Abstract
Cis,syndiotacticA-alt-B copolymers, where A and B are two enantiomerically pure trans-2,3-disubstituted-5,6-norbornenes with “opposite” chiralities, can be prepared with stereogenic-at-metal initiators of the type M(NR)(CHR′)(OR”)(pyrrolide). Formation of a high percentage of alternating AB copolymer linkages relies on an inversion of chirality at the metal with each propagating step and a relatively fast formation of an AB sequence as a consequence of a preferred diastereomeric relationship between the chirality at the metal and the chirality of the monomer. This approach to formation of an alternating AB copolymer contrasts dramatically with the principle of forming AB copolymers from achiral monomers and catalysts.
Short abstract
A racemic chiral olefin metathesis initiator will polymerize two enantiomerically pure, structurally similar norbornenes (A and B) that have “opposite” chiralities to yield cis,syndiotacticA-alt-B copolymers.
Introduction
Copolymers in which monomers A and B are incorporated in an alternating manner, poly(A-alt-B), are rare.1−5 Examples are alternating AB copolymers formed from CO and olefins or CO2 and epoxides. In these cases alternation is greatly assisted by the fact that one partner (CO or CO2) does not itself polymerize. A few alternating AB copolymers have been formed through ring-opening metathesis polymerization (ROMP) of cyclic olefin monomers,6−28 but in these circumstances both A and B usually can be homopolymerized and the stereochemistry of the C=C bond in the polymer is not fixed. One exception is the alternating AB copolymer which has all trans C=C bonds and <5% AA errors formed from a norbornene-like monomer (B) that is slow to homopolymerize and cyclooctene or cycloheptene (A). The most successful initiators are of the type Mo(NR)(CHCMe2Ph)[OCMe(CF3)2]2 (R = 2,6-Me2C6H3 or 2,6-i-Pr2C6H3), two well-defined alkylidene initiators that contain Mo or W out of many that have proven useful for preparing stereoregular polymers from norbornenes and norbornadienes.29−35
 |
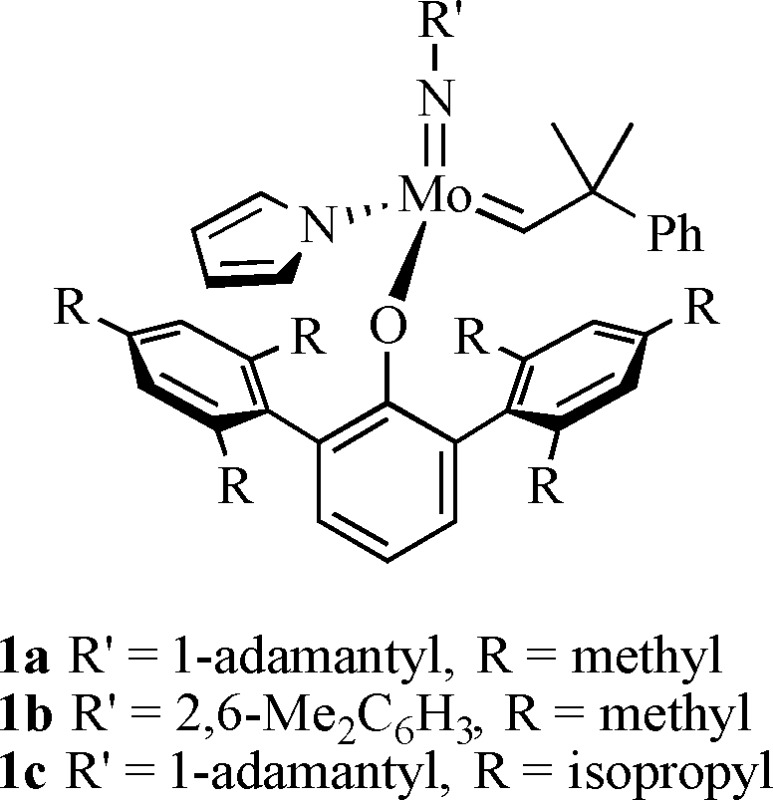
1 |
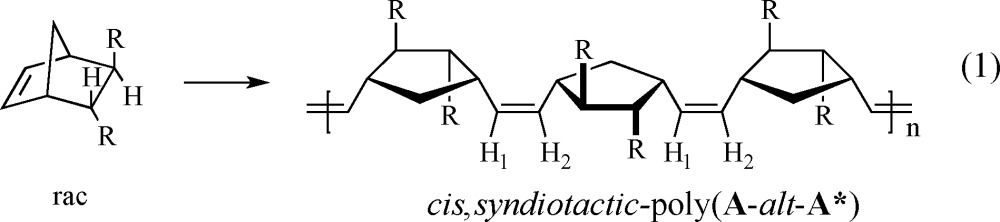
Among the well-defined Mo or W initiators are those that contain a stereogenic metal, e.g., 1a–1c. These initiators can produce a special category of stereoregular A-alt-B copolymers made from a racemic chiral monomer where A and B are enantiomers. These “A-alt-A*” copolymers have a basic cis, syndiotactic structure (eq 1), which is readily proven through 1H and 13C NMR studies.36,37 Only one example of a stereoregular A-alt-A* copolymer has been reported in the older literature.38,39 The cis structure is formed when 1a or 1b reacts with monomer to yield all cis metallacycles in trigonal bipyramidal (TBP) intermediates in which the terphenoxide and the imido ligands are in apical positions, while syndiotacticity results from an inversion of chirality at the metal center with each step in the polymerization. Inversion of chirality at the metal forces the olefin to approach first one side of the M=C bond and then the other. Incorporation of enantiomers in an alternating fashion is a consequence of one enantiomer of the racemic monomer reacting more rapidly with one enantiomer (at the metal center) of each propagating species. We have called this mode of control of polymer structure “stereogenic metal control”; although the chirality of the chain end nearest the metal that results from last inserted monomer is not necessarily irrelevant, the determining feature is the lowest energy diastereomeric combination of chirality at the metal and chirality of the monomer. The “errors” in the cis,syndiotactic-poly(A-alt-A*) structure arise through formation of AA and A*A*cis,syndiotactic and trans,isotactic dyads. Trans,isotactic dyads arise through formation of a trans metallacyclobutane intermediate (instead of a cis metallacyclobutane), which “flips over” before opening, a rearrangement that preserves the configuration at the metal and leads to a trans C=C linkage.37 This mechanistic proposal is based on the fact that polymerization of (+)-DCMNBE (DCMNBE = 2,3-dicarbomethoxynorbornene) by 1a yields a polymer that contains ∼75% trans,isotactic dyads and 25% cis,syndiotactic dyads, while 1c yields a polymer that contains ∼92% trans,isotactic dyads and ∼8% cis,syndiotactic dyads (Figure 1).37 The olefinic protons in trans,isotactic polymer are inequivalent and on the same C=C bond (and therefore coupled to each other with JHH ∼ 16 Hz37) while the olefinic protons in cis,syndiotactic polymer are inequivalent and on different C=C bonds (and therefore not coupled to each other). It was also shown that W(O)(CH-t-Bu)(OHMT)(Pyr)(PMe2Ph) polymerizes (+)-DCMNBE to give only cis,syndiotactic-poly[(+)-DCMNBE].
