Summary
Objective
Metabolic dysfunction characterized by insulin resistance (IR) is an important risk factor for type‐2 diabetes and coronary artery disease (CAD). The aim of this study was to determine if clinical lifestyle interventions differing in scope and intensity improve IR, defined by the lipoprotein IR (LPIR) score, in individuals differing in the severity of metabolic dysfunction.
Methods
Subjects with diagnosed type‐2 diabetes, CAD or significant risk factors participated in one of two clinical lifestyle modification interventions: (i) intensive non‐randomized programme with a strict vegetarian diet (n = 90 participants, 90 matched controls) or (ii) moderate randomized trial following a Mediterranean‐style diet (n = 89 subjects, 58 controls). On‐treatment and intention‐to‐treat analyses assessed changes over 1 year in LPIR, lipoprotein profiles and metabolic risk factors in intervention participants and controls in both programmes.
Results
In the on‐treatment analysis, both interventions led to weight loss: [−8.9% (95% CI, −10.3 to −7.4), intensive programme; −2.8% (95% CI, −3.8 to −1.9), moderate programme; adjusted P < 0.001] and a decrease in the LPIR score [−13.3% (95% CI, −18.2 to −8.3), intensive; −8.8% (95% CI, −12.9 to −4.7), moderate; adjusted P < 0.01] compared with respective controls. Of the six lipoprotein parameters comprising LPIR, only large very‐low‐density lipoprotein particle concentrations decreased significantly in participants compared with controls in both programmes [−26.3% (95% CI, −43.0 to −9.6), intensive; −14.2% (95% CI, −27.4 to −1.0), moderate; P < 0.05]. Intention‐to‐treat analysis confirmed and strengthened the primary results.
Conclusion
A stringent lifestyle modification intervention with a vegetarian diet and a moderate lifestyle modification intervention following a Mediterranean diet were both effective for improving IR defined by the LPIR score.
Keywords: Insulin resistance, lifestyle modification, lipoproteins, weight loss
Introduction
An estimated 86 million adults in the USA suffer from preclinical diabetes 1, which accounts for more than $44 billion in annual healthcare costs 2. Individuals with prediabetes and its sequelae represent a large population at risk for development of type‐2 diabetes and coronary artery disease (CAD) 3 that could benefit from early intervention; however, a large proportion of these patients go undiagnosed. Enhanced prevention before onset of overt disease through lifestyle modification is crucial to reducing health complications and the economic burden attributable to cardiometabolic disease 4.
The pathogenesis of type‐2 diabetes and CAD remains obscure because of the interaction of multiple metabolic pathways. Lifestyle modification focusing on nutrition and physical activity has shown substantial health benefits by improving body weight and a variety of cardiometabolic risk factors 5, 6. Intensive lifestyle interventions, following guidelines established in the Multicenter Lifestyle Demonstration Project 7, have positive effects on vascular health by altering plasma lipoproteins 8, improving circulating CAD biomarkers 9 and favourably modulating gene expression in peripheral blood 10. Likewise, moderate lifestyle changes may be effective for ameliorating cardiovascular (CV) risk 11. Although lifestyle modification interventions are effective in mediating risk through traditional pathways, little is known about the effects of lifestyle modification on lipid metabolism or the significance of lipoprotein responses in long‐term type‐2 diabetes and CAD risk reduction.
Insulin resistance (IR) is a key feature of metabolic dysfunction in prediabetes characterized by decreased tissue sensitivity to insulin and a compensatory increase in insulin secretion. Current methods for defining IR, including glycemic status, clamp techniques and oral glucose tolerance tests, haemoglobin A1C and homeostatic model assessment, vary widely in sensitivity and may be confounded by age, gender and physical activity 12. Lipoprotein particle characteristics measured by nuclear magnetic resonance (NMR) are recognized as important predictors of CV and metabolic risk 13 because lipoprotein profiles have been associated with progression of low‐grade coronary artery lesions 14 and CAD severity independent of age and standard lipid measurements 15. Six lipoprotein parameters showing the strongest association with IR 16 and prediabetes have been used to derive the lipoprotein IR (LPIR) score 17, a surrogate measure for assessing IR status that may prove useful for identifying patients at risk for developing type‐2 diabetes and CAD. The LPIR score was developed using homeostasis model assessment of IR in 4972 non‐diabetic subjects from the Multi‐Ethnic Study of Atherosclerosis and verified independently using glucose disposal rates measured during hyperinsulinemic–euglycemic clamps 18. Recent reports show that the LPIR score, which reflects lipoprotein derangements of IR, is associated with incident type‐2 diabetes independent of established risk factors 19, 20.
In this study, we hypothesized that lifestyle modification can be effective for improving IR in individuals differing in the severity of metabolic dysfunction. We used NMR spectroscopy to examine changes in lipoprotein subclasses in two clinical lifestyle modification interventions differing in scope and intensity to improve our understanding of how lifestyle changes impact IR defined by the LPIR score. Our objectives were to (i) assess changes in traditional metabolic risk factors, (ii) measure response of lipoprotein subclasses and (iii) determine changes in IR over 1 year using the LPIR score.
Methods
Human studies
Participation in both lifestyle modification interventions was voluntary, and all participants provided written informed consent. The interventions were performed according to the Declaration of Helsinki, were approved by the Chesapeake Institutional Review Board, Center for IRB Intelligence, Columbia, Maryland (Pro00009375 and Pro00009404) and are registered as NCT01805492 and NCT02136758 at ClinicalTrials.gov.
Intensive lifestyle modification intervention
The intensive intervention was a year‐long prospective, non‐randomized clinical programme, based on the Dr Dean Ornish Program for Reversing Heart Disease, designed to stabilize or reverse progression of heart disease through comprehensive changes in lifestyle. Entry criteria included physician‐diagnosed diabetes or CAD (stable angina, angioplasty, ≥50% luminal narrowing on coronary angiogram, acute myocardial infarction, bypass surgery or stent placement) or presence of two or more metabolic risk factors: obesity (body mass index [BMI] ≥30), hypertension (systolic blood pressure [BP] ≥140 mmHg or diastolic BP ≥90 mmHg), borderline or high total cholesterol (≥200 mg dL−1) or family history of heart disease in parents or siblings. Individuals with a known history of autoimmune disease or a systemic/chronic disease requiring chemotherapy or long‐term treatment were excluded from participation. Although subjects were required to abstain from smoking for at least 3 months prior to and during the programme, compliance was not verified through cotinine assays. The programme was conducted from January 2004 to February 2009.
