Abstract
Background:
Emerging evidence indicates that NLRP3 inflammasome-induced inflammation plays a crucial role in the pathogenesis of depression. Thus, inhibition of NLRP3 inflammasome activation may offer a therapeutic benefit in the treatment of depression. Fluoxetine, a widely used antidepressant, has been shown to have potential antiinflammatory activity, but the underlying mechanisms remain obscure.
Methods:
We used a chronic mild stress model and cultured primary macrophage/microglia to investigate the effects of fluoxetine on NLRP3 inflammasome and its underlying mechanisms.
Results:
We demonstrated that fluoxetine significantly suppressed NLRP3 inflammasome activation, subsequent caspase-1 cleavage, and interleukin-1β secretion in both peripheral macrophages and central microglia. We further found that fluoxetine reduced reactive oxygen species production, attenuated the phosphorylation of double-stranded RNA-dependent protein kinase, and inhibited the association of protein kinase with NLRP3. These data indicate that fluoxetine inhibits the activation of NLRP3 inflammasome via downregulating reactive oxygen species-protein kinase-NLRP3 signaling pathway. Correspondingly, in vivo data showed that fluoxetine also suppressed NLRP3 inflammasome activation in hippocampus and macrophages of chronic mild stress mice and alleviated chronic mild stress-induced depression-like behavior.
Conclusions:
Our findings reveal that fluoxetine confers an antidepressant effect partly through inhibition of peripheral and central NLRP3 inflammasome activation and suggest the potential clinical use of fluoxetine in NLRP3 inflammasome-driven inflammatory diseases such as depression.
Keywords: fluoxetine, reactive oxygen species, NLRP3 inflammasome, depression, double-stranded RNA-dependent protein kinase
Introduction
Depression is a severe psychiatric disorder with a lifetime prevalence in excess of 15% and is the fourth leading cause of disability worldwide (DelMastro et al.,2015). Several lines of evidence have demonstrated that NLRP3 inflammasome plays a pivotal role in the pathophysiology of depression (Alcocer-Gomez and Cordero, 2014; Alcocer-Gomez et al., 2014; Choi and Ryter, 2014; Zhang et al., 2015). NLRP3 inflammasome has been found to be activated in patients with major depressive disorder (Alcocer-Gomez et al., 2015) and in rodent models of depression (Zhang et al., 2014), whereas antidepressant treatment could suppress NLRP3 inflammasome activation (Lu et al., 2014; Liu et al., 2015; Xue et al., 2015; Li et al., 2016). Stress-induced depressive behaviors require a functional NLRP3 inflammasome (Pan et al., 2014). NLRP3 deletion inhibits these stress-like effects (Zhang et al., 2015). These findings indicate that NLRP3 inflammasome could be a target for new therapeutic interventions to treat depression in patients.
NLRP3 inflammasome is an intracellular multiprotein complex that consists of nod-like receptor protein 3, adaptor protein ASC, and procaspase-1 precursor (Schroder and Tschopp, 2014). This complex is noted for its broad array of activating stimuli, which include bacterial, fungal, and viral components; endogenous danger signals such as extracellular ATP; and environmental microparticles such as silica crystals (Martinon et al., 2006; Rathinam et al., 2012). NLRP3 inflammasome activation leads to the maturation of caspase-1 and the processing of its substrates, interleukin (IL)-1β and IL-18 (Shao et al., 2015). Normal activation of the NLRP3 inflammasome contributes to host defense (Jo et al., 2015). However, excessive activation of the NLRP3 inflammasome participates in the pathogenesis of a spectrum of diseases, including type 2 diabetes (Kim et al., 2016), rheumatoid arthritis (Choulaki et al., 2015), Alzheimer’s disease (Lamkanfi and Dixit, 2014), and depression (Pan et al., 2014). Thus, the functional manipulation of the NLRP3 inflammasome has been demonstrated to be a promising therapeutic strategy for these diseases (Di Virgilio, 2014; Xue et al., 2015).
Fluoxetine, a selective serotonin reuptake inhibitor antidepressant, is commonly prescribed for treating depression due to its tolerability and safety (Montgomery, 1998; Garrison and Levin, 2000). Increasing evidence indicates that fluoxetine inhibits the production of proinflammatory cytokines including IL-1β, but the underlying mechanisms are still poorly understood (Liu et al., 2013; Branco-de-Almeida et al., 2014). Since the IL-1β secretion is tightly controlled by NLRP3 inflammasome, we thus hypothesize that fluoxetine might regulate NLRP3 inflammasome activation. To test this possibility, we prepared a chronic mild stress (CMS) model and cultured primary macrophage/microglia to investigate the effects of fluoxetine on NLRP3 inflammasome and its underlying mechanisms in vitro and in vivo. It was found that fluoxetine suppressed NLRP3 inflammasome activation via inhibiting the reactive oxygen species (ROS)-double-stranded RNA-dependent protein kinase (PKR)-NLRP3 signaling pathway in peripheral macrophages and central microglia. Furthermore, fluoxetine also inhibited NLRP3 inflammasome activation in hippocampus and macrophages of CMS mice. Our findings suggest fluoxetine may have promising clinical effects in NLRP3 inflammasome-driven inflammatory diseases such as depression.