Figure 1.
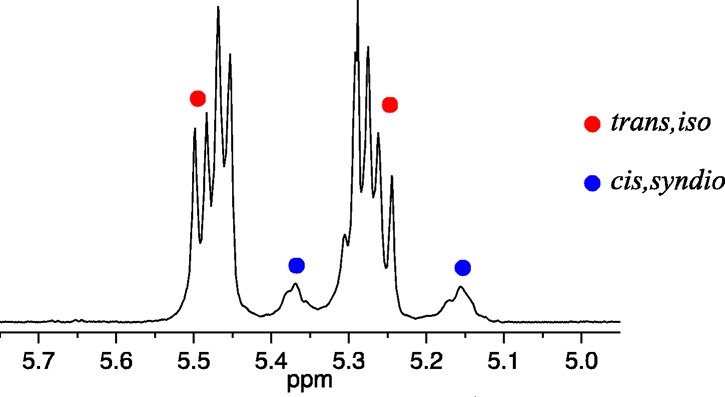
Olefinic region of the 1H NMR spectrum of the poly[(R,R)-DCMNBE] prepared from initiator 1c. Reprinted with permission from ref (37). Copyright 2012 American Chemical Society.
An interesting question is whether stereogenic metal control will direct formation of a copolymer where A and B are not strictly enantiomers, but have similar structures and reactivities toward homopolymerization, are enantiomerically pure, and have “opposite” chirality. If A and B are significantly different chemically, the resulting polymer could be further manipulated through selective reactions that involve one of the two components within the polymer. We show here that several such cis,syndiotacticA-alt-B copolymers can be prepared with Mo (primarily) and W alkylidene initiators.
Results and Discussion
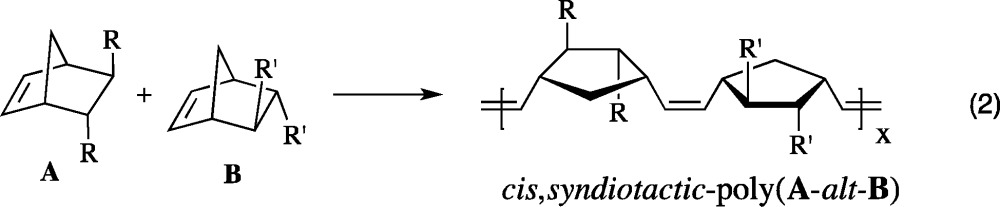
Initial screening experiments employed the four monomers shown in Figure 2, where the A monomers have the (2R,3R) configuration and the B monomers have the (2S,3S) configuration. The 13C NMR spectra of cis,syndiotactic-poly(A-alt-B) should reveal four different olefinic carbon resonances, and 1H NMR spectra could reveal up to four first order resonances for four different olefinic protons that are coupled pairwise (eq 2). (Overlap of proton resonances could result in non first order 1H NMR spectra.) Racemic A1 is known to be polymerized by 1a to give cis,syndiotactic-poly[A1(R,R)-alt-A1(S,S)];37 we find that cis,syndiotactic,alt polymers are also formed from racemic A2, B1, and B2 (see Supporting Information).
 |
2 |
Figure 2.
First four monomers employed in this study.
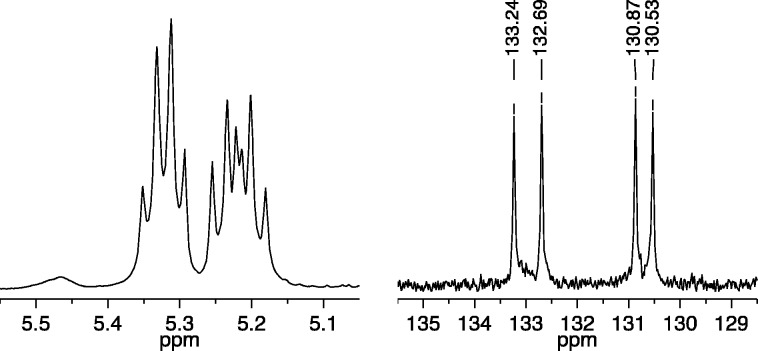
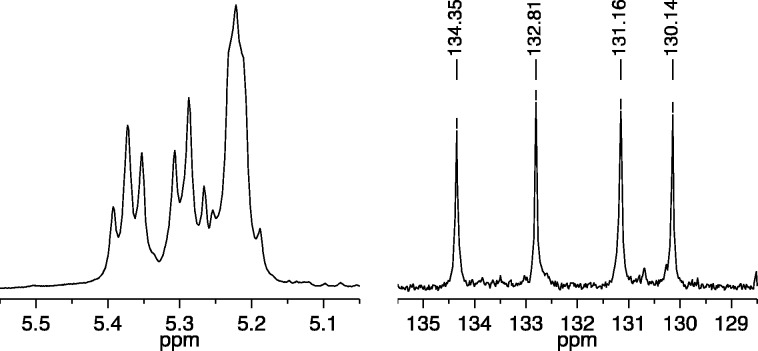
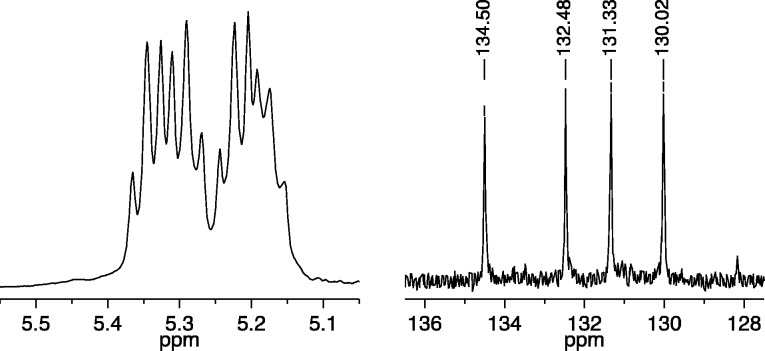
Copolymerization of a mixture of 25 equiv of A1 and 25 equiv of B2 with 1a (0.1 M in toluene-d8) as the initiator was complete within seconds to give cis,syndiotactic-poly(A1-alt-B2). Its partial 13C NMR spectrum in CDCl3 showed primarily four different olefinic resonances (Figure 3, right), while its 1H NMR spectrum showed four overlapping first order (pseudo triplet) olefinic proton resonances (Figure 3, left). (See Supporting Information for details.) The broad resonance shown between 5.45 and 5.50 ppm in Figure 3 we propose is half of the pattern that arises from trans,isotacticA1A1 and B2B2 “errors” (see Figure 1). The other half of the pattern, along with any (minor) pattern that is characteristic of cis,syndiotacticA1A1 and B2B2 errors (see Figure 1), is buried under the main pattern of four triplets for cis,syndiotactic-poly(A1-alt-B2) around 5.30 ppm. If we assume that only trans,isotacticA1A1 and B2B2 dyad resonances are present under the main four triplet resonance, we can estimate that ∼94% of the polymer contains cis,syndiotactic-poly(A1-alt-B2) dyads. The olefinic carbon resonances for any errors cannot be identified reliably in the partial carbon NMR spectrum shown in Figure 3.
