Abstract
Insecticide selectivity to natural enemies is an important concern in integrated pest management programs. Although there is a wide range of information concerning pesticide lethal and sublethal effects on contaminated surfaces, little is known when the route of exposure occurs at a trophic level. This study evaluates this route of pesticide intake on the omnivorous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae) for the first time. Under laboratory conditions, prey treated with six insecticides (flubendiamide, spirotetramat, deltamethrin, flonicamid, metaflumizone, and sulfoxaflor) were offered to N. tenuis adults for 3 days. Mortality (24, 48, and 72 h after treatment), offspring production (third until eighth day) and longevity were documented. Metaflumizone and sulfoxaflor were classified as moderately harmful products because although the percentage of mortality was only 28 and 36%, respectively, both products caused a severe decrease in offspring production and longevity. Flonicamid and flubendiamide were classified as slightly harmful products; although they did not have a lethal effect, sublethal impact was important on the parameters studied. Spirotetramat and deltamethrin were insecticides categorized as harmless. This information could be useful for selecting the most appropriate insecticides to control pests in tomato crops in which N. tenuis is a relevant biological control agent.
Keywords: side effect, trophic contamination, insecticide toxicity, omnivorous predator, IOBC classification
Integrated pest management (IPM) programs help to decrease chemical contamination on crops, combat pest resistance against pesticides and meet the demands of policy makers (less residue on food) (Gentz et al. 2010, Lu et al. 2012, Asplen et al. 2015). However, the use of pesticides, in particular insecticides and acaricides, is still important in IPM although new products with distinct modes of action and improved safety profiles are replacing older products (Guedes et al. 2016). These modern pesticides are often applied coinciding with the release of biological control agents (Wheeler 2002, Grafton-Cardwell et al. 2005). For this reason, the side effects of newly developed pesticides on the most important biological agents in each crop must be studied. The optimum is a pesticide being toxic to the targeted pests but harmless or with limited effects on the targeted pests’ natural enemies.
Chemical products can affect beneficials in two ways: 1) by having acute toxicity (mortality or lethal effects), and 2) by causing physiological or behavioral changes in individuals that survive after being in contact with the product (sublethal effects) (Lee 2000, Desneux et al. 2007). The pesticide risk assessment to natural enemies is typically based on lethal effects. As such, for pesticide registration under the European Union, the first tier testing consisted on the determination of exclusively this kind of effects on two sensitive indicator species (the braconid Aphidius rhopalosiphi DeStephani-Pérez and the phytoseiid Typhlodromus pyri Secheuten) after exposure to pesticides on inert surfaces in the laboratory. Only when detrimental effects are detected, both lethal and sublethal effects are studied in the second and final tier testing under more realistic conditions (Candolfi et al. 2001). However, many modern studies highlight the importance of evaluating sublethal effects that can also greatly affect the efficacy of natural enemies in controlling pests (Desneux et al. 2007; Arnó and Gabarra 2011; Biondi et al. 2013, 2015; Pan et al. 2014; Fernández et al. 2015).
Additionally, different methods to assess the toxicity of pesticides can be considered along with the multiple possible routes of toxicant entry into natural enemies including (Croft 1990, Ruberson et al. 1998): 1) topical assays, which are used to reproduce the direct exposure of natural enemy to spray droplets (Bengochea et al. 2014); 2) residual toxicity tests, which use plant substrates or inert surfaces to represent the contact with residues on the crop (Biondi et al. 2015); 3) treated plants, in which pesticides that enter plant tissues can contaminate resources, such as nectar or plant sap, that are important in the nutrition of many natural enemies (Wanumen et al. 2016); and 4) treated prey or hosts because toxicants can move across trophic levels (Planes et al. 2013). The importance of these different routes of exposure to terrestrial organisms depends on a range of factors, such as the intrinsic properties of insecticides (e.g., contact and ingestion activity, or systemic properties) and the biological and ecological characteristics of natural enemies (i.e., parasitoid or predator). In the specific case of predators, a major route of exposure to pesticides is through ingestion of contaminated prey (He et al. 2012). However, there are few studies of predators that have documented pesticide contamination at trophic level compared with those that study residual or contact exposition (IOBC 2016).
Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae) is a generalist predator of the Mediterranean Basin (Arnó et al. 2009, Moreno-Ripoll et al. 2014) commonly used in biological control programs for three of the most important pests of tomato crops: Tuta absoluta (Meyrick) (Lepidoptera: Gelechidae), whiteflies such as Bemisia tabaci (Gennadius) and Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) (Mollá et al. 2011, Calvo et al. 2012, Zappalà et al. 2013). In addition, N. tenuis also preys on other pests, such as thrips, aphids, mites, and lepidopteran pests (Arnó et al. 2010, Pérez-Hedo and Urbaneja 2015).