MATERIALS AND METHODS
Animals and Reagents
Male C57BL/6 mice (8–12 weeks) were maintained in specific pathogen-free facilities at the Animal Care Facility of Nanjing Medical University. All experimental procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all animals were treated according to protocols approved by Institutional Animal Care and Use Committee of Nanjing Medical University.
The following antibodies were used: goat anti-NLRP3 (sc-34410, Santa Cruz Biotechnology), rabbit anti-Caspase-1 (sc-514, Santa Cruz Biotechnology), goat anti-IL-1β (AF-401-NA, R&D), rabbit anti-phospho-PKR (Thr446) (11280, Signalway antibody), and rabbit anti-PKR (21272, Signalway antibody). Lipopolysaccharide (LPS) (Escherichia coli, 0111:B4), ATP, and fluoxetine were from Sigma. ROS-specific fluorescent probe CM-H2DCFDA was from Invitrogen. IL-1β ELISA kits was obtained from R&D Systems.
Cell Culture and Stimulation
Bone marrow-derived macrophages (BMDMs) were derived from tibia and femoral bone marrow cells and cultured for 7 days in Dulbecco’s modified essential media complemented with 10% fetal bovine serum, 1% penicillin/streptomycin (vol/vol), and 50nM GMSF. Cortical mixed glial cultures were generated from male C57BL/6 mice postnatal day 0 to 3 pups. Microglia were isolated by collecting the floating fraction of 10-day-old mixed glial cultures following 1 hour on a rotary shaker at 37°C at 250rpm and plated in Dulbecco’s modified essential media containing 4.5g/L glucose, 2mM l-glutamine, 1mM sodium pyruvate, 10% fetal bovine serum, and 1% penicillin/streptomycin (vol/vol) on poly-d-lysine-coated 24-well culture plates. The purity of BMDMs and microglial cultures was >95% as determined with immunocytochemistry. Macrophages, microglia, RAW264.7 cells were stimulated with LPS (100ng/mL) for 6 hours and then pulsed with 5mM ATP for 30 minutes. In the experiments, cells were pretreated with the indicated concentrations of fluoxetine for 1 hour before LPS stimulation. Cell extracts and precipitated supernatants were analyzed by immunoblot and ELISA.
Cell Viability Assay
Cell viability was determined by the tetrazolium salt 3-[4,5-dimethylthiazol-2-yl] -2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) assay. BMDMs were plated into 96-well culture plates at a density of 5×104 cells/mL with 200mL culture medium per well. Following treatment with different concentrations of fluoxetine, 20mL MTT solution (5mg/mL) was added to each well and incubated at 37°C for 4 hours. The medium was aspirated and 200mL dimethyl sulfoxide was added. The absorbance value was measured using a multi-well spectrophotometer (Bio-Rad) at 490nm.
CMS Procedure
Individually housed male mice were allowed to acclimate for 1 week and then were subjected to 5 weeks of stressors, which were mild and unpredictable in nature, duration, and frequency. Stressors included inversion of day/night light cycle, soiled cage bedding, 45° tilted cage, restraint, overnight food and water deprivation, and pairing with another stressed animal. All of the stressors were shown in Table 1. Sucrose preference and the body weight of each animal were evaluated weekly until the end of the CMS. All the mice treated with CMS for 4 weeks when depressive behaviors occurred began receiving fluoxetine (10mg/kg/d i.p) administration for 4 weeks. The CMS procedure was continued during the entire fluoxetine treatment period. The concentration of fluoxetine was consistent with previous research (Yang et al., 2013).
Table 1.
The Stressors of a CMS Procedure
| Stressor | Description | Duration (h) |
|---|---|---|
| Tilted caging | Each cage tilted to a 45-degree angle | 18 |
| Wet caging | 200mL water in 100g sawdust bedding | 12 |
| Paired caging | Put mouse into cage of strange rat | 12 |
| Water/food deprivation | Deprivation water and food | 12 |
| Restraint stress | The mice are kept in closed and ventilated tubes | 4 |
| Intermittent illumination | 2-h/2-h light/dark cycle | 12 |
| Continuous illumination | Continuous illumination in dark cycle | 12 |
Sucrose Preference Test
Mice were given the choice to drink from 2 bottles for 10 hours; one contained a sucrose solution (1%) and the other contained only tap water. To prevent possible effects of side preference in drinking behavior, the positions of the bottles in the cage were switched after 5 hours. The animals were deprived of water for 12 hours before the test. The consumption of tap water, sucrose solution, and total intake of liquids was estimated simultaneously in the control and experimental groups by weighing the bottles. The preference for sucrose was measured as a percentage of the consumed sucrose solution relative to the total amount of liquid intake.
Tail Suspension Test
Mice tails were wrapped with tape from the base to the tip, covering about four-fifths of its length and fixed upside down on the hook. The immobility time of each mouse was recorded over a 6-minute period. Mice were considered immobile only when they hung passively and completely motionless. The time of immobility of the tail suspended mice during the last 4 minutes was measured with TailSuspScan (Clever Sys).
