Abstract
Background:
Acutely elevated cortisol levels in healthy humans impair autobiographical memory recall and alter hemodynamic responses of the amygdala to emotionally valenced stimuli. It is hypothesized that the effects of the cortisol on cognition are influenced by the ratio of mineralocorticoid receptor to glucocorticoid receptor occupation. The current study examined the effects of acutely blocking mineralocorticoid receptors and glucocorticoid receptors separately on 2 processes known to be affected by altering levels of cortisol: the specificity of autobiographical memory recall, and the amygdala hemodynamic response to sad and happy faces.
Methods:
We employed a within-subjects design in which 10 healthy male participants received placebo, the mineralocorticoid receptor antagonist spironolactone (600mg) alone, and the glucocorticoid receptor antagonist mifepristone (600mg) alone in a randomized, counter-balanced order separated by 1-week drug-free periods.
Results:
On autobiographical memory testing, mineralocorticoid receptor antagonism impaired, while glucocorticoid receptor antagonism improved, recall relative to placebo, as evinced by changes in the percent of specific memories recalled. During fMRI, the amygdala hemodynamic response to masked sad faces was greater under both mineralocorticoid receptor and glucocorticoid receptor antagonism relative to placebo, while the response to masked happy faces was attenuated only during mineralocorticoid receptor antagonism relative to placebo.
Conclusions:
These data suggest both mineralocorticoid receptor and glucocorticoid receptor antagonism (and potentially any deviation from the normal physiological mineralocorticoid receptor/glucocorticoid receptor ratio achieved under the circadian pattern) enhances amygdala-based processing of sad stimuli and may shift the emotional processing bias away from the normative processing bias and towards the negative valence. In contrast, autobiographical memory was enhanced by conditions of reduced glucocorticoid receptor occupancy.
Keywords: cortisol, mineralocorticoid, glucocorticoid, autobiographical memory, amygdala
Introduction
Cortisol is a hormone secreted by the adrenal gland that influences a wide range of physiological functions, including glucose metabolism, energy distribution, stress responses, and cognitive performance (Belanoff et al., 2001; Abercrombie et al., 2003). We previously reported that acutely increasing cortisol levels impaired autobiographical memory (AM) recall in healthy humans, as high-dose hydrocortisone (0.45mg/kg i.v.) resulted in fewer specific memories (memory of a single event that occurred at an identifiable time and place) and more categorical memories (summaries of recurring events without reference to a single occurrence) relative to placebo (Young et al., 2011). The impairing effects of elevated cortisol concentrations on this memory system have been consistently reported (Buss et al., 2004).
Cortisol binds to mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs), which are densely located in the human amygdala (Wang et al., 2014); in rodents, activation of these receptors using corticosterone robustly influences the electrophysiological activity of amygdala neurons (Duvarci and Pare, 2007). Notably, in soldiers studied before and following deployment, predeployment GR density in peripheral blood mononuclear cells negatively correlated with predeployment amygdala activity and also predicted increased amygdala activity observed following deployment (Geuze et al., 2012). Furthermore, elevated cortisol levels (0.45mg/kg i.v.) increased the blood oxygen level-dependent (BOLD) amygdala response to presentations of sad facial stimuli in men (Erickson et al., 2005) and basal cortisol levels correlated positively with amygdala activity while viewing emotionally valenced relative to neutral pictures (van Stegeren et al., 2007). However, distinct results have been reported under other behavioral conditions and glucocorticoid doses; a low-dose injection of hydrocortisone (10mg) decreased amygdala activity during rest (Lovallo et al., 2010), and high-dose (40mg) hydrocortisone decreased amygdala activity relative to placebo during reward anticipation while performing the monetary incentive delay task (Montoya et al., 2014). These contrasting results may depend upon the differences in stimulus valence and in the cortisol concentrations achieved.
The effects of cortisol on memory and emotional processing are believed to follow an inverted U-shape function based on the ratio of MR to GR occupation (de Kloet et al., 1999; Lupien et al., 2007). The affinity of cortisol for MRs is 6- to 10-fold higher than for GRs. Moreover, as cortisol concentrations increase during the peak phase in the circadian cycle or during stress, MRs become saturated and cortisol binds to an increasingly greater fraction of GRs (Reul and De Kloet, 1985). When most MRs and a relatively small proportion of GRs are activated, cognitive function is enhanced (de Kloet et al., 1999; Lupien et al., 2007). This condition represents the top of the inverted U-shape function as the optimal MR/GR ratio for these cognitive functions. On the other hand, when circulating levels of glucocorticoids are significantly decreased (i.e., both MR and GR occupancy is low and the MR/GR occupation ratio is high) or increased (i.e., GR occupancy is high and the MR/GR occupation ratio is low), cognitive function is relatively impaired (de Kloet et al., 1999; Lupien et al., 2007). These are the extremes of the inverted U-shape function.
The majority of support for the MR/GR occupation hypothesis comes from studies that correlated or increased cortisol levels experimentally. In contrast, few studies have assessed the effects of experimentally reducing MR and GR function in humans, although such data are crucial for fully characterizing the effect of varying glucocorticoid hormone function on memory and emotional processing. Research into the effects of acute MR antagonism showed that oral spironolactone administration impaired working memory (Cornelisse et al., 2011) and prevented stress-induced changes in functional connectivity assessed using a classification paradigm (Schwabe et al., 2013) and a socially evaluated cold-pressure task (Vogel et al., 2015). Although the effect of acute GR antagonism has not been studied using neuroimaging, one study examined the effects of acute MR and GR antagonism using oral administration of 400mg of spironolactone or 200mg of mifepristone, respectively, on recall of emotional stories and pictures learned 3 days previously. All participants demonstrated significant forgetting over the 3 days, but MR antagonism increased the number of items forgotten for emotional stimuli (suggesting an impairing effect on memory recall), and GR antagonism did not alter recall for text but resulted in fewer forgotten details of the pictures, suggesting either no effect or an enhancing effect on memory (Rimmele et al., 2013).
Thus, the goal of the current study was to examine the effects of acutely blocking the MR and GR separately on 2 processes known to be affected by altering levels of cortisol: AM recall, and the amygdala BOLD response to emotional faces. Testing occurred during the morning hours, based on the expectation that assessments of the effects of blocking corticosteroid receptors would prove more sensitive during the relatively higher cortisol concentrations during that phase of the diurnal pattern. We expect to extend the results of Rimmele et al. (2013) on declarative memory by testing the differential effects of MR and GR antagonism on AM recall. We thus hypothesized that AM would be impaired following MR antagonism and improved following GR antagonism. With regard to amygdala activity, as increasing cortisol (increasing GR occupation and the GR/MR occupancy ratio) reportedly increased the amygdala response to negative stimuli (Erickson et al., 2005) and decreased the response to positive stimuli (Montoya et al., 2014), we predicted MR antagonism (i.e., raising the GR/MR occupancy ratio) would result in increased amygdala activity to sad and decreased to happy faces, while GR antagonism would result in decreased amygdala activity to sad and increased activity to happy faces.