The intervention focused on four areas of lifestyle: diet (low‐fat, high‐carbohydrate vegetarian with <10% of calories from fat), aerobic exercise (≥3 h week−1), stress management (1 h day−1), and group support (1–2 sessions/week). During the first 12 weeks, participants met with clinical staff three times in the first week (16.5 h total) and two times per week (10 h week−1) during the remaining weeks. Clinical staff included a registered dietitian for nutrition counselling, exercise physiologist for exercise training, certified yoga instructor for stress management and licensed psychologist for group support. During the remaining portion of the programme, participants were primarily self‐directed but were required to visit the clinic once a week for group support and stress management sessions. Total time commitment was 566.8 ± 74.4 h (10.9 ± 1.4 h week−1), excluding compliance reporting.
To achieve a balanced distribution of risk factors between intervention and control participants in a non‐randomized clinical trial, non‐intervention controls were prospectively matched to intervention participants based on gender, age (±5 years) at entry and presence or absence of physician‐diagnosed CAD. Control subjects received standard care from their primary physicians but did not participate in any component of the lifestyle programmes or receive any advice or counselling beyond routine care information regarding healthy lifestyle behaviours.
Moderate lifestyle modification intervention
A prospective, randomized trial based on the Walter Reed Integrative Cardiac Health Project Lifestyle Program was designed to investigate the efficacy of moderate lifestyle modification for improving clinical status in individuals with CAD or risk factors that promote metabolic disease. The Integrative Cardiac Health Project programme was developed to assess and ameliorate CV risk through changes in lifestyle behaviours in women and men who are eligible for care in the Department of Defense Healthcare System 21. Participants were recruited from the regional community and randomized (~3:2 allocation ratio) to either the lifestyle intervention arm or usual care (control) group. The programme was conducted from January 2010 to August 2013.
Participants were required to be ≥18 years of age and have at least one of the following risk factors: fasting blood glucose ≥100 mg dL−1, physician diagnosis of diabetes/prediabetes or currently taking anti‐glycemic medications; overweight or obese (BMI ≥25); total cholesterol ≥200 mg dL−1, documented history of hypercholesterolemia or currently taking lipid‐lowering medications; high‐density lipoprotein (HDL) ≤ 44 mg dL−1; low‐density lipoprotein (LDL) ≥130 mg dL−1 or documented history of hyperlipidemia; elevated triglycerides (≥200 mg dL−1); systolic BP ≥130 mmHg or diastolic BP ≥ 85 mmHg, documented diagnosis of hypertension or current use of antihypertensive medications; family history of CAD in a first degree relative; physician‐diagnosed stroke; history of smoking; post‐traumatic stress disorder or at risk for post‐traumatic stress disorder; insomnia (≤5 h of sleep/night) or sleep apnea. Exclusion criteria were unstable coronary syndromes, refractory congestive heart failure, uncontrolled arrhythmia or high‐grade uncorrected cardiac conduction abnormalities; significant left main stenosis (>50%) or an ejection fraction <35% with no revascularization; dietary or physical contraindications to components of the intervention that would preclude compliance; history of substance abuse without self‐certification of abstinence for at least 3 months.
The intervention group participated in a 3‐month therapeutic education and lifestyle workshop in which they developed individualized lifestyle plans to reduce metabolic risk. Lifestyle plans focused on a Mediterranean‐style diet that included moderate carbohydrate and animal/vegetable fat, weight loss, strength and endurance, and stress reduction. Participants first attended a 4‐h orientation that outlined objectives, requirements and expectations and then met individually with a registered dietitian, exercise physiologist, stress management instructor and psychologist to learn effective strategies for integrating healthy changes into their current lifestyle. Subjects met monthly with each specialty provider to receive reinforcement for implementing recommended lifestyle changes and guidance for maintaining success on their own. Total time commitment was 199.7 ± 175.2 h (3.8 ± 3.4 h week−1), excluding compliance reporting. Over the next 9 months, participants received additional instruction for integrating healthy behaviours into daily life through monthly contact with an integrative health coach.
Participants enrolling in the moderate lifestyle intervention were randomly assigned to the treatment or control arm to minimize selection bias and confounding. Control subjects received standard care from their primary physicians but did not participate in any component of the lifestyle programmes or receive any advice or counselling beyond routine care information regarding healthy lifestyle behaviours.
Clinical measurements
Clinical examinations were held at baseline (pretreatment) and 1 year (post‐intervention) to collect fasting blood samples for lipid profiles and information on anthropometry, BP, physical fitness, mental health, family history and medication use. All interviews and data instruments were administered by trained clinical staff. Medical history was extracted from participants' medical records. Daily caloric intake and nutrient composition were calculated from 3‐day dietary recall questionnaires using Food Processor® v10.10 (ESHA Research, Salem, OR, USA). Height and weight were measured on a combined scale (Cardinal Scale, Webb City, MO, USA). BP was recorded using a mercury sphygmomanometer on the right arm of seated, relaxed subjects. Standard lipid assays were performed on fasting plasma samples using an AEROSET™ clinical chemistry system (Abbott Laboratories, Abbott Park, IL, USA). To monitor treatment fidelity, all subjects self‐reported their daily diet, exercise and stress management frequency/duration, group support meeting attendance and any smoking activity on a weekly basis.
Lipoprotein subclass measurements
Lipoprotein subclass concentrations and mean particle diameters were measured by NMR spectroscopy at LipoScience (now LabCorp, Raleigh, NC, USA) and calculated using the LipoProfile‐3 algorithm following previously published methods 18. Six lipoprotein measures, including large very‐low‐density lipoprotein (VLDL) particle concentration, VLDL size and small LDL concentration, which show a positive association with development of IR, and large HDL concentration, HDL size and LDL size, which are negatively associated (protective) with IR, were used to derive the LPIR score, with values ranging from 0 (most insulin sensitive) to 100 (most insulin resistant) (Figure S1). Inter‐assay and intra‐assay coefficients of variation (%CV) for these lipoprotein measurements have been well characterized and are generally <6% 22.
On‐treatment analysis
Flow diagrams showing enrollment, attrition and programme completion for both lifestyle modification interventions are presented in Figure 1. On‐treatment analysis for the intensive intervention included 90 participants with matched controls who completed 1 year; for the moderate intervention, there were 89 randomized participants and 58 controls with complete baseline and 1‐year data.
Figure 1.