Forced Swim Test
Mice were individually forced to swim in an open cylindrical container (diameter, 15cm; height, 25cm) containing 14cm of water at room temperature (about 22±1°C) for 6 minutes. A mouse was judged to be immobile when it floated in an upright position and made only small movements to keep its head above water. The duration of immobility was recorded during the last 4 minutes of the 6-minute testing period by TailSuspScan (Clever Sys). The immobility observed in this test is considered to reflect a state of despair.
ELISA
Cell culture supernatants and serum were assayed for IL-1β with ELISA kits from R&D Systems according to the manufacturer’s instructions.
ROS Detection
Intracellular ROS was measured with the ROS-specific fluorescent probe CM-H2DCFDA. BMDMs were incubated with 25 μM H2DCF-DA for 30 minutes and washed twice with PBS. Fluorescence was measured at OD485-530 using a multi-well spectrophotometer. The ROS production intensity of untreated control cells was arbitrarily set to 100%.
Coimmunoprecipitation
The cell lysates from BMDMs were immunoprecipitated with anti-NLRP3 antibody followed by protein A/G plus agarose (Santa Cruz Biotechnology). After washing, bound proteins were eluted from the beads and analyzed by immunoblot for PKR.
Western-Blotting Analysis
Cell lysates were homogenized in lyses buffer (Beyotime, China) and protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA). The analysis of protein was performed according to standard SDS-PAGE. Proteins were separated on 10% to 15% Tris-HCl polyacrylamide gels (Bio-Rad) and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking, the blots were incubated with specific antibodies as described above in Tris-buffered saline with Tween 20 overnight at 4°C, and then with horseradish peroxidase conjugated secondary antibodies. Immunoreactive bands were detected by enhanced chemiluminescence plus detection reagent (Pierce, Rockford, IL) and analyzed using an Omega 16ic Chemiluminescence Imaging System (Ultra-Lum, CA).
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between multiple groups were made using a 2-tailed, unequal-variance Student’s t test, or ANOVA followed by Tukey’s posthoc analysis. Differences were considered significant at P < .05.
RESULTS
Fluoxetine Inhibits NLRP3 Inflammasome Activation in Macrophages
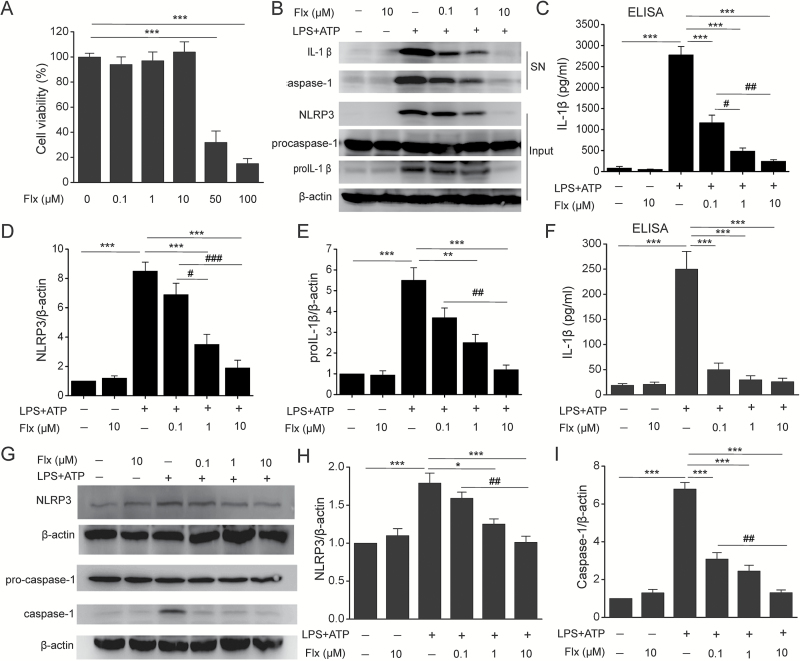
MTT assay was performed to determine its cytotoxicity to BMDMs and found fluoxetine (0.1 μM, 1 μM, and 10 μΜ) treatment had no effect on cell viability (Figure 1A) (F=0.462, P=.717). To determine the effects of fluoxetine on NLRP3 inflammasome activation, LPS-primed BMDMs were pretreated with fluoxetine before ATP challenge. Fluoxetine indeed reduced NLRP3-dependent caspase-1 activation and IL-1β maturation in a concentration-dependent manner (Figure 1B-C) (F=82.249, P<.01). It has been reported that fluoxetine can repress LPS-induced NF-κB activation (Du et al., 2014), so we then examined whether fluoxetine had an impact on priming for inflammasome activation. As shown in Figure 1D-E, fluoxetine significantly decreased LPS-induced NLRP3 (F=34.599, P<.01) and pro-IL-1β expression (F=24.202, P<.01). The observed inhibitory effects of fluoxetine on NLRP3 inflammasome activation were also confirmed in RAW264.7 macrophages (Figures 1F-I, F=32.306, P<.01). These results demonstrate that fluoxetine inhibits NLRP3 inflammasome activation in macrophages.