METHODS
Participants
Ten psychiatrically and medically healthy, right-handed males participated in the current study (age 28±5 years). Volunteers recruited from the community via advertisements underwent the Structural Clinical Interview for DSM-IV disorders (First et al., 2002) at the Laureate Institute for Brain Research (LIBR).
Exclusion criteria included general MRI exclusions, major medical/neurological disorders, exposure to medication likely to influence cerebral function/blood flow/endocrine status within 3 weeks, meeting DSM-IV criteria for drug/alcohol abuse within the previous 1 year or for alcohol/drug dependence (excepting nicotine) within the lifetime, or current or past history of any major psychiatric disorder. Individuals who worked evening or night shifts were excluded, because diurnal cortisol patterns reportedly are altered in these individuals (Griefahn and Robens, 2010). After receiving a complete explanation of the study procedures, all participants provided written informed consent as approved by the Western IRB. Participants received financial compensation for their participation.
Anxiety and depressive symptoms were rated at each baseline material pick-up visit and on each scan day using the State-Trait Anxiety Inventory (Spielberger et al., 1970), the Hamilton Rating Scale for Depression (21-item; Hamilton, 1960), and the Profile of Mood States (McNair et al., 1971).
Pharmacological Procedure
To block the MR, 600mg spironolactone (Aldactone provided by The Apothecary Shoppe, Tulsa, OK) was used (Delyani, 2000), while to block the GR, 600mg mifepristone (Mifeprex provided by Athenium Pharmaceuticals, LLC, New York, NY) was selected (Munden and Schmidt, 1992). A placebo was also used (provided by The Apothecary Shoppe). The placebo and study drugs were each encapsulated to achieve identical appearance by The Apothecary Shoppe. Only male participants were included to reduce variability of the results in this relatively small sample and because previous studies suggested changes in behavior and brain activity following acute cortisol manipulations were greater in males than females (Stark et al., 2006; Young et al., 2011).
Participants completed 3 study visits during which they received placebo/placebo (6 placebo capsules; 3 at midnight, 3 at 5 am), mifepristone/placebo (3 x 200-mg capsules at midnight and 3 placebo capsules at 5 am), and spironolactone/spironolactone (6 x 200-mg pills: 3 at midnight, 3 at 5 am) in a randomized, counter-balanced order under double-blind conditions. Each visit was separated by a 1-week interval. Participants came to LIBR at 3 pm the day before each study day to pick up a pill alarm with 2 compartments labeled with which time to open each compartment. Each compartment contained 3 capsules for a total of 6 capsules/night, which they would take at midnight and 5 am before returning to LIBR at 7 am. The dosing times were based on previous pharmacological studies that found that mifepristone administered at midnight markedly raises cortisol levels when assessed between 8 am and noon (Gaillard et al., 1984), while spironolactone markedly elevates cortisol levels when administered in 2 doses separated by 5 hours and tested 30 minutes to 4 hours later (Young et al., 1998). Furthermore, mifepristone continued to prevent GR release of heat-shock protein and translocation of the receptor complex 24 hours after administration (Raux-Demay et al., 1990), and active metabolites of spironolactone believed to be responsible for the antimineralocoticoid activity have a halflife of 14 hours (Gardiner et al., 1989). Therefore, we are confident this procedure did result in appropriate receptor blockade during testing. Participants were provided with a pill alarm that signaled the dosing times. To further ensure compliance, participants were instructed to leave a telephone message at a central number at the time of ingestion. If 2 messages were not received at the appropriate times, the participant would be excluded from the study (however, in no instance did this occur). No participant reported experiencing any adverse effect to any of the medications.
Cortisol Measurements
A baseline blood sample was obtained at 3 pm the day before each study session and assayed for cortisol. The morning of the study, an i.v. cannula was inserted into a forearm vein, and between 7:30 am and 10 am 10 ccs of blood was collected at 30-minute intervals. The i.v. fluids were administered between each blood draw to keep the line open, and an initial blood sample was discarded to avoid dilution with these fluids. Plasma cortisol levels were obtained from these samples. All blood samples were stored at -80°C until assay. Assays were performed using a cortisol enzyme immunoassay kit for total cortisol.
fMRI Task and Processing
fMRI scanning commenced at 8 am and was performed on a 3T GE Discovery MR750 scanner and 8-channel receive-only head coil. Gradient-recalled, echoplanar imaging with sensitivity encoding was used for fMRI with the following parameters: repetition time=2000ms, echo time=27ms, sensitivity encoding acceleration=2, flip angle=90°, matrix=96×96, field-of-view =24cm, 39 axial slices, voxel size=2.5×2.5×2.9mm3. The first 3 images of each run were discarded to allow for steady-state tissue magnetization. High-resolution T1 weighted anatomical MRI scans (repetition time/echo time=5ms/1.93ms, flip angle=8°, matrix=256×256, field-of-view=24cm, slice thickness=1.2mm, 120 axial slices) also were acquired for co-registration with the echoplanar imaging series.
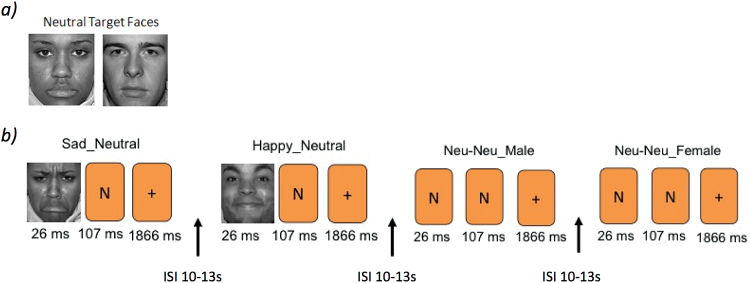
Participants performed a backward masking task (Victor et al., 2010). Prior to each of two 9-minute 8-second runs, participants were shown 2 neutral target faces and instructed to remember the faces for the next scan run and respond as quickly as possible to indicate if the presented face matched one of the target faces based on identity (not emotional expression). Two faces were presented for each stimulus, a “masked” face for 26ms, immediately followed by a “masking face” for 107ms, and a fixation cross for 1866ms. In total, 48 stimuli (6 types [sad/neutral, happy/neutral, neutral/sad, neutral/happy, neutral/neutral female, neutral/neutral male] x 8 presentations each) were presented for each run in a pseudo-randomized, mixed-trial design (Figure 1), with the emotional pairings predetermined (e.g., happy/neutral) and the program Optseq (Dale, 1999) used to randomly determine the order of face presentation with an equal number of target faces presented in each position. Each event type was gender matched. A 10 to 13-second interstimulus interval served as a baseline comparison. Three different stimulus face sets equivalent on ratings of valence were counterbalanced and used for the 3 experimental sessions. Faces were selected from the NimStim Set of Facial Expressions (Tottenham et al., 2009).