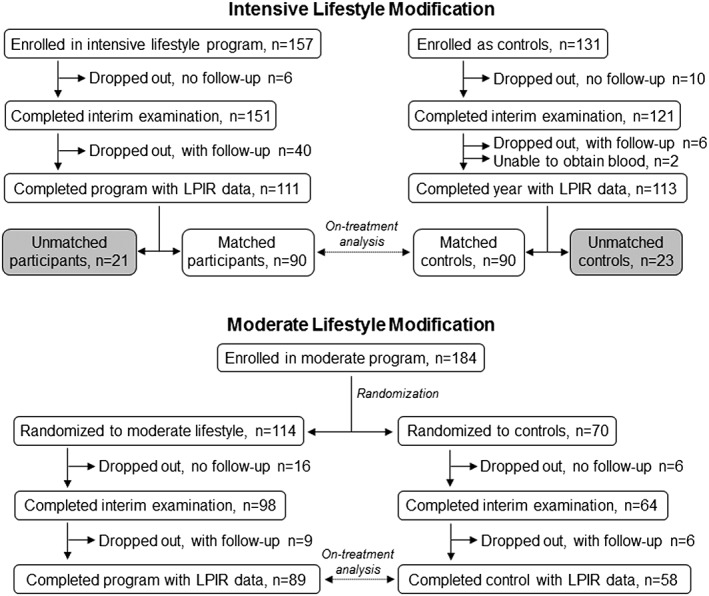
Participant enrollment, attrition and completion in the intensive (top) and moderate (bottom) lifestyle modification interventions.
Intention‐to‐treat analysis
To better establish the validity of our results, we conducted an intention‐to‐treat analysis. Participants were allowed to continue in both lifestyle modification interventions even if they did not meet target compliance in one or more areas or failed to fully comply with reporting requirements. To account for potential bias, we included all participants and controls regardless of compliance, programme completion or matched/unmatched status and focused on the primary outcomes of interest – LPIR score and lipoprotein components. We used a multiple imputation procedure with five Monte Carlo simulations to estimate missing outcome values based on other known covariates, including interim outcomes and the distribution of known data 23. Each simulated data set was then analysed by linear regression and results were combined to yield the intention‐to‐treat analysis P values. Final sample sizes were 157 intensive lifestyle participants, 131 controls; 114 moderate lifestyle participants, 70 controls.
Statistical analysis
The on‐treatment analysis was conducted using r statistical software (v3.1.1). All significance tests were two‐sided, and significance was set at α = 0.05. A Wilcoxon signed‐rank test or matched pairs t‐test compared baseline variables between intensive lifestyle participants and matched controls. A Wilcoxon rank‐sum test or independent samples t‐test was used to compare baseline variables between moderate lifestyle subjects and randomized controls. An independent samples t‐test or chi‐squared test was used to compare baseline variables between participants in the intensive and moderate lifestyle modification interventions. Regression modelling adjusted for metabolic risk factors and changes in lipid‐lowering medications was used to assess changes in LPIR score, lipoproteins and risk factors from baseline to 1 year in the intervention and control groups in both interventions. Data for certain variables were log‐transformed or square root‐transformed to achieve normally distributed and homoscedastic residuals in regression modelling. Repeated measures analysis of variance was used to assess the potential influence of medication use on risk factor response.
Results
Intensive lifestyle
Baseline characteristics of intensive lifestyle modification participants are presented in Table S1. Average age at baseline was 60.3 years (range 40.7–79.8). At entry, many subjects had clinically relevant disorders: 63% were clinically obese, 57% had hypertension, 46% had high cholesterol and 46% had severe IR (LPIR ≥75). Over the course of 1 year, the intensive intervention resulted in significant reductions in the number of obese (63% down to 40%, P = 0.003), hypertensive (57% to 37%, P = 0.008), dyslipidemic (46% to 30%, P = 0.007) and severely insulin‐resistant (46% to 28%, P = 0.003) participants.
All lipoprotein components of the LPIR score, dietary measures and metabolic risk factors did not differ significantly at baseline between participants who completed the intervention (n = 111) and those who dropped out (n = 46), but dropouts tended to be younger (54.3 ± 10.7 vs. 60.9 ± 8.8 years of age; P < 0.001) and heavier (107.1 ± 32.1 vs. 95.5 ± 21.3 kg; P = 0.046) than completers (Table S2). Subjects excluded because of non‐matching (n = 21) had higher levels of large HDL particles (4.9 ± 2.4 vs. 3.8 ± 2.4 µmol L−1; P = 0.031), larger average HDL size (9.0 ± 0.4 vs. 8.8 ± 0.4 nm; P = 0.025) and higher HDL cholesterol (50.4 ± 12.1 vs. 44.3 ± 12.7 mg dL−1; P = 0.021) than participants included in the on‐treatment analysis (Table S3).
Participants had a more atherogenic lipoprotein profile at entry than controls despite the prospective matching strategy but achieved significant improvement in IR, as measured by the LPIR score (−13.3% [95% CI, −18.2 to −8.3]; P = 0.008 adjusted for confounding variables), compared with matched controls (Table 1). Large VLDL particles (−26.3% [95% CI, −43.0 to −9.6]; adjusted P = 0.004) and VLDL size (−7.6% [95% CI, −10.6 to −4.5]; adjusted P = 0.009), lipoprotein parameters positively associated with development of IR, also showed a significant decrease compared with controls. Participants with severe IR (LPIR ≥75 at baseline; n = 41) faired significantly better during the intervention (−19.4%; adjusted P < 0.001) than subjects with less severe IR (baseline LPIR <75; −5.5%).
Table 1.