Figure 1.
Fluoxetine inhibited NLRP3 inflammasome activation in bone marrow-derived macrophages (BMDMs) and RAW264.7 macrophages. BMDMs and RAW264.7 macrophages were incubated with indicated concentrations of fluoxetine (Flx, 0.1 μM, 1 μM, 10 μM) for 1 hour, then stimulated with LPS (100ng/mL) followed by 30 minutes of ATP (5mM) treatment. (A) The cytotoxicity of fluoxetine (Flx, 0.1 μM, 1 μM, 10 μM, 50 μM, 100 μM) was measured by 3-[4,5-dimethylthiazol-2-yl] -2,5-diphenyltetrazolium bromide (MTT) assay (*** P<.001 vs Flx 0 μM). There were no significant differences among Flx 0 μM, Flx 0.1 μM, Flx 1 μM, and Flx 10 μM groups. (B) Immunoblot analysis of interleukin (IL)-1β and cleaved caspase-1 (p20) in culture supernatants (SN) of BMDMs and immunoblot analysis of the NLRP3, proIL-1β, and procaspase-1 in lysates of those cells (input). (C) ELISA of IL-1β in supernatants of BMDMs. (D-E) Quantitative analysis of NLRP3 (D) and proIL-1β (E) in BMDMs. (F) ELISA of IL-1β secretion in supernatants of RAW264.7 macrophages. (G-I) Representative immunoblots (G) and quantitative analysis of NLRP3 (H) and caspase-1 (I) in RAW264.7 macrophages. Data are represented as means ± SEM from 2 replicates in 3 independent experiments. * P<.05, ** P<.01, *** P<.001 vs LPS+ATP; # P<.05, ## P<.01, ### P<.001 vs LPS+ATP+Flx 0.1 μM. There were no significant differences between -LPS-ATP and -LPS-ATP + Flx 10 μM.
Fluoxetine Suppresses NLRP3 Inflammasome Activation in Microglia
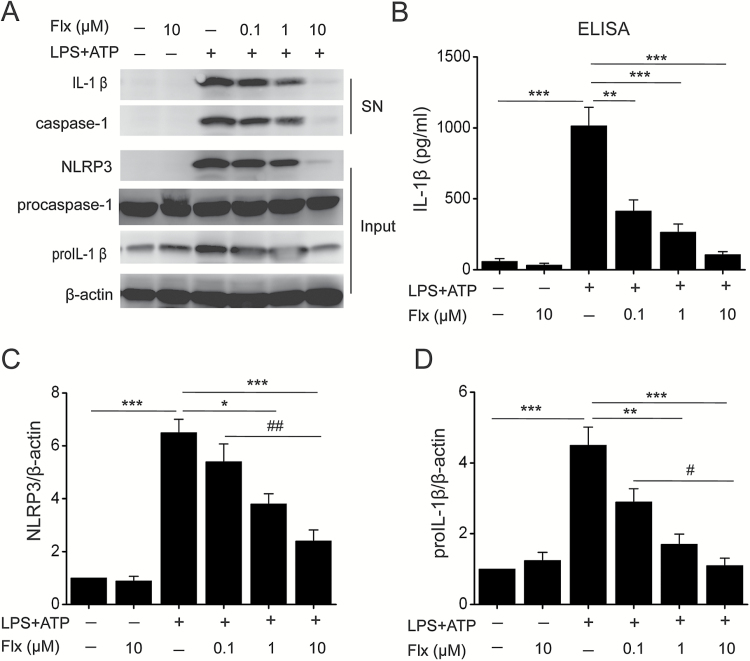
Microglia, the resident macrophages of the CNS, are the major sites contributing to the inflammatory responses in the brain (Kim et al., 2015) and play a crucial role in the pathogenesis of depression (Dheen et al., 2007; Irwin et al., 2012; Brites and Fernandes, 2015; Yirmiya et al., 2015). Therefore, we then sought to study the regulation of NLRP3 inflammasome by fluoxetine in microglia. Similar to the results obtained in the macrophages, fluoxetine inhibited ATP-induced caspase-1 activation and IL-1β production (Figure 2A-B) (F=29.815, P<.01) in microglia. Moreover, fluoxetine also reduced the expression of NLRP3 (F=30.162, P<.01) and pro-IL-1β (Figure 2C-D, F=19.732, P<.01). These data indicate that fluoxetine indeed can block NLRP3 inflammasome activation in microglia.
Figure 2.
Fluoxetine suppressed NLRP3 inflammasome activation in microglia. Microglia were incubated with indicated concentrations of fluoxetine (Flx, 0.1 μM, 1 μM, 10 μM) for 1 hour, then stimulated with LPS (100ng/mL) followed by 30 minutes of ATP (5mM) treatment. (A) Immunoblot analysis of interleukin (IL)-1β and cleaved caspase-1 (p20) in culture supernatants (SN) of microglia and immunoblot analysis of the NLRP3, proIL-1β, and procaspase-1 in lysates of those cells (input). (B) ELISA of IL-1β in supernatants of microglia. (C-D) Quantitative analysis of NLRP3 (C) and proIL-1β (D) in microglia. Data are represented as means ± SEM from 2 replicates in 3 independent experiments. * P<.05, ** P<.01, *** P<.001 vs LPS+ATP; # P<.05, ## P<.01 vs LPS+ATP+Flx 0.1 μM. There were no significant differences between -LPS-ATP and -LPS-ATP + Flx 10 μM.
