Abstract
Background:
Inflammatory processes could underlie mood disorders. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMP) are inflammation-related molecules. The current study sought an association between mood disorders and systemic levels of MMPs and TIMPs.
Methods:
Serum was obtained from patients with mood disorders (n=21) and patients with schizophrenia (n=13) scheduled to undergo electroconvulsive therapy. Serum was also obtained from healthy controls (n=40). Clinical symptoms were assessed by the Hamilton Rating Score for Depression and the Brief Psychiatric Rating Scale. Serum levels of MMPs and TIMPs were quantified by ELISA.
Results:
The serum levels of MMP-2 in mood disorder patients, but not in schizophrenia patients, prior to the first electroconvulsive therapy session (baseline) was significantly lower than that of healthy controls. At baseline, levels of MMP-9 and TIMP-2, -1 were not different between patients with mood disorder and schizophrenia and healthy controls. After a course of electroconvulsive therapy, MMP-2 levels were significantly increased in mood disorder patients, but MMP-9 levels were significantly decreased in both mood disorder and schizophrenia patients. In mood disorder patients, there was a significant negative correlation between depressive symptoms and serum levels of MMP-2 and a positive correlation between depressive symptoms and MMP-9. In addition, alterations of serum levels of MMP-2 and MMP-9 were significantly correlated each other and were associated with certain depressive symptoms.
Conclusion:
A change in inflammatory homeostasis, as indicated by MMP-2 and MMP-9, could be related to mood disorders, and these markers appear to be sensitive to electroconvulsive therapy.
Keywords: MMP-2, MMP-9, mood disorders, schizophrenia, electroconvulsive therapy
Introduction
Matrix metalloproteinases (MMPs) are zinc-dependent proteases that are involved not only in the degradation and remodeling of the extracellular matrix but also in processing bioactive molecules, including cell surface receptors, neurotrophic factors, chemokines, and cytokines (Kim and Joh, 2012). MMPs are indispensable enzymes for various physiological processes such as embryogenesis, morphogenesis, tissue reconstruction, and regulation of inflammatory processes under a variety of conditions (Nissinen and Kahari, 2014). MMPs belong to a family of more than 25 enzymes, and each has their own activating cascade with MMP-2 and MMP-9 at the core. Among MMPs, gelatinases, including MMP-2 and MMP-9, are similar in structure and are able to enzymatically process various substrates of the extracellular matrix, such as gelatin, collagen I and collagen IV (Sbardella et al., 2012). Constitutively expressed in most cell types, MMP-2 has a prohomeostatic property, whereas MMP-9 is expressed at low levels in most cell types but is highly responsive to stimulation and has proinflammatory properties (Van den Steen et al., 2002; Sbardella et al., 2012).
These enzymes are also known to be related to pathological processes such as cancer, neurodegenerative disorders, arthritis, and cardiovascular diseases (Sbardella et al., 2012). In the central nervous system, MMPs play important roles in synaptogenesis, synaptic plasticity, and long-term potentiation (Ethell and Ethell, 2007). In contrast, there are limited studies of the role of MMPs in psychiatric disorders.
The MMPs are endogenously inhibited by the tissue inhibitors of MMPs (TIMPs). In humans, 4 TIMP species have been reported. Of these, the C-terminal domain of TIMP-1 and TIMP-2 binds to the hemopexin of the proenzymes of MMP-9 and MMP-2, respectively (Carlo, 2014). This indicates that TIMP-1 and TIMP-2 are major individual inhibitors of MMP-9 and MMP-2 (Goldberg et al., 1992).
Mood disorders (MDs) have been recognized as having a strong association with chronic inflammatory states (Rosenblat et al., 2014). In depression, fatigue and somatic symptoms such as gastrointestinal symptoms, autonomic symptoms, and flu-like malaise are significantly associated with signs of an activated inflammatory pathway (Maes et al., 2012). Several studies have reported an association between depressive symptoms and state-dependent elevated levels of inflammatory markers, such as interleukin-1 beta, interleuki-6, and tumor necrosis factor-alpha (Felger and Lotrich, 2013; Rosenblat et al., 2014). MMPs process cytokines, which leads to alterations of their activity (Van Lint and Libert, 2007). At the same time, the expression of MMPs, MMP-9 in particular, is regulated by cytokines (Harkness et al., 2000). This indicates that cytokines and MMPs regulate each other in a bidirectional manner.
The current study sought to find an association between MD and inflammation-related markers. The main MMPs, MMP-2 and MMP-9, and their inhibitors, TIMP-2 and TIMP-1, were quantified in patients with MD. First, serum levels of MMP-2, MMP-9, TIMP-2, and TIMP-1 from patients with MD who had depressive episodes and were eligible for electroconvulsive therapy (ECT) were compared with healthy controls and patients with schizophrenia-spectrum disorders (SCZ), who also had psychotic episodes and were also eligible for ECT. Serum levels of MMP and TIMP were then compared before and after a course of ECT in patients with MD and SCZ. Finally, an association between serum levels of MMPs and TIMPs with clinical symptoms in patients with MD and SCZ was determined.