N. tenuis is a voracious predator, although it has a zoophytophagous diet. It needs plants as a source of water and supplementary nutrients and also as an egg-laying substrate (endophytic oviposition) (De Puysseleyr et al. 2012). At high densities damage on tomato crops has been reported (Biondi et al. 2016). However, plants are a suboptimal food source for N. tenuis. According to Urbaneja et al. (2005) this predator is not able to complete its biological cycle without prey on eggplant, pepper or tomato plants. Different behavior has been observed on the alternative host plant Sesamum indicum (L.) (Pedaliaceae) (Biondi et al. 2016). Sanchez (2008) reported that N. tenuis showed a typical predator dynamic because its abundance increased as whitefly densities increased and decreased after whitefly numbers dropped. These results suggest that this predator exploits plants when arthropod prey are scarce, except during its early nymphal stage, when it has a higher affinity for plants than for prey (Calvo and Urbaneja 2004). In addition, Perez-Hedo et al. (2015) have documented that the phytophagous behavior of this mirid benefits indirectly the tomato plants by activating a defense response (jasmonic acid pathway) which makes the plants more attractive to the whitefly parasitoid Encarsia formosa Gahan (Hymenoptera: Aphelinidae) than to B. tabaci. Therefore, because N. tenuis is an active and generalist predator, the probability of it feeding on prey contaminated with pesticides is high. In light of this, it is important to determine which insecticides have the potential to reduce the biological parameters of this predator by this route of entry. Towards that goal, the purpose of this study was to evaluate trophic contamination in N. tenuis adults, taking into account both lethal (mortality) and sublethal (offspring production and longevity) effects of six modern insecticides: flubendiamide, spirotetramat, deltamethrin, flonicamid, metaflumizone, and sulfoxaflor considering that to our knowledge, there are no other studies concerning the impact of these pesticides on this mirid at a trophic level.
Materials and Methods
The bioassays for this study were carried out at the Laboratory of Crop Protection, Department of Vegetal Production, Politechnical University of Madrid, Spain. Laboratory bioassay conditions were controlled in a climatic chamber at 25 ± 2 °C, with a relative humidity of 75 ± 10% and a photoperiod of 16:8 (L:D) h.
Insects
N. tenuis adults were obtained from the laboratory-reared populations. Initially, 1,000 insects were supplied by Agrobio (La Mojonera, Almeria, Spain) as NESIDIOcontrol. Insects were distributed in two ventilated plastic cages (40 cm length × 30 cm height × 30 cm deep). As prey, 5 g of Ephestia kuehniella Zeller (Lepidoptera:Pyralidae) (EPHEScontrol Agrobio) eggs were supplied in four plastic cylindrical containers (3.5 cm in diameter × 1 cm height). As a source of water and oviposition substrate, we used 500 g of fresh green beans randomly distributed inside each cage. To maintain the rearing conditions, the oviposition substrate was replaced every 2 days until the death of 80% of the adults. The rearing was maintained under the same environmental conditions as the bioassays. Before the experiments, three consecutive generations of N. tenuis were reared in the laboratory.
Insecticides
Five commercial insecticide formulations and a technical product (sulfoxaflor) were tested. The evaluated products are all recommended for pest control of tomato crops in Spain (MAGRAMA 2015), except sulfoxaflor, which was recently included in Annex 1 of the European directive 91/414/EEC (OJEC 2016). All tests were performed with fresh insecticide solutions prepared with distilled water at (in the case of sulfoxaflor) the maximum concentration recommended by the manufacturer or (for all others) in accordance with their Spanish registrations for use in tomato crops (MAGRAMA 2015). The concentrations of active ingredient tested, mode of action (IRAC 2013) and commercial name were as follows: flubendiamide, 60 mg a.i. L−1, ryanodine receptor modulator (Fenos 24% WG; Bayer Cropscience S.L.); spirotetramat, 75 mg a.i. L−1, inhibitor of acetyl CoA carboxylase (Movento 15% OD; Cropscience S.L.); deltamethrin, 12.5 mg a.i. L−1, sodium channel modulators (Decis 2.5% EW; Bayer S.A.); flonicamid, 60 mg a.i. L−1 selective, homopteran feeding blockers (Teppeki 50% WG; BioScience S.A.); metaflumizone, 240 mg a.i. L−1 voltage-dependent sodium channel blockers (Alverde 24% SC; Basf); and sulfoxaflor, 60 mg a.i. L−1, nicotinic acethylcholine receptor (nAChR) agonist (Technical product, Dow Agroscience S.L.). Distilled water was used as a control.
Evaluation of predator consumption of treated eggs
This experiment was conducted to determine whether treated preys were ingested by N. tenuis for the 3 days of exposition, in order to detect repellent or antifeedant action of the insecticides evaluated. E. kuehniella eggs were used as prey in the experiments because N. tenuis has good demographic parameters when fed on this factitious prey (Molla et al. 2014). At one moment, 0.05 g of E. kuehniella eggs were dipped for 25 s in insecticide or water solutions (test groups and control). We verified that the eggs were fully wet by the solution using a stereomicroscope. After the eggs dried, they were distributed in sets of 50 on black plastic cards using tragacanth gum (Manuel Riesgo S.A, Spain) at 3% as an adhesive substrate (30 cards per treatment). Were used as experimental units ventilated plastic cages (12 cm in diameter × 5 cm height, with 5.5 cm in diameter ventilation holes covered with mesh on the top). Inside them, a pair of N. tenuis adults (48–72-h old) was released and fed with treated eggs over 3 days (one black plastic card per day), using a ratio of 50 treated eggs/pair of predators/day. A fresh green bean segment (3 cm) sealed with parafilm (Manuel Riesgo, Bemis, USA) at the terminal parts was used as a water source. Ten replicates were performed per each compound and for control. Daily, the pair’s consumption of the 50 eggs was evaluated.
Evaluation of lethal and sublethal effects of insecticides
To provide contaminated prey for 3 days, 2 g of E. kuehniella eggs were dipped for 1 minute in each treatment solution. After the eggs dried, they were distributed in sets of 0.2 g and placed in a circular plastic feeder (1 cm in diameter). We used the same cages previously described with a green bean segment included. Fifteen N. tenuis adults (48–72-h old) were introduced into each cage and fed with the treated eggs for 3 days. Adult mortality (lethal effect) was evaluated during this period. Each treatment has five replicates. Subsequently, the adults were fed with untreated prey and evaluations of offspring production and longevity parameters were conducted.
