Summary
Background
Alternate‐day‐fasting (ADF) has been proposed as an effective dieting method. Studies have found that it also can increase life span in rodents, and reduce inflammation in humans. The aim of this paper was to systematically review the efficacy of ADF compared to very‐low‐calorie dieting (VLCD) in terms of weight loss, and reduction of fat mass and fat‐free mass.
Methods
Systematic review: PubMed literature searches were performed. Fixed review procedures were applied. Studies were evaluated for quality. Twenty‐eight studies were included. Meta‐analysis: 10/28 studies (four ADF and six matched VLCD) were further analyzed.
Results
After adjustment for BMI and duration, there was no significant difference in mean body weight loss (VLCD 0.88 kg more weight loss than ADF, 95% CI: −4.32, 2.56) or fat‐free mass (VLCD 1.69 kg more fat‐free mass loss than ADF, 95% CI: −3.62, 0.23); there was a significant difference observed in fat mass (ADF 3.31 kg more fat mass loss than VLCD, 95% CI: 0.05, 6.56). Meta‐analysis showed that, among ADF studies, the pooled change in body weight, fat mass and fat‐free mass was 4.30 kg (95% CI: 3.41, 5.20), 4.06 kg (95% CI: 2.99, 5.13) and 0.72 kg (95% CI: −0.07, 1.51), respectively, while among VLCD studies, the pooled change was 6.28 kg (95% CI: 6.08, 6.49), 4.22 kg (95% CI: 3.95, 4.50) and 2.24 kg (95% CI: 1.95, 2.52), respectively.
Conclusions
Our results from both the systematic review and the meta‐analysis suggest that ADF is an efficacious dietary method, and may be superior to VLCD for some patients because of ease of compliance, greater fat‐mass loss and relative preservation of fat‐free mass. Head‐to‐head randomized clinical trials are needed to further assess relative efficacy of these two approaches.
Keywords: Alternate‐day fasting, fat mass, obesity, very‐low‐calorie diet
Introduction
In 2014, there were more than 1.9 billion adults classified as overweight, of which 600 million were classified as obese (11% of men and 15% of women). These figures are double what they were in the 1980s, a clear indication that obesity is spreading widely across the globe. The obesity epidemic is now associated with more deaths globally than underweight 1.
A widely used strategy to lose weight is caloric restriction (CR) 2. A common CR method that became popular in the 1980s was very‐low‐calorie dieting (VLCD) 3. VLCD is by definition prescribing fewer than 800 calories to be consumed daily 4. Although VLCD has been shown to be an effective weight loss strategy in the short term 3, it has also been argued that its risk of weight regain is greater due to its association with binge eating disorder 5, behavioural fatigue 6 and loss of fat free mass 7.
Intermittent CR regimens were designed to improve adherence 8. An intermittent CR regimen that has recently emerged is alternate‐day‐fasting (ADF). With ADF, there is a fasting day, during which typically 25% of the dieter's estimated energy needs (typically <800 calories/day) are consumed, followed by a normal feeding day when food and liquids are consumed ad libitum 9. ADF has been associated with lower risk of weight regain, perhaps due to better compliance 10 and with relative preservation of fat‐free mass 7.
Aside from the possible weight control benefits, at least in some animal studies, fasting or ADF appears to have multiple health‐promoting effects, notably related to aging, cancer, inflammation and neurodegeneration 11. With respect to aging, one study has suggested that ADF can increase the life span of rodents by up to 80%. The possible mechanism of this effect is that fasting decreases glucose levels and insulin‐like growth factor (IGF‐1), which are aging promoters 12. With respect to cancer, a study has suggested that ADF greatly reduces the incidence of lymphoma in mice 13, and another study found that fasting one day per week may delay oncogenesis in p53‐missing mice 14. In addition, a recent study concluded that by reducing glucose levels, insulin and ketone bodies, intermittent fasting may create a difficult environment for cancer cells to survive, thus improving the action of chemotherapeutic agents 15.
In humans, a recent study has shown that intermittent fasting reduces oxidative stress and inflammation and it improves cellular glucose uptake and insulin sensitivity 11. In addition, two studies have shown that fasting may improve neurologic function in the following ways: by increasing the levels of antioxidants, neurotropic factors and protein chaperones, and reducing the level of pro‐inflammatory markers 16, and by upregulating the expression of synaptic plasticity‐related proteins, as well as anti‐apoptotic pathways 17.
In terms of body composition, a study showed that intermittent fasting reduces body weight, fat mass, waist circumference and blood pressure 18. The same authors stated that the metabolic effects of intermittent fasting include reductions in total cholesterol, LDL and triglycerides, as well as improvement of satiety through reductions in Leptin and Resistin, and increases in Adiponectin 18.
A recent review comparing weight loss reported using daily CR and intermittent CR found that intermittent CR was as effective as daily CR in reducing body weight as well as in preserving fat‐free mass 7. In the present review, we compared two types of severely energy‐restricted diets, achieved through either daily energy restriction or every‐other‐day energy restriction.
We systematically reviewed the efficacy of ADF diets compared to VLCD (the control) in terms of weight loss, and reduction of fat mass and fat‐free mass. Our objective was to explore the hypothesis that ADF could be an effective alternative to more restrictive dieting approaches, namely VLCD.
