Abstract
Background:
This meta-analysis of randomized controlled trials aimed to examine the advantages of long-acting injectable antipsychotics over placebo or oral medications regarding efficacy and safety for patients with bipolar disorder.
Methods:
Two categorical meta-analyses of randomized controlled trials were performed to compare study-defined relapse rate (primary), discontinuation rates, and individual adverse events: (1) risperidone-long-acting injectable vs placebo, and (2) long-acting injectable antipsychotics vs oral medications.
Results:
We identified 7 randomized controlled trials (n=1016; long-acting injectable antipsychotics [flupenthixol (1 randomized controlled trial) and risperidone (6 randomized controlled trials)=449]; oral medications [mood stabilizers, antidepressants, antipsychotic, or any combination of these agents=283]; and placebo=284). Risperidone-long-acting injectable antipsychotic was superior to placebo for study-defined relapse rate (risk ratio=0.63, P<.0001), relapse of manic symptoms (risk ratio=0.42, P<.00001), and all-cause discontinuation (risk ratio=0.75, P=.007). Risperidone-long-acting injectable was associated with higher incidence of prolactin-related adverse events (risk ratio=4.82, P=.001) and weight gain (risk ratio=3.80, P<.0001) than placebo. The pooled long-acting injectable antipsychotics did not outperform oral medications regarding primary outcome but with significant heterogeneity (I2=74%). Sensitivity analysis, including only studies with rapid cycling or high frequency of relapse patients, revealed that long-acting injectable antipsychotics were superior compared to oral medications (I2=0%, RR=0.58, P=.0004). However, the comparators in this sensitivity analysis did not include second-generation antipsychotic monotherapy. In sensitivity analysis, including only studies with second-generation antipsychotic monotherapy as the comparator, long-acting injectable antipsychotics did not outperform second-generation antipsychotic monotherapy. Risperidone-long-acting injectable was also associated with higher incidence of prolactin-related adverse events than oral medications (RR=2.66, P=.03).
Conclusions:
Long-acting injectable antipsychotics appear beneficial for relapse prevention in patients with rapid cycling. Furthermore, randomized controlled trials comparing long-acting injectable antipsychotics and oral second-generation antipsychotic using larger samples of rapid cycling patients are warranted.
Keywords: long-acting injectable antipsychotics, bipolar disorder, efficacy, safety, systematic review, meta-analysis
Introduction
Bipolar disorder is a potentially lifelong and disabling condition characterized by episodes of mania or hypomania and episodes of depressed mood (Grunze et al., 2013; Kendall et al., 2014). Bipolar disorder is associated with an excess mortality including an increased risk of suicide (Grunze et al., 2013; Kendall et al., 2014). An estimated 25% to 50% of patients with bipolar disorder are reported to attempt suicide at least once (Jamison, 2000). A recent network meta-analysis showed that adherence to pharmacological treatment is critical for effective control of depressive and manic symptoms (Miura et al., 2014). Thus, adherence to medication is essential for people with bipolar disorder to respond satisfactorily to the treatment (Grunze et al., 2013; Kendall et al., 2014). However, adherence to pharmacotherapy is often poor in chronic psychiatric illnesses, including bipolar disorder (Anderson et al., 2012; Geddes and Miklowitz, 2013; Zullig et al., 2013). The frequency of nonadherence in bipolar disorder patients is estimated to range between 10% and 60% (Gigante et al., 2012). Nonadherence increases the risk of relapse and suicide (Samalin et al., 2014) as well as risk of rehospitalization (Gigante et al., 2012).
Long-acting injectable (LAI) antipsychotics (LAI-APs) are considered to possess several benefits compared with oral antipsychotics, including more stable blood levels, consistent bioavailability, predictable medication adherence, and an improved pharmacokinetic profile, all of which allow for use of lower dosages (Spanarello and La Ferla, 2014). Consequently, the LAI-APs are expected to have greater relapse prevention for psychiatric disorders including bipolar disorder and schizophrenia (Grunze et al., 2013; Priebe et al., 2013; Yatham et al., 2013; Kendall et al., 2014; NICE, 2014) and reduce the incidence of adverse events (Spanarello and La Ferla, 2014) in comparison with oral antipsychotics. In light of the recent network meta-analysis of bipolar disorder indicating that continuous antipsychotic treatment is effective for preventing relapse (Miura et al., 2014), we hypothesized that LAI-APs are superior to oral medications, including oral antipsychotic and mood stabilizers, regarding efficacy. In fact, the United States Food and Drug Administration approved the use of risperidone-LAI as both monotherapy and adjunct therapy to lithium or valproate for the maintenance treatment of bipolar I disorder in 2009. To date, 7 randomized controlled trials (RCTs) have been conducted on LAI-APs for the treatment of bipolar disorder (Ahlfors et al., 1981; Yatham et al., 2007; Macfadden et al., 2009; Chengappa et al., 2010; Quiroz et al., 2010; Bobo et al., 2011; Vieta et al., 2012). However, although the results of a systematic review and meta-analyses are considered to present a higher level of evidence than individual trials (Higgins and Green, 2011), there has been no systematic review and meta-analysis of LAI-APs regarding efficacy, tolerability, or safety for patients with bipolar disorder. A meta-analysis can increase the statistical power for group comparisons and overcome the limitation of sample size in underpowered studies (Higgins and Green, 2011). To synthesize the available trial evidence, we conducted a systematic review and meta-analysis of RCTs comparing LAI-APs to placebo and oral medications for bipolar disorder. The meta-analysis was designed to assess the benefits and drawbacks of LAI-APs by comparing efficacy, discontinuation rates, and adverse events to placebo and oral medication groups.