Directly after removing treated eggs, twelve couples of N. tenuis per treatment were randomly selected to study the offspring production parameter and the remaining adults were used to study the longevity. To evaluate the offspring production, couples were isolated (12 replicates) into a cylindrical plastic cage (9 cm in diameter, 3 cm height) with E. kuehniella eggs offered ad libitum and a fresh green bean as oviposition substrate. The oviposition substrate was changed every 2 days over 6 days (3 changes total); each fresh green bean containing N. tenuis eggs was transferred into a plastic cup (11 cm in diameter). After 11 days, the number of emerging nymphs was recorded. To assess the longevity parameter, mortality was recorded daily until the death of all individuals and was calculated with the mean values of five replicates per treatment. The overall experiment was repeated twice.
Data analysis
One-way analysis of variance (ANOVA) using the Stagraphics program was used to determine significant (P < 0.05) differences among treatments. When the same experiment was performed twice, a mixed-model factorial ANOVA was performed first with treatments as fixed factor and experiment at random factor. Since experiments were found to be homogenous the weighted averages of ten replicates for mortality and longevity and 24 replicates for offspring were used for statistics. When necessary, data expressed as percentages were transformed by arcsin(sqrt(x/100)) to meet the assumptions of the analysis. Afterward, the means were separated by the Least Significant Difference (LSD) test. If any of the assumptions of the analysis of variance were violated after appropriate transformations, the effect of treatments was analysed by means of a non-parametric Kruskal-Wallis test followed by a Dunn test to determine significant differences among treatments.
For the treatments that were significantly different from the controls, mortality (Mc) was corrected according to Schneider-Orelli’s formula (Püntener 1981) and reductions in offspring production (R1) or longevity (R2) were corrected by calculating the ratios between treatments and controls. The total effect (E) of each insecticide was evaluated using an equation based on Van de Veire et al. (2002): E = 100 – [(100 − Mc) × R1 × R2]
When no significant differences were found, the corrected value for mortality was assigned as zero and for sublethal effects was assigned as 1 (Planes et al. 2013). The pesticides were classified using the resulting values according to the four toxicity categories proposed by the International Organization for Biological Control (IOBC) for laboratory tests: Class 1, harmless (E < 30% reduction); Class 2, slightly harmful (E = 30–79% reduction); Class 3, moderately harmful (E = 80–99% reduction), and Class 4, harmful (E > 99% reduction) (Hassan et al. 1994; Sterk et al. 1999).
The proportion of living insects over time was fitted by a Weibull function (Pinder et al. 1978), whose general form is S(t) = e−(t/b)ß, where S(t) = probability of surviving to a given age, t = the time expressed in days, b is a parameter that describes the scale, and ß is a parameter that describes the shape of the curve. The shape parameter controls the rate of change of the age-specific mortality rate, and therefore, the general form of the survivorship curve (Pinder et al. 1978). Comparisons of survival data were made by means of a Wilcoxon test using a significance level of P < 0.05. Statistical analyses were performed using Statgraphics (Virginia, USA) Centurion XVI (Stat-Point Technologies 2009) and model fitting operations were performed using Tablecurve 2D 5.01 (SYSTAT 2002).
Results
Evaluation of predator consumption of treated eggs
The daily N. tenuis consumption of eggs was similar in all the different treatments (Table 1). We did not observe significant differences using statistical analyses at 24 h (H = 7.51; P = 0.2758) or at 72 h (H = 11.74; P = 0.0684); however, a significant effect was observed at 48 h (H = 13.60; P = 0.0344), but the subsequent Dunn test revealed no differences among treatments (P > 0.05).
Table 1.
Evaluation of predator consumption of treated E. kuehniella eggs
| Compounds | Consumption (%)a |
|||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | Total | |
| Control | 99.4 ± 0.6a | 96.8 ± 1.7 a | 95.8 ± 1.4 a | 97.3 ± 0.9 a |
| Flubendiamide | 95.4 ± 4.6 a | 96.0 ± 3.6 a | 98.2 ± 1.3 a | 95.8 ± 3.4 a |
| Spirotetramat | 89.0 ± 5.1 a | 82.4 ± 7.2 a | 83.3 ± 11.0 a | 85.5 ± 4.4 a |
| Deltamethrin | 96.8 ± 1.9 a | 99.2 ± 0.6 a | 88.0 ± 7.4 a | 94.7 ± 2.7 a |
| Flonicamid | 97.1 ± 2.1 a | 98.0 ± 1.6 a | 90.7 ± 6.4 a | 95.2 ± 2.4 a |
| Metaflumizone | 96.2 ± 2.5 a | 90.2 ± 8.9 a | 77.0 ± 14.1 a | 89.2 ± 6.9 a |
| Sulfoxaflor | 89.8 ± 9.7 a | 76.0 ± 10.4 a | 85.7 ± 3.3 a | 81.9 ± 8.3 a |
a Eggs were changed daily. Data are the mean of ten replicates ± SE. Means within columns followed by the same letter are not significantly different (Kruskal-Wallis test followed by a Dunn test, P > 0.05).