Baseline and 1‐year changes in the LPIR score and other cardiovascular risk factors in intensive lifestyle modification intervention participants (n = 90) and matched controls (n = 90)
| Baseline | Change (%) in 1 year | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Controls | Participants | P value† | Controls | Participants | P value‡ | |
| LPIR score | 56.5 ± 19.5 | 68.4 ± 18.7 | <0.001 | −1.8 | −13.3** | 0.008 | |
| Atherogenic components of LPIR | |||||||
| Large VLDL, nmol L−1 § | 6.5 ± 7.0 | 9.5 ± 7.5 | 0.002 | −10.3 | −26.3** | 0.004 | |
| VLDL size, nm§ | 51.1 ± 7.3 | 55.2 ± 8.6 | <0.001 | −1.6 | −7.6** | 0.009 | |
| Small LDL, nmol L−1 # | 711 ± 306 | 862 ± 342 | 0.004 | −1.8 | −7.4 | 0.413 | |
| Protective components of LPIR | |||||||
| Large HDL, µmol L−1 # | 5.1 ± 2.8 | 3.8 ± 2.4 | <0.001 | −0.4 | +9.9 | 0.406 | |
| HDL size, nm | 9.0 ± 0.4 | 8.8 ± 0.4 | 0.018 | −0.1 | +0.5 | 0.431 | |
| LDL size, nm | 20.5 ± 0.5 | 20.3 ± 0.7 | 0.025 | +0.3 | +0.3 | 0.247 | |
| Dietary measures | |||||||
| Calories, kcal§ | 1887 ± 580 | 2077 ± 768 | 0.088 | −8.4* | −15.3* | 0.723 | |
| Carbohydrates, g | 237 ± 72 | 281 ± 106 | 0.005 | −6.3* | +12.7** | <0.001 | |
| Fat, g§ | 67.3 ± 35.5 | 69.4 ± 41.8 | 0.629 | −9.9 | −61.7** | <0.001 | |
| Saturated fat, g§ | 21.7 ± 12.6 | 22.0 ± 15.2 | 0.369 | −11.0 | −73.6** | <0.001 | |
| Cardiovascular risk factors | |||||||
| Weight (kg) | 83.6 ± 16.1 | 96.1 ± 22.0 | <0.001 | +0.5 | −8.9** | <0.001 | |
| BMI, kg m−2 | 28.5 ± 4.5 | 33.4 ± 7.4 | <0.001 | +0.5 | −8.9** | <0.001 | |
| Diastolic BP, mmHg | 79.5 ± 9.9 | 80.3 ± 10.1 | 0.543 | −1.8 | −5.9** | 0.011 | |
| Lipids | |||||||
| HDL, mg dL−1 | 49.8 ± 13.1 | 44.3 ± 12.7 | <0.001 | −3.4* | −2.9 | 0.730 | |
| LDL, mg dL−1 § | 109 ± 35 | 112 ± 40 | 0.894 | +1.5 | −4.5 | 0.238 | |
| Total cholesterol, mg dL−1 | 188 ± 45 | 192 ± 47 | 0.484 | −0.1 | −5.2* | 0.027 | |
| Triglycerides, mg dL−1 § | 148 ± 98 | 180 ± 92 | 0.002 | −0.5 | −11.0* | 0.321 | |
| Triglyceride/HDL ratio§ | 3.4 ± 3.4 | 4.5 ± 2.9 | <0.001 | +5.8 | −11.2 | 0.500 | |
Values are mean ± SD.
Comparison of baseline values based on a paired t‐test or Wilcoxon signed‐rank test.
Change from baseline to 1 year between groups based on regression modelling and adjustment for confounding variables.
Data were log‐transformed for regression modelling.
Data were square root‐transformed for regression modelling.
Change from baseline to 1 year within each group: P < 0.05.
Change from baseline to 1 year within each group: P < 0.001.
BMI, body mass index; BP, blood pressure; HDL, high‐density cholesterol; LDL, low‐density cholesterol; LPIR, lipoprotein insulin resistance; VLDL, very‐low‐density cholesterol.
There was a clear overall health benefit from the intervention as evidenced by significant improvements in CV risk factors. Participants reduced their dietary fat consumption (−61.7% [95% CI, −74.9 to −48.6]; adjusted P < 0.001) and showed significant reductions in BMI (−8.9% [95% CI, −10.3 to −7.4]; adjusted P < 0.001), body weight (−8.9% [95% CI, −10.3 to −7.4]; adjusted P < 0.001), diastolic BP (−5.9% [95% CI, −8.9 to −2.9]; adjusted P = 0.011) and total cholesterol (−5.2% [95% CI, −8.3 to −2.1]; adjusted P = 0.027) compared with matched controls (Table 1).
Moderate lifestyle
Baseline characteristics of moderate lifestyle patients are presented in Table S1. Average age was 61.5 years (range 33.9–86.2). There were no baseline differences between intervention subjects and randomized controls. At entry, 51% of patients were clinically obese, 44% had hypertension, 29% had high cholesterol and 18% had severe IR, but by the end of treatment, the percentage of obese patients had declined to 42% (P = 0.049), hypertensive patients down to 26% (P = 0.010), dyslipidemic to 25% (P = 0.198) and severely insulin resistant to 15% (P = 0.486). Dropouts (n = 25) were younger (56.0 ± 11.2 vs. 61.5 ± 10.1 years of age; P = 0.034) and had higher concentrations of large VLDL particles (6.4 ± 5.0 vs. 4.7 ± 5.0 nmol L−1; P = 0.031), higher triglycerides (171 ± 80 vs. 136 ± 66 mg dL−1; P = 0.044) and a higher triglyceride/HDL ratio (4.3 ± 2.8 vs. 3.1 ± 1.9; P = 0.037) than completers (Table S4).
Participants in the moderate lifestyle modification intervention also achieved significant reduction in IR (LPIR score −8.8% [95% CI, −12.9 to −4.7]; adjusted P = 0.001) compared with randomized controls (Table 2). Significant improvements were observed for large VLDL particles (−14.2% [95% CI, −27.4 to −1.0]; adjusted P = 0.024), HDL size (+1.2% [95% CI, +0.5 to +1.9]; P = 0.046) and the following CV risk factors: dietary fat intake (−12.8% [95% CI, −23.2 to −2.4]; P = 0.044), BMI (−2.8% [95% CI, −3.8 to −1.9]; P < 0.001), body weight (−2.8% [95% CI, −3.8 to −1.9]; P < 0.001) and diastolic BP (−6.3% [95% CI, −9.3 to −3.4]; P = 0.047). Changes in LPIR were similar for patients with severe IR (−6.2%; n = 16) and mild IR (−9.8%; adjusted P = 0.486) at entry.
Table 2.