METHODS
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Moher et al., 2009) (supplementary Table 1: PRISMA Checklist).
Search Strategy and Inclusion Criteria of Studies
To identify relevant RCTs, 2 authors (T.K. and K.O.) independently searched MEDLINE, Cochrane Library, and PsycINFO, without language restrictions, from inception to March 26, 2016 using the following search strategy: (bipolar disorder, mania, manic, hypomania, hypo-mania, rapid cycle, rapid-cycle, or bipolar depression) AND (randomized, random, or randomly) AND (depot, decanoate, enanthate, long acting injectable, microsphere, once monthly, palmitate, or pamoate). Two authors (T.K. and K.O.) independently assessed inclusion/exclusion criteria and selected studies. The references of included articles and review articles were also searched for citations of additional relevant published and unpublished research, such as conference abstracts. We also searched the clinical trial registries (ClinicalTrials.gov; http://clinicaltrials.gov/) and WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/search/en/) to include RCTs as comprehensively as possible and to minimize the possibility of publication bias.
Data Synthesis and Outcome Measures
The primary outcome was study-defined relapse rate of any mood symptom (supplementary Table 2). Secondary outcome measures were study-defined relapse rate of manic/hypomanic/mixed symptoms or depressive symptoms, number of episodes (any mood symptoms, manic/hypomanic/mixed symptoms, or depressive symptoms) (supplementary Table 2), score on the Young Mania Rating Scale (Young et al., 1978), Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), or Clinical Global Impression-Severity (CGI-S) scale (Guy and Bonato, 1970) at the study endpoint, discontinuation rates, and individual adverse events.
Data Extraction
Two authors (T.K. and K.O.) independently extracted data from the included studies. We used intention-to-treat or modified intention-to-treat analysis. When data required for the meta-analysis were missing, we contacted the study investigators and requested unpublished data. The following 2 categorical meta-analyses of RCTs were performed for evaluating each outcome: (1) risperidone-LAI vs the placebo, and (2) individual and pooled LAI-APs vs oral medications, including oral antipsychotics, mood stabilizers, antidepressants, or any combination of these agents.
Meta-Analytic Methods
These meta-analyses were conducted using Review Manager Software (Version 5.3 for Windows, Cochrane Collaboration, http://tech.cochrane.org/Revman). The random effects model was chosen because of potential heterogeneity across studies. The risk ratio (RR) was estimated along with its 95% CI for each meta-analysis. In this study, when the RR showed significant differences between groups for efficacy or adverse events, the number needed to treat or harm (NNT or NNH) was calculated from the risk difference (RD) using the formula NNT=1/RD or NNH=1/RD. For continuous data, weighted mean differences (WMDs) were used. When outcomes with different metrics were combined, standardized mean differences were used (DerSimonian and Laird, 1986). We also planned to investigate study heterogeneity using the chi-square test of homogeneity (P < .05) together with the I2 statistic, considering I2 ≥ 50% indicative of considerable heterogeneity (Higgins et al., 2003). In cases with I2 ≥ 50% for the primary outcome, we performed sensitivity analyses to determine the reasons for the heterogeneity. We also assessed the methodological qualities of the articles included according to the Cochrane Risk of Bias Criteria (Cochrane Collaboration, http://www.cochrane.org/).