Evaluation of lethal and sublethal effects of insecticides
The results of the tests for insecticidal effects on mortality, offspring production and longevity of N. tenuis adults are shown in Table 2. At 24 h, mortality percentages were very low (under 6%) in all the treatments although LSD tests revealed significant differences between the sulfoxaflor treatment and the control (F = 2.41; df = 6,63; P = 0.0366). Cumulative mortality produced by this neonicotinoid increased slightly (by 18%) at 48 h (F = 10.80; df = 6,63; P < 0.0001). Insects treated with flonicamid also showed significantly greater mortality at 48 h, although this effect was not observed at 72 h. Finally, at 72 h, sulfoxaflor and metaflumizone produced maximum values of mortality, 36 and 28%, respectively (H = 42.73; P < 0.0001).
Table 2.
Effects on mortality, offspring production and longevity in N. tenuis when adults fed of E. kuehniella insecticide-contaminated eggs, using the maximum field recommended concentration for tomato crop
| Compounds | Lethal effect mortality (%)a |
Sublethal effects |
Total effect (%) [IOBC category] | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | Offspring/fem./dayb | Longevity (days)c | ||
| Control | 0.0 ± 0.0 a | 0.7 ± 0.7 a | 0.7 ± 0.7 a | 6.0 ± 0.5 a | 17.7 ± 0.6 a | – |
| Flubendiamide | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 2.0 ± 1.0 a | 4.0 ± 0.4 bcd | 15.1 ± 1.1 ab | 32.8 [2] |
| Spirotetramat | 1.3 ± 1.3 a | 1.3 ± 1.3 a | 2.7 ± 2.0 a | 5.3 ± 0.7 ab | 14.5 ± 0.8 b | 18.1 [1] |
| Deltamethrin | 0.7 ± 0.7 a | 1.3 ± 0.9 a | 2.7 ± 1.5 a | 4.3 ± 0.7 bc | 15.2 ± 0.9 ab | 29.1 [1] |
| Flonicamid | 3.3 ± 1.5 ab | 5.3 ± 1.7 b | 6.7 ± 1.7 ab | 3.4 ± 0.5 cd | 13.0 ± 0.7 b | 58.6 [2] |
| Metaflumizone | 2.7 ± 2.0 ab | 3.3 ± 2.0 ab | 28.0 ± 7.3 b | 2.6 ± 0.6 d | 7.9 ± 1.0 c | 85.9 [3] |
| Sulfoxaflor | 5.3 ± 1.7 b | 18.0 ± 4.2 c | 36.0 ± 6.4 b | 2.6 ± 0.4 d | 7.4 ± 1.0 c | 88.2 [3] |
a,c Data are mean of 10 replicates ± SE.
b Data are the mean of 24 replicates ± SE.
IOBC Toxicity categories for laboratory test: 1) harmless (<30%); 2) slightly harmful (30–79%); 3) moderately harmful (80–99%); and 4) harmful (>99%). Within a column, data followed by different letter are significantly different (P < 0.05).
All insecticides except spirotetramat caused a significant reduction in the offspring production of N. tenuis with respect to the controls (F = 5.88; df = 6,161; P < 0.0001). Metaflumizone and sulfoxaflor reduced this reproductive parameter more severely than flubendiamide, deltamethrin, or flonicamid.
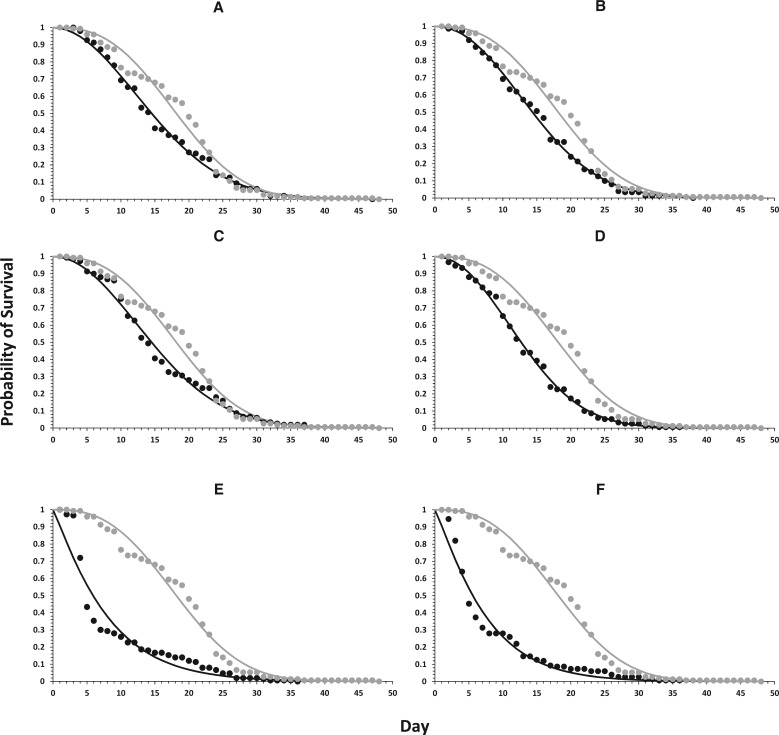
Predator longevity was slightly diminished in comparison with control treatment (17.7 days) when fed on prey treated with spirotetramat and flonicamid (14.5 and 13 days), but again, a higher reduction was observed for the metaflumizone and sulfoxaflor treatments (7.9 and 7.4 days, respectively) (F = 18.88; df = 6,63; P < 0.0001). In concordance with these results, the Wilcoxon test revealed significant differences in the survival curves among treatments (χ2 = 323.187; P < 0.0001) (Table 3; Fig. 1). When the scale (b) and shape (β) parameters of the Weibull function are considered, two groups of insecticides can be established. Thus, metaflumizone and sulfoxaflor showed similar values for b and β and according to those, no significant differences were observed in their survival curves (χ2 = 1.49381; P = 0.221624). Similarly, flubendiamide, spirotetramat, deltamethrin and flonicamid presented similar values for those parameters, and no significant differences were observed in their survival curves (χ2 = 6.62121; P = 0.085001). However, both groups had survival curves significantly different from those of the control (χ2 = 137.799; P < 0.0001, for metaflumizone and sulfoxaflor vs. control; χ2 = 28.3878; P < 0.0001, for flubendiamide, spirotetramat, deltamethrin, and flonicamid vs. control).