Figure 3.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A1-alt-B2) (olefinic resonances only).
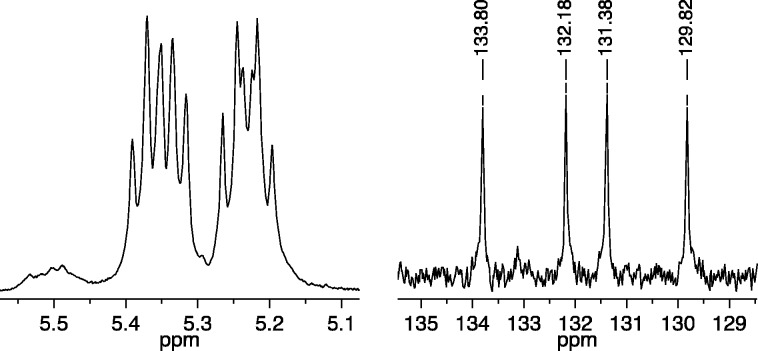
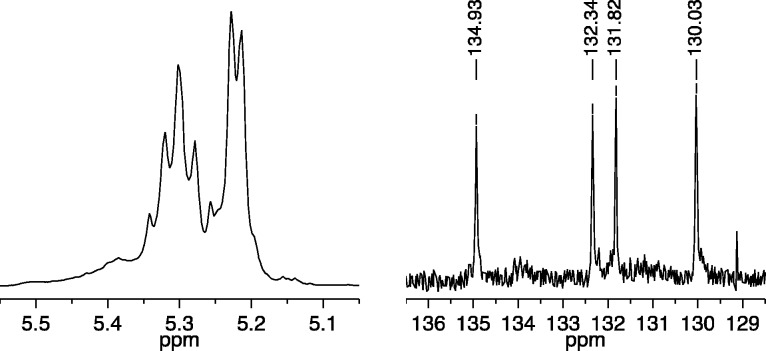
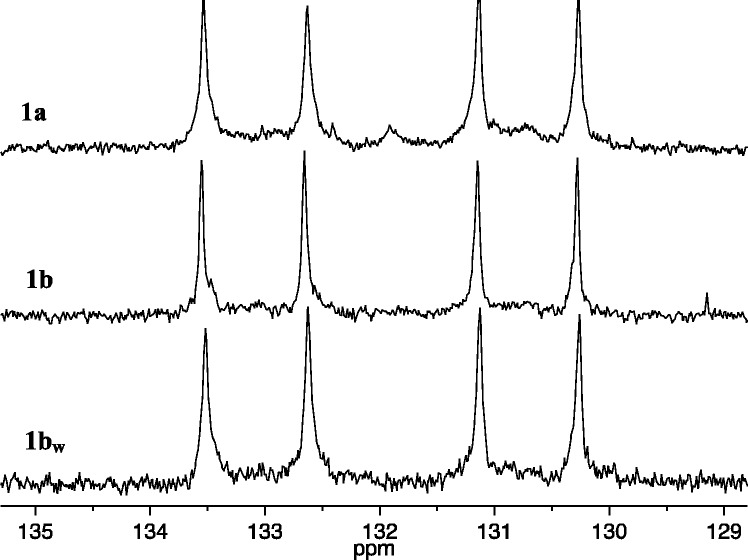
Copolymerization of 25 equiv of (R,R)-2,3-(CO2Me)2-norbornene (A1) and 25 equiv (S,S)-2,3-(CO2CH2CF3)2-norbornene (B1) with 1a (0.1 M in toluene-d8) as the initiator was also complete within seconds. The 13C NMR spectrum of the resulting polymer again showed primarily four different olefinic carbon resonances (Figure 4, right), while its 1H NMR spectrum showed four overlapping first order (pseudo triplet) olefinic proton resonances (Figure 4, left), consistent with the formation of cis,syndiotactic-poly(A1-alt-B1). A virtually identical cis,syndiotactic-poly(A1-alt-B1) polymer was prepared employing 1b as the initiator (see Supporting Information). It is clear from the spectra in Figure 5 that this cis,syndiotacticA1-alt-B1 copolymer contains more trans,isotactic errors than the cis,syndiotacticA1-alt-B2 copolymer described above, most likely as a consequence of the more significant differences in reactivity between A1 and B1 than between A1 and B2. The 5.50 ppm resonance was integrated, and the % cis,syndiotactic-poly(A1-alt-B1) dyads were calculated to be ∼90%. Two olefinic carbon resonances for the A1A1 and B1B1 “errors” in this case can be seen at ∼133.0 and 128.3 ppm (Figure 4). The 19F NMR spectrum of cis,syndiotactic-poly(A1-alt-B1) also reveals two types of overlapping fluorine resonances for A1B1 and B1B1 errors (see Supporting Information), integration of which suggests that the % cis,syndiotactic-poly(A1-alt-B1) dyads is ∼80%.