Figure 1.
Design of the backward masking task. (a) Two neutral target faces were shown prior to the start of each run in which participants were instructed to remember and determine whether subsequent face presentations matched the identity of. b) Two faces were shown as part of each trail presentation. Examples of the masked face events types (SN, HN, NN) are shown with “N” placeholders to indicate the presentation of a neutral face. HN,happy/neutral face presentation; ISI,interstimulus interval; NN, neutral/neutral face presentation; SN,sad/neutral face presentation.
Image preprocessing and analysis were performed using AFNI (http://afni.nimh.nih.gov) and consisted of despiking, slice acquisition time correction, and within-subject realignment. The anatomical image was registered to the first functional image then spatially normalized to the TT_N27 template using Advanced Normalization Tools with SyN method (Avants et al., 2008). The estimated warping parameter was used to normalize the functional images. The template image was resampled to 1.75mm3 isotropic voxels so that the spatially normalized image had a voxel size of 1.75mm3. Images were smoothed using a 4-mm full-width half-maximum Gaussian kernel, and the signal time course was scaled to percent signal change relative to the mean signal across time in each voxel. Using 3dDeconvolve for each participant, the hemodynamic response to each event type was modeled with a delta function at the event onset and convolved with the gamma-variate hemodynamic response function. Regressors modeling the task, motion parameters, and 4th-order polynomial regressors were used in the model. Because the masked and unmasked faces for each stimulus pair were presented too closely in time to model the response to each component separately, the data were modeled as event-related correlates of the combined stimulus pairs and these pairs were the main effects of interest: presentation of sad/neutral (SN), happy/neutral (HN), and neutral/neutral (NN) faces. Events with target faces of sad and happy in the unmasked position were also modeled separately and included in the design matrix.
We defined regions-of-interest for the amygdala using Talairach amygdala masks within AFNI. Because of the small sample size, we combined the left and right amygdala masks into a single mask to increase our power to detect differences. For each participant, the 3dDeconvolve output was resampled, and the amygdala mask applied to calculate the percent signal change within the region-of-interest for SN-NN and HN-NN face presentation. The resulting percent signal change values were entered into a repeated-measures ANOVA with the repeated measure of contrast (HN-NN, SN-NN) and drug (placebo, MR antagonist, GR antagonist) using SYSTAT 13 (Systat Software Inc). A whole-brain ANOVA was also performed with the significance criterion set at P<.05 corrected (determined using AFNI 3dClustSim at voxel P<.001, cluster size >10).
Memory Testing
Following the fMRI, participants completed 2 memory tests starting at 9 am.
AM Test
The AM Test (Williams et al., 1986) is a cued memory test in which subjects are presented with positively, negatively, and neutrally valenced cue words and instructed to recall a specific AM following each cue word. Three different cue word sets were administered in a counterbalanced order on the 3 test days using words equivalent in valence and salience. The AMT included 18 cue words, with 6 each of neutral (e.g., pottery), positive (e.g., sunny), and negative (e.g., grave) valence. Participants had 60s to retrieve a unique memory for each cue. When a memory was recalled, the response time was recorded with a stopwatch and the participant indicated the valence of the retrieved memory. Memories were coded according to their level of specificity according to standard definitions in the AM literature (Williams et al., 2007). A specific memory was defined as a memory of an event that occurred at an identifiable time and location and did not last longer than 24 hours (i.e., “My calculus final last fall”). A categorical memory was defined as a summary of a recurring event without reference to a single occurrence (i.e. “I took a lot of tests in college”). The determination of whether the memory was specific, categorical, or other was made while the rater was blind to condition. All responses were rated by a single rater (K.Y.), and an independent rater scored 40% of responses; inter-rater reliability agreement was 90.3%.
Declarative Memory Test
A declarative memory test was administered to assess the specificity of the results to the AM system. This test involved oral presentation of 12 neutral words over 3 immediate recall trials. Three different lists were created and administration was counterbalanced between participants. After a 20-minute interval during which the AMT was administered, free recall of the list was again assessed (delayed recall).
Statistical Analysis
Using SYSTAT, repeated-measures ANOVAs were used to analyze total cortisol concentrations, the percent of specific and categorical memories recalled and the reaction time to recall these memories, and the number of words recalled during immediate and delayed recall. Time and condition were the repeated measures for the plasma analysis; condition, valence, and specificity for the AM analysis; and condition and recall delay for the declarative memory test. Paired samples t tests were performed to examine the significant differences in the ANOVAs and were corrected for multiple comparisons (Bonferroni).
RESULTS
Cortisol
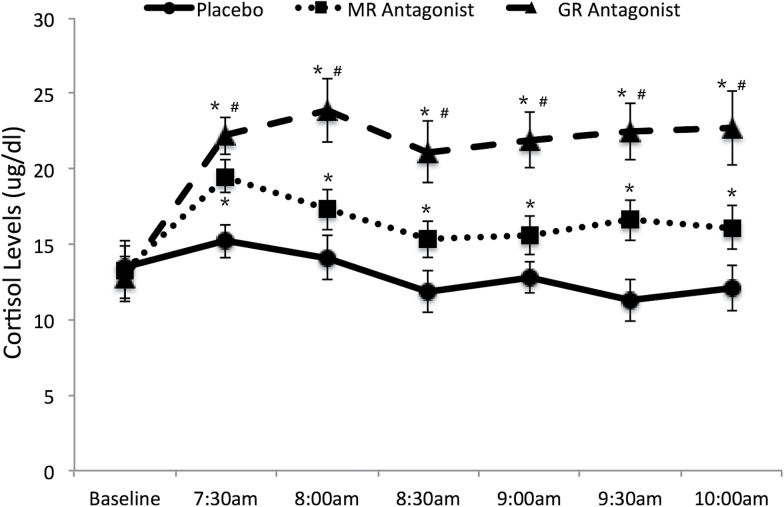
The results of the repeated-measures ANOVA of the within-subjects factor time (baseline, 7:30 am, 8 am, 8:30 am, 9 am, 9:30 am, 10 am) and condition for free cortisol levels appear in Figure 2. There was a main effect of condition (F(2,18)=9.57, P=.003) and time (F(6,54)=8.57, P<.001), and a condition x time interaction (F(12,108)=4.40, P<.001). Paired t tests revealed that all conditions significantly differed from each other at all time points after baseline (ts(9)>7.24, Pscorrected<.001), but did not differ at the baseline blood draw (ts(9) <1.07, Ps>.32; corrected Pscorrected =1.00). The time x condition interaction revealed that while cortisol levels following both MR and GR antagonism increased at all time points relative to baseline (ts(9)>3,91, Ps<.004), cortisol levels did not change from baseline in the placebo condition (ts(9)<0.1.31, Ps<.23).