Methods
Data sources
Literature searches were conducted using PubMed, with the time frame of publication 1 January 2000 to 30 September 2015. An example of search commands is detailed as follows:
(Caloric restriction [tiab] OR VLED [tiab] OR LED [tiab] OR very low energy diet [tiab] OR low energy diet [tiab] OR LCD [tiab] OR 25% energy deficit weight loss diet [tiab] OR calorie restriction [tiab] OR Modifast [tiab] OR very‐low‐calorie diet [tiab] OR dietary restriction [tiab] OR daily energy restriction [tiab] OR VLCD [tiab] OR energy restriction [tiab] OR low calorie [tiab] OR very low calorie dietary intervention [tiab] OR continuous energy restriction [tiab] OR CER [tiab] OR continuous diet[tiab] OR alternate day fasting[tiab] OR ADF[tiab] OR every other day fast[tiab] OR ADMF[tiab] OR ad libitum every other day[tiab] OR ADCR diet[tiab] OR alternate day calorie restriction[tiab] OR modified alternate fasting[tiab] OR intermittent fasting[tiab] OR diet in every other day[tiab] ) AND "weight loss"[Mesh]
Study selection and criteria
The following exclusion criteria were applied: case reports, letters, comments, reviews or animal studies; languages other than English; and publication date other than from 1 January 2000 to 30 September 2015. The following inclusion criteria were applied: adults aged 18–70 years, with BMI ≥25 kg/m2, good general state of health (i.e. without a diagnosed condition), on only very‐low‐energy diets (<800 calories/d) for VLCD studies and interventions lasting between 3 and 12 weeks.
Data extraction
Studies were selected for data extraction if they met the above inclusion and exclusion criteria and reported at least weight loss data. We only included study arms where diet only was used; we excluded any arms that used physical activity or drugs to ensure better comparability between study interventions. Sample size and intervention length were recorded. Characteristics of the initial study sample (e.g. age, sex and weight), baseline body weight, fat mass, fat‐free mass and waist circumference were captured when available. Conversion of units to keep data comparable was implemented when necessary. Two reviewers independently reviewed the studies, extracted data and then resolved disparities by agreement (Supplementary Table S1, available as Supplementary data at OSP online).
Quality assessment
Two reviewers used the Downs and Black quality checklist to assess the risk of study bias (ROB) for each included study 19: (i) low ROB: when a study fulfilled all the following criteria: stated the objective, described the main outcomes, described the characteristics of the enrolled subjects, clearly described the interventions, described the main findings, randomized the subjects to the intervention groups, concealed the intervention assignments until recruitment was complete and partially or fully described the distribution of potential confounders in each treatment group; (ii) moderate ROB: if a study did not fulfil one of the above criteria, or if such could not be verified and (iii) high ROB: if a study did not fulfil more than one of the above criteria.
Statistics and meta‐analysis
All statistical analysis were pre‐specified. The dietary interventions were grouped into two sets: ADF and VLCD. Study‐level data were summarized using descriptive statistics. If not reported, the standard error of means were computed using the following formula: SE = SD/ √n. STATA 14 was used both for the meta‐analysis and the meta‐regression. The random‐effects meta‐analysis approach was performed to estimate the overall difference in each intervention. Meta‐regression models were used to adjust for BMI and length of intervention. The residual maximum‐likelihood method was used to estimate the additive (between‐study) component of variance Tau2. Heterogeneity was tested using I2 test. The p‐values calculated by Monte Carlo permutation test were used to address multiple testing. A statistically significant difference was defined by a p‐value less than 0.05.
Results
Data retrieval
A flow chart showing the systematic review process is provided in Figure 1. The initial search resulted in 2,357 publications. After applying the exclusion criteria, 627 remained. The full text of these studies was then retrieved, and after screening for inclusion criteria, data were extracted from 28 studies, 6 ADF and 22 VLCD studies. Four articles contributed to more than one arm of the analysis. Only the 10 studies that reported the change with standard errors for all three outcomes of interest (i.e. weight loss, fat mass and fat‐free mass were included in the meta‐analysis) (Figure 1).
Figure 1.
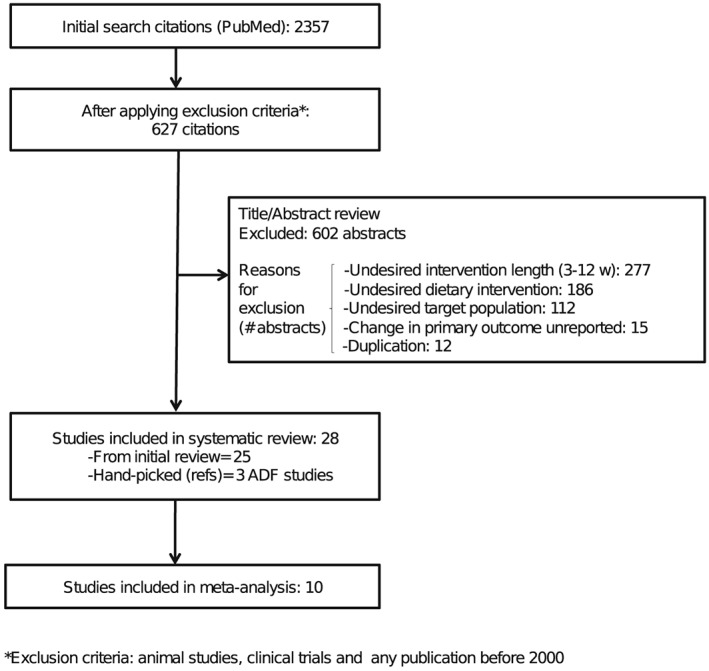
Flow chart of the systematic review process.