Methods
Subjects
This study was conducted at the Department of Psychiatry of the National Hospital Organization Kure Medical Center between January 2011 and April 2013. Thirty-four patients (12 men and 22 women, mean age ± SD=54.1±16.1) were recruited among inpatients planning to receive ECT based on the guidelines of the American Psychiatric Association (2001). ECT is often proscribed when a patient exhibits episodes of severe major depression, psychosis, and catatonia or has shown insufficient improvement with prescribed pharmacotherapy treatment (American Psychiatric Association, 2001). All patients were diagnosed according to DSM-IV-TR. Twenty-one patients were diagnosed as having MD, either major depressive disorders (n=11) or bipolar disorders (n=10), with a current episode of major depression. Thirteen patients were diagnosed with SCZ, either schizophrenia (n=10) or schizoaffective disorder (n=3), with symptoms currently exacerbated to psychosis, such as delusions, and catatonia. Various types of schizophrenia were diagnosed, including catatonic (n=5), paranoid (n=4), and undifferentiated (n=1). Patients with a past or present history of substance abuse, substance dependence, significant neurological illness, or any other significant medical illness were excluded from participation in the current study. A clinical psychiatrist recommended ECT to each patient according to standards recommended by the American Psychiatric Association task force and based on the patient’s urgency and severity of illness.
Forty healthy subjects (14 men and 26 women, mean age 54.2±13.9 years), with no history of past or current mental disorders were recruited as a control group.
After procedures were fully explained, written informed consent was obtained from all subjects to participate in the study. The current study was approved by the Ethics Committee of National Hospital Organization Kure Medical Center.
ECT Treatment Procedures
ECT was performed according to procedures from a previous report (Shibasaki et al., 2015). Before undergoing ECT, each patient was screened for general health through a physical and neurological examination, blood and urine tests, electrocardiogram, chest X-ray film, and a cerebral computed tomography scan. Anesthesia was induced with i.v. thiamylal sodium (2–3mg/kg, i.v.) and suxamethonium chloride (0.5–1mg/kg, i.v.). The ECT device used was the Thymatron System IV brief pulse square wave apparatus (Somatics Inc). Electrodes were positioned bilaterally on the frontal-temporal region. Only one adequate seizure was required for each session, which was defined as an electroencephalographic seizure lasting more than 25 seconds with a high-amplitude, slow wave, and postictal suppression. The initial stimulus dose was determined using the half-age method (Petrides and Fink, 1996). If an adequate electroencephalographic seizure occurred in one session, the stimulus energy of the next session remained the same. When a missed or inadequate seizure occurred, the patient was restimulated after a 20- to 30-second pause and the stimulus increased 1.5- to 2.0-fold. The maximum number of stimulations for each treatment session was 2. ECT was administered a maximum of 3 times per week. If any adverse effects (eg, cognitive dysfunction, delirium) occurred, the frequency of the ECT schedule was reduced to once or twice per week. ECT continued until the patient was asymptomatic or the attending psychiatrist determined that the patient had obtained the maximum benefit within 3 to 15 sessions. After the purpose and the ECT procedure were described in detail, written informed consent was obtained from patients or caregivers of patients prior to initiating ECT.
Most of patients with MD (20/21 patients) received antidepressant pharmacotherapy before ECT: duloxetine (20–60mg/d; n=8), mirtazapine (15–45mg/d; n=8), paroxetine (20–40mg/d; n=4), sertraline (25–100mg/d; n=2), mianserin (10–60mg/d; n=5), trazodone (25–75mg/d; n=3), imipramine (50mg/d; n=1), or nortriptyline (100mg/d; n=2). Nine subjects were on a stable dose of antidepressant during ECT, 8 subjects had antidepressant dosage decreased during the course of ECT, and 3 subjects had antidepressant dosage increased during the course of ECT. One patient did not receive any antidepressants while undergoing the course of ECT. Eleven of 21 patients with MD also took an antipsychotic medication (either olanzapine or quetiapine) before the course of ECT to control either psychomotor agitation or psychotic symptoms.
Eleven of 13 patients with SCZ received antipsychotic pharmacotherapy before the course of ECT: risperidone (1.5–12mg/d; n=7), olanzapine (10–20mg/d; n=4), quetiapine (300–675mg/d; n=4), zotepine (150–400mg/d; n=3), blonanserin (12–24mg/d; n=2), aripiprazole (24mg/d; n=1), haloperidol (30mg/d; n=1), chlorpromazine (450mg/d; n=1), or i.v. haloperidol (5–10mg/d; n=3). During the course of ECT, 3 subjects were on a stable dose of antipsychotics, 7 subjects had antipsychotics dosage decreased, and 3 subjects had antipsychotics dosage increased.
Mood stabilizers were withdrawn prior to the first ECT session. The use of benzodiazepines was permitted during the study period. Routine psychotherapy was practiced regularly.