Figure 4.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A1-alt-B1) (olefinic resonances only).
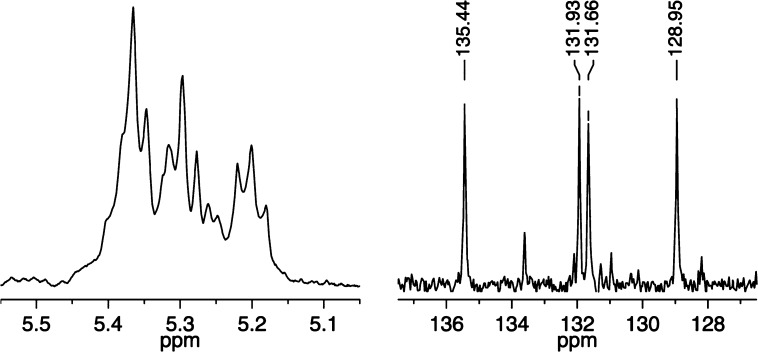
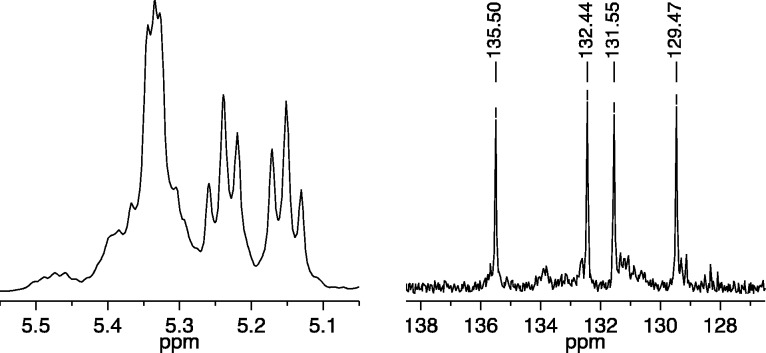
Figure 5.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A2-alt-B2) (olefinic resonances only).
A third cis,syndiotactic polymer was prepared through copolymerization of a mixture of 25 equiv of (R,R)-2,3-(CH2OAc)2-norbornene (A2) and 25 equiv of (S,S)-2,3-(CO2Et)2-norbornene (B2) with 1b as the initiator (Figure 5). The 13C NMR spectrum showed primarily four different olefinic carbon resonances, while the 1H NMR spectrum showed two essentially first order triplet resonances for protons coupled to one another, along with a second order resonance at ∼5.23 ppm for two coupled olefinic protons. On the basis of the carbon NMR spectrum we can estimate the number of A2A2 and B2B2 errors to be on the order of 5%.
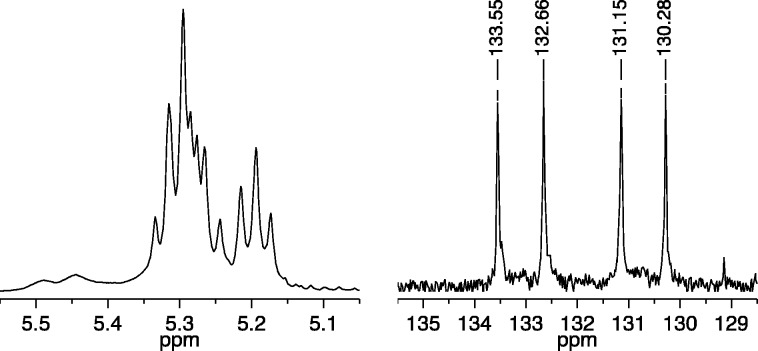
A fourth example is cis,syndiotactic-poly(A2-alt-B1). The 1H NMR spectrum of cis,syndiotactic-poly(A2-alt-B1) (Figure 6) provides little evidence that the polymer is relatively regular. However, inspection of the 13C NMR spectrum shows primarily four olefinic resonances, consistent with a relatively high percentage (estimated ∼90%) of the proposed structure. The complexity seen in the 1H NMR spectrum can be traced to the overlap and second order nature of the proton resonances. At least four carbon resonances for A2A2 and B1B1 errors can be seen in the 13C NMR spectrum.
Figure 6.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A2-alt-B1) (olefinic resonances only).
Twelve other Mo and W initiators were explored for making cis,syndiotactic-poly(A1-alt-B1), but none was as efficient as 1a or 1b, at least according to proton NMR spectroscopy (see Supporting Information for details). The failure of more than two initiators (so far) to produce cis,syndiotacticA-alt-B copolymers of the type described here is not surprising if one considers the complexity of the stereoregular ROMP reaction29 and the need to control formation of A1A1 and B1B1 errors. The requirements that the metal has a stereogenic center, that its configuration must switch with each insertion of A or B, and that the polymerization be controlled primarily by the chirality of the stereogenic metal are demanding.
Four additional enantiomerically pure monomers (Figure 7) were prepared, and five AxBy combinations were found to give copolymers with >90% alternating AB dyads using 1b as the initiator, according to their 1H and 13C NMR spectra (see Supporting Information for a complete list of reactions employing Ax and By). The percentage of trans,isotactic and/or cis,syndiotactic errors was estimated to be in the range of 5–10%.
Figure 7.
Four additional monomers.