Characteristics of study participants
A total of 1,193 study participants were included in the analyses, 132 who underwent ADF and 1,060 who underwent VLCD. For ADF studies reporting this information, mean age of participants was 42.33 years, 92.42% were female, mean BMI was 33.17 kg/m2, mean baseline body weight was 90.28 kg, mean fat mass was 38.06 kg, mean fat‐free mass was 48.32 kg and mean waist circumference was 97.70 cm. For the VLCD studies, mean age of participants was 40.04 years, 67.76% were female, mean BMI was 31.15 kg/m2, mean baseline body weight was 83.55 kg, mean fat mass was 31.34 kg, mean fat‐free mass was 44.52 kg and mean waist circumference was 100.56 cm (Table 1A).
Table 1A.
Patient characteristics
| Patient characteristics | ADF (132) | VLCD (1,060) |
|---|---|---|
| Age (years) | 42.33 | 40.04 |
| Females (%) | 92.42 | 67.76 |
| BMI (kg/m2) | 33.17 | 31.15 |
| Body weight (kg) | 90.28 | 83.55 |
| Fat mass (kg) | 38.06 | 31.34 |
| Fat‐free mass (kg) | 48.32 | 44.52 |
| Waist circumference (cm) | 97.70 | 100.56 |
ADF, alternate‐day fasting; BMI, body mass index; VLCD, very‐low‐calorie‐diets.
Characteristics of included studies
For the included ADF studies, one was published between 2000 and 2010, and five between 2011 and 2015. Two studies had intervention durations of 3–8 weeks, and four 9–12 weeks. Four studies were conducted in North America and two in Asia. Of the included VLCD studies, 14 were published between 2000 and 2010, and eight between 2011 and 2015. Eighteen had intervention durations of 3–8 weeks, and four 9–12 weeks. Nineteen were conducted in Europe and three in Australia (Table 1B).
Table 1B.
Study characteristics
| Study characteristics | Number of studies (ADF) | Number of participants | Number of studies (VLCD) | Number of participants |
|---|---|---|---|---|
| Publication year | ||||
| 2000–2010 | 1 | 16 | 14 | 883 |
| 2011–2015 | 5 | 116 | 8 | 177 |
| Intervention length | ||||
| 3–8 weeks | 2 | 41 | 18 | 917 |
| 9–12 weeks | 4 | 91 | 4 | 143 |
| Study location | ||||
| North America | 4 | 91 | ||
| Europe | 19 | 1,000 | ||
| Asia | 2 | 41 | ||
| Other | 3 | 60 | ||
ADF, alternate‐day fasting; VLCD, very‐low‐calorie‐diets.
Of the 28 studies included in the systematic review (Supplementary Table S2, available as Supporting Information at OSP online), after applying quality assessment based on Downs and Black criteria 19, 20 had high ROB, 7 had moderate ROB and 1 had low ROB. Of the 10 studies used in the meta‐analysis, 5 had high ROB and 5 had moderate ROB.
Missing data
Gender reporting was missing in three studies, age reporting in six and BMI reporting in one study. Baseline body weight was missing in 3 studies, fat mass in 11, fat‐free mass in 18 and waist circumference in 12 studies.
Characteristics of the studies included in the meta‐analysis
Table 2A details the 10 studies included in the meta‐analysis: 4 ADF and 6 VLCD. Mean age of the 662 participants included in the meta‐analysis was 44.69 ± 1.9 years, mean BMI was 31.31 kg/m2 and 78.06% were females. ADF studies were all grant funded, while all of the VLCD studies were industry funded.
Table 2A.