Table 3.
Parameters estimated for the Weibull function describing the survivorship of N. tenuis adults, after being contaminated via the food chain, with six insecticides
| Treatment | ba ± SE | ßb ± SE | R2 |
|---|---|---|---|
| Control | 20.8 ± 0.2 | 2.7 ± 0.2 | 0.988 |
| Flubendiamide | 17.5 ± 0.1 | 1.9 ± 0.0 | 0.996 |
| Spirotetramat | 17.1 ± 0.1 | 2.1 ± 0.0 | 0.997 |
| Deltamethrin | 17.4 ± 0.2 | 2.0 ± 0.1 | 0.993 |
| Flonicamid | 15.1 ± 0.1 | 2.1 ± 0.0 | 0.997 |
| Metaflumizone | 8.2 ± 0.4 | 1.1 ± 0.1 | 0.929 |
| Sulfoxaflor | 7.7 ± 0.2 | 1.1 ± 0.1 | 0.969 |
a b, parameter that describes the scale of the Weibull function.
b ß parameter that describes the shape of the Weibull function.
Fig. 1.
Survival probability of N. tenuis adults fed with E. kuehniella eggs treated with (A) Flubendiamide, (B) Spirotetramat, (C) Deltamethrin, (D) Flonicamid, (E) Metaflumizone, and (F) Sulfoxaflor. Within each graph, • represents the observed values for each insecticide and • represents the observed values for the control. Lines correspond with the line of best fit according to the Weibull function.
The IOBC toxicity insecticide classifications were based on their total effect. Their values were assessed as follows (Table 2): spirotetramat (harmless) < deltamethrin (harmless) < flubendiamide (slightly harmful) < flonicamid (slightly harmful) < metaflumizone (moderately harmful) < sulfoxaflor (moderately harmful).
Discussion
This study shows the differences between six evaluated insecticides in terms of their toxicity to N. tenuis. Our results are based on both lethal (acute toxicity) and sublethal (offspring production and longevity) effects on adults fed with treated prey for 3 days. Spirotetramat and deltamethrin showed no non-target activity, flubendiamide, and, notably flonicamid produced a moderate impact on adult predators, while metaflumizone and sulfoxaflor were clearly detrimental to N. tenuis adults exposed to these insecticides at trophic level.
Pesticides may interfere with the feeding behavior of exposed insects due to their repellent or antifeedant properties (Desneux et al. 2007). Flonicamid, flubendiamide, and metaflumizone are described as insecticides with antifeedant properties (Tomlin 2009). In our assays, we did not detect any of such effects because N. tenuis consumed a high percentage of eggs similar in all treatments and control, although the insects could also feed on a fresh been segment included in the arena. This natural enemy can survive for several days feeding only on plants when prey is scarce (Urbaneja et al. 2005).
In general, there is little information about the non-target toxicity of these insecticides when the uptake is by ingestion. In this study, spirotetramat did not produce mortality or decrease the offspring production of N. tenuis. Only a small reduction of survival was observed, and spirotetramat was therefore categorized as harmless. A study performed with spirotetramat on larvae and adults of the predator Cryptolaemus montrouzieri Mulsant (Coleoptera: Coccinelidae) also confirmed the safety of this insecticide via the food chain (Planes et al. 2013). The insecticide did not affect adult survival until 40 days after the treatment, larval and pupal mortality, fecundity, and egg hatching.
Flubendiamide is a synthetic diamide insecticide with a high activity against Lepidoptera but without evidence of activity in other insect orders (Gentz et al. 2010). Nevertheless, flubendiamide produced a slight reduction of N. tenuis offspring production. This insecticide did not cause mortality or sublethal effects (feeding, life span and reproduction) on Bombus impatiens Cresson (Hymenoptera: Apidae) when ingested in a honey solution. Gontijo et al. (2015) studied the impact of diamide chlorantraniliprole, a pesticide with a similar mode of action, on Orius insidiosus (Say) (Hemiptera: Anthocoridae). They reported a lack of lethal effects when the natural enemy was fed on treated emerged sunflower seeds as a unique water source, although a delay of oviposition onset was observed. However, when adults of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) were exposed to treated sunflower during their maturation period, chlorantraniliprole reduced the subsequent survival and fecundity of the predator (Gontijo et al. 2014).
In our work, deltamethrin reduced only offspring production of N. tenuis. Mohaghegh et al. (2000) studied deltamethrin toxicity on Podisus maculiventris (Say) (Hemiptera: Pentatomidae) by direct ingestion of treated drinking water. After exposure of 48 h to a field dose, neither acute effects nor sublethal effects in longevity or overall reproduction were observed. However, a longer preoviposition period for adults was reported, which may reflect the fact that reproduction was initially affected by deltamethrin similarly to our observations for N. tenuis (offspring production was evaluated for 6 days after treatment). Also in concordance with our results, flonicamid had no direct toxicity on fourth-instar larvae and females of Adalia bipunctata (L.) (Coleoptera: Coccinellidae) when fed with treated aphids for 3 days (Jalali et al. 2009); however, this study solely considered lethal effects.