The ROS-PKR-NLRP3 Signaling Pathway Mediates the Inhibitory Effect of Fluoxetine on NLRP3 Inflammasome in Macrophages
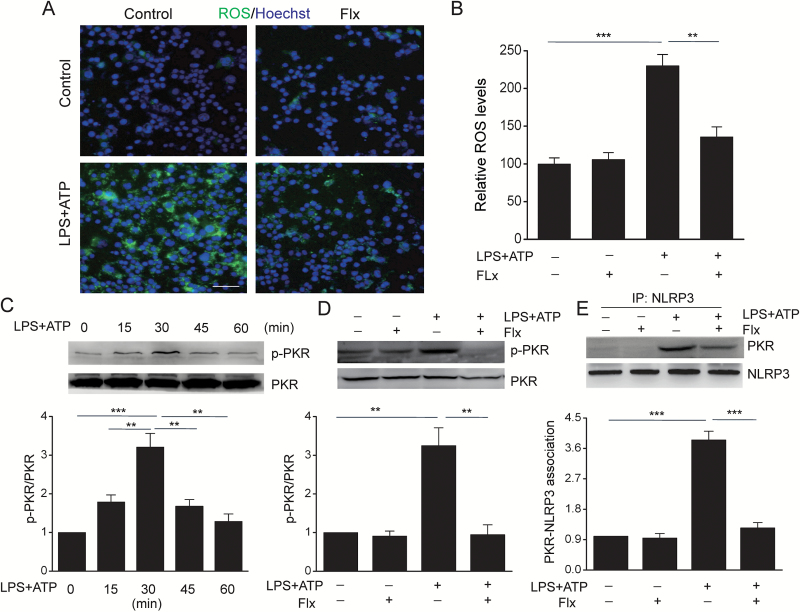
Our preceding data have strongly demonstrated that fluoxetine inhibited NLRP3 inflammasome. To dissect the underlying molecular mechanisms, we first measured the ROS production, which is believed to be a common NLRP3 activator (Martinon, 2015). Fluoxetine significantly decreased ATP-induced ROS production as detected by the fluorescence of CM-H2DCFDA in BMDMs (Figure 3A-B) (F=26.807, P<.01). It has been demonstrated that ROS induces double-stranded PKR phosphorylation and activates NLRP3 (Lu et al., 2013). We then determined if fluoxetine could alter PKR activity. As shown in Figure 3C, PKR was effectively phosphorylated in BMDMs following ATP stimulation at 30 minutes (F=16.5, P<.01). Pretreatment with fluoxetine significantly reduced the phosphorylation of PKR (Figure 3D) (F=18.146, P=.001) and the interaction between PKR and NLRP3 (Figure 3E) (F=73.049, P<.01). These data indicate that fluoxetine blocks NLRP3 inflammasome by inhibiting ROS-PKR-NLRP3 signaling pathway.
Figure 3.
Fluoxetine reduced ATP-induced ROS production, RNA-dependent protein kinase (PKR) phosphorylation and the association of PKR with NLRP3 in macrophages. Bone marrow-derived macrophages (BMDMs) were pretreated with fluoxetine (Flx, 10 μM) for 1 hour, then stimulation with LPS+ATP. (A) Cells were stained with H2DCF-DA labeling and images captured with a fluorescence microscope. (B) The quantitative analysis of ROS levels by a Titertek Fluoroskan II microtiter fluorescence plate reader. (C) LPS-primed BMDMs were stimulated with ATP at indicated time points and phosphorylated and total PKR were determined by Western-blot analysis (** P<.01, *** P<.001 vs LPS+ATP 30min). (D-E) BMDMs were pretreated with fluoxetine (Flx, 10 μM) for 1h, followed by stimulation with LPS+ATP for 30 minutes. (D) Representative immunoblots and quantitative analysis of phosphorylated and total PKR. (E) Immunoprecipitation and immunoblot analysis of the PKR-NLRP3 interaction. Data are represented as means ± SEM from 2 replicates in 3 independent experiments. ** P<.01, *** P<.001 vs LPS+ATP. There were no significant differences among -LPS-ATP, -LPS-ATP+Flx, and +LPS+ATP+Flx.