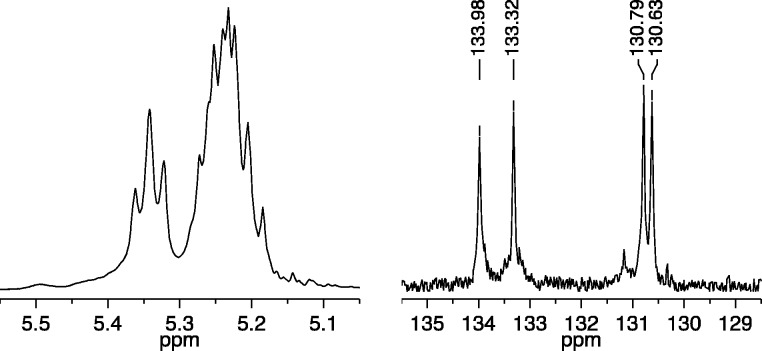
Copolymerization of A2 and B3 ((S,S)-(CO2-t-Bu)2-norbornene) using 1b yielded a CDCl3-soluble polymer whose 1H NMR spectrum showed primarily two resonances, a triplet at 5.34 ppm and a second order resonance at 5.23 ppm that integrated to three times its relative intensity (Figure 8). Weak and broad resonances near 5.50 and 5.45 can be attributed to trans,isotactic errors. The presence of primarily four olefinic resonances in the 13C NMR spectrum at 134.0, 133.3, 130.8, and 130.6 ppm suggests that the cis,syndiotactic-poly(A2-alt-B3) structure is of the order of 90%.
Figure 8.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A2-alt-B3) (olefinic resonances only).
Copolymerization of A1 and (S,S)-(CH2OAc)2-norbornene (B4) proceeded smoothly to give another CDCl3 soluble polymer. The 1H NMR spectrum of the isolated polymer showed two pairs of overlapping olefinic proton resonances and weak resonances at 5.45–5.50 for trans,isotactic errors (Figure 9). However, the 13C NMR spectrum showed primarily four olefinic resonances, which confirm that the polymer has largely the cis,syndiotactic,alt structure. Given the successful copolymerization of (R,R)-(CH2OAc)2-norbornene (A2) with (S,S)-(CO2Et)2-norbornene (B2; Figure 5), the formation of cis,syndiotactic-poly(A1-alt-B4) is not surprising. The resonance attributed to trans,isotactic errors is much more pronounced when 1a is used as the initiator (see Supporting Information).
Figure 9.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A1-alt-B4) (olefinic resonances only).
The copolymer derived from A3 ((R,R)-(CO2-t-Bu)2-norbornene) and B5 ((S,S)-(CH2OMe)2-norbornene) showed two higher ordered olefinic proton resonances of equal intensity in CDCl3, (Figure 10) along with resonances for A3A3 and B5B5 errors. The two overlapping proton resonances were not well-resolved, making assessment of the errors in the polymer structure difficult. However, the 13C NMR spectrum showed primarily four olefinic resonances, consistent with formation of largely cis,syndiotactic-poly(A3-alt-B5). Copolymerization of A1 and B5 gives cis,syndiotactic-poly(A1-alt-B5) (Figure 11), the proton NMR spectrum of which resembles that of cis,syndiotactic-poly(A2-alt-B2) (Figure 5). The second order olefinic proton resonance at 5.33 ppm was shifted to higher frequency with respect to two coupled triplet proton resonances, revealing the resonances for trans,isotactic errors. Copolymerization of A3 with B2 gave cis,syndiotactic-poly(A3-alt-B2) (Figure 12), which contains <10% errors.
Figure 10.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A3-alt-B5) (olefinic resonances only).
Figure 11.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A1-alt-B5) (olefinic resonances only).
Figure 12.
1H NMR (500 MHz, CDCl3, left) and 13C NMR (125 MHz, CDCl3, right) spectra of cis,syndiotactic-poly(A3-alt-B2) (olefinic resonances only).
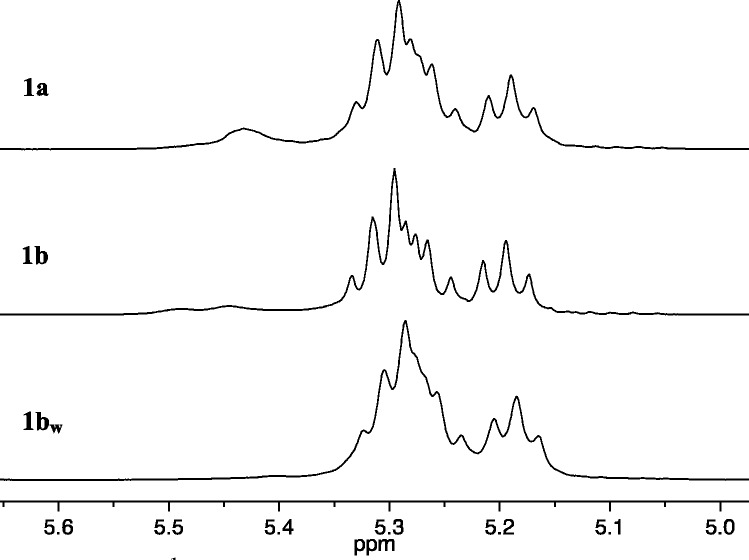
It is important to establish whether the tungsten analogue of 1b (1bw) is an equally efficient catalyst. Addition of 50 equiv of rac-DCBNBE (DCBNBE = 2,3-dicarbo-t-butoxynorbornene) to a toluene solution of 1bw led to full consumption of the monomer within 10 min. Only two pseudo triplet olefinic proton resonances (3JHH = 10 Hz) are present in the 1H NMR spectrum of the resulting polymer (Figure 13a). The 13C NMR spectrum is also sharp and free of any significant fine structure associated with structural irregularities (see Supporting Information). These results are consistent with a cis,syndiotactic,alt structure for the polymer. The two small broad resonances assigned to trans,isotactic dyads, in the olefin region of poly(rac-DCBNBE) prepared from 1a, are absent from the spectrum of poly(rac-DCBNBE) prepared from 1bw. The reason is that polymerization of (S,S)-DCBNBE with 1bw gives cis,syndiotactic-poly[(S,S)-DCBNBE] with the 1H NMR spectrum shown in Figure 13b; there is no evidence for a trans,isotactic structure. The olefinic proton resonances of cis,syndiotactic-poly[(S,S)-DCBNBE] are located in the middle of the olefinic proton resonances for poly(rac-DCBNBE) (Figure 13a), which makes it difficult to assess the percentage of microstructural errors formed in this copolymer using 1H NMR spectroscopy. However, when racemic DCENBE (DCENBE = 2,3-dicarboethoxynorbornene) and rac-DCMNBE are polymerized by 1bw under similar conditions, the olefinic proton triplet resonances are broadened, Figures 13c and 13e. The positions of the minor component within the olefinic proton resonances of poly(rac-DCENBE) and poly(rac-DCMNBE) are visible and can be unambiguously ascribed to cis,syndiotactic dyads in the largely cis,syndiotactic,alt structure (compare Figures 13c–13e).