Metaflumizone and sulfoxaflor were the most noxious products to N. tenuis. Metaflumizone is more active when ingested than by contact, and it controls a broad range of pests across the orders Lepidoptera, Coleoptera, Hemiptera, Homoptera, Hymenoptera, Diptera, etc. (Tomlin 2009). In our work, its acute toxicity was not high, although an important detrimental impact was observed in offspring production and survival. On the contrary, it was moderately harmful to N. tenuis in a residual laboratory test, causing a significant mortality after 3 days of exposition to fresh residues both on inert substrate (60.9% of corrected mortality) or on plant leaves (82.8%) (Wanumen et al. 2016).
Sulfoxaflor belongs to a novel class of insecticides, the sulfoximines, which are aimed to control of a broad range of sap-feeding insect pests (Babcock et al. 2011). By ingest of contaminated prey, this insecticide showed a small lethal effect. On the contrary, fresh and aged residues of sulfoxaflor produced a 100% of mortality on N. tenuis in laboratory tests (Wanumen et al. 2016). Thiamethoxam and imidacloprid are products classified, according to their modes of action, into the same group that includes sulfoxaflor, nAChR competitive modulators (IRAC 2013). Tillman (2006) reported an important lethal effect when thiamethoxam was included in a gel-like food offered to Nezara viridula (Hemiptera: Pentatomidae) (L.) nymphs and adults. Torres and Ruberson (2004) also report that neonicotinoid treatments (imidacloprid and thiamethoxam) produced a high mortality of Podisus nigrispinus (Dallas) (Hemiptera: Pentatomidae) nymphs by ingestion (via treated drinking water). He et al. (2012) observed an important effect on the voracity and functional response of Serangium japonicum Chapin (Coleoptera: Coccinellidae) adults after being fed with B. tabaci eggs contaminated with imidacloprid. However, in that case, the predator made a fast recovery after exposure ceased.
The effectiveness of IPM programs can be optimized only through a detailed understanding of the predators’ biological responses to the pesticides with which they will be integrated. Nevertheless, there are few studies about trophic contamination of predators. Furthermore, the sublethal effects of pesticides on natural enemies are rarely taken into account when IPM programs are established (Desneux et al. 2007). This work provides a better understanding of the impact of several modern insecticides associated with contaminated prey exposure. Although translating laboratory results to field conditions is always difficult, all the insecticides studied in this work are mainly applied as foliar sprays in tomato crops and are used against pests known to be prey species of N. tenuis. This mirid predator spends much of its time at the tops of crop canopies, and it is a generalist and voracious predator. Therefore, its probability of intoxication by trophic contamination is high. In addition, exposure to insecticide-treated prey may be discontinuous because natural enemies likely alternate foraging in treated and untreated areas. However, we think that this consideration should be tempered because the time of exposure in our work was only a small fraction of N. tenuis life span. Moreover, the selected prey in this study consisted of lepidopteran eggs, but other prey, such as B. tabaci nymphs, may contain higher concentrations of insecticides.
In summary, our results suggest that spirotetramat and deltamethrin are harmless to N. tenuis via treated prey, while flubendiamide produces a slight reduction in predator fitness that could disappear in a less intensive exposure scenario. However, for flonicamid, and particularly for metaflumizone and sulfoxaflor, the negative impact through trophic contamination must be taken into account to preserve augmentative releases of N. tenuis in tomato crops.
Acknowledgments
A. Wanumen acknowledges to “Research and Development and Innovation Projects in Science, Technology and Innovation and Research Groups in Colombia” (COLCIENCIAS) by the Francisco José de Caldas doctoral fellowship. This work was partially supported by the Spanish Ministry of Education and Culture (project AGL2010-22196-C02-02 and AGL2013-47603-C2-1-R to Elisa Viñuela).