Figure 2.
Mean cortisol levels (μg/dL) for each condition. Error bars indicate ± 1 SEM. *Difference from placebo at pcorrected<.05. #Difference from the MR antagonist sprionolactone at pcorrected<.05.
Amygdala Region of Interest Analysis
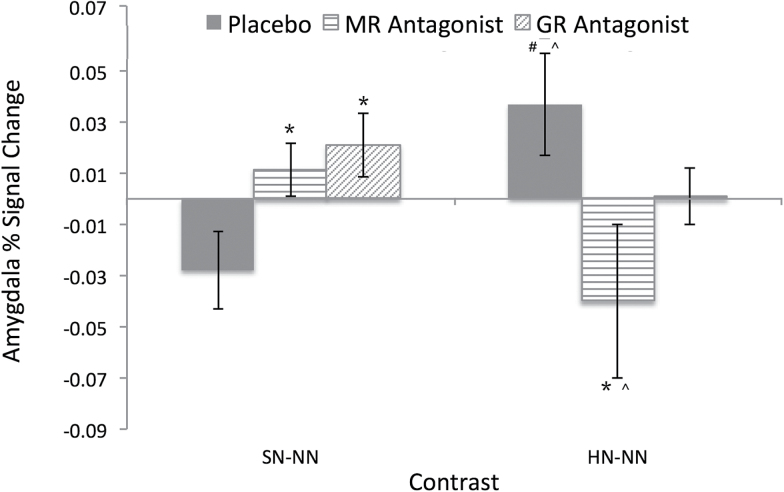
The results of the repeated-measures ANOVA of the within-subjects factor of valence contrast (SN-NN, HN-NN) and condition for the amygdala BOLD response appear in Figure 3. There were no main effects for valence (F(1,9)=0.01, P=.95) or condition (F(2,18)=0.37, P=.69), but the valence x condition interaction was significant (F(2,18)=2.24, P=.03). Follow-up paired t tests revealed that for the SN-NN contrast, both MR and GR antagonism resulted in increased amygdala BOLD activity relative to placebo (ts(9)>7.24, Pscorrected<.001, ds>1.79), while the BOLD response under MR and GR antagonism did not differ(t(9)=1.05, pcorrected=.96, d=0.15). For the HN-NN contrast, the amygdala BOLD activity during MR antagonism was significantly less than that under placebo and GR antagonism (ts(9)5.40>, Pscorrected<.004, ds>1.43), while the BOLD response under placebo and GR antagonism did not differ r(t(9)=0.32, pcorrected=1.00, d=0.40). Additional follow-up tests revealed that under placebo, the amygdala response was greater during the HN-NN contrast than under the SN-NN contrast (t(9)=6.56, pcorrected<.001, d=2.67), while during MR antagonism the BOLD response was greater during the SN-NN contrast than the HN-NN contrast (t(9)=5.65, pcorrected=.001, d=1.94) and the response during GR antagonism did not differ between the valence contrasts (t(9)=0.60, pcorrected=1.00, d=0.30).
Figure 3.
Amygdala percent signal change for SN-NN and HN-NN face presentations for each condition. *Difference from placebo at pcorrected<.05. #Difference from the MR antagonist sprionolactone at pcorrected<.05. ^Difference from the SN-NN condition at pcorrected<.05. HN,happy/neutral face presentation; NN, neutral/neutral face presentation; SN,sad/neutral face presentation.
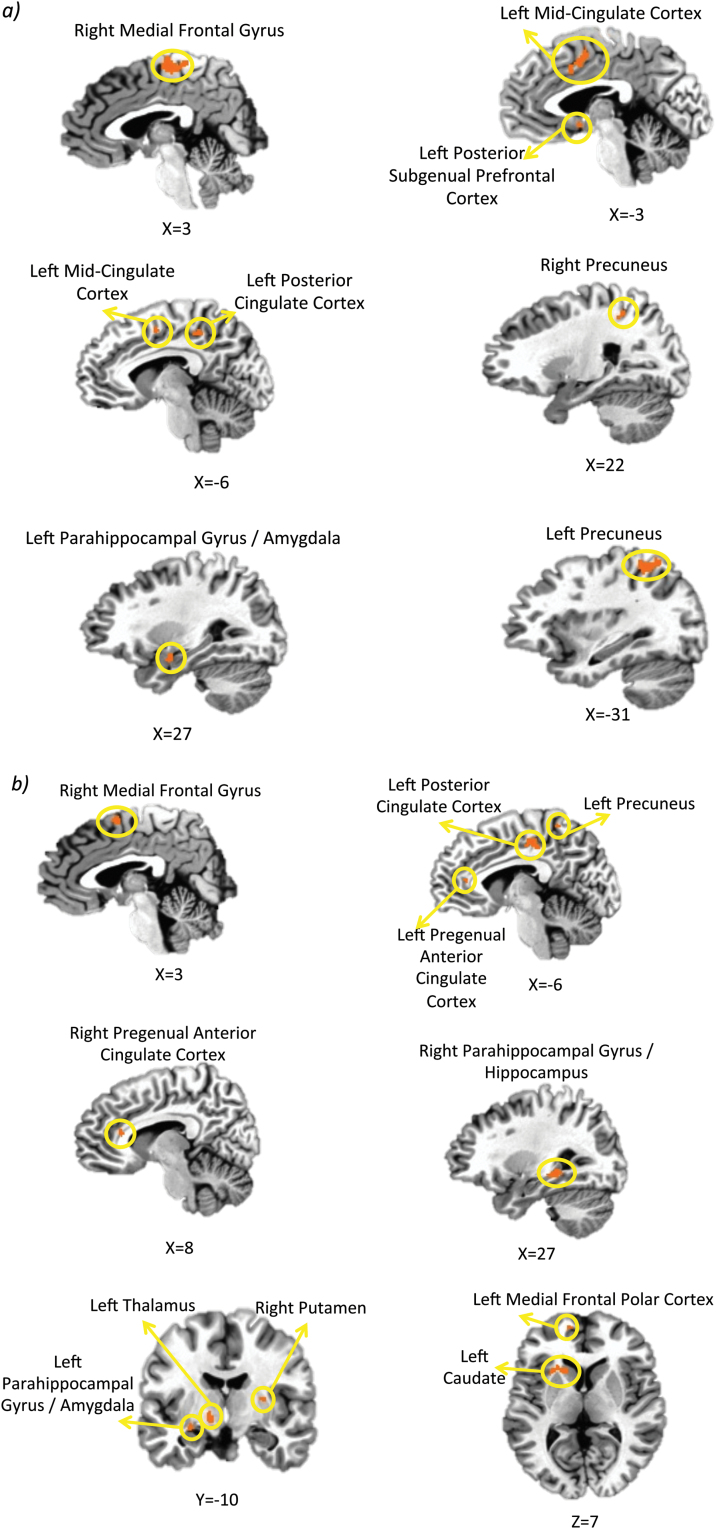
Exploratory Whole Brain Analysis
Table 1 and Figure 4 show the results of the posthoc whole brain analysis. For the SN-NN contrast, GR antagonism increased the hemodynamic response relative to placebo and MR antagonism in left precuneus, mid-cingulate cortex, posterior subgenual prefrontal cortex, posterior cingulate cortex, caudate, right medial frontal gyrus, and precuneus. For the HN-NN contrast, the BOLD response during both MR and GR antagonism were attenuated relative to those obtained under placebo in left thalamus and caudate, right putamen, bilateral parahippocampal gyrus, and medial prefrontal gyrus. GR antagonism resulted in an increased BOLD response relative to placebo and MR antagonism in the left posterior cingulate cortex, precuneus, and bilateral pregenual anterior cingulate cortex.