Assessment of Clinical Symptoms
Clinical symptomatic scores were assessed using the 17-item (Hamilton Rating Score for Depression [HAMD]) for patients with MD and the Brief Psychiatric Rating Scale (BPRS) for patients with SCZ. HAMD 17-items were assigned to the following 5 subscales: core symptom (items 1, 2, 7, 8, 10, 13); sleep (items 4, 5, 6); activity (items 7, 8); psychic anxiety (items 9, 10); and somatic anxiety (items 11, 12, 13) in accordance with a previous report (Seretti et al., 1999). Each patient’s symptoms were assessed prior to the first ECT session (baseline, pre-ECT) and a day after the last ECT session (post-ECT) by the same psychiatrist. Responders to ECT were defined as demonstrating a 50% decrease in HRSD score in MD patients or a 30% decrease in BPRS score in SCZ patients.
MMPs and TIMPs Assay
Venous blood samples were taken in the morning (between 7:00 and 8:00 am) at pre-ECT and post-ECT. Blood samples were drawn into anticoagulant-free tubes. They were kept at room temperature for 1 hour, and serum was separated by centrifugation at 3000rpm for 15 minutes at 4°C. Serum samples were stored at -80°C until assay. Serum levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 were measured using ELISA (Quantikine ELISA, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical Analysis
The data are shown as the mean ± SD. The normal distribution of data was tested by the Shapiro-Wilk and the Kolmogorov-Smirnov tests, and statistical analysis was performed by nonparametric tests. A Kruskal-Wallis test was used to evaluate the significant difference in the parameters (clinical and laboratory values) among the 3 groups (MD, SCZ, and control). The Bonferroni test was performed as the posthoc test. Significant differences in the parameters between the 2 groups of patients with MD and SCZ were evaluated using the Mann-Whitney U-test. A chi-square test was used for categorical variables. A Wilcoxon signed-rank test was used to evaluate the statistical differences in the parameters between pre-ECT and post-ECT. Spearman’s correlation evaluated the possible relationship between the clinical symptoms and serum levels. The receiver operating characteristic (ROC) curves for the assessment of specificity and sensitivity of MMPs and TIMPs were calculated to discriminate between the MD group and the control group. The areas under the ROC curve (AUC) were calculated for each MMPs and TIMPs as well. Statistical significance after logistic regression between the 2 AUCs was determined. Statistical analysis was conducted by logistic regression, analyzing diagnosis value of single and combined detection of MMPs. Statistical significance was defined as P < .05. Analyses were performed using SPSS version 22.0 for Windows (IBM Japan Corporation, Tokyo, Japan).
Results
Clinical Data
The clinical data of the 3 groups (MD, SCZ, and control) are presented in Table 1. Gender and age did not significantly differ between the 3 groups. Patients with MD were significantly older and their duration of illness was shorter compared with the SCZ group. Duration of current episode, number of ECTs, and duration of the ECT course did not differ between the MD and SCZ groups. There were no differences between the dose equivalence of imipramine of pre-ECT and post-ECT in the MD group and also no differences between the dose equivalence of chlorpromazine of pre-ECT and post-ECT in the SCZ group.
Table 1.
Subject Clinical Data
| MD | SCZ | Controls | P | |
|---|---|---|---|---|
| (n=21) | (n=13) | (n=40) | ||
| Gender, female | 15 (71.4%) | 7 (53.8%) | 26 (65.0%) | .580a |
| Age (y) | 58.5±15.4 | 46.9±15.1 | 54.2±13.9 | .163b |
| Age of onset (y) | 51.2±16.8 | 31.4±15.6 | .002c | |
| Subtype of MD | ||||
| Major depressive disorder | 11 (52.4%) | |||
| Bipolar disorder | 10 (47.6%) | |||
| Subtype of SCZ | ||||
| Paranoid type | 4 (30.8%) | |||
| Catatonic type | 5 (38.5%) | |||
| Undifferentiated type | 1 (7.7%) | |||
| Schizoaffective disorder | 3 (23.1%) | |||
| Duration of illness (y) | 7.4±8.4 | 15.5±12.1 | .029c | |
| Duration of current episode (mo) | 6.7±6.6 | 4.6±3.7 | .400c | |
| HAMD score at pre-ECT | 19.6±8.9 | |||
| HAMD score at post-ECT | 3.0±2.5 | <.001d | ||
| BPRS score at pre-ECT | 53.8±13.3 | |||
| BPRS score at post-ECT | 28.5±4.9 | .001d | ||
| IMI equivalence at pre-ECT (mg/d) | 175.0±109.1 | |||
| IMI equivalence at post-ECT (mg/d) | 149.4±93.6 | .153d | ||
| CPZ equivalence at pre-ECT (mg/d) | 1325.2±813.5 | |||
| CPZ equivalence at post-ECT (mg/d) | 1124.2±717.8 | .110d | ||
| Number of ECT (sessions) | 9.8±3.3 | 10.1±3.3 | .780c | |
| Duration of ECT course (d) | 27.4±11.6 | 25.2±6.5 | .462c | |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CPZ, chlorpromazine; ECT, electroconvulsive therapy; HAMD, Hamilton Rating Score for Depression; IMI, imipramine; MD, mood disorder; SCZ: schizophrenia-spectrum disorder.