References Cited
- Arnó J., Castañé C., Riudavets J., Gabarra R. 2010.. Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull. Entomol. Res. 100: 105–115. [DOI] [PubMed] [Google Scholar]
- Arnó J., Gabarra R. 2011.. Side effect of selected insecticides on the Tuta absoluta (Lepidoptera: Gelechiidae) predators Macrolophus pygmaeus and Nesidiocoris tenuis (Hemiptera:Miridae). J. Pest Sci. 84: 513–520. [Google Scholar]
- Arnó J., Sorribas R., Prat M., Matas M., Pozo C., Rodriguez D., Garreta A., Gómez A., Gabarra R. 2009.. Tuta absoluta, a new pest in IPM tomatoes in the northeast of Spain. OILB-WPRS Bull. 49: 203–208. [Google Scholar]
- Asplen M. K., Anfora G., Biondi A., Choi D., Chu D., Daane K. M., Gilbert P., Gutierrez A. P., Hoelmer K. A. 2015.. Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J. Pest Sci. 88: 469–494. [Google Scholar]
- Babcock J. M., Gerwick C. B., Huang J. X., Loso M. R., Nakamura G., Nolting S. P., Rogers R. B., Sparks T. C., Thomas J., Watson G. B., Zhu Y. 2011.. Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag. Sci. 67: 328–334. [DOI] [PubMed] [Google Scholar]
- Bengochea P., Budia F., Viñuela E., Medina P. 2014.. Are kaolin and copper treatments safe to the olive fruit fly parasitoid Psyttalia concolor? J. Pest Sci. 87: 351–359. [Google Scholar]
- Biondi A., Zappalà L., Stark J. D., Desneux N. 2013.. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS One 8: e76548.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi A., Campolo O., Desneux N., Siscaro G., Palmeri V., Zappalà L. 2015.. Life stage-dependent susceptibility of Aphytis melinus DeBach (Hymenoptera: Aphelinidae) to two pesticides commonly used in citrus orchards. Chemosphere 128: 142–147. [DOI] [PubMed] [Google Scholar]
- Biondi A., Zappalà L., Di Mauro A., Tropea Garzia G., Russo A., Desneux N., Siscaro G. 2016.. Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? BioControl 61: 79–90. [Google Scholar]
- Calvo F. J., Bolckmans K., Belda J. E. 2012.. Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaco control in tomato. BioControl 57: 809–881. [Google Scholar]
- Calvo J., Urbaneja A. 2004.. Nesidiocoris tenuis, un aliado para el control biológico de la mosca blanca. Horticult. Int. 44: 20–25. [Google Scholar]
- Candolfi M. P., Barrett K. L., Campbell P. J., Foster R., Grandy N., Lewis M.-C., Oomen P. A., Schmuck R., Vogt H. 2001.. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non-target artrhropods. ESCORT 2 Workshop (European standard characteristics of non-target arthropod regulatory testing). Wageningen, The Netherlands, 21–23 March, 2000.
- Croft B. 1990.. Arthropod biological control agents and pesticides. John Wiley & Sons, New York, USA.
- De Puysseleyr V., De Man S., Hofte M., De Clercq P. 2012.. Plantless rearing of the zoophytophagous bug Nesidiocoris tenuis. BioControl 47: 101–113. [Google Scholar]
- Desneux N., Decourtye A., Delpuech J. M. 2007.. The sublethal effects of pesticides on beneficial organisms. Annu. Rev. Entomol. 52: 81–106. [DOI] [PubMed] [Google Scholar]
- Fernández M. M., Medina P., Fereres A., Smagghe G., Viñuela E. 2015.. Are mummies and adults of Eretmocerus mundus (Hymenoptera:Aphelinidae) compatible with modern insecticides? J. Econ. Entomol. 108: 2268–2277. [DOI] [PubMed] [Google Scholar]
- Gentz M., Murdoch G., King G. 2010.. Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest mangement. Biol. Control 52: 208–215. [Google Scholar]
- Gontijo P., Moscardini V., Michaud J. P., Carvalho G. 2014.. Non-target effects of chlorantraniliprole and thiamethoxam on Chrysoperla carnea when employed as sunflower seed treatments. J. Pest Sci. 87: 711–719. [Google Scholar]
- Gontijo P., Moscardini V., Michaud J. P., Carvalho G. 2015.. A Non-target effects of two sunflower seed treatments on Orius insidiosus (Hemiptera: Anthocoridae). Pest Manag. Sci. 71: 515–522. [DOI] [PubMed] [Google Scholar]
- Grafton-Cardwell E. E., Godfrey L. D., Chaney W. E., Bentley W. J. 2005.. Various novel insecticides are less toxic to humans, more specific to key pests. California Agric. 59: 29–34. [Google Scholar]
- Guedes R.N.C., Smagghe G., Stark J. D., Desneux N. 2016.. Pesticide-induced in arthropod pests for optimized IPM programs. Annu. Rev. Entomol. 61: [DOI] [PubMed] [Google Scholar]
- Hassan S. A., Bigler F., Bogenshutz H., Brun J., Calis J.N.M. 1994.. Results of the sixth joint pesticide testing programme of the IOBC/WPRS-working group “Pesticides and Beneficial Organisms”. Entomophaga 39: 107–119. [Google Scholar]
- He Y., Zhao J., Zheng Y., Desneux N., Wu K. 2012.. Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 21: 1291–1300. [DOI] [PubMed] [Google Scholar]
- IOBC. International Organisation for Biological and Integrated Control. 2016.. Pesticide side effect database. (https://www.iobc-wprs.org/ip_ipm/IOBC_Pesticide_Side_Effect_Database.html) (accesed 2 June 2016).
- IRAC. 2013.. Insecticide Resistance Action Committee. IRAC MoA classification scheme. Version 7.2. http://www.irac-online.org (accessed 28 September 2013).
- Jalali M., Van Leeuwen T., Tirry L., De Clercq P. 2009.. Toxicity of selected insecticides to the two-spot ladybird Adalia bipunctata. Phytoparasitica 37: 323–326. [Google Scholar]
- Lee C. Y. 2000.. Sublethal effects of insecticides on longevity, fecundity and behaviour of insects pests. A review . J. BioSci. 11: 107–112. [Google Scholar]
- Lu Y., Wu K., Jiang Y., Guo Y., Desneux N. 2012.. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487: 362–365. [DOI] [PubMed] [Google Scholar]
- MAGRAMA. 2015.. Ministry of Agriculture, Food and Environment. European community list of active substances included, excluded and under evaluation. http://www.magra-ma.gob.es/agricultura/pags/fitos/registro/fichas/pdf/ListaþSustanciasþactivasþaceptadasþexcluidasþ(abrilþ14).pdf (accessed 11 June 2013).