Table 1.
Regions Where the Blood Oxygen Level Dependence Signal (BOLD) Measured Using fMRI Differed between Drugs in Response to Implicitly Presented Sad (SN-NN) and Happy (HN-NN) Face Stimuli
| SN-NN | |||||
|---|---|---|---|---|---|
| % Signal Change | |||||
| Area | x,y,za | Cluster Sizeb | Placebo | MR Antagonist | GR Antagonist |
| SN-NN | |||||
| R Medial Frontal G | 1, -15, 58 | 112 | -0.04 (0.01) | 0.02 (0.01) | 0.06 (0.02) |
| L Mid-cingulate C | -4, 1, 42 | 30 | -0.02 (0.01) | -0.04 (0.01) | 0.06 (0.01) |
| L Posterior subgenual PFC | -3, 4, -7 | 11 | -0.07 (0.02) | -0.01 (0.01) | 0.05 (0.01) |
| L PCC | -8, -38, 42 | 21 | 0.02 (0.01) | 0.03 (0.01) | 0.06 (0.02) |
| L Precuneus | -31, -47, 49 | 231 | 0.04 (0.02) | 0.02 (0.01) | 0.05 (0.01) |
| R Precuneus | 22, -46, 48 | 27 | 0.02 (0.01) | 0.02 (0.01) | 0.04 (0.01) |
| R Amygdala / PHG | 27, -4, -15 | 24 | -0.04 (0.01) | 0.02 (0.01) | 0.03 (0.01) |
| HN-NN | |||||
| L Medial Frontal Polar C | -8, 53, 6 | 39 | 0.07 (0.02) | -0.01 (0.01) | 0.05 (0.01) |
| R Medial Frontal G | 1, 1, 56 | 57 | 0.06 (0.01) | -0.01 (0.01) | 0.02 (0.01) |
| L Pregenual ACC | -8, 39, 13 | 19 | 0.03 (0.01) | -0.01 (0.01) | 0.05 (0.02) |
| R Pregenual ACC | 8, 29, 14 | 13 | 0.02 (0.01) | -0.02 (0.04) | 0.04 (0.01) |
| L PCC | -6, -24, 42 | 64 | -0.01 (0.01) | -0.02 (0.01) | 0.06 (0.01) |
| L Precuneus | -8, -45, 58 | 40 | -0.05 (0.01) | 0.03 (0.01) | 0.05 (0.01) |
| L Caudate | -15, 15, 7 | 76 | 0.05 (0.01) | 0.02 (0.01) | 0.04 (0.01) |
| R Putamen | 25, -11, 11 | 53 | 0.07 (0.02) | 0.01 (0.01) | 0.04 (0.01) |
| L Amygdala | -25, -10, -11 | 25 | 0.05 (0.01) | -0.01 (0.01) | 0.03 (0.01) |
| R PHG / Hippocampus | 27, -31, -5 | 83 | 0.04 (0.01) | -0.05 (0.01) | 0.02 (0.01) |
| L Thalamus | -11, -8, -3 | 63 | 0.06 (0.02) | 0.03 (0.01) | 0.02 (0.01) |
Abbreviations: ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; PFC = prefrontal cortex; PHG = parahippocampal gyrus; C= cortex; G = gyrus; L = left; R = right
aCoordinates correspond to the stereotaxic array by Talairach and Tournoux (1988). bCluster size refers to the number of contiguous voxels for which the voxel F statistic corresponds to corrected P<0.05.
Figure 4.
Regional differences in hemodynamic activity under different conditions. Regions where differential hemodynamic activity was observed at pcorrected<.05 during (a) implicitly presented sad (SN-NN) and (b) happy (HN-NN) face stimuli.
AM Performance
Table 2 shows the results of the repeated-measures ANOVA of the within-subjects factors of specificity (specific, categorical, other), valence (positive, negative), and condition for the percent of autobiographical memories recalled and the reaction time to recall these memories. There was a main effect of valence (F(1,9)=244, P<.001), with more memories recalled that were positive than negative in valence (t(9)=6.51, pcorrected<.001, d=4.07). There was also a main effect of specificity (F(2,18)=16.3, P<.001), with more specific memories recalled than categorical or other(ts(9)>4.78, pcorrected <.004, ds>4.04).
Table 2.
Percent of Memories Recalled for Each Autobiographical and Declarative Memory Variable for Each Condition
| Placebo | MR Antagonist | GR Antagonist | |
|---|---|---|---|
| Autobiographical Memory | |||
| Specific total | 67.4 (7.12) | 60.7 (9.90)*# | 73.2 (5.34)* |
| Positive | 72.0 (7.44) | 68.8 (7.72) | 70.8 (3.22) |
| Negative | 28.0 (7.08) | 31.2 (6.67) | 29.2 (2.44) |
| Categorical total | 21.1 (1.44) | 17.7 (2.14)* | 17.8 (1.21)* |
| Positive | 81.6 (3.94) | 81.3 (4.15) | 81.3 (3.61) |
| Negative | 18.4 (3.65) | 18.7 (1.36) | 18.7 (1.22) |
| Other total | 11.5 (2.94) | 21.6 (3.36)*# | 9.00 (1.44) |
| Positive | 79.5 (3.64) | 77.5 (3.78) | 80.1 (4.12) |
| Negative | 20.5 (2.93) | 22.5 (2.23) | 19.9 (1.83) |
| Positive total | 77.6 (9.87) | 73.8 (8.24) | 76.3 (5.28) |
| Negative total | 22.4 (2.41) | 26.2 (2.22) | 23.7 (1.81) |
| Declarative Memory | |||
| Immediate Recall 1 | 55.0 (15.1) | 53.3 (11.7) | 51.1 (14.5) |
| Immediate Recall 2 | 70.0 (16.5) | 69.2 (10.6) | 66.0 (15.1) |
| Immediate Recall 3 | 79.2 (15.1) | 79.7 (13.3) | 75.0 (16.6) |
| Delayed Recall | 67.0 (18.7) | 65.7 (20.1) | 62.5 (20.3) |
Numbers in parentheses indicate 1 SDM. *Difference from placebo at pcorrected<.05. #Difference from the GR antagonist mifepristone at pcorrected<.05.