Baseline and 1‐year changes in the LPIR score and other cardiovascular risk factors in moderate lifestyle modification intervention participants (n = 89) and randomized controls (n = 58)
| Baseline | Change (%) in 1 year | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Controls | Participants | P value† | Controls | Participants | P value‡ | |
| LPIR score | 51.4 ± 23.4 | 53.9 ± 20.5 | 0.625 | +3.7 | −8.8** | 0.001 | |
| Atherogenic components of LPIR | |||||||
| Large VLDL, nmol L−1 § | 4.1 ± 4.3 | 4.7 ± 5.0 | 0.402 | +3.8 | −14.2* | 0.024 | |
| VLDL size, nm§ | 48.1 ± 6.6 | 49.2 ± 5.9 | 0.357 | +2.7 | −1.0 | 0.117 | |
| Small LDL, nmol L−1 # | 774 ± 390 | 771 ± 326 | 0.940 | −5.6 | −12.7** | 0.178 | |
| Protective components of LPIR | |||||||
| Large HDL, µmol L−1 # | 5.4 ± 3.6 | 5.2 ± 3.1 | 0.995 | +0.1 | +10.4* | 0.085 | |
| HDL size, nm | 9.0 ± 0.5 | 8.9 ± 0.4 | 0.973 | +0.1 | +1.2* | 0.046 | |
| LDL size, nm | 20.6 ± 0.6 | 20.5 ± 0.5 | 0.548 | −0.1 | +0.3 | 0.407 | |
| Dietary measures | |||||||
| Calories, kcal§ | 1947 ± 644 | 1802 ± 598 | 0.147 | −10.1* | −9.8* | 0.193 | |
| Carbohydrates, g | 249 ± 81 | 233 ± 83 | 0.201 | −12.0* | −8.2* | 0.859 | |
| Fat, g§ | 71.1 ± 32.5 | 62.8 ± 29.4 | 0.121 | −8.4 | −12.8* | 0.044 | |
| Saturated fat, g§ | 22.3 ± 11.0 | 19.7 ± 9.1 | 0.162 | −1.8 | −10.4 | 0.020 | |
| Cardiovascular risk factors | |||||||
| Weight (kg) | 86.5 ± 20.0 | 89.1 ± 21.0 | 0.453 | 0.0 | −2.8** | <0.001 | |
| BMI, kg m−2 | 31.1 ± 6.5 | 31.5 ± 6.5 | 0.721 | 0.0 | −2.8** | <0.001 | |
| Diastolic BP, mmHg | 78.9 ± 11.0 | 80.0 ± 11.5 | 0.377 | −1.6 | −6.3** | 0.047 | |
| Lipids | |||||||
| HDL, mg dL−1 | 50.2 ± 14.5 | 47.8 ± 12.3 | 0.561 | −2.3* | +0.2 | 0.102 | |
| LDL, mg dL−1 § | 116 ± 30 | 111 ± 31 | 0.273 | −4.8 | −3.8 | 0.761 | |
| Total cholesterol, mg dL−1 | 192 ± 36 | 185 ± 39 | 0.266 | −4.3* | −3.6* | 0.921 | |
| Triglycerides, mg dL−1 § | 130 ± 64 | 136 ± 66 | 0.532 | −3.0 | −10.6* | 0.128 | |
| Triglyceride/HDL ratio§ | 2.9 ± 1.7 | 3.1 ± 1.9 | 0.353 | +0.3 | −10.1* | 0.108 | |
Values are mean ± SD.
Comparison of baseline values based on an independent samples t‐test or Wilcoxon rank‐sum test.
Change from baseline to 1 year between groups based on regression modelling and adjustment for confounding variables.
Data were log‐transformed for regression modelling.
Data were square root‐transformed for regression modelling.
Change from baseline to 1 year within each group: P < 0.05.
Change from baseline to 1 year within each group: P < 0.001.
BMI, body mass index; BP, blood pressure; HDL, high‐density cholesterol; LDL, low‐density cholesterol; LPIR, lipoprotein insulin resistance; VLDL, very‐low‐density cholesterol.
Intention‐to‐treat analysis
Conclusions from the intention‐to‐treat analysis were nearly identical to those from the on‐treatment analysis (Table 3). In the intensive lifestyle modification intervention, the LPIR score, large VLDL particle concentrations and VLDL size showed a significant reduction (P < 0.001). With moderate lifestyle, changes in LPIR, large VLDL particles, large HDL particles and HDL size were significant (P < 0.05) in the intention‐to‐treat analysis.
Table 3.
One‐year changes in the LPIR score and other lipoproteins in the intervention and control arms of the intensive and moderate lifestyle modification interventions for the on‐treatment and intention‐to‐treat analyses
| On‐treatment | Intention‐to‐treat | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Controls change (%) in 1 year | Participants change (%) in 1 year | P value† | Controls change (%) in 1 year | Participants change (%) in 1 year | P value‡ | |
| Intensive lifestyle | (n = 90) | (n = 90) | (n = 131) | (n = 157) | |||
| LPIR score | −1.8 | −13.3** | 0.008 | −0.3 | −13.2** | <0.001 | |
| Atherogenic components of LPIR | |||||||
| Large VLDL, nmol L−1 § | −10.3 | −26.3** | 0.004 | −0.5 | −26.5** | <0.001 | |
| VLDL size, nm§ | −1.6 | −7.6** | 0.009 | −0.3 | −7.8** | <0.001 | |
| Small LDL, nmol L−1 # | −1.8 | −7.4 | 0.413 | −2.4 | −8.8* | 0.710 | |
| Protective components of LPIR | |||||||
| Large HDL, µmol L−1 # | −0.4 | +9.9 | 0.406 | +0.6 | +5.4 | 0.705 | |
| HDL size, nm | −0.1 | +0.5 | 0.431 | 0.0 | +0.5 | 0.351 | |
| LDL size, nm | +0.3 | +0.3 | 0.247 | +0.3 | +0.2 | 0.223 | |
| Moderate lifestyle | (n = 58) | (n = 89) | (n = 70) | (n = 114) | |||
| LPIR score | +3.7 | −8.8** | 0.001 | +2.0 | −6.4** | 0.003 | |
| Atherogenic components of LPIR | |||||||
| Large VLDL, nmol L−1 § | +3.8 | −14.2* | 0.024 | −0.2 | −10.5* | 0.040 | |
| VLDL size, nm§ | +2.7 | −1.0 | 0.117 | +1.1 | −0.1 | 0.335 | |
| Small LDL, nmol L−1 # | −5.6 | −12.7** | 0.178 | −4.9 | −9.0* | 0.308 | |
| Protective components of LPIR | |||||||
| Large HDL, µmol L−1 # | +0.1 | +10.4* | 0.085 | −1.3 | +8.6* | 0.029 | |
| HDL size, nm | +0.1 | +1.2* | 0.046 | 0.0 | +0.9* | 0.031 | |
| LDL size, nm | −0.1 | +0.3 | 0.407 | −0.1 | +0.2 | 0.188 | |
Change from baseline to follow‐up between groups based on regression modelling and adjustment for confounding variables.
Change from baseline to follow‐up between groups based on pooled results of regression models obtained via Monte Carlo simulation.
Data were log‐transformed for regression modelling.
Data were square root‐transformed for regression modelling.
Change from baseline to follow‐up between groups: P < 0.05.
Change from baseline to follow‐up between groups: P < 0.001.
HDL, high‐density cholesterol; LDL, low‐density cholesterol; LPIR, lipoprotein insulin resistance; VLDL, very‐low‐density cholesterol.