Figure 13.
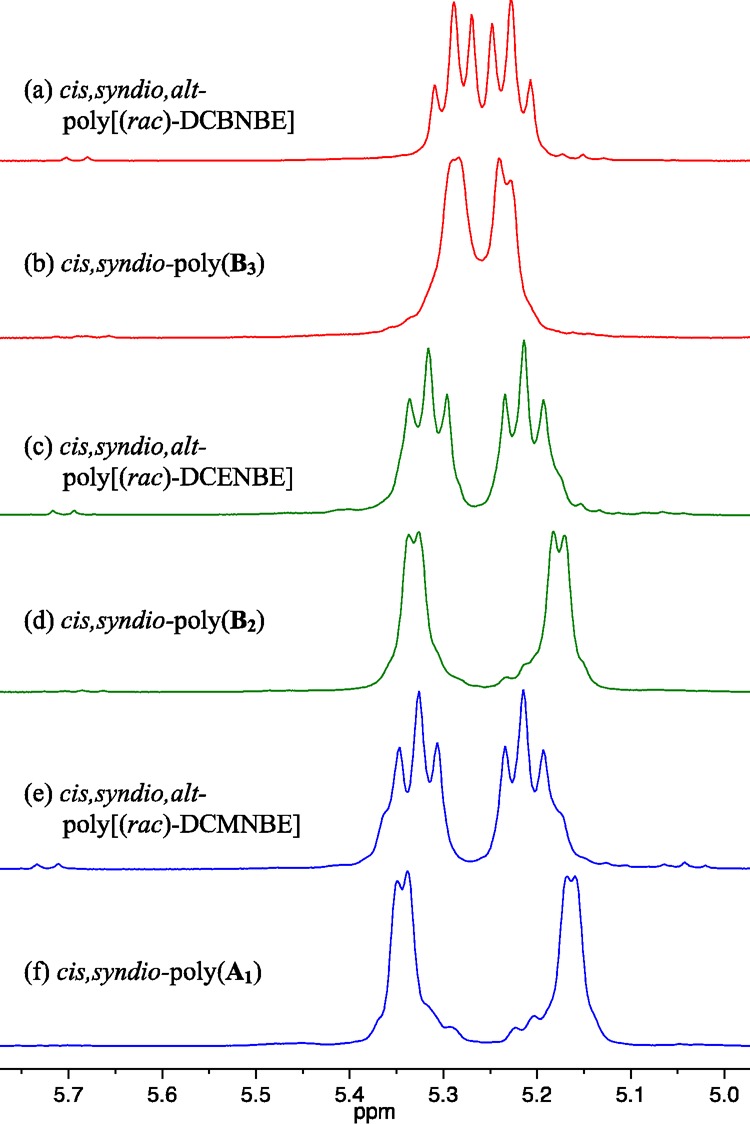
1H NMR (500 MHz, CDCl3) spectra for cis,syndiotactic polymers synthesized using 1bw (olefinic resonances only).
As reported previously,37 (+)-DCMNBE and rac-DCMNBE are polymerized at approximately the same rate using W(O)(CHCMe3)(OHMT)(Pyr)(PMe2Ph). In contrast, (−)-DCBNBE and rac-DCBNBE are polymerized at different rates. The sharp olefinic resonances in the 1H and 13C spectra of poly(rac-DCBNBE) prepared from 1a as the initiator are consistent with a lower percentage of cis,syndiotactic errors. A similar trend is seen when one inspects the 1H and 13C NMR spectra of poly(rac-DCBNBE) using 1bw (Figure 13a).
The proton and carbon NMR spectra of the A3-alt-B2 copolymers derived from 1a, 1b, and 1bw are compared in Figures 14 and 15. Initiators 1b and 1bw appear to yield the highest percentages of cis,syndiotacticA3-alt-B2 structures with trans,isotactic errors being formed when 1b is employed and cis,syndiotactic errors being formed when 1bw is employed.
Figure 14.
13C NMR spectra (125 MHz, CDCl3) of cis,syndiotactic-poly(A3-alt-B2) (olefinic resonances only) formed with initiators 1a, 1b, and 1bw.
Figure 15.
1H NMR spectra (500 MHz, CDCl3) of cis,syndiotactic-poly(A3-alt-B2) (olefinic resonances only) formed with initiators 1a, 1b, and 1bw.
Conclusions
Cis,syndiotacticA-alt-B copolymers, where A and B are two enantiomerically pure trans-2,3-disubstituted-5,6-norbornenes with “opposite” chiralities, can be prepared with stereogenic-at-metal initiators of the type M(NR)(CHR′)(OHMT)(pyrrolide) (R = 1-adamantyl or 2,6-Me2C6H3; R′ = CMe2Ph; M = Mo or W). The errors when Mo initiators are employed are primarily trans,isotacticAA and BB dyads, while the errors when a W initiator is employed are cis,syndiotacticAA and BB dyads. Formation of a high percentage of alternating AB copolymer linkages relies on an inversion of chirality at the metal center with each propagating step and faster formation of an AB sequence than an AA or BB sequence as a consequence of a preferred diastereomeric relationship between the chirality at the metal and the chirality of the monomer.
Experimental Section
Representative Polymerization
A mixture of 21.0 mg (0.1 mmol, 25 equiv) of A1 and 34.6 mg (0.1 mmol, 25 equiv) of B1 in 0.5 mL of toluene-d8 was added to a solution of 3.0 mg (0.004 mmol) of 1b in 0.5 mL of toluene-d8. The reaction mixture thickened within seconds. 1H NMR spectroscopy was used to monitor the course of the reaction. Once complete, the reaction mixture was exposed to air and poured into 35 mL of MeOH. The precipitated cis,syndiotactic-poly(A1B1) was allowed to settle. The solvent was decanted, and the polymer was dried in vacuo.
Acknowledgments
We are grateful to the Department of Energy (DE-FG02-86ER13564) for research support. R.R.S. thanks Timothy M. Swager for use of his ATR-FTIR.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.6b00200.