RESULTS
Study Characteristics
Of 198 hits, we removed 151 duplicates, 33 references based on abstract/title review, and 7 articles after full-text review (6 review articles and 1 same study), retaining 7 RCTs (Ahlfors et al., 1981; Yatham et al., 2007; Macfadden et al., 2009; Chengappa et al., 2010; Quiroz et al., 2010; Bobo et al., 2011; Vieta et al., 2012) (supplementary Figure 1). Moreover, we did not retrieve any additional RCTs by searching the review articles and the clinical trial registries. Thus, the meta-analysis included 7 RCTs (n=1016; LAI-APs [flupenthixol and risperidone, 449]; oral medication [mood stabilizers, antidepressants, antipsychotic, or any combination of these agents, 283]; and placebo, 284). The details of each study are described in Table 1. One trial tested flupenthixol decanoate (25 subjects) (Ahlfors et al., 1981) and 6 tested risperidone-LAI (424 subjects). Four of the 7 RCTs examined risperidone-LAI added to usual treatments (mood stabilizers, antidepressants, antipsychotics, or any combination of these agents). As the comparator group, 2 studies used various oral second-generation antipsychotics (SGAs, 51 subjects) (Yatham et al., 2007; Chengappa et al., 2010) and 1 used olanzapine (131 subjects) (Vieta et al., 2012). Both the mean and median duration of studies was 15 months. Two of the 7 RCTs included only patients with rapid cycling (Bobo et al., 2011) or high frequency of relapse (Macfadden et al., 2009) (Table 1). All 7 RCTs were industry sponsored. Three of the 7 RCTs were double blind. The methodological quality of each RCT based on Cochrane risk-of-bias criteria are shown in supplementary Figures 2 and 3.
Table 1.
Study, Patients, and Treatment Regimens in Randomized, Controlled Trials Included in the Current Meta-analysis
| Study, Country, Funding | Total n | Blind | Duration | Inclusion Criteria | Drug | n | Mean Age(% Male) | History of Illness | Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Ahlfors 1981, MC, industry |
42 | OP | 18 m | Bipolar or unipolar recurrent manic-depressive illness. Pt experienced ≥3 mood episodes of sufficient severity to require treatment during the last 5 y before the enrollment. | Flup-DEC: 10, 15, or 20mg/3 wk | 25 | 50 (45.5) |
DI≥5 | Mean episode frequency: Flup- DEC=Li Mean % time ill: Flup- DEC=Li |
| Li: serum level = 0.8~1.0 mmol/L (12h after the last intake) | 17 | ||||||||
| Bobo 2011, USA, industry |
50 | OP | 12 m | Rapid cycling BDI and BDII (DSM-IV-TR, MINI- confirmed). Age=18–64. YMRS ≥8 or HAMD17 ≥8 and a history of ≥4 symptomatic relapses in the y prior to study, with ≥1 symptomatic relapse in the previous 6 m. TAU: no medication, monotherapy (MS, AP, or AD) and combination therapy (MS+AP, MS+MS, MS+AD, AP+AD, AD+AD, MS+AP+AD, or other) | RIS-LAI (27.0±10.4mg/2 wks)+TAU | 25 | 42.8±8.7 (30) | DI=26.4±7.3 | Mean any-cause relapse events: RIS-LAI+TAU =TAU, Mean time spent in an any- cause relapse event: RIS- LAI+TAU =TAU |
| TAU | 25 | 38.2±10.2 (36) | DI=22.3±9.7 | ||||||
| Chengappa 2010, USA, industry |
50 | OP | 15 m | BDI and BDII (DSM-IV-TR). Age≥18. Pt had hypomanic, manic or mixed episode (YMRS>15). TAU: CAR, LAM, Li, VAL or any combination of these agents (unchanged during the study). | RIS-LAI (25, 37.5, or 50mg/2wk)+TAU | 25 | 40±9.2 (30) | NR | Number of negative clinical events: RIS- LAI+TAU>SGA+TAU |
| SGA (ARI: 15–30mg/d, OLA: 15–25mg/d, QUE: 300–700mg/d, or ZIP: 160mg/d) +TAU | 25 | 39.4±10.9 (28) | |||||||
| Macfadden 2009, USA and India, industry |
124 | DB | 12 m | BDI and BDII (DSM-IV-TR). Age=18–70. Pt had ≥4 mood episodes (i.e. high frequency of relapses; defined as an event requiring psychiatric intervention) in the past 12 m. TAU: LAM, Li, TOP, VAL, SNRI, SSRI, TCA, anxiolytics, or any combination of these agents (unchanged during the study). | RIS-LAI [25 (67.7%), 37.5 (27.7%), or 50 (4.6%) mg/2 wk] +TAU | 65 | 40.0±11.8 (70.8) | AAD=28.3±9.4 | Time to recurrence: RIS-LAI+TAU >PBO+TAU |
| PBO+TAU | 59 | 37.6±12.0 (72.9) | AAD=24.6±8.4 | ||||||
| Quiroz 2010, MC, industry |
303 | DB | 24 m | BDI (DSM-IV-TR). Age=18–65. Acute manic or mixed episode (YMRS ≥20) or were stable (CGI-S ≤3) on APs or MDs. Pt experienced ≥2 mood episodes during the 2 y preceding enrollment. Stable pt had one episode within 4 m of enrollment. BMI=17–33. Pt who maintained response to open-label RIS-LAI for 26wk were randomly allocated to PBO-injections or to continue RIS-LAI for up to 24 m. | RIS-LAI: 12.5, 25 (77%), 37.5, or 50mg/2 wkPBO | 154