Medication usage
In regression modelling, improvement in the LPIR score and large VLDL particles remained significant after adjustment for changes in lipid‐lowering medications in both lifestyle interventions. Likewise, lipoprotein changes in the subset of participants who were not taking or did not change the brand or dosage of lipid‐lowering medications during the intervention were similar to lipoprotein changes in all patients (Table S5), indicating that medication use did not have significant effects on the LPIR score or lipoprotein response during lifestyle intervention.
Discussion
Lifestyle modification interventions differing in dietary stringency, exercise intensity and time commitment improved IR and related lipoprotein parameters in patients with cardiometabolic disease or adverse risk factor profiles. Despite baseline differences in disease severity, patients in both lifestyle modification interventions significantly improved their IR status, as measured by the lipoprotein‐derived LPIR score, independent of other risk factors including BMI and triglyceride levels. The intensive lifestyle modification intervention focusing on a low‐fat diet and the moderate lifestyle modification intervention with a Mediterranean diet also led to significant reductions in several cardiometabolic risk factors, including dietary fat and saturated fat intake, body weight and BMI, and diastolic BP. These results were confirmed and strengthened in an intention‐to‐treat analysis.
Insulin resistance increases risk of developing type‐2 diabetes and is an independent risk factor for major CV events in patients with pre‐existing arterial disease 24, 25. In addition to influencing development of clinical disease and mortality 26, IR is now recognized to play an important role in preclinical (silent) CAD. Recent studies indicate that IR is associated with asymptomatic myocardial perfusion defects in normotensive adults with preclinical diabetes 27 and is linked to angiographically documented silent CAD in patients with type‐2 diabetes 28. A growing body of research suggests that altered glycemic control affects myocardial metabolism 29 and that abnormal myocardial metabolism plays a significant role in the development of cardiac dysfunction 30. Therefore, ameliorating IR should be considered a key target for metabolic interventions in patients at risk for diabetes and CAD.
Recent meta‐analyses have shown that lifestyle interventions promoting a healthy diet and physical activity are effective for improving cardiometabolic health and preventing progression to type‐2 diabetes 31, 32. However, a plethora of lifestyle interventions are available that differ substantially in behavioural change strategy 33, which may affect efficacy and long‐term adherence 34. Research suggests that intensive interventions are usually more effective in reducing CV risk factors and preventing progression to diabetes 31, 35. In this study, we show that a clinically intensive intervention (~11 h week−1) and a moderate lifestyle intervention, requiring ~4 h week−1 and focusing on personalized lifestyle changes through patient education 36, were both effective for improving IR in the majority of participants. For subjects with severe IR at entry, intensive lifestyle changes may be superior to moderate changes for ameliorating IR.
Combining patients from both lifestyle interventions allowed us to examine how basic overall changes in diet, exercise and body weight during lifestyle modification affect IR. Scatter plots showed a significant positive correlation between changes in the LPIR score and overall changes in BMI, but no significant correlation with fat consumption or exercise (Figure 2). Although research suggests that diet 37 and exercise 38 influence plasma lipoproteins, our results indicate that weight loss should be the focus of a comprehensive healthy lifestyle intervention for improving IR and reducing CV risk.
Figure 2.
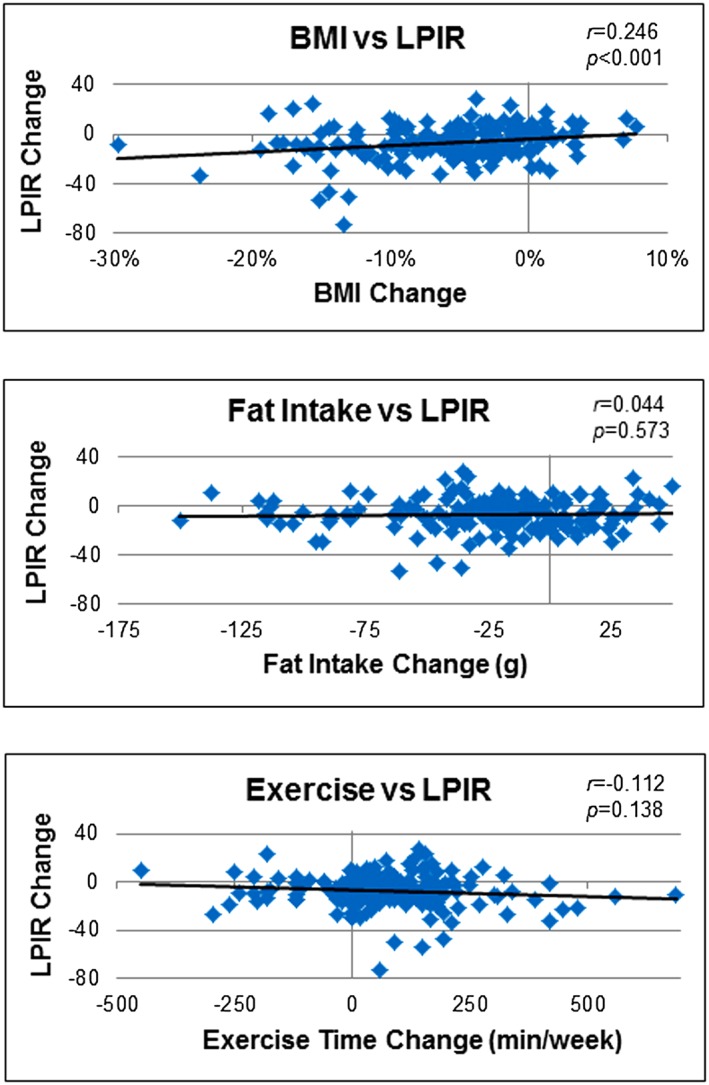
Scatter plots showing correlations between change in insulin resistance (LPIR score) and overall change in selected variables during lifestyle modification.
Impaired insulin sensitivity and adiposity may contribute to occlusive vascular disease through an increase in the concentration of large triglyceride‐rich VLDL particles 39, which have been positively associated with coronary artery calcification 40, 41 and severity of CAD 15. Large VLDLs may be more important for atherogenic risk than medium or small VLDLs because large lipid‐enriched VLDL particles are more efficiently catabolized to small dense LDLs 42, which more easily penetrate the endothelial wall, thereby enhancing accumulation of triglycerides and cholesterol ester in the vascular intima. Patients in both lifestyle programmes showed a significant reduction in circulating large VLDL particles, which suggests a potential mechanism by which improvement in IR may inhibit atherosclerotic involvement and reduce cardiometabolic risk.