Fluoxetine Suppresses NLRP3 Inflammasome Activation via Inhibiting the Association of PKR with NLRP3 in Hippocampus of CMS Mice
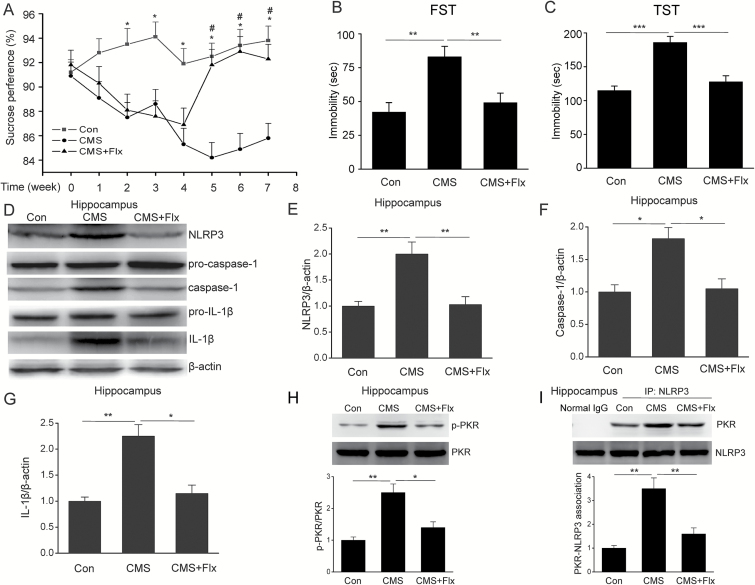
Increasing evidence suggest that NLRP3 inflammasome is involved in the pathogenesis of depression (Alcocer-Gomez et al., 2015; Zhang et al., 2015). We then wanted to know whether fluoxetine can prevent NLRP3 inflammasome-driven inflammation in CMS-induced model of depression. To address this question, we prepared the CMS model and found that fluoxetine administration significantly improved depressive behavior, including increased sucrose preference (Figure 4A) (F=11.923, P<.01) and decreased duration of immobility time in TST (Figure 4B) (F=21.342, P<.01) and FST (Figure 4C) (F=9.106, P<.01). Meanwhile, CMS significantly increased NLRP3 expression, caspase-1 cleavage and IL-1β production in hippocampus, and this effect was reversed by fluoxetine treatment (Figures 4D-G, F=11.698, P<.01). In addition, we also found that fluoxetine significantly inhibited the CMS-induced PKR phosphorylation (Figure 4H) (F=15.799, P<.01) and an association between PKR-NLRP3 (Figure 4I) (F=18.402, P=.001) in hippocampus. These data suggest that fluoxetine indeed inhibits CMS-induced NLRP3 inflammasome activation via suppression of the interaction between PKR and NLRP3 in mice.
Figure 4.
Fluoxetine inhibited chronic mild stress (CMS)-induced NLRP3 inflammasome activation via suppression of the association RNA-dependent protein kinase (PKR) with NLRP3 in hippocampus of mice. (A) Sucrose preference (n=18/groups; * P<.05, # P<.05 vs CMS). (B) Tail suspension test (n=18/groups). (C) Forced swim test (n=18/groups). Representative immunoblots (D) and quantitative analysis of NLRP3 (E), caspase-1 (F) and IL-1β (G) in hippocampus of CMS mice (n=4/groups). (H) Representative immunoblots and quantitative analysis of phosphorylated and total PKR (n=4/groups). (I) Immunoprecipitation and immunoblot analysis of the PKR-NLRP3 interaction (n=4/groups). Data are represented as means ± SEM. * P<.05, ** P<.01 vs CMS.
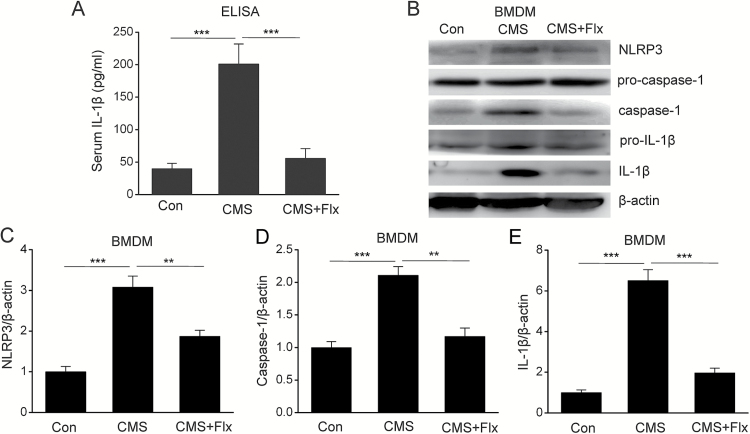
Fluoxetine Inhibits NLRP3 Inflammasome Activation in Macrophages of CMS Mice
Several lines of evidence have demonstrated that peripheral inflammation plays an important role in the progression of depression (Reader et al., 2015). Since macrophages are the main cells responsible for peripheral inflammation, we presume that fluoxetine might regulate NLRP3 inflammasome activation in macrophages in a CMS-induced model of depression. To verify this hypothesis, we first measured the levels of IL-1β in serum and found that fluoxetine treatment significantly decreased serum IL-1β production in the CMS model (Figure 5A) (F=18.937, P<.01). As IL-1β mature is dependent on NLRP3 inflammasome, we then cultured the BMDMs from CMS mice to investigate the impact of fluoxetine on NLRP3 inflammasome. As shown in Figures 5B-E, the expression of NLRP3 (F=29.21, P<.01), caspase-1 activation (F=25.201, P<.01), and subsequent IL-1β mature (F=73.957, P<.01) in BMDMs is significantly increased in CMS mice, and this enhancement was abolished by fluoxetine treatment. These results indicate that fluoxetine could inhibit CMS-induced NLRP3 inflammasome activation in a macrophage in model of depression.
