrobattista E. Coiled coil interactions for the targeting of liposomes for nucleic acid delivery. Nanoscale 2016, 8, 8955–8965. 10.1039/C6NR00711B. [DOI] [PubMed] [Google Scholar]
- Kanasty R.; Dorkin J. R.; Vegas A.; Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- Wittrup A.; Ai A.; Liu X.; Hamar P.; Trifonova R.; Charisse K.; Manoharan M.; Kirchhausen T.; Lieberman J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015, 33, 870. 10.1038/nbt.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. W.; Jiang X. M.; Ashley C.; Brinker C. J. Electrostatically mediated liposome fusion and lipid exchange with a nanoparticle-supported bilayer for control of surface charge, drug containment, and delivery. J. Am. Chem. Soc. 2009, 131, 7567–7569. 10.1021/ja902039y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. W.; Stace-Naughton A.; Jiang X. M.; Brinker C. J. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J. Am. Chem. Soc. 2009, 131, 1354–1355. 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. E.; Carnes E. C.; Epler K. E.; Padilla D. P.; Phillips G. K.; Castillo R. E.; Wilkinson D. C.; Wilkinson B. S.; Burgard C. A.; Kalinich R. M.; Townson J. L.; Chackerian B.; Willman C. L.; Peabody D. S.; Wharton W.; Brinker C. J. Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. ACS Nano 2012, 6, 2174–2188. 10.1021/nn204102q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. S.; Situ A.; Kang Y. A.; Villabroza K. R.; Liao Y. P.; Chang C. H.; Donahue T.; Nel A. E.; Meng H. Irinotecan delivery by lipid-coated mesoporous silica nanoparticles shows improved efficacy and safety over liposomes for pancreatic cancer. ACS Nano 2016, 10, 2702–2715. 10.1021/acsnano.5b07781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda V.; Engelke H.; Sauer A.; Arcizet D.; Brauchle C.; Radler J.; Bein T. Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Lett. 2010, 10, 2484–2492. 10.1021/nl100991w. [DOI] [PubMed] [Google Scholar]
- Wang K.; Huang Q.; Qiu F.; Sui M. Non-viral delivery systems for the application in p53 cancer gene therapy. Curr. Med. Chem. 2015, 22, 4118–4136. 10.2174/0929867322666151001121601. [DOI] [PubMed] [Google Scholar]
- Saito Y.; Nakaoka T.; Saito H. MicroRNA-34a as a therapeutic agent against human cancer. J. Clin. Med. 2015, 4, 1951–1959. 10.3390/jcm4111951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.; Askes S. H.; Bonnet S.; Kros A.; Campbell F. Temporal control of membrane fusion through photolabile PEGylation of liposome membranes. Angew. Chem., Int. Ed. 2016, 55, 1396–1400. 10.1002/anie.201509673. [DOI] [PubMed] [Google Scholar]
- Voskuhl J.; Wendeln C.; Versluis F.; Fritz E. C.; Roling O.; Zope H.; Schulz C.; Rinnen S.; Arlinghaus H. F.; Ravoo B. J.; Kros A. Immobilization of liposomes and vesicles on patterned surfaces by a peptide coiled-coil binding motif. Angew. Chem., Int. Ed. 2012, 51, 12616–12620. 10.1002/anie.201204836. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Cai S.; Peterson B. R. Practical synthesis of 3beta-amino-5-cholestene and related 3beta-halides involving i-steroid and retro-i-steroid rearrangements. Org. Lett. 2009, 11, 567–570. 10.1021/ol802343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.