Data show the mean ± SD or number (%).
a Comparison between 3 groups by the chi-square test.
b Comparison between 3 groups by the Kruskal Wallis test.
c Comparison between 2 patients groups by the Mann-Whitney U-test.
d Comparison between scores at pre-ECT and those at post-ECT by Wilcoxon signed-rank test.
Serum Levels of MMPs and TIMPs at Pre-ECT in the MD Group Compared with the SCZ Group and Control Group
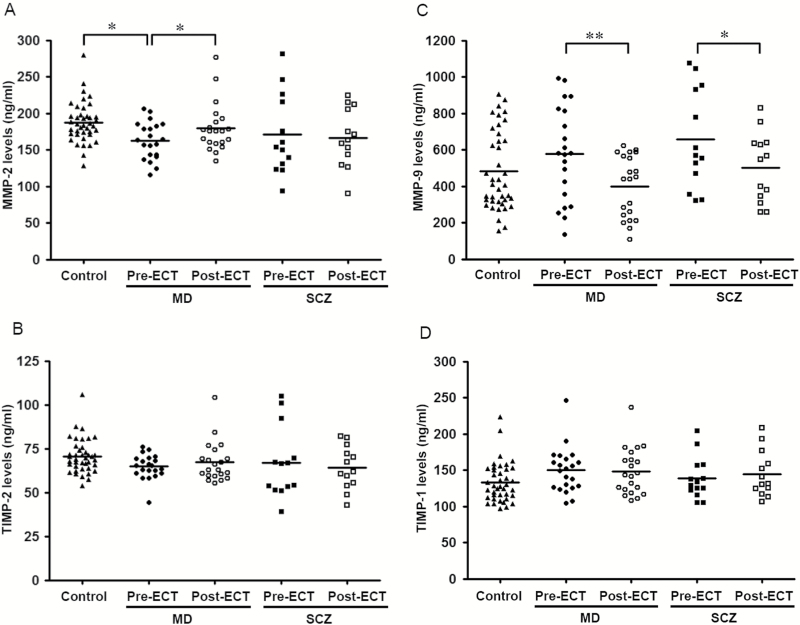
Serum levels of MMP-2 in the MD group with depressive episodes at pre-ECT were significantly lower than those of the control group (Figure 1A; P=.023), whereas there were no significant differences for the serum levels of TIMP-2, MMP-9, and TIMP-1 between the MD group and the control group (Figure 1B-D). Serum levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the MD group at pre-ECT were not significant different compared with those of the SCZ group with psychotic episodes at pre-ECT. Additionally, serum levels of MMPs and TIMPs were not significantly different between MD patients with either MD (unipolar) or bipolar disorder (bipolar) at pre-ECT (MMP-2, unipolar=165.9±25.0ng/mL vs bipolar=159.4±26.8ng/mL, P=.512; TIMP-2, 63.1±7.6ng/mL vs 66.6±6.2ng/mL, P=.259; MMP-9, 553.9±218.8ng/mL vs 604.6±310.5ng/mL, P=.668; TIMP-1, 150.5±39.4ng/mL vs 148.0±23.2ng/mL, P=.571).
Figure 1.
Scatter plot of serum levels of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs). MMP-2 (A), TIMP-2 (B), MMP-9 (C), and TIMP-1 (D) in controls (▲), mood disorders (MD) group before a course of ECT (pre-ECT, ●) and after a course of ECT (post-ECT, ○), and schizophrenia-spectrum disorders (SCZ) group pre-ECT (■) and post-ECT (□). The horizontal bars represent the mean values.
In the ROC curve analysis of diagnosis, the AUC of MMP-2, MMP-9, TIMP-1, and TIMP-2 at pre-ECT between the MD and control groups were 0.738, 0.603, 0.674, and 0.675, respectively. When combing MMP-9 and MMP-2, the combined AUC was 0.745.
Alterations of Serum Levels of MMPs and TIMPs in the MD and SCZ Groups over the Course of ECT
The scores for depressive symptoms evaluated by HAMD in the MD group and the scores of psychotic symptoms evaluated by BPRS in the SCZ group significantly decreased following ECT (Table 1). All patients with MD were responders to ECT. Eleven of 13 patients with SCZ (84.6%) were responders to ECT. Both nonresponding SCZ patients were diagnosed with paranoid-type SCZ.
There was a statistically significant increase in serum levels of MMP-2 in the MD group over the course of ECT (P=.046) but no significant change observed in the SCZ group (P=.650) (Figure 1A). There were statistically significant decreases in serum levels of MMP-9 in both the MD and SCZ groups following ECT (MD group, P=.003; SCZ group, P=.019) (Figure 1C). Among the 2 nonresponders to ECT in the SCZ group, one patient had an increase in serum level of MMP-9 and the other had a decrease in serum level of MMP-9 after the course of ECT (data not shown).