Figure 5.
Fluoxetine inhibited NLRP3 inflammasome activation in bone marrow-derived macrophages (BMDMs) of chronic mild stress (CMS) mice. (A) Serum interleukin (IL)-1β levels by ELISA (n=18/groups). Representative immunoblots (B) and quantitative analysis of NLRP3 (C), caspase-1 (D), and IL-1β (E) in BMDMs of CMS mice (n=4/groups). Data are represented as means ± SEM. ** P<.01, *** P<.001 vs CMS.
Discussion
The most important finding presented here is that fluoxetine inhibits the NLRP3 inflammasome activation in vitro and in vivo. Activation of the NLRP3 inflammasome requires 2 signals (Franchi et al., 2014; Elliott and Sutterwala, 2015). The first signal is induced by endogenous molecules or microbial that activates NF-κB to increase the expression of NLRP3 and proIL-1β, which is a prerequisite for inflammasome activation (Bauernfeind et al., 2009). The second signal directly activates the NLRP3 inflammasome to induce caspase-1 cleavage, leading to the maturation of the proinflammatory cytokines IL-1β and IL-18 (Schroder and Tschopp, 2014). In the present study, we found that fluoxetine reduced LPS-induced expression of NLRP3 and proIL-1β. We also observed that fluoxetine suppressed activation of caspase-1 and the maturation of IL-1β in response to NLRP3 activators such as ATP. These data indicate that fluoxetine inhibits both the priming (signal 1) and the activation of the NLRP3 inflammasome (signal 2).
Our studies have further revealed the molecular mechanism underlying the regulation of NLRP3 inflammasome activation by fluoxetine. The production of ROS is believed to be a common activator of the NLRP3 inflammasome (Martinon, 2015). It has been demonstrated that ROS can enhance PKR phosphorylation (Pyo et al., 2008), which plays a crucial role in the activation of NLRP3 inflammasome (Lu et al., 2013). In this study, we found that fluoxetine not only decreased the ROS production but also reduced the activity of PKR and PKR-NLRP3 association in macrophages. These data demonstrate that fluoxetine inhibits the activation of NLRP3 inflammasome through controlling the production of ROS and the association of NLRP3 with PKR. Fluoxetine reduces the level of ROS, which attenuates the phosphorylation of PKR and the PKR-NLRP3 interaction and subsequently inhibits the NLRP3 inflammasome activation. Taken together, these findings indicate that the ROS-PKR-NLRP3 signal pathway contributes to the inhibition of the NLRP3 inflammasome activation by fluoxetine.
In addition, we provide evidence that fluoxetine ameliorates CMS-induced depressive behavior via inhibiting peripheral and central NLRP3 inflammasome activation. Several studies demonstrate that NLRP3 inflammasome plays a critical role in the pathogenesis of depression (Alcocer-Gomez et al., 2014; Deng et al., 2015). Thus, the NLRP3 inflammasome may represent a novel therapeutic target for depression. In this study, we used a CMS-induced anhedonia model of depression and found that CMS led to activation of the NLRP3 inflammasome in the hippocampus and BMDMs. Fluoxetine treatment abolished CMS-induced activation of NLRP3 inflammasome, accompanied by improved depressive behavior including a significant increase in the sucrose preference and decrease in the duration of immobility time. These data suggest that suppression of peripheral and central NLRP3 inflammasome activation may contribute to the improved depressive behaviors observed in fluoxetine-treated mice.
In conclusion, our study demonstrates that fluoxetine suppresses CMS-induced NLRP3 inflammasome activation in the hippocampus and in BMDMs, accompanied by improvement of depressive behavior. Furthermore, fluoxetine inhibits the activation of NLRP3 inflammasome via downregulating ROS-PKR-NLRP3 signaling pathway in macrophages and microglia (Figure 6). Collectively, our findings reveal that fluoxetine confers an antidepressant effect partly through inhibition of peripheral and central NLRP3 inflammasome activation and suggest the promising clinical use of fluoxetine in NLRP3 inflammasome-driven inflammatory diseases such as depression.
Figure 6.
Schematic layout of showing fluoxetine inhibits NLRP3 inflammasome activation via ROS- RNA-dependent protein kinase-NLRP3 signaling pathway in depression. Fluoxetine decreases the level of ROS, which attenuates PKR phosphorylation and the interaction of PKR with NLRP3, and subsequently inhibits the NLRP3 inflammasome activation.
Statement of Interest
None.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 81473196 and 81573403).