Studies included in the meta‐analysis
| Name | Sample size (n) | Females(%) | Age * (years) | Baseline BMI* | Length (weeks) | Change in body weight* (kg) | Change in fat mass* (kg) | Change in fat‐free mass* (kg) |
|---|---|---|---|---|---|---|---|---|
| ADF | ||||||||
| Varady, 2013 20 | 15 | 66 | 47 ± 3.0 | 26 ± 1.0 | 12 | 5.20 ± 0.9 | 3.60 ± 0.7 | 1.60 ± 0.5 |
| Bhutani, 2013 21 | 25 | 96 | 40 ± 2.0 | 35 ± 1.0 | 12 | 3.00 ± 1.0 | 2.00 ± 1.0 | 1.00 ± 1.0 |
| Klemple, 2013 (HF) 9 | 17 | 100 | 42 ± 3.0 | 35 ± 0.7 | 8 | 4.30 ± 1.0 | 5.40 ± 1.5 | 1.10 ± 1.3 |
| Klemple, 2013 (LF) 9 | 18 | 100 | 43 ± 2.0 | 36 ± 0.7 | 8 | 3.70 ± 0.7 | 4.20 ± 0.6 | 0.50 ± 0.7 |
| Varady, 2009 10 | 16 | 75 | 46 ± 2.0 | 34 ± 1.0 | 8 | 5.60 ± 1.0 | 5.40 ± 0.8 | 0.10 ± 0.1 |
| VLCD | ||||||||
| Munro, 2013 (placebo) 22 | 19 | 79 | 47 ± 2.0 | 34 ± 0.8 | 4 | 5.79 ± 0.4 | 4.19 ± 0.4 | 1.33 ± 0.4 |
| Munro, 2013 (fish oil)22 | 20 | 75 | 45 ± 2.0 | 31 ± 0.6 | 4 | 6.12 ± 0.3 | 4.36 ± 0.3 | 1.68 ± 0.3 |
| Westerterp‐P, 2005† 23 | 76 | 70 | 28 ± 0.3 | 4 | 5.90 ± 0.2 | 5.00 ± 0.2 | 2.50 ± 0.5 | |
| Lejeune, 2005 24 | 113 | 45 ± 1.0 | 29 ± 0.2 | 4 | 6.30 ± 0.3 | 4.00 ± 0.3 | 2.30 ± 0.2 | |
| Westerterp‐P, 2004 25 | 148 | 44 ± 0.8 | 30 ± 0.2 | 4 | 6.40 ± 0.1 | 3.90 ± 0.3 | 2.50 ± 0.2 | |
| Kovacs, 2004 26 | 104 | 75 | 30 ± 0.3 | 4 | 6.40 ± 0.3 | 4.00 ± 0.3 | 2.40 ± 0.3 | |
| Lejeune, 2003 27 | 91 | 29 ± 0.3 | 4 | 6.60 ± 0.2 | 4.10 ± 0.2 | 2.50 ± 0.2 | ||
Mean + SEM.
Numbers were computed from one figure of the article.
ADF, alternate‐day fasting; BMI, body mass index; HF, high fat group; LF, low fat group; VLCD, very‐low‐calorie‐diets.
Meta‐analysis results
Unadjusted values show that compared to the VLCD studies, ADF participants had a smaller loss in body weight (−1.99 kg, 95% confidence interval [CI]: −2.94, −1.04) and a smaller loss in fat‐free mass (−1.60 kg, 95% CI: −2.40, −0.80), while no significant change was observed between diet interventions in fat mass (−0.16 kg, 95% CI: −1.19, 0.87) (Table 2B).
Table 2B.
Meta‐regression results
| Variables | Unadjusted (95% CI) | P‐value | Adjusted (95% CI) | P‐value |
|---|---|---|---|---|
| Body weight (kg) | ||||
| ADF | −1.99 (−2.94, −1.04) | <0.01 | −0.88 (−4.32, 2.56) | 0.57 |
| BMI | −0.08 (−0.26, 0.09) | 0.28 | ||
| Length | −0.14 (−0.68, 0.40) | 0.56 | ||
| Fat mass (kg) | ||||
| ADF | −0.16 (−1.19, 0.87) | 0.73 | 3.31 (0.05, 6.56) | 0.05 |
| BMI | −0.10 (−0.27, 0.06) | 0.19 | ||
| Length | −0.57 (−1.08, −0.05) | 0.03 | ||
| Fat‐free mass (kg) | ||||
| ADF | −1.60 (−2.40, −0.80) | <0.01 | −1.69 (−3.62, 0.23) | 0.07 |
| BMI | −0.18 (−0.33, −0.03) | 0.02 | ||
| Length | 0.06 (−0.28, 0.41) | 0.68 | ||
ADF, alternate‐day fasting; BMI, body mass index; CI, confidence interval; VLCD, very‐low‐calorie‐diets.
After adjustment for BMI and duration of the dietary intervention, there was no significant difference between interventions in body weight (−0.88 kg, 95% CI: −4.32, 2.56) or fat‐free mass (−1.69 kg, 95% CI: −3.62, 0.23) (Table 2B). There was a significant difference between interventions in that the adjusted loss of fat mass on the ADF regimens was 3.31 kg greater than on the VLCD regimens (95% CI: 0.05, 6.56).
Regarding change in body weight, Figure 2 shows that, among ADF studies, the smallest reduction was 3.00 kg (95% CI: 1.04, 4.96) and the greatest reduction was 5.60 kg (95% CI: 3.64, 7.56). The pooled change was 4.30 kg (95% CI: 3.41, 5.20). Among the VLCD studies, the smallest reduction in body weight was 5.79 kg (95% CI: 4.92, 6.65) and greatest reduction was 6.40 kg (95% CI: 5.81, 6.98). The pooled change was 6.28 kg (95% CI: 6.08, 6.49).
Figure 2.
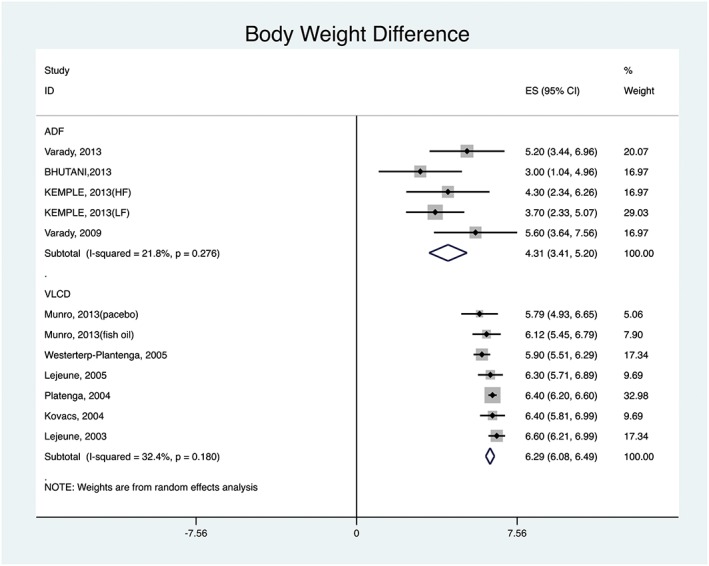
ADF versus VLCD: body weight difference pooled results.