Serum levels of TIMP-2 and TIMP-1 did not significantly change over the course of ECT in both the MD and SCZ groups (Figure 1B, D).
Relationship between Serum Levels of MMPs and TIMPs at Pre-ECT and Post-ECT and Subject Characteristics of the MD Group
No significant correlations were observed in the MD group between subject characteristics (gender, age, age of onset, duration of illness, duration of current episode, or dose of medication) and serum levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 at either pre-ECT or post-ECT (data not shown).
Correlation between Serum Levels of MMPs and TIMPs and Clinical Symptomatic Scores in the MD and SCZ Groups
Correlation coefficients were calculated between serum levels of MMPs and TIMPs and clinical symptomatic scores evaluated by HAMD for MD and by BPRS for SCZ, combining results from pre-ECT and post-ECT (Table 2). In the SCZ group, there was no significant correlation between serum levels of MMPs, TIMPs, and total BPRS scores (Table 2). In the MD group, serum levels of TIMP-1 and TIMP-2 did not correlate with total HAMD score (Table 2). Furthermore, no significant correlations were observed between TIMPs and subscale HAMD scores (Table 3).
Table 2.
Correlation between MMP and TIMP Serum Levels and Clinical Symptomatic Scores of MD and SCZ Groups
| Total HAMD Score for MD | Total BPRS Score for SCZ | |||
|---|---|---|---|---|
| Rho | P | Rho | P | |
| MMP-2 | -0.316 | .041 * | -0.241 | .236 |
| TIMP-2 | -0.039 | .806 | -0.209 | .305 |
| MMP-9 | 0.415 | .006 ** | 0.260 | .200 |
| TIMP-1 | 0.087 | .584 | -0.256 | .206 |
Abbreviations: BPRS: Brief Psychiatric Rating Scale; HAMD, Hamilton Rating Score for Depression; MD, mood disorder; MMP, matrix metalloproteinase; SCZ, schizophrenia-spectrum disorder; TIMP, tissue inhibitor of MMP.
*P < .05, **P < .01.
Table 3.
Correlation between MMP and TIMP Serum Levels and Subscale HAMD Scores in MD Groups
| Core symptom | Sleep | Activity | Psychic Anxiety | Somatic Anxiety | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rho | P | Rho | P | Rho | P | Rho | P | Rho | P | |
| MMP-2 | -0.339 | .028* | -0.191 | .225 | -0.322 | .038* | -0.180 | .253 | -0.350 | .023* |
| TIMP-2 | -0.085 | .593 | 0.072 | .650 | -0.124 | .434 | -0.025 | .875 | -0.022 | .891 |
| MMP-9 | 0.399 | .009** | 0.169 | .285 | 0.457 | .020* | 0.316 | .041* | 0.363 | .018* |
| TIMP-1 | 0.049 | .759 | 0.078 | .625 | 0.047 | .766 | -0.018 | .911 | 0.204 | .195 |
Abbreviations: HAMD, Hamilton Rating Score for Depression; MD, mood disorder; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of MMP.
*P < .05, **P < .01.
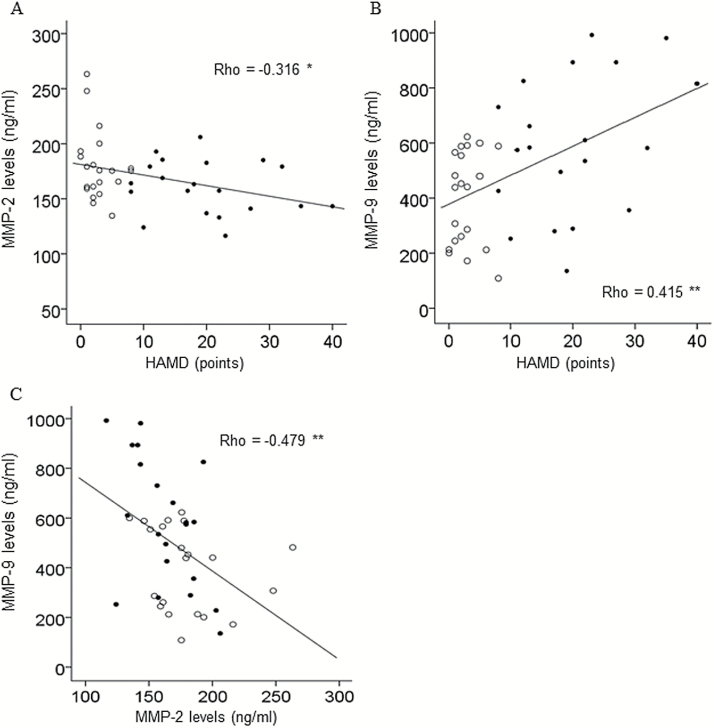
However, in the MD group, there was a significant negative correlation between serum levels of MMP-2 and total HAMD score (P=.041) (Table 2; Figure 2A) and a significant positive correlation between serum levels of MMP-9 and total HAMD score (P=.006) (Table 2; Figure 2B). Furthermore, negative correlations between serum levels of MMP-2 and subscale HAMD scores (core symptom, activity, somatic anxiety) were observed as well as positive correlations between serum levels of MMP-9 and the same subscale HAMD scores (core symptom, activity, psychic anxiety, somatic anxiety) as observed with MMP-2 (Table 3). A significant negative correlation between serum levels of MMP-2 and MMP-9 was observed in the MD group (P=.001, Rho=-0.479), but not in the SCZ group (P=.368, Rho=-0.184) or in the control group (P=.592, Rho=0.087) (Figure 2C).