Experimental details for all reactions and all supporting NMR characterization of polymers (PDF)
Author Contributions
E.S.J. and J.M.J. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Odian G.Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2004. [Google Scholar]
- Coates G. W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. 10.1021/cr990286u. [DOI] [PubMed] [Google Scholar]
- Cheng M.; Lobkovsky E. B.; Coates G. W. Catalytic Reactions Involving C1 Feedstocks: New High-Activity Zn(II)-Based Catalysts for the Alternating Copolymerization of Carbon Dioxide and Epoxides. J. Am. Chem. Soc. 1998, 120, 11018–11019. 10.1021/ja982601k. [DOI] [Google Scholar]
- Super M.; Berluche E.; Costello C.; Beckman E. Copolymerization of 1,2-Epoxycyclohexane and Carbon Dioxide Using Carbon Diioxide as Both Reactant and Solvent. Macromolecules 1997, 30, 368–372. 10.1021/ma960755j. [DOI] [Google Scholar]
- Darensbourg D. J.; Holtcamp M. W. Catalytic Activity of Zinc(II) Phenoxides Which Possess Readily Accessible Coordination Sites. Copolymerization and Terpolymerization of Epoxides and Carbon Dioxide. Macromolecules 1995, 28, 7577–7579. 10.1021/ma00126a043. [DOI] [Google Scholar]
- Vehlow K.; Wang D.; Buchmeiser M. R.; Blechert S. Alternating Copolymerizations Using a Grubbs-Type Initiator with an Unsymmetrical, Chiral N-Heterocyclic Carbene Ligand. Angew. Chem., Int. Ed. 2008, 47, 2615–2618. 10.1002/anie.200704822. [DOI] [PubMed] [Google Scholar]
- Song A.; Parker K. A.; Sampson N. S. Synthesis of Copolymers by Alternating ROMP (AROMP). J. Am. Chem. Soc. 2009, 131, 3444–3445. 10.1021/ja809661k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A.; Parker K. A.; Sampson N. S. Cyclic Alternating Ring-Opening Metathesis Polymerization (CAROMP). Rapid Access to Functionalized Cyclic Polymers. Org. Lett. 2010, 12, 3729–3731. 10.1021/ol101432m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutthasupa S.; Shiotsuki M.; Masuda T.; Sanda F. Alternating Ring-Opening Metathesis Copolymerization of Amino Acid Derived Norbornene Monomers Carrying Nonprotected Carboxy and Amino Groups Based on Acid-Base Interaction. J. Am. Chem. Soc. 2009, 131, 10546–10551. 10.1021/ja903248c. [DOI] [PubMed] [Google Scholar]
- Nakade H.; Ilker M. F.; Jordan B. J.; Uzun O.; LaPointe N. L.; Coughlin E. B.; Rotello V. M. Duplex strand formation using alternating copolymers. Chem. Commun. 2005, 3271–3273. 10.1039/b502929e. [DOI] [PubMed] [Google Scholar]
- Lichtenheldt M.; Wang D.; Vehlow K.; Reinhardt I.; Kühnel C.; Decker U.; Blechert S.; Buchmeiser M. R. Alternating Ring-Opening Metathesis Copolymerization by Grubbs-Type Initiators with Unsymmetrical N-Heterocyclic Carbenes. Chem. - Eur. J. 2009, 15, 9451–9457. 10.1002/chem.200900384. [DOI] [PubMed] [Google Scholar]
- Vehlow K.; Lichtenheldt M.; Wang D.; Blechert S.; Buchmeiser M. R. Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-ene with cis-Cyclooctene and Cyclopentene. Macromol. Symp. 2010, 296, 44–48. 10.1002/masy.201051007. [DOI] [Google Scholar]
- Ilker M. F.; Coughlin E. B. Alternating Copolymerizations of Polar and Nonpolar Cyclic Olefins by Ring-Opening Metathesis Polymerization. Macromolecules 2002, 35, 54–58. 10.1021/ma011394x. [DOI] [Google Scholar]
- Bornand M.; Torker S.; Chen P. Mechanistically Designed Dual-Site Catalysts for the Alternating ROMP of Norbornene and Cyclooctene. Organometallics 2007, 26, 3585–3596. 10.1021/om700321a. [DOI] [Google Scholar]
- Romulus J.; Tan L.; Weck M.; Sampson N. S. Alternating Ring-Opening Metathesis Polymerization Copolymers Containing Charge-Transfer Units. ACS Macro Lett. 2013, 2, 749–752. 10.1021/mz4002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeffler C. S.; Grubbs R. H. Catalyst-Dependent Routes to Ring-Opening Metathesis Alternating Copolymers of Substituted Oxanorbornenes and Cyclooctene. Macromolecules 2013, 46, 3288–3292. 10.1021/ma400141c. [DOI] [Google Scholar]
- Abbas M.; Wappel J.; Slugovc C. Alternating Diene Metathesis Polycondensation (ALTMET) – Opitimizing Catalyst Loading. Macromol. Symp. 2012, 311, 122–125. 10.1002/masy.201000095. [DOI] [Google Scholar]
- Buchmeiser M. R.; Ahmad I.; Gurram V.; Kumar P. S. Pseudo-Halide and Nitrate Derivatives of Grubbs andGrubbs-Hoveyda Initiators: Some Structural Features Related to the Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-ene with Cyclic Olefins. Macromolecules 2011, 44, 4098–4106. 10.1021/ma200995m. [DOI] [Google Scholar]
- Demel S.; Slugovc C.; Stelzer F.; Fodor-Csorba K.; Galli G. Alternating Diene Metathesis Polycondensation (ALTMET) – AVersatile Tool for the Preparation of Perfectly Alternating AB Copolymers. Macromol. Rapid Commun. 2003, 24, 636–641. 10.1002/marc.200350007. [DOI] [Google Scholar]
- Choi T.-L.; Rutenberg I. M.; Grubbs R. H. Synthesis of A,B-Alternating Copolymers by Ring-Opening-Insertion-Metathesis Polymerization. Angew. Chem., Int. Ed. 2002, 41, 3839–3841. . [DOI] [PubMed] [Google Scholar]