With regard to change in fat mass, Figure 3 shows that, among ADF studies, the smallest reduction was 2.00 kg (95% CI: 0.04, 3.96) and the greatest was 5.40 kg (95% CI: 3.83, 6.97). The pooled change was 4.06 kg (95% CI: 2.99, 5.13). For the VLCD studies, the smallest reduction in fat mass was 3.90 kg (95% CI: 3.31, 4.49) and greatest was 5.00 kg (95% CI: 4.41, 5.59). The pooled change was 4.22 kg (95% CI: 3.95, 4.50).
Figure 3.
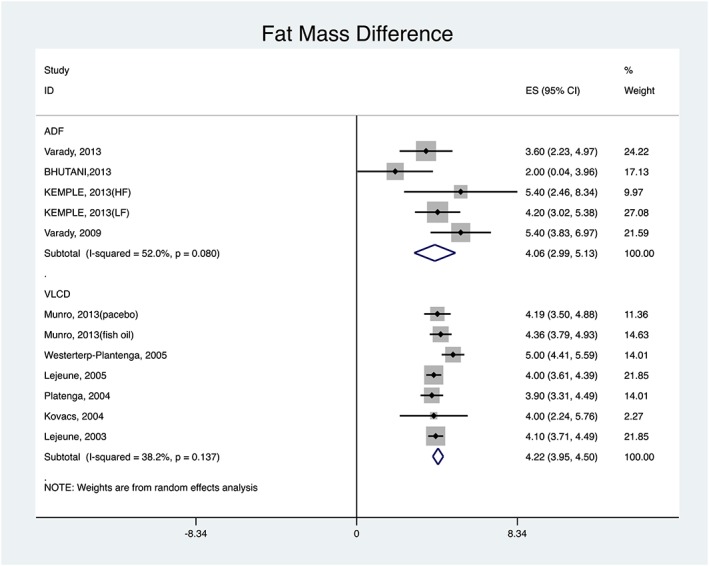
ADF versus VLCD: fat mass difference pooled results.
For change in fat‐free mass, Figure 4 shows that, among ADF studies, the smallest reduction was 0.10 kg (95% CI: −0.1, 0.30) and the greatest 1.60 kg (95% CI: 0.62, 2.58). The pooled change was 0.72 kg (95% CI: −0.07, 1.51). Among the VLCD studies, the smallest reduction was 1.33 kg (95% CI: 0.59, 2.07) and the greatest 2.50 kg (95% CI: 2.10, 2.89). The pooled change was 2.24 kg (95% CI: 1.95, 2.52).
Figure 4.
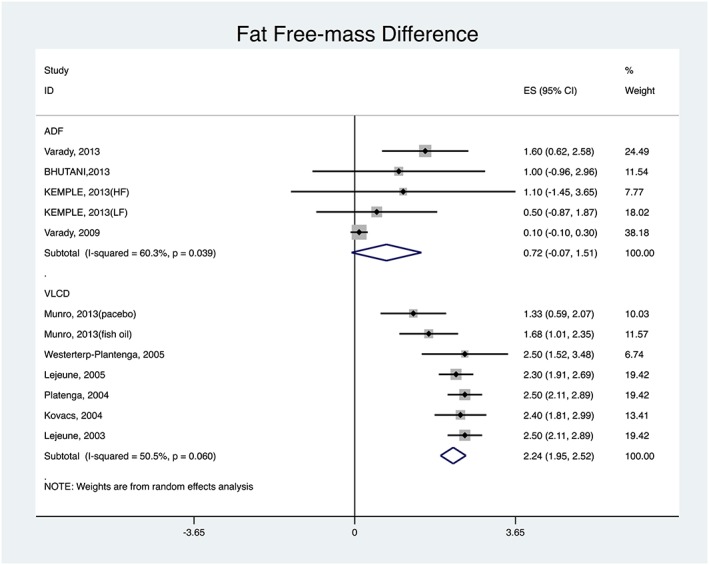
ADF versus VLCD: fat‐free mass difference pooled results.
Discussion
This is the first paper to perform a systematic review together with a meta‐analysis comparing ADF and VLCD regimens in terms of weight, fat mass and fat‐free mass reduction.
Our meta‐analysis shows that both dietary interventions are efficacious, resulting in substantial body weight loss. Although the magnitude of weight loss by VLCD is somewhat greater, ADF results in greater relative reduction of fat mass and lesser reduction of fat‐free mass.