Figure 2.
Correlation between serum levels of matrix metalloproteinases (MMPs) and total Hamilton Rating Scale for Depression (HAMD) score in patients with mood disorders (MD) before and after a course of ECT. (A) Significant negative correlation between serum levels of MMP-2 and total HAMD score. (B) Significant positive correlation between serum levels of MMP-9 and total HAMD score. (C) Significant negative correlation between serum levels of MMP-2 and MMP-9. Pre-ECT values (●) and post-ECT values (○); n=21, *P < .05
Discussion
The current study demonstrated that serum levels of MMP-2 were specifically reduced in MD patients with depressive symptoms applicable to ECT and that serum levels of both MMP-2 and MMP-9 were significantly altered in opposing directions in a depressive state-dependent manner following ECT. In addition, alterations of MMP-2 and MMP-9 appear to be associated with certain depressive symptoms such as not only core symptom, but also somatic anxiety and activity.
The current study is the first to demonstrate a significant negative association between serum levels of MMP-2 and clinical scores of depressive symptoms during the course of ECT in MD patients. A previous proteomic study demonstrated that the plasma levels of MMP-2 in depressive patients were significantly lower than those of control subjects, and plasma levels of MMP-2 in schizophrenic patients were the same as those of control subjects (Domenici et al., 2010). The study, however, measured MMPs at only one time point, utilized a proteomics method, and did not evaluate patient symptoms. Despite these limitations, the previous findings parallel the current findings.
It is currently unknown why circulating levels of MMP-2 declined and are associated with levels of MMP-9 in the MD patients in a depressive state. MMP-2 is constitutively expressed in almost all human tissues, but mainly by endothelial and epithelial cells and fibroblasts (Sbardella et al., 2012). Within the central nervous system, astrocytes are a major source of MMP-2 and presumably drive physiological remodeling of the blood brain barrier (BBB) (del Zoppo et al., 2007; Candelario-Jalil et al., 2011). MMP-2 is detectable in significant serum concentrations under physiological conditions and is linked to homeostatic functioning (Sbardella et al., 2012). The current study showed a reduction of MMP-2 in MD patients before ECT and an increase in MMP-2 after a course of ECT. A number of studies have demonstrated significant reductions of glia, mainly astrocytes, in the postmortem brain of MD patients (Ongur et al., 1998; Cotter et al., 2001; Gittins and Harrison, 2011). Another postmortem brain study demonstrated that coverage of blood vessels by astrocyte endfeet in the prefrontal cortex was significantly reduced in patients with MD (Rybakowski et al., 2013). The decline in glia within brain vasculature areas associated with mood function suggests the possibility of reductions in microenvironmental cellular components of the BBB, which is composed of astrocytes and vascular endothelial cells, in MD patients. A clinical finding pointed out that serum levels of MMP-2 were decreased in patients suffering from subarachnoid hemorrhage, in which the BBB is in fact compromised (Horstmann et al., 2006). Decreased MMP-2 in the circulation in MD patients, then, suggests a small compromise of the BBB compared with a gross compromise observed with subarachnoid hemorrhage. The increase of serum levels of MMP-2 in MD patients after the ECT course could be associated with the remodeling of gliovascular units, which is supported by previous ECT findings showing gliogenesis and angiogenesis (Hellsten et al., 2005; Wennstrom et al., 2006; Bouckaert et al., 2014). Further, this MMP-2-related phenomenon could lead to increased incidences of inflammatory states such as production of MMP-9 through a disruption in the BBB in the brain of MD patients, because expression of MMP-9 is regulated not only by immune cells in response to inflammatory events (Van den Steen et al., 2002; Sbardella et al., 2012) but also directly by MMP-2 activity (Dzwonek et al., 2004; Romi et al., 2012). Thus, future studies will be needed to clearly establish evidence of a MMP-2-related “dysregulated BBB” and concurrent alteration of MMP-9 in MD patients by examining samples from patients such as postmortem brain and cerebrospinal fluid.