While there was no interaction with valence (Fs<1.08, Ps>.36), there was a condition x specificity interaction (F(4,36)=12.7, P=.001). For specific memory recall, during GR antagonism participants recalled significantly more specific memories than while under placebo (t(9)=3.57, pcorrected=.002, d=0.50), while during MR antagonism participants recalled significantly less specific memories than while under placebo (t(9)=3.00, pcorrected=.01, d=1.02). The difference between the percent of specific memories recalled during MR and GR antagonism was also significant (t(9)=6.09, pcorrected<.001, d=1.06). With regard to categorical memories, participants recalled fewer categorical memories during both MR and GR antagonism relative to placebo (ts(9) >7.43, Pscorrected<.001, ds>0.94), but did not differ from each other (t(9)=0.13, pcorrected=1.00, d=0.25). With regard to the other memory types, participants recalled more of these memory types during MR relative to placebo or GR antagonism (ts(9) >7.15 Pscorrected<.01, ds>3.22), which did not differ from each other (t(9)=0.01, pcorrected=1.00, d=0.05).
There was no significant main effect or interaction under any condition on reaction time (Fs<2.17, Ps>0.32).
Declarative Memory Performance
Table 2 also shows the results of the repeated-measures ANOVA of the within-subjects factors of delay (immediate 1, immediate 2, immediate 3, delayed), and condition for the percent of words recalled from the declarative memory task. There was a main effect of delay (F(3,27)=33.3, P<.001), with immediate recall at time 1 being worse than in any of the other recall conditions (ts(9)>4.19, Pscorrected<.001, ds>1.65), which did not differ from each other (t(9) <1.91, pcorrected>.28, d<0.40). There was no main effect of condition (F(2,18)=2.09, P=.16) nor was there a condition x delay interaction (F(6,54)=0.89, P=.51).
Mood Ratings
There was no difference in participants’ baseline mood ratings prior to any treatment condition (all ts(9)<0.98 Ps>.37), no difference in participants’ ratings obtained across the different drug conditions (ts(9)<1.31, Ps>.22), and no change between the baseline and session ratings for any drug condition (ts(9) <1.50, Ps>.17) (Table 3).
Table 3.
Mood Ratings for Each Condition at Baseline and Following Completion of a Study Visit
| Placebo | MR Antagonist | GR Antagonist | ||||
|---|---|---|---|---|---|---|
| Baseline | Study Visit | Baseline | Study Visit | Baseline | Study Visit | |
| POMS Depressiona | 0.20 (0.42) | 0.10 (0.32) | 0.30 (0.95) | 0.10 (0.32) | 0.60 (1.35) | 0.40 (0.97) |
| POMS Totalb | -14.4 (7.82) | -11.0 (12.8) | -11.2 (14.5) | -8.00 (13.57) | -14.0 (8.03) | -11.5 (9.57) |
| STAI Statec | 23.8 (5.12) | 24.3 (1.72) | 24.3 (6.43) | 25.4 (5.91) | 24.5 (5.21) | 25.0 (4.37) |
| HDRSd | 0.80 (1.14) | 0.50 (0.97) | 0.70 (1.25) | |||
Abbreviations: HDRS = Hamilton Depression Rating Scale (21 item); POMS = Profile of Mood States; STAI = State/Trait Anxiety Inventory.
Numbers in parenthesis indicate 1 SDM.
a POMS depression subscale scores can range from 0 to 60, with higher scores indicating worsening depression.
b POMS Total mood disturbance scores can range from -32 to 200, with higher scores indicating worsening mood.
c STAI scores can range from 20 to 80, with higher scores indicating worsening anxiety.
d HDRS scores can range from 0 to 66, with higher scores indicating worsening clinical depression, and a score of 7 or greater indicating clinically significant depression.
Discussion
We demonstrated differential effects of blocking the MR versus GR on AM recall and neural processing of implicitly presented emotional facial expressions in healthy men. Both MR and GR antagonism increased the amygdala BOLD response to the sad valence relative to placebo. In contrast, AM was enhanced by GR antagonism and impaired by MR antagonism. These changes were not associated with alterations in mood or anxiety symptoms, suggesting they occurred independently of current mood state.
Our hypotheses were confirmed regarding AM recall; blocking the MR impaired AM recall as evinced by fewer specific memories recalled, while blocking the GR (thereby decreasing GR occupation and presumably shifting participants into the higher range of MR/GR occupation) improved AM recall evinced by more specific and fewer categorical AMs. These findings are consistent with previous results on the effects of MR and GR antagonism on long-term memory retrieval (Rimmele et al., 2013) and extend these results to the AM system. These results occurred independently of the valence of the retrieved memory. While Rimmele et al. (2013) reported that the effect was only for emotional and not for neutral material, we were unable to investigate whether this was true for AM recall, as no participant rated a memory as being neutral in valence. Results are also consistent with preclinical literature showing MR antagonism impairing memory and GR antagonism improving contextual and recognition memory (Revsin et al., 2009; Ninomiya et al., 2010).
The extent to which the effects we observed on the AM system may extend to other types of memory remains unclear, as there was no significant effect of receptor antagonism on declarative memory for neutral words. Notably, these latter data appear consistent with the findings of Rimmele et al. (2013), who found that the effects of MR and GR antagonism were limited to emotionally valenced words and pictures but did not extend to the neutral stimuli. Therefore, it is conceivable that the lack of effect observed on declarative non-AM pertains specifically to the testing of neutral words and different results may on declarative memory may have been obtained had emotional words had been included.
With regard to amygdala activity during the implicit presentation of emotional faces, our hypotheses were partially supported. Firstly, when tested under placebo, participants manifested the normative biased amygdala response towards happy faces and away from sad faces in the absence of pharmacological manipulation (Victor et al., 2010). Secondly, as predicted, MR antagonism increased the amygdala response to sad faces but decreased the response to happy faces, mimicking the effects observed when cortisol levels are experimentally increased and GR occupation increases (Erickson et al., 2005; Montoya et al., 2014). However, contrary to our predictions, GR antagonism also significantly increased the amygdala response to sad faces and nonsignificantly decreased the response to happy faces. These results suggest that any deviation from the physiological MR/GR ratio achieved under the circadian peak in cortisol release increases the amygdala’s response to negative stimuli and shifts the emotional bias toward the negative valence (Browning et al., 2010). As this is the first fMRI study to date to look at the effects of acute GR antagonism, future studies examining different doses at different times of day are needed to fully explain these results.