While magnitude of initial weight loss is greater using VLCD, studies have shown, however, that VLCD may increase the risk of headache, fatigue, dizziness, hair loss, constipation and dehydration. As a result, it requires regular medical supervision 28, 29, 30, 31. Also, VLCD has been associated with an increased risk of developing gallstones; one study showed that after 8 weeks of VLCD, 25% of patients developed gallstones and 6% required cholecystectomy 32. VLCD may also be associated with development of binge eating disorder. Following a VLCD regimen one study showed that the disorder developed among 62% of subjects, but decreased among 39% 33. However, Wadden et al. (1994) found no difference in binge‐eating occurrence or in weight loss among binge eaters versus non‐binge eaters who were both following a VLCD regimen. Their hypothesis was that binge eaters might find VLCD easier to follow than conventional calorie‐restrictive diets 34.
In their meta‐analysis, Tsai and Wadden (2006) concluded that VLCD may be a viable option to lose weight in the short term; however, they found that patients failed to maintain 15 to 25% of VLCD‐associated initial weight loss, due to difficult compliance, adaptive hormonal changes and our toxic food environment 3.
We found that ADF may be as effective as even the very restrictive VLCD with respect to fat‐mass reduction, and provides relative preservation of fat‐free mass. Two recent reviews have evaluated the effects of intermittent diets versus daily CR on weight loss, fat mass and fat‐free mass 7, 35. One (non‐systematic) reported fat‐free mass preservation by the intermittent diets 7, while the other one (systematic) reported no difference between the diets 35.
In our review, efficacy of ADF for weight‐ and fat‐mass reduction was an expected finding, and it is most likely explained by the substantial overall decrease in energy intake that adherence to alternate‐day energy restriction will provide. It is noteworthy, however, that as prescribed, VLCD would provide somewhat greater levels of energy restriction than ADF, as it generally provides a >50% reduction in estimated energy needs 4. This implies logically that in order for mean weight and fat loss on ADF regimens to be as good as that achieved on VLCD regimens, average compliance with ADF must have been superior to compliance with VLCD.
On the other hand, preservation of fat‐free mass, at the cost of fat mass during weight reduction 18 is unexpected with the level of energy restriction that ADF prescribes. To our knowledge, this is the first review to report this finding. The mechanism for this effect, if confirmed by other studies, is unclear, although it is possible that either the fasting period is brief enough that there is less loss of lean tissue in the first place, or that the days where full energy needs are met allow for recovery of fat‐free mass by rebuilding lean tissue lost on the fasting days.
Several studies have reported that ADF did not appear to cause a hyperphagic response on the feeding day 9, 20, 21, which may have facilitated the subjects' ability to maintain a substantial level of mean energy restriction. In line with this, Klempel et al. (2010) conducted a modified ADF study and found that, on feeding days, subjects only consumed 95% of their calculated energy intake 36. ADF has also been found to decrease hunger and increase satiety and dieting satisfaction in 8 to 12‐week studies, all of which may enhance the adherence to the diet 7, 9. The possible mediators of these appetite effects include reductions in Leptin and Resistin, and increases in Adiponectin 18. While a counter‐intuitive approach to weight control, it has been argued that ADF regimens may achieve relatively high levels of dietary adherence because they require energy restriction only every other day 10 and do not require a change in the types of food consumed, but rather a change in the pattern of consumption 9. Varady et al. (2009) reported ADF efficacy for weight reduction even during self‐implementation periods 10. For all the reasons presented earlier, we consider ADF a viable alternative approach to weight control, as also suggested by others 37.
As with any diet for weight loss, ADF reduction in body weight was related to the level of adherence to this dietary intervention 18, 38, 39. Its efficacy in at least one of the reviewed studies was maximized when combined with exercise at least three times per week 21. However, a very recent study by Barnoski et al. (2015) – who examined whether ADF improves eating behaviours in a way that promotes successful weight loss and weight loss maintenance – observed a reduction in body weight even without a change in physical activity 40. The same study observed no changes in appetite ratings (hunger, satisfaction and fullness), dietary restraint, emotional eating, uncontrolled eating or self‐efficacy in the ADF or the calorie restriction groups as compared to the control. The authors concluded that the role of beneficial eating behaviours in body weight reduction through ADF or CR remains unclear 40, hence warranting further research.
In line with other recent studies, the first finding of our review is that ADF appears to be an effective strategy for initial weight loss (at least the first eight weeks) in overweight and subjects with obesity. But most importantly, our review suggests that ADF may be superior to daily CR in terms of type of weight lost (fat vs. non‐fat), and adherence.
Health risks may also be affected favourably by ADF. Waist circumference, a marker of visceral obesity which is associated with coronary heart disease and diabetes 41, 42, was reduced by 4–10% from baseline in the studies reviewed, and correlated with overall weight reduction 7, 10, 43. Adherence to this regimen was also associated with a decrease in triglycerides, total cholesterol and LDL. These effects were also correlated with the reduction in body weight and visceral fat. The possible mechanism by which ADF alters lipids is via an increase in oxidation of free fatty acids during periods of weight loss, while free fatty acid synthesis is reduced 44. This leads to a reduction in very low density lipoprotein (VLDL) synthesis by the liver and thus reduced circulating levels of LDL 45. Thus, adherence to an ADF diet may be cardio protective.