In the current study, the serum levels of MMP-9 in MD patients was not significantly different from that of the control group before ECT, but MMP-9 significantly decreased following ECT. In addition, a significant positive correlation was observed between MMP-9 and HAMD scores. Similar to the current findings, a previous study found that HAMD scores and serum levels of MMP-9 were positively correlated and there was no difference between depressed patients and controls in serum levels of MMP-9 (Yoshida et al., 2012). Others, however, have found that plasma levels of MMP-9 in depressed patients were significantly higher than those of the controls in a proteomic study (Domenici et al., 2010), and serum levels of MMP-9 in depressed young patients with bipolar disorder were significantly higher than those of the controls, which remained elevated even following drug treatment (Rybakowski et al., 2013). Possible reasons for the discrepancy between the current findings and the findings of increased MMP-9 in depressed patients include differences in subject age, severity of symptoms, choice of symptom rating scale, and treatment protocol. It is also possible that circulating MMP-9 is considerably sensitive to psychosocial stress even in healthy individuals (Garvin et al., 2009).
The mechanism by which ECT reduces serum levels of MMP-9 is unknown. An inflammatory state has been proposed as the substrate of MD, since circulating proinflammatory cytokines increase with the severity of mood disturbance symptoms (Felger and Lotrich, 2013; Rosenblat et al., 2014) and cytokine levels decrease after the ECT course (Hestad et al., 2003; Rotter et al., 2013). It is possible that in a depressive episode, the homeostatic state maintained by the immune system could be altered by changes in expression of proinflammatory cytokines and MMPs, and these alterations could be normalized through ECT treatment. The decreased MMP-9 levels in both MD and SCZ groups following ECT could reflect a common involvement of MMP-9 in psychiatric disorders. Fragile X syndrome, psychiatric disorders associated with anxiety, compulsive-repetitive behaviors, and social communication deficits have been associated with changes in MMP-9 levels. Knockout of the MMP-9 gene improved symptoms in a mouse model of Fragile X syndrome and reduced dendritic spine abnormalities (Sidhu et al., 2014). A clinical trial of the antibiotic minocycline decreased MMP-9 levels and appeared to attenuate the symptoms of Fragile X syndrome (Dziembowska et al., 2013). These findings, combined with the current findings, suggest that decreasing MMP-9 alleviates some types of psychiatric disorders.
As biomarkers for MD, ROC analysis in the current study demonstrated that the AUC for both MMPs and TIMPs prior to ECT was found to be 0.745 at its peak, which does not suggest that either ligand would have high diagnostic value as a biomarker. Because serum levels of MMP-2 was negatively correlated to certain depressive symptoms, to which MMP-9 was positively correlated, both MMP-2 and MMP-9 could serve as biomarkers for specific depressive states or symptoms with greater accuracy if possibly combined with other inflammatory ligands such as cytokines.
To place the current findings into perspective, there are a few limitations that should be mentioned. While the total sample size of the current study was small, significant correlations were nonetheless observed. Expanding the cohorts could reveal significant changes and correlations in MMP and TIMP. Second, it is unclear if blood concentrations of MMP and TIMP reflect those in the brain. There are some reports that found no correlation between blood and CSF concentrations of MMPs (Horstmann et al., 2010; Niebroj-Dobosz et al., 2010). Therefore, it is possible that the dynamics of MMPs, as well as TIMP, in the brain are different from those in peripheral tissues. To overcome this issue, direct assessment of MMPs in CSF from MD patients could be highly useful. Finally, the effect of medications on serum MMPs levels is not well understood. Although there were no statistically significantly differences before and after ECT on the equivalence of both imipramine and chlorpromazine, patients were allowed to use medication during the course of ECT. A future investigation could include drug-naïve patients to evaluate the effects of medications on MMP, at least before treatments.
Conclusion
Serum levels of MMP-2 were significantly decreased in MD patients with depressive episodes, and a course of ECT in these patients significantly increased MMP-2 but decreased MMP-9. Also in MD patients, there were significant correlations between depressive symptoms and serum levels of MMP-2 and MMP-9, in opposite directions, in response to ECT. The findings suggest that alteration of homeostasis linked to inflammation lead to changes in levels of MMP-2 and MMP-9, which occurs in a disease-specific manner and depend on the severity of depressive symptoms, which are in turn sensitive to ECT treatment.
Interest Statement
Shigeto Yamawaki MD, PhD has received donation for research from Astellas, Pfizer and MSD honoraria for lectures from Glaxo Smith Kline, Astellas, Daiichi Sankyo, Japan Eli Lilly, Takeda, Otsuka, Eisai, Dai Nippon Sumitomo, Shionogi, Meiji Seika, Mochida, and Pfizer. No financial support for this manuscript was received from any pharmaceutical companies.
Acknowledgments
We are grateful to our colleagues for their tireless and expert assistance: Dr. Hideo Kobayakawa, Dr. Keigo Nakatsu, and Dr. Motonobu Nakamura. We also thank Dr. Yasutaka Fujita for establishing the patients’ data base and Dr. Aldric Hama for editorial assistance.
This work was supported in part by Grants-in-Aid from the Japan Society for the Promotion of Science (grant number: 15K09819) and grants from the SENSHIN Medical Research Foundation.