References
- Alcocer-Gomez E, Cordero MD. (2014) NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci Ther 20:294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcocer-Gomez E, de Miguel M, Casas-Barquero N, Nunez-Vasco J, Sanchez-Alcazar JA, Fernandez-Rodriguez A, Cordero MD. (2014) NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun 36:111–117. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, Ryffel B, Apetoh L, Ghiringhelli F, Bullon P, Sanchez-Alcazar JA, Carrion AM, Cordero MD. (2015) Stress-Induced depressive behaviors require a functional NLRP3 inflammasome. Mol Neurobiol 2015 Sep 11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco-de-Almeida LS, Franco GC, Castro ML, Dos Santos JG, Anbinder AL, Cortelli SC, Kajiya M, Kawai T, Rosalen PL. (2014) Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J Periodontol 83:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites D, Fernandes A. (2015) Neuroinflammation and Depression: microglia activation, extracellular microvesicles and microrna dysregulation. Front Cell Neurosci. Advance online publication. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AJ, Ryter SW. (2014) Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Mol Cells 37:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choulaki C, Papadaki G, Repa A, Kampouraki E, Kambas K, Ritis K, Bertsias G, Boumpas DT, Sidiropoulos P. (2015) Enhanced activity of NLRP3 inflammasome in peripheral blood cells of patients with active rheumatoid arthritis. Arthritis Res Ther. Advance online publication. doi: 10.1186/s13075-015-0775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelMastro K, Hellem T, Kim N, Kondo D, Sung YH, Renshaw PF. (2015) Incidence of major depressive episode correlates with elevation of substate region of residence. J Affect Disord 129:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XY, Xue JS, Li HY, Ma ZQ, Fu Q, Qu R, Ma SP. (2015) Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav 152:264–271. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. (2007) Microglial activation and its implications in thebrain diseases. Curr Med Chem 14:1189–1197. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. (2014) The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev 65:872–905. [DOI] [PubMed] [Google Scholar]
- Du RW, Du RH, Bu WG. (2014) beta-Arrestin 2 mediates the anti-inflammatory effects of fluoxetine in lipopolysaccharide-stimulated microglial cells. J Neuroimmune Pharmacol 9:582–590. [DOI] [PubMed] [Google Scholar]
- Elliott EI, Sutterwala FS. (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Munoz-Planillo R, Nunez G. (2014) Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison GD, Levin GM. (2000) Factors affecting prescribing of the newer antidepressants. Ann Pharmacother 34:10–14. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Wang JM, Chen S, Brinton RD. (2012) Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Front Endocrinol. Advance online publication. doi: 10.3389/fendo.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo EK, Kim JK, Shin DM, Sasakawa C. (2015) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. Advance online publication. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kim N, Yenari MA. (2015) Mechanisms and potential therapeutic applications of microglial activation after brain injury. CNS Neurosci Ther 21:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wang W, Okla M, Kang I, Moreau R, Chung S. (2016) Suppression of NLRP3 inflammasome by gamma-tocotrienol ameliorates type 2 diabetes. J Lipid Res 57:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. (2014) Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28:137–161. [DOI] [PubMed] [Google Scholar]
- Li R, Wang X, Qin T, Qu R, Ma S. (2016) Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1beta production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res 296:318–325. [DOI] [PubMed] [Google Scholar]
- Liu B, Xu C, Wu X, Liu F, Du Y, Sun J, Tao J, Dong J. (2015) Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 294:193–205. [DOI] [PubMed] [Google Scholar]
- Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. (2013) Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology 61:592–599. [DOI] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. (2013) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Yang JZ, Geng F, Ding JH, Hu G. (2014) Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. Int J Neuropsychopharmacol 17:1501–1510. [DOI] [PubMed] [Google Scholar]
- Martinon F. (2015) Signaling by ROS drives inflammasome activation. Eur J Immunol 40:616–619. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241. [DOI] [PubMed] [Google Scholar]
- Montgomery SA. (1998) Efficacy and safety of the selective serotonin reuptake inhibitors in treating depression in elderly patients. Int Clin Psychopharmacol, 13:S49–54. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD. (2014) Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41:90–100. [DOI] [PubMed] [Google Scholar]
- Pyo CW, Lee SH, Choi SY. (2008) Oxidative stress induces PKR-dependent apoptosis via IFN-gamma activation signaling in Jurkat T cells. Biochem Biophys Res Commun 377:1001–1006. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Fitzgerald KA. (2012) Regulation of inflammasome signaling. Nat Immunol 13:333–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. (2015) Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. (2014) The inflammasomes. Cell 140:821–832. [DOI] [PubMed] [Google Scholar]
- Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C. (2015) NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. Advance online publication. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Li H, Deng X, Ma Z, Fu Q, Ma S. (2015) L-Menthone confers antidepressant-like effects in an unpredictable chronic mild stress mouse model via NLRP3 inflammasome-mediated inflammatory cytokines and central neurotransmitters. Pharmacol Biochem Behav 134:42–48. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Huang X, Zhao FF, Xu Q, Hu G. (2013) Iptakalim enhances adult mouse hippocampal neurogenesis via opening Kir6.1-composed K-ATP channels expressed in neural stem cells. CNS Neurosci Ther 18:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R. (2015) Depression as a microglial disease. Trends Neurosci 38:637–658. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, Zhou JR, Sun DY, Huang AJ, Wang X, Wang YX, Jiang CL. (2014) Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther 20:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, Wang W, Wang YX, Jiang CL. (2015) NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int J Neuropsychopharmacol. 20;18 doi: 10.1093/ijnp/pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]