This review was limited by the small number of ADF studies published to date, necessitating the inclusion of non‐randomized clinical trials. Furthermore, of the studies that were included in this review, there was an over representation of women on the ADF regimens (92%). Glucose response in women is adversely affected following a fast 46; therefore, it is possible that gender differences in physiologic response to the ADF regimen may have affected our analysis. Animal studies have suggested that gender plays an important role in the evolutionary adaptation to fasting. One study found that only female mice demonstrated increases in arousal and reduced Gherlin, suggesting that women may stand to achieve greater benefit from ADF 47. While these differences, on a physiologic level, are important in determining which diet may be most appropriate for a given patient, it is important to note that no gender differences in weight loss have yet been shown in human studies 48. Better responses to the ADF have, however, been shown in older individuals and Caucasians, but we were unable to assess this from the papers included in this review 48.
Conclusion
Among individuals with obesity, ADF is an efficacious dietary method, and may be superior to VLCD for some patients because of ease of compliance, greater fat‐mass loss and relative preservation of fat‐free mass. However, further studies comparing ADF to VLCD (ideally head‐to‐head randomized clinical trials) that also control for patient characteristics, are needed to confirm the efficaciousness of these two approaches for weight loss, and to determine if ADF is better suited to certain populations. This information is of interest to health care providers and dietitians, as well as individuals with obesity seeking effective and potentially easier to follow methods to lose weight.
Conflict of interests
None declared.
Author contributions
BA, AGA, JK and DS did the literature search and the systematic review of the studies. AGA did the quality assessment. BA and AA did the statistical analysis and the meta‐analysis. BA and LC wrote the manuscript. BA, AGA, AA, KC, AU and LC contributed to the interpretation and discussion of the results and reviewed and edited drafts of the manuscript.
Funding
BA was funded by a postdoctoral fellowship from Saudi Arabian Cultural Mission (SACM).
Acknowledgements
Our sincere gratitude to Dr. John McGready and Gayene Yenokyan for their help with data analysis, and the Saudi Arabian Cultural Mission (SACM) for financial support. Our gratitude to Drs. John McGready and Gayene Yenokyan from the Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics, for their help with data analysis.
Alhamdan, B. A. , Garcia‐Alvarez, A. , Alzahrnai, A. H. , Karanxha, J. , Stretchberry, D. R. , Contrera, K. J. , Utria, A. F. , and Cheskin, L. J. (2016) Alternate‐day versus daily energy restriction diets: which is more effective for weight loss? A systematic review and meta‐analysis. Obesity Science & Practice, 2: 293–302. doi: 10.1002/osp4.52.
References
- 1. World Health Organization . Obesity and Overweight. URL http://www.who.int/mediacentre/factsheets/fs311/en/. Updated January 2015. (accessed May 20 2015).
- 2. Del Corral P, Chandler‐Laney PC, Casazza K, Gower BA, Hunter GR. Effect of dietary adherence with or without exercise on weight loss: a mechanistic approach to a global problem. J Clin Endocrinol Metab 2009; 94: 1602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai AG, Wadden TA. The Evolution of Very‐Low‐Calorie Diets: An Update and Meta‐analysis. Obesity 2006; 14: 1283–93. [DOI] [PubMed] [Google Scholar]
- 4. Oberhauser F, Schulte DM, Faust M, et al. Weight loss due to a very low calorie diet differentially affects insulin sensitivity and interleukin‐6 serum levels in nondiabetic obese human subjects. Horm Metab Res 2012; 44: 465–470. [DOI] [PubMed] [Google Scholar]
- 5. Telch CF, Agras WS. The effects of a very low calorie diet on binge eating. Behav Ther 1993; 24: 177–93. [Google Scholar]
- 6. Smith DE, Wing RR. Diminished weight loss and behavioral compliance during repeated diets in obese patients with type II diabetes. Health Psychol 1991; 10: 378. [DOI] [PubMed] [Google Scholar]
- 7. Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev 2011; 12: e593–601. [DOI] [PubMed] [Google Scholar]
- 8. Varady KA, Hellerstein MK. Alternate‐day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr 2007; 86: 7–13. [DOI] [PubMed] [Google Scholar]
- 9. Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high‐fat diet produces similar weight loss and cardio‐protection as ADF with a low‐fat diet. Metabolism 2013; 62: 137–143. [DOI] [PubMed] [Google Scholar]
- 10. Varady KA, Bhutani S, Church EC, Klempel MC. Short‐term modified alternate‐day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr 2009; 90: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 11. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab 2014; 19: 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene‐disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell 2009; 8: 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Descamps O, Riondel J, Ducros V, Roussel AM. Mitochondrial production of reactive oxygen species and incidence of age‐associated lymphoma in OF1 mice: effect of alternate‐day fasting. Mech Ageing Dev 2005; 126: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 14. Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult‐onset calorie restriction and fasting delay spontaneous tumorigenesis in p53‐deficient mice. Carcinogenesis 2002; 23: 817–822. [DOI] [PubMed] [Google Scholar]