Similar to the findings in the amygdala, the whole brain results also suggest that both MR and GR antagonism increase the response to negative stimuli and decrease the response to positive stimuli within multiple cortico-limbic structures that form part of the extended medial prefrontal/viseromotor network (Price and Drevets, 2012). Notably, some regions involved in memory recall (hippocampus; McClelland et al., 1995), self-focused processing (precuneus; Cavanna and Trimble, 2006), posterior cingulate (Brewer et al., 2013), and emotional processing and regulation (anterior cingulate, medial prefrontal cortex; Phillips et al., 2003) showed increased BOLD activity under both MR and GR antagonism relative to placebo during unconscious processing of sad faces, suggesting more neural resources are devoted to the automatic, implicit processing of negative environmental stimuli. Most notably, however, the regional data implicate the amygdala, anterior cingulate cortex, and posterior subgenual prefrontal cortical regions (infralimbic) regions that have been shown to play major roles in the regulation of glucocorticoid hormone release under stress in rodents (Herman and Cullinan, 1997), monkeys (Jahn et al., 2010), and humans (Boehringer et al., 2015).
The same regions were differentially affected by MR and GR antagonism during masked happy face processing, with the addition of striatal regions (putamen, caudate), and the thalamus, except the direction of the effect was the opposite as that seen with sad faces; MR and GR antagonism reduced the response of these regions to happy faces relative to placebo, consistent with a shift away from the normative positive emotional processing bias. It is unclear whether such findings would extend to other emotional stimuli, since the implicitly presented face stimuli reveal an unconscious, automatic processing bias but are not aversive or rewarding per se. In contrast, studies performed in animal models have generally examined the effect of pharmacological manipulation under stressful and aversive conditions (i.e, foot shock conditioning, Morris Water Maze, avoidance responses; de Kloet et al., 1999). In humans, one study found high-dose cortisol administration attenuated the hemodynamic responses within the striatum and amygdala to monetary reward anticipation (Montoya et al., 2014). This condition would presumably increase GR occupancy, thereby decreasing the MR/GR ratio, and is therefore compatible with the current findings that decreasing the MR/GR ratio via MR antagonism also reduces the brain’s response to rewarding or positive stimuli.
Interestingly, the results found while participants received the MR antagonist resemble the results seen in patients with major depressive disorder (MDD) in multiple respects. It is well established that MDD patients recall fewer specific and more categorical AMs (Williams et al., 2007). Furthermore, previous work with the backward masking task found MDD participants had an increased amygdala response to masked sad, and a decreased response to masked happy face presentation relative to controls (Victor et al., 2010). Notably, previous studies found that at least a subset of MDD patients have chronically elevated levels of cortisol into the range (18–20 μg/dL) of that obtained under GR antagonism in the current study (Gibbons and McHugh, 1962), and this increased cortisol in MDD is associated with decreased MR mRNA and an imbalance in the MR/GR ratio (López et al., 1998).
These data, taken together with the findings of the current study, suggest the hypothesis that in MDD the deficits in AM recall may be driven by increased occupancy of the GR, and that GR antagonists may improve AM recall in MDD. The data further suggest that either elevated or reduced MR/GR occupancy ratios may shift the emotional processing bias away from the normative bias. Notably, mood did not change in the current study, suggesting the mood effects of MDD may be independent of acute changes in MR/GR ratio in site occupancy. However, it is possible that prolonged exposure may be necessary for changes in mood to occur. This hypothesis is supported by data from patients with Cushing’s Disease, which show that chronic elevations of cortisol release can produce depressive symptoms (Starkman et al., 1981). Therefore, our design may not have been of a duration sufficient to detect mood changes in healthy individuals. Furthermore, studies examining changes in depressive symptoms following treatment with the GR antagonist mifepristone have produced inconsistent results, with some reporting no clinical improvement, and others reporting significant improvements in depression symptoms (Gallagher and Young, 2006). To our knowledge no study has assessed cognitive function in MDD patients following mifepristone treatment. The only study to examine such a measure was performed in patients with bipolar disorder, which found improved spatial working memory and verbal fluency in these patients following mifepristone relative to placebo administration, while only small improvements were observed in clinical ratings (Young et al., 2004). Thus, further studies are warranted to characterize the potential effects of GR antagonism on cognitive performance and emotional processing in MDD.
Several limitations of the study merit comment. First, the sample size was relatively small. However, because this was a repeated-measures design, each participant served as his own control, thereby reducing the amount of error arising from natural variance between individuals and increasing the statistical power (Hoyle, 1999). The effect sizes observed for significant effects were also large, further supporting the validity of the results in this relatively small sample (Friston, 2012). Second, the generalizability of our results was limited by our inclusion of only males. Gender-specific effects of cortisol on regional brain activation have been previously reported (Merz et al., 2010), and it is possible that the pattern of results observed in the current study are limited to men. Future research is needed to examine the effects of GR antagonism in women. Third, while we hypothesize that our results are related to the ratio of MR/GR occupation, we could not measure this ratio directly in the human brain in vivo and thus cannot definitively conclude that MR/GR occupation was altered in the hypothesized manner. Finally, the pharmacological manipulation we used to assess the effects of GR antagonism was not entirely selective, as mifepristone also exerts partial agonist effects at progesterone receptors (Spitz and Bardin, 1993).
In conclusion, our results show that GR antagonism improves AM and increases the amygdala’s response to sad faces during the backward masking task in healthy men. MR antagonism impairs AM but also induces a negativity bias during the backward masking task as evinced by an increased amygdala response to sad faces and a decreased response to happy faces. Our results support the inverted U-shape function with respect to the MR/GR ratio for implicit emotional processing. We anticipate, however, that within the physiological range of cortisol levels, there is likely to be a relatively broad range in the MR/GR occupancy ratio in which cognition and emotional processing are “optimal” and that by using receptor antagonists we have tested such processes outside of this optimal range.
Statement of Interest
W.C.D. is currently an employee of and holds equity in Janssen Pharmaceuticals, LLC, of Johnson & Johnson, Inc. The other authors have no financial conflicts of interest or disclosures to declare.
Acknowledgments
This work was supported by the Laureate Institute for Brain Research and The William K. Warren Foundation. The funders had no influence on the design or conduct of the study, collection, management, analyses, or interpretation of the data, or in the preparation, review or approval of the manuscript.