- Mohaghegh J., De Clercq P., Tirry L. 2000.. Toxicity of Selected Insecticides to the Spined Soldier Bug, Podisus maculiventris (Heteroptera: Pentatomidae). Biocontrol. Sci. Techn. 10: 33–40. [Google Scholar]
- Mollá O., Gonzáles-Cabrera J., Urbaneja A. 2011.. The combined use of Bacillus thuringienses and Nesidiocoris tenuis against the tomato borer Tuta absoluta. BioControl 56: 883–891. [Google Scholar]
- Mollá O., Biondi A., Alonso-Valiente M., Urbaneja A. 2014.. A comparative life history study of towo mirid bugs preying on Tuta absoluta and Ephestia Kuehniella eggs on tomato crops: implications for biological control. Biocontrol 59: 175–183. [Google Scholar]
- Moreno-Ripoll R., Gabarra R., Symondson W.O.C., King R. A., Agustí N. 2014.. Do the interactions among natural enemies compromise the biological control Bemisia tabaci? J. Pest Sci. 87: 133–141. [Google Scholar]
- OJEC. 2016.. Official Journal of the European Union. Regulation 1107/2009 of the European Parliament and Council of 25 August 2016, L. 199, 8-11.
- Pan H., Liu Y., Liu B., Lu Y., Xu X., Qian X., Wu K., Desneux N. 2014.. Lethal and sublethal effects of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, on the mirid bug Apolygus lucorum. J. Pest Sci. 87: 731–738. [Google Scholar]
- Pérez-Hedo M., Urbaneja A. 2015.. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. 88: 65–73. [Google Scholar]
- Pérez-Hedo M., Urbaneja-Bernat P., Jaques J. A., Flors V., Urbaneja A. 2015.. Defensive plant responses induced by Nesidiocoris tenuis (Hemiptera: Miridae) on tomato plants. J. Pest Sci. 88: 543–554. [Google Scholar]
- Pinder J. E., III, Wiener J. G., Smith M. H. 1978.. The Weibull distribution: a new method of summarizing survivorship data. Ecology 59: 175–179. [Google Scholar]
- Planes L., Catalán J., Tena A., Pocuna J. L., Jacas J. A., Izquierdo J., Urbaneja A. 2013.. Lethal and sublethal effects of spirotetramat on the mealbug destroyer, Cryptolaemus montrouzieri. J. Pest Sci. 86: 321–327. [Google Scholar]
- Püntener W. 1981.. Manual for field trials in plant protection, 2nd ed. Syngenta International AG, Switzerland. [Google Scholar]
- Ruberson J. R., Nemoto H., Hirose Y. 1998.. Pesticides and conservation of natural enemies in pest management, pp. 207–220. In Barbosa P., (ed.), Conservation biological control. Academic Press, San Diego, USA. [Google Scholar]
- Sanchez J. 2008.. Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric. For. Entomol. 10: 75–80. [Google Scholar]
- Stat-Point Technologies, Inc. 2016.. Statgraphics centurion: data analysis and statistical software (actualized 2009). (http://www.statgraphics.net/wp-content/uploads/2015/03/Centurion-XVI-Manual-Principal.pdf) (accessed 25 August 2016).
- Sterk G., Hassan S., Baillod M., Bakker F., Bigler F., Blumel S., Bogenschutz H., Boller E., Bromand B., Brun J., et al. 1999.. Results of the seventh joint pesticide testing programmer carried out by the IOBC/WPRS-Working Group “Pesticides and Beneficial Organisms”. BioControl 44: 99–117. [Google Scholar]
- SYSTAT. 2002.. SYSTAT for Windows, version 10.2. SYSTAT software Inc, California. [Google Scholar]
- Tillman P. 2006.. Susceptibility of pest Nezara viridula (Heteroptera:Pentatomidae) and parasitoid Thrichopoda pennipes (Diptera: Tachinidae) to selected insecticides. J. Econ. Entomol. 99: 648–657. [DOI] [PubMed] [Google Scholar]
- Tomlin C. 2009.. The pesticide manual. A world compendium. Ed. British. Crop. Production Council (BCPC), Hampshire.
- Torres J., Ruberson J. 2004.. Toxicity of thiamethoxam and imidacloprid to Podisus nigrispinus (Dallas) (Heteroptera:Pentatomidae) nymphs associated to aphid and whitefly control in cotton. Neotrop. Entomol. 33: 99–106. [Google Scholar]
- Urbaneja A., Tapia G., Stansly P. 2005.. Influence of host plant and prey availability on developmental time and survivorship of Nesidiocoris tenuis (Het.: Miridae). BioControl. Sci. Techn. 15: 513–518. [Google Scholar]
- Van de Veire M., Sterk G., Van der Staaij M., Ramakers P., Tirry L. 2002.. Sequential testing scheme for the assessment of the side-effects of plant protection products on the predatory bug Orius laevigatus. BioControl 47: 101–113. [Google Scholar]
- Wanumen A. C., Carvalho G. A., Medina P., Viñuela E., Adán A. 2016.. Residual acute toxicity of some modern insecticides toward two mirid predators of tomato pests. J. Econ. Entomol. 109: 1049–1085. [DOI] [PubMed] [Google Scholar]
- Wheeler W. B. 2002.. Role of research and regulation in 50 years of pest management in agriculture. J. Agric. Food Chem. 50: 4151–4155. [DOI] [PubMed] [Google Scholar]
- Zappalà L., Biondi A., Alma A., Al-Jboory I. J., Arnò J., Bayram A., Chailleux A., El-Arnaouty A., Gerling D., Guenaoui Y., et al. 2013.. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle-East, and their potential use in pest control strategies. J. Pest Sci. 86: 635–647. [Google Scholar]