- 15. Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 2012; 4: 124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol 2010; 67: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh R, Manchanda S, Kaur T, et al. Middle age onset short‐term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 2015; 16: 775–788. DOI: 10.1007/s10522-015-9603-y. [DOI] [PubMed] [Google Scholar]
- 18. Bhutani SKM, Berger RA, Varady KA. Improvements in coronary heart disease risk indicators by alternate‐day fasting involve adipose tissue modulations. Obesity (Silver Spring) 2010; 18: 2152–2159. [DOI] [PubMed] [Google Scholar]
- 19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J 2013; 12: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 2013; 21: 1370–1379. [DOI] [PubMed] [Google Scholar]
- 22. Munro IA, Garg ML. Prior supplementation with long chain omega‐3 polyunsaturated fatty acids promotes weight loss in obese adults: a double‐blinded randomised controlled trial. Food Funct 2013; 4: 650–658. [DOI] [PubMed] [Google Scholar]
- 23. Westerterp‐Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 2005; 13: 1195–1204. [DOI] [PubMed] [Google Scholar]
- 24. Lejeune MP, Kovacs EM, Westerterp‐Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. Br J Nutr 2005; 93: 281–289. [DOI] [PubMed] [Google Scholar]
- 25. Westerterp‐Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord 2004; 28: 57–64. [DOI] [PubMed] [Google Scholar]
- 26. Kovacs EM, Lejeune MP, Nijs I, Westerterp‐Plantenga MS. Effects of green tea on weight maintenance after body‐weight loss. Br J Nutr 2004; 91: 431–437. [DOI] [PubMed] [Google Scholar]
- 27. Lejeune MP, Kovacs EM, Westerterp‐Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body‐weight loss in human subjects. Br J Nut 2003; 90: 651–659. [DOI] [PubMed] [Google Scholar]
- 28. National Task Force on the Prevention and Treatment of Obesity, National Institutes of Health . Very low‐calorie diets. JAMA 1993; 270: 967–74. [PubMed] [Google Scholar]
- 29. Mustajoki P, Pekkarinen T. Very low energy diets in the treatment of obesity. Obes Rev 2001; 2: 61–72. [DOI] [PubMed] [Google Scholar]
- 30. Saris WH. Very‐low‐calorie diets and sustained weight loss. Obes Res 2001; 9: 295–301S. [DOI] [PubMed] [Google Scholar]
- 31. Wadden TA, Stunkard AJ, Brownell KD. Very low calorie diets: their efficacy, safety, and future. Ann Intern Med 1983; 99: 675–84. [DOI] [PubMed] [Google Scholar]
- 32. Liddle RA, Goldstein RB, Saxton J. Gallstone formation during weight‐reduction dieting. Arch Intern Med 1989; 149: 1750–3. [PubMed] [Google Scholar]
- 33. Telch CF, Agras WS. The effects of a very low calorie diet on binge eating. Behav Ther 1993; 24: 177–93. [Google Scholar]
- 34. Wadden TA, Foster GD, Letizia KA. One‐year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol 1994; 62: 165. [DOI] [PubMed] [Google Scholar]
- 35. Seimon RV, Roekenes JA, Zibellini J, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol 2015; pii: S0303–7207(15)30080‐0. DOI: 10.1016/j.mce.2015.09.014[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36. Klempel MC, Bhutani S, Fitzgibbon M, Freels S, Varady KA. Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. J Nutr 2010; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnstone A. Fasting for weight loss: an effective strategy or latest dieting trend&quest. Int J Obes 2015; 39: 727–33. [DOI] [PubMed] [Google Scholar]
- 38. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005; 293: 43–53. [DOI] [PubMed] [Google Scholar]
- 39. Warziski MT, Sereika SM, Styn MA, Music E, Burke LE. Changes in self‐efficacy and dietary adherence: the impact on weight loss in the PREFER study. J Behav Med 2008; 31: 81–92. [DOI] [PubMed] [Google Scholar]
- 40. Barnoski A, Klempel MC, Bhutani S et al. Modulation in Eating Behaviors by Alternate Day Fasting Versus Daily Calorie Restriction: Impact on Weight Loss and Weight Maintenance Success. Obesity Society Annual Meeting, abstract T‐OR‐2018, Los Angeles, CA November 4, 2015.
- 41. Scaglione R, Di Chiara T, Cariello T, Licata G. Visceral obesity and metabolic syndrome: two faces of the same medal? Intern Emerg Med 2010; 5: 111–119. [DOI] [PubMed] [Google Scholar]
- 42. Garruti G, Depalo R, Vita MG, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online 2009; 19: 552–563. [DOI] [PubMed] [Google Scholar]
- 43. Eshghinia S, Gadgevich GM. Effect of short‐term modified alternate‐day fasting on the lipid metabolism in obese women. Iran J Diabetes Obes 2011; 3: 1–5. [Google Scholar]
- 44. Poynten AM, Markovic TP, Maclean EL, et al. Fat oxidation, body composition and insulin sensitivity in diabetic and normoglycaemic obese adults 5 years after weight loss. Int J Obes Relat Metab Disord 2003; 27: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 45. Kudchodkar BJ, Sodhi HS, Mason DT, Borhani NO. Effects of acute caloric restriction on cholesterol metabolism in man. Am J Clin Nutr 1977; 30: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 46. Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res 2005; 13: 574–581. [DOI] [PubMed] [Google Scholar]
- 47. Martin B, Pearson M, Kebejian L, et al. Sex‐dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology 2007; 148: 4318–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varady KA, Hoddy KK, Kroeger CM, et al. Determinants of weight loss success with alternate day fasting. Obes Res Clin Pract 2015; pii: S1871–403X(15)00134‐9. DOI: 10.1016/j.orcp.2015.08.020. [DOI] [PubMed] [Google Scholar]