- Al Samak B.; Amir-Ebrahimi V.; Corry D. G.; Hamilton J. G.; Rigby S.; Rooney J. J.; Thompson J. M. Dramatic solvent effects on ring-opening metathesis polymerization of cycloalkenes. J. Mol. Catal. A: Chem. 2000, 160, 13–21. 10.1016/S1381-1169(00)00228-4. [DOI] [Google Scholar]
- Konzelman J.; Wagener K. B. Acyclic Diene Metathesis (ADMET) Polymerization. Synthesis of Perfectly Alternating Copolymers from a Single Monomer. Macromolecules 1996, 29, 7657–7660. 10.1021/ma960854x. [DOI] [Google Scholar]
- Wu Z.; Grubbs R. H. Preparation of Alternating Copolymers from the Ring-Opening Metathesis Polymerization of 3-Methylcyclobutene and 3,3-Dimethylcyclobutene. Macromolecules 1995, 28, 3502–3508. 10.1021/ma00114a002. [DOI] [Google Scholar]
- Ding L.; Zheng X.-Q.; Lu R.; An J.; Qiu J. Perfectly AB-alternating copolymers via alternating diene metathesis polymerization: one-step synthesis, characterization and properties. Polym. Int. 2014, 63, 997–1002. 10.1002/pi.4599. [DOI] [Google Scholar]
- Tan L.; Parker K. A.; Sampson N. S. A Bicyclo[4.2.0]octene-Derived Monomer Provides Completely Linear Alternating Copolymers via Alternating Ring-Opening Metathesis Polymerization (AROMP). Macromolecules 2014, 47, 6572–6579. 10.1021/ma5012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-K.; Bang K.-T.; Hess A.; Grubbs R. H.; Choi T.-L. Multiple Olefin Metathesis Polymerization That Combines All Three Olefin Metathesis Transformations: Ring-Opening, Ring-Closing, and Cross Metathesis. J. Am. Chem. Soc. 2015, 137, 9262–9265. 10.1021/jacs.5b06033. [DOI] [PubMed] [Google Scholar]
- Elling B. R.; Xia Y. Living Alternating Ring-Opening Metathesis Polymerization Based on Single Monomer Additions. J. Am. Chem. Soc. 2015, 137, 9922–9926. 10.1021/jacs.5b05497. [DOI] [PubMed] [Google Scholar]
- Tan L.; Li G.; Parker K. A.; Sampson N. S. Ru-Catalyzed Isomerization Provides Access to Alternating Copolymers via Ring-Opening Metathesis Polymerization. Macromolecules 2015, 48, 4793–4800. 10.1021/acs.macromol.5b01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock R. R. Synthesis of Stereoregular Polymers through Ring-Opening Metathesis Polymerization. Acc. Chem. Res. 2014, 47, 2457–2466. 10.1021/ar500139s. [DOI] [PubMed] [Google Scholar]
- Forrest W. P.; Weis J. G.; John J. M.; Axtell J. C.; Simpson J. H.; Swager T. M.; Schrock R. R. Stereospecific Ring-Opening Metathesis Polymerization of Norbornadienes Employing Tungsten Oxo Alkylidene Initiators. J. Am. Chem. Soc. 2014, 136, 10910–10913. 10.1021/ja506446n. [DOI] [PubMed] [Google Scholar]
- Jeong H.; John J. M.; Schrock R. R.; Hoveyda A. H. Synthesis of Alternating trans-AB Copolymers Employing Ring-Opening Metathesis Polymerization Initiated by Molybdenum Alkylidenes. J. Am. Chem. Soc. 2015, 137, 2239–2242. 10.1021/jacs.5b00221. [DOI] [PubMed] [Google Scholar]
- Jeong H.; Ng V. W. L.; Börner J.; Schrock R. R. Stereoselective Ring-Opening Metathesis Polymerization (ROMP) of Methyl-N-(1-phenylethyl)-2-azabicyclo[2.2.1]hept-5-ene-3-carboxylate by Molybdenum and Tungsten Initiators. Macromolecules 2015, 48, 2006–2012. 10.1021/acs.macromol.5b00264. [DOI] [Google Scholar]
- Autenrieth B.; Jeong H.; Forrest W. P.; Axtell J. C.; Ota A.; Lehr T.; Buchmeiser M. R.; Schrock R. R. Stereospecific Ring-Opening Metathesis Polymerization (ROMP) of Endo-Dicyclopentadiene by Molybdenum and Tungsten Catalysts. Macromolecules 2015, 48, 2480–2492. 10.1021/acs.macromol.5b00123. [DOI] [Google Scholar]
- Autenrieth B.; Schrock R. R. Stereospecific Ring-Opening Metathesis Polymerization (ROMP) of Norbornene and Tetracyclododecene by Mo and W Initiators. Macromolecules 2015, 48, 2493–2503. 10.1021/acs.macromol.5b00161. [DOI] [Google Scholar]
- Hyvl J.; Autenrieth B.; Schrock R. R. Proof of Tacticity of ROMP Polymers Through Post Polymerization Modification. Macromolecules 2015, 48, 3148–3152. 10.1021/acs.macromol.5b00477. [DOI] [Google Scholar]
- Flook M. M.; Ng V. W. L.; Schrock R. R. Synthesis of cis,syndiotactic ROMP Polymers Containing Alternating Enantiomers. J. Am. Chem. Soc. 2011, 133, 1784–1786. 10.1021/ja110949f. [DOI] [PubMed] [Google Scholar]
- Flook M. M.; Börner J.; Kilyanek S.; Gerber L. C. H.; Schrock R. R. Five-Coordinate Rearrangements of Metallacyclobutane Intermediates During Ring-Opening Metathesis Polymerization (ROMP) of 2,3-Dicarboalkoxynorbornenes by Molybdenum and Tungsten Monoalkoxide Pyrrolide (MAP) Initiators. Organometallics 2012, 31, 6231–6243. 10.1021/om300530p. [DOI] [Google Scholar]
- Hamilton J. G.; Ivin K. J.; Rooney J. J. 13C N.m.r. Spectra of Ring-opened Polymers of 1-Methylbicyclo[2.2.1]hept-2-ene and their Hydrogenated Products Brit. Br. Polym. J. 1984, 16, 21–33. 10.1002/pi.4980160106. [DOI] [Google Scholar]
- Hamilton J. G.; Ivin K. J.; Rooney J. J.; Waring L. C. Alternating Copolymerization of Enantiomers of 1-Methylbicyclo[2.2.1]hept-2-ene by a Metathesis Catalyst. J. Chem. Soc., Chem. Commun. 1983, 159–161. 10.1039/c39830000159. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.