References
- American Psychiatric Association (2001) The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging (A Task Force Report of the American Psychiatric Association), 2nd ed. Washington DC: American Psychiatric Press. [Google Scholar]
- Bouckaert F, Sienaert P, Obbels J, Dols A, Vandenbulcke M, Stek M, Bolwig T. (2014) ECT: its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J ECT 30:143–151. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA. (2011) Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke 42:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo DIA. (2014) Matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 and -2 in sera and urine of patients with renal carcinoma. Oncol Lett 7:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. (2007) Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 38:646–651. [DOI] [PubMed] [Google Scholar]
- Domenici E, Wille DR, Tozzi F, Prokopenko I, Miller S, McKeown A, Brittain C, Rujescu D, Giegling I, Turck CW, Holsboer F, Bullmore ET, Middleton L, Merlo-Pich E, Alexander RC, Muglia P. (2010) Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One 5:e9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, Durbin-Johnson B, Hagerman RJ, Tassone F. (2013) High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am J Med Genet A 161:1897–1903. [DOI] [PubMed] [Google Scholar]
- Dzwonek J, Rylski M, Kaczmarek L. (2004) Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett 567:129–135. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. (2007) Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res 85:2813–2823. [DOI] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin P, Nilsson L, Carstensen J, Jonasson L, Kristenson M. (2009) Plasma levels of matrix metalloproteinase-9 are independently associated with psychosocial factors in a middle-aged normal population. Psychosom Med 71:292–300. [DOI] [PubMed] [Google Scholar]
- Gittins RA, Harrison PJ. (2011) A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord 133:328–332. [DOI] [PubMed] [Google Scholar]
- Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. (1992) Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem 267:4583–4591. [PubMed] [Google Scholar]
- Harkness KA, Adamson P, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. (2000) Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain 123:698–709. [DOI] [PubMed] [Google Scholar]
- Hellsten J, West MJ, Arvidsson A, Ekstrand J, Jansson L, Wennstrom M, Tingstrom A. (2005) Electroconvulsive seizures induce angiogenesis in adult rat hippocampus. Biol Psychiatry 58:871–878. [DOI] [PubMed] [Google Scholar]
- Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. (2003) Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J ECT 19:183–188. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Su Y, Koziol J, Meyding-Lamade U, Nagel S, Wagner S. (2006) MMP-2 and MMP-9 levels in peripheral blood after subarachnoid hemorrhage. J Neurol Sci 251:82–86. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Budig L, Gardner H, Koziol J, Deuschle M, Schilling C, Wagner S. (2010) Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer’s disease. Int Psychogeriatr 22:966–972. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. (2012) Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol Ther (Seoul) 20:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Ringel K. (2012) Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psychiatry 36:169–175. [DOI] [PubMed] [Google Scholar]
- Niebroj-Dobosz I, Janik P, Sokolowska B, Kwiecinski H. (2010) Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Eur J Neurol 17:226–231. [DOI] [PubMed] [Google Scholar]
- Nissinen L, Kahari VM. (2014) Matrix metalloproteinases in inflammation. Biochim Biophys Acta 1840:2571–2580. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A 95:13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides G, Fink M. (1996) The “half-age” stimulation strategy for ECT dosing. Convuls Ther 12:138–146. [PubMed] [Google Scholar]
- Romi F, Helgeland G, Gilhus NE. (2012) Serum levels of matrix metalloproteinases: implications in clinical neurology. Eur Neurol 67:121–128. [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. (2014) Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 53:23–34. [DOI] [PubMed] [Google Scholar]
- Rotter A, Biermann T, Stark C, Decker A, Demling J, Zimmermann R, Sperling W, Kornhuber J, Henkel A. (2013) Changes of cytokine profiles during electroconvulsive therapy in patients with major depression. J ECT 29:162–169. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Remlinger-Molenda A, Czech-Kucharska A, Wojcicka M, Michalak M, Losy J. (2013) Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J Affect Disord 146:286–289. [DOI] [PubMed] [Google Scholar]
- Sbardella D, Fasciglione GF, Gioia M, Ciaccio C, Tundo GR, Marini S, Coletta M. (2012) Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Aspects Med 33:119–208. [DOI] [PubMed] [Google Scholar]
- Seretti A, Cusin C, Lattuada E, Di Bella D, Catalano M, Smeraldi E. (1999) Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry 4:280–283. [DOI] [PubMed] [Google Scholar]
- Shibasaki C, Takebayashi M, Fujita Y, Yamawaki S. (2015) Factors associated with the risk of relapse in schizophrenic patients after a response to electroconvulsive therapy: a retrospective study. Neuropsychiatr Dis Treat 11:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. (2014) Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci 34:9867–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. (2002) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37:375–536. [DOI] [PubMed] [Google Scholar]
- Van Lint P, Libert C. (2007) Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukocyte Biol 82:1375–1381. [DOI] [PubMed] [Google Scholar]
- Wennstrom M, Hellsten J, Ekstrand J, Lindgren H, Tingstrom A. (2006) Corticosterone-induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol Psychiatry 59:178–186. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K. (2012) Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One 7:e42676. [DOI] [PMC free article] [PubMed] [Google Scholar]