Lipid‐lowering medications such as statins and fibrates reduce plasma VLDL concentrations by inhibiting hepatic secretion and stimulating catabolism of triglyceride‐rich lipoproteins 43. Because >50% of our participants were taking prescription agents to manage hyperlipidemia at baseline, we examined the potential impact of changes in lipid‐lowering medications on LPIR and lipoprotein profiles during lifestyle modification. We found that (i) changes in the LPIR score and large VLDL particle concentrations remained significant after adjustment for changes in lipid‐lowering medication use in both lifestyle interventions and (ii) results of participants who were not taking or did not change the brand or dosage of medications during the study were similar to those in all participants. These observations support our conclusion that medication use did not have a significant impact on LPIR or lipoprotein response during lifestyle modification.
Metabolic risk reduction through intensive lifestyle modification involved demanding behavioural changes that required motivation and an excessive time commitment, which may restrict the applicability and generalizability of an intensive intervention outside a controlled clinical environment. Accordingly, it was impractical to use a randomized design for the intensive lifestyle intervention, and matched controls differed significantly from participants at entry for several metabolic risk factors, including LPIR score, lipoprotein profiles and body weight. However, well‐designed case–control studies may be similar to randomized trials for estimating treatment effects 44. Nevertheless, a randomized trial with moderate lifestyle changes and broader clinical applicability was similarly effective for improving IR defined by the LPIR score. Participants were not fully compliant in all programme areas and dropout rates exceeded 20% in both lifestyle interventions, which may have biased our interpretation of intervention efficacy in the on‐treatment analyses, but an intention‐to‐treat analysis that included all participants validated and reinforced results of the main analyses. Although changes in numerous lipid‐lowering medications did not substantially affect LPIR response to lifestyle modification, other medications with secondary effects on lipoproteins may have influenced the results. In addition, lipoprotein parameters comprising LPIR may be affected by hypertriglyceridemic conditions unrelated to IR. Finally, NMR spectroscopy cannot distinguish between VLDL particles and chylomicron remnants of similar size; thus, other remnant particles may have interfered with our quantitation of VLDL.
In conclusion, clinical lifestyle interventions that favourably modify lipoprotein profiles may ameliorate IR and reduce risk for diabetic and CV complications. In this study, we showed that detailed lipoprotein profiling may provide insight into mechanisms underlying changes in vascular atherogenicity that occur with lifestyle modification and improve our understanding of lifestyle influences on dyslipidemia and insulin sensitivity. Although additional research is needed to determine factors influencing long‐term changes in IR, physicians should consider the benefits of moderate lifestyle changes for improving insulin sensitivity when developing treatment regimens for patients at risk for diabetes and CAD. An increased understanding of lifestyle influences on lipoproteins and IR may be useful for developing tailored therapies for maintaining optimal cardiometabolic health.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Author contributions
D. L. E., M. N. V. and R. J. M. E. conceived and designed the study and interpreted data; N. S. C. conducted statistical analysis and assisted with data interpretation; H. L. B. collected patient samples and performed biochemical analyses and M. K. and M. N. V. revised the manuscript for intellectual content. All authors contributed to writing and approved the final manuscript. D. L. E. had primary responsibility for final content.
Funding
This study was supported by the US Army Medical Research and Materiel Command (MRMC)/Telemedicine and Advanced Technology Research Center (TATRC) and Henry M. Jackson Foundation (W81XWH‐10‐2‐0080). Identification of specific products or scientific instrumentation does not constitute endorsement by the authors, Department of Defense or any component agency. Opinions and assertions expressed herein are private views of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense or US Government.
Supporting information
Table S1 Baseline characteristics of participants in intensive and moderate lifestyle modification
Table S2 Baseline characteristics in intensive lifestyle modification participants: dropouts vs completers
Table S3 Baseline characteristics in intensive lifestyle modification participants: unmatched vs matched
Table S4 Baseline characteristics in moderate lifestyle modification participants: dropouts vs completers
Table S5 Effects of lipid‐lowering medications on the LPIR score and lipoproteins
Figure S1 Atherogenicity of the six lipoprotein characteristics associated with insulin resistance and type‐2 diabetes risk that were used to calculate the LPIR score (22).
Supporting info item
Acknowledgements
Contributions of Amy Burke, RN, BSN; Mary Jane Haberkorn, RN; Kathleen Prazich; Fran Lechak, MS, RD, LDN; Angie Rokita, BS; Judith Sullivan, ERYT; Gary Pagano, MS; Tammy McCall, RN; and Kelly Warshel, MD are gratefully acknowledged. We especially thank the participants. Thomas M. Morgan, MD and Margery A. Connelly, PhD provided insightful comments.
Ellsworth, D. L. , Costantino, N. S. , Blackburn, H. L. , Engler, R. J. M. , Kashani, M. , and Vernalis, M. N. (2016) Lifestyle modification interventions differing in intensity and dietary stringency improve insulin resistance through changes in lipoprotein profiles. Obesity Science & Practice, 2: 282–292. doi: 10.1002/osp4.54.