References
- Abercrombie H, Kalin N, Thurow M, Rosenkranz M, Davidson R. (2003) Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci 117:505–516. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanoff JK, Gross K, Yager A, Schatzberg AF. (2001) Corticosteroids and cognition. J Psychiatr Res 35:127–145. [DOI] [PubMed] [Google Scholar]
- Boehringer A, Tost H, Haddad L, Lederbogen F, Wust S, Schwarz E, Meyer-Lindenberg A. (2015) Neural correlates of the cortisol awakening response in humans. Neuropsychopharmacology 40:2278–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. (2013) What about the “self” is processed in the posterior cingulate cortex? Front Hum Neurosci 7:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Harmer CJ. (2010) The modification of attentional bias to emotional information: a review of the techniques, mechanisms, and relevance to emotional disorders. Cogn Affect Behav Neurosci 10:8–20. [DOI] [PubMed] [Google Scholar]
- Buss C, Wolf O, Witt J, Hellhammer D. (2004) Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology 29:1093–1096. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Cornelisse S, Joels M, Smeets T. (2011) A randomized trial on mineralocorticoid receptor blockade in men: effects on stress responses, selective attention, and memory. Neuropsychopharmacology 36:2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. (1999) Optimal experimental design for event-related fMRI. Hum Brain Mapp 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. (1999) Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22:422–426. [DOI] [PubMed] [Google Scholar]
- Delyani JA. (2000) Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int 57:1408–1411. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. (2007) Glucocorticoids enhance the excitability of princiap basolateral amygdala neurons. J Neurosci 27:4482–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Wood SE, Furey ML, Nugent AC, Mah L, Schulkin J, Drevets WC. (2005) Differential effects of hydrocortisone on amygdala habituation to emotional facial expressions: an fMRI study. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: New York State Psychiatric Institute, Biometrics Research. [Google Scholar]
- Friston K. (2012) Ten ironic rules for non-statistical reviewers. Neuroimage 61:1300–1310. [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE. (1984) RU 486: a steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci U S A 81:3879–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P, Young AH. (2006) Mifepristone (RU-486) treatment for depression and psychosis: a review of the therapeutic implications. Neuropsychiatr Dis Treat 2:33–42. [PMC free article] [PubMed] [Google Scholar]
- Gardiner P, Schrode K, Quinlan D, Martin BK, Boreham DR, Rogers MS, Stubbs K, Smith M, Karim A. (1989) Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites. J Clin Parmacol 29:342–347. [DOI] [PubMed] [Google Scholar]
- Geuze E, van Wingen GA, van Zuiden M, Rademaker AR, Vermetten E, Kavelaars A, Fernandez G, Heijnen CJ. (2012) Glucorcoiticoid receptor number predicts increase in amygdala activity after sever stress. Psychoneuroendocrinology 37:1837–1844. [DOI] [PubMed] [Google Scholar]
- Gibbons JL, McHugh P. (1962) Plasma cortisol in depressive illness. J Psychiatr Res 1:162–171. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Robens S. (2010) The normalization of the cortisol awakening response and of the cortisol shift profile across consecutive night shifts--an experimental study. Psychoneuroendocrinology 35:1501–1509. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84. [DOI] [PubMed] [Google Scholar]
- Hoyle RH. (1999) Statistical strategies for small sample research. SAGE Publications, Inc. [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. (2010) Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychiatry 67:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J, Chalmers D, Little K, Watson S. (1998) Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry 43:547–573. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT. (2010) Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology 35:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S, Maheu F, Tu M, Fiocco A, Schramek T. (2007) The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65:209–237. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. (1995) Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102:419–457. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropplemen L. (1971) Edits manual: profile of mood states. San Diego: Educational and Industrial Testing Services. [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. (2010) Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35:33–46. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J. (2014) Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology 47:31–42. [DOI] [PubMed] [Google Scholar]
- Munden PM, Schmidt TJ. (1992) Mifepristone blocks specific glucocorticoid receptor binding in rabbit iris-ciliary body. Arch Ophthalmol 110:703–705. [DOI] [PubMed] [Google Scholar]
- Ninomiya EM, Martynhak BJ, Zanoveli JM, Correia D, da Cunha C, Andreatini R. (2010) Spironolactone and low-dose dexamethasone enhance extinction of contextual fear conditioning. Prog Neuropsychopharmacol Biol Psychiatry 34:1229–1235. [DOI] [PubMed] [Google Scholar]
- Phillips M, Drevets W, Rauch S, Lane R. (2003) Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 54:504–514. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. (2012) Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 16:61–71. [DOI] [PubMed] [Google Scholar]
- Raux-Demay MC, Pierret T, Bouvier d’Yvoire M, Bertagna X, Girard F. (1990) Transient inhibition of RU 486 antiglucocorticoid action by dexamethasone. J Clin Endoncrinol Metab 70:230–233. [DOI] [PubMed] [Google Scholar]
- Reul J, De Kloet E. (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505. [DOI] [PubMed] [Google Scholar]
- Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS. (2009) Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology 34:747–758. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Besedovsky L, Lange T, Born J. (2013) Blocking mineralocorticoid receptors impairs, blocking glucocorticoid receptors enhances memory retrieval in humans. Neuropsychopharmacology 38:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Hoffken O, Wolf OT. (2013) Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol Psychiatry 74:801–808. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. (1970) Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spitz IM, Bardin CW. (1993) Mifepristone (RU 486)--a modulator of progestin and glucocorticoid action. N Engl J Med 329:404–412. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf O, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. (2006) Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage 32:1290–1298. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Schteingart DE, Schork MA. (1981) Depressed mood and other psychiatric manifestations of Cushing’s syndrome: relationship to hormone levels. Psychosom Med 43:3–18. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. (2009) The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. (2007) Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem 87:57–66. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. (2010) Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 67:1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Klumpers F, Krugers HJ, Fang Z, Oplaat KT, Oitzl MS, Joels M, Fernandez G. (2015) Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology 40:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Verweij EW, Krugers HJ, Joels M, Swaab DF, Lucassen PJ. (2014) Distribution of the glucorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct Funct 219:1615–1626. [DOI] [PubMed] [Google Scholar]
- Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, Dalgleish T. (2007) Autobiographical memory specificity and emotional disorder. Psychol Bull 133:122–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Maiman LA, Broadbent DN, Kotok D, Lawrence RA, Longfield LA, Mangold AH, Mayer SJ, Powell KR, Sayre JW, et al. (1986) Educational strategies to improve compliance with an antibiotic regimen. Am J Dis Child 140:216–220. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. (2004) Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology 29:1538–1545. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. (1998) The role of mineralocorticoid receptors in hypothalamic-pituitary-adrenal axis regulation in humans. J Clin Endocrinol Metab 83:3339–3345. [DOI] [PubMed] [Google Scholar]
- Young K, Drevets WC, Schulkin J, Erickson K. (2011) Dose-dependent effects of hydrocortisone infusion on autobiographical memory recall. Behav Neurosci 125:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]