References
- 1. Centers for Disease Control and Prevention . National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, 2014. Available at: http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html
- 2. Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2014; 37: 3172–3179. [DOI] [PubMed] [Google Scholar]
- 3. Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Endocrinol Metab Clin North Am 2008; 37: 581–601. [DOI] [PubMed] [Google Scholar]
- 4. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2960–2984. [DOI] [PubMed] [Google Scholar]
- 5. Nordmann AJ, Suter‐Zimmermann K, Bucher HC, et al. Meta‐analysis comparing Mediterranean to low‐fat diets for modification of cardiovascular risk factors. Am J Med 2011; 124: 841–851. [DOI] [PubMed] [Google Scholar]
- 6. Baillot A, Romain AJ, Boisvert‐Vigneault K, et al. Effects of lifestyle interventions that include a physical activity component in class II and III obese individuals: a systematic review and meta‐analysis. PLoS One 2015; 10 e0119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot 2010; 24: 260–266. [DOI] [PubMed] [Google Scholar]
- 8. Decewicz DJ, Neatrour DM, Burke A, et al. Effects of cardiovascular lifestyle change on lipoprotein subclass profiles defined by nuclear magnetic resonance spectroscopy. Lipids Health Dis 2009; 8: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chainani‐Wu N, Weidner G, Purnell DM, et al. Changes in emerging cardiac biomarkers after an intensive lifestyle intervention. Am J Cardiol 2011; 108: 498–507. [DOI] [PubMed] [Google Scholar]
- 10. Ellsworth DL, Croft DT Jr, Weyandt J, et al. Intensive cardiovascular risk reduction induces sustainable changes in expression of genes and pathways important to vascular function. Circ Cardiovasc Genet 2014; 7: 151–160. [DOI] [PubMed] [Google Scholar]
- 11. Taggart J, Williams A, Dennis S, et al. A systematic review of interventions in primary care to improve health literacy for chronic disease behavioral risk factors. BMC Fam Pract 2012; 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antuna‐Puente B, Disse E, Rabasa‐Lhoret R, Laville M, Capeau J, Bastard JP. How can we measure insulin sensitivity/resistance? Diabetes Metab 2011; 37: 179–188. [DOI] [PubMed] [Google Scholar]
- 13. Blake GJ, Otvos JD, Rifai N, Ridker PM. Low‐density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation 2002; 106: 1930–1937. [DOI] [PubMed] [Google Scholar]
- 14. Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS): treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol 1996; 16: 697–704. [DOI] [PubMed] [Google Scholar]
- 15. Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol 1998; 18: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 16. Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003; 52: 453–462. [DOI] [PubMed] [Google Scholar]
- 17. Frazier‐Wood AC, Garvey WT, Dall T, Honigberg R, Pourfarzib R. Opportunities for using lipoprotein subclass profile by nuclear magnetic resonance spectroscopy in assessing insulin resistance and diabetes prediction. Metab Syndr Relat Disord 2012; 10: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shalaurova I, Connelly MA, Garvey WT, Otvos JD. Lipoprotein insulin resistance index: a lipoprotein particle‐derived measure of insulin resistance. Metab Syndr Relat Disord 2014; 12: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mackey RH, Mora S, Bertoni AG, et al. Lipoprotein particles and incident type 2 diabetes in the multi‐ethnic study of atherosclerosis. Diabetes Care 2015; 38: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016; 1: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kashani M, Eliasson AH, Walizer EM, et al. Early empowerment strategies boost self‐efficacy to improve cardiovascular health behaviors. Glob J Health Sci 2016; 8: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006; 26: 847–870. [DOI] [PubMed] [Google Scholar]
- 23. van Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 24. Tenenbaum A, Adler Y, Boyko V, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J 2007; 153: 559–565. [DOI] [PubMed] [Google Scholar]
- 25. Verhagen SN, Wassink AM, van der Graaf Y, Gorter PM, Visseren FL. SMART Study Group. Insulin resistance increases the occurrence of new cardiovascular events in patients with manifest arterial disease without known diabetes. The SMART study. Cardiovasc Diabetol 2011; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuliani G, Morieri ML, Volpato S, et al. Insulin resistance and systemic inflammation, but not metabolic syndrome phenotype, predict 9 years mortality in older adults. Atherosclerosis 2014; 235: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nasr G, Sliem H. Silent ischemia in relation to insulin resistance in normotensive prediabetic adults: early detection by single photon emission computed tomography (SPECT). Int J Cardiovasc Imaging 2011; 27: 335–341. [DOI] [PubMed] [Google Scholar]
- 28. Gazzaruso C, Solerte SB, De Amici E, et al. Association of the metabolic syndrome and insulin resistance with silent myocardial ischemia in patients with type 2 diabetes mellitus. Am J Cardiol 2006; 97: 236–239. [DOI] [PubMed] [Google Scholar]
- 29. Rosano GM, Vitale C, Fragasso G. Metabolic therapy for patients with diabetes mellitus and coronary artery disease. Am J Cardiol 2006; 98: 14J–18J. [DOI] [PubMed] [Google Scholar]
- 30. Banerjee S, Peterson LR. Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr Cardiol Rep 2007; 9: 143–149. [DOI] [PubMed] [Google Scholar]
- 31. Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015; 163: 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glechner A, Harreiter J, Gartlehner G, et al. Sex‐specific differences in diabetes prevention: a systematic review and meta‐analysis. Diabetologia 2015; 58: 242–254. [DOI] [PubMed] [Google Scholar]
- 33. Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract 2011; 91: 1–12. [DOI] [PubMed] [Google Scholar]
- 34. Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev 2011; 12: 912–934. [DOI] [PubMed] [Google Scholar]
- 35. Thomas GN, Jiang CQ, Taheri S, et al. A systematic review of lifestyle modification and glucose intolerance in the prevention of type 2 diabetes. Curr Diabetes Rev 2010; 6: 378–387. [DOI] [PubMed] [Google Scholar]
- 36. Coppola A, Sasso L, Bagnasco A, Giustina A, Gazzaruso C. The role of patient education in the prevention and management of type 2 diabetes: an overview. Endocrine 2016; 53: 18–27. [DOI] [PubMed] [Google Scholar]
- 37. Lamarche B, Couture P. Dietary fatty acids, dietary patterns, and lipoprotein metabolism. Curr Opin Lipidol 2015; 26: 42–47. [DOI] [PubMed] [Google Scholar]
- 38. Sarzynski MA, Burton J, Rankinen T, et al. The effects of exercise on the lipoprotein subclass profile: a meta‐analysis of 10 interventions. Atherosclerosis 2015; 243: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goff DC Jr, D'Agostino RB Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism 2005; 54: 264–270. [DOI] [PubMed] [Google Scholar]
- 40. Colhoun HM, Otvos JD, Rubens MB, Taskinen MR, Underwood SR, Fuller JH. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes 2002; 51: 1949–1956. [DOI] [PubMed] [Google Scholar]
- 41. Mackey RH, Kuller LH, Sutton‐Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol 2002; 90(suppl): 71i–76i. [DOI] [PubMed] [Google Scholar]
- 42. Schreier L, Berg G, Zago V, Gonzalez AI, Wikinski R. Kinetics of in vitro lipolysis of human very low‐density lipoprotein by lipoprotein lipase. Nutr Metab Cardiovasc Dis 2002; 12: 13–18. [PubMed] [Google Scholar]
- 43. Guerin M, Lassel TS, Le Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler Thromb Vasc Biol 2000; 20: 189–197. [DOI] [PubMed] [Google Scholar]
- 44. Ioannidis JP, Haidich AB, Pappa M, et al. Comparison of evidence of treatment effects in randomized and non‐randomized studies. JAMA 2001; 286: 821–830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of participants in intensive and moderate lifestyle modification
Table S2 Baseline characteristics in intensive lifestyle modification participants: dropouts vs completers
Table S3 Baseline characteristics in intensive lifestyle modification participants: unmatched vs matched
Table S4 Baseline characteristics in moderate lifestyle modification participants: dropouts vs completers
Table S5 Effects of lipid‐lowering medications on the LPIR score and lipoproteins
Figure S1 Atherogenicity of the six lipoprotein characteristics associated with insulin resistance and type‐2 diabetes risk that were used to calculate the LPIR score (22).
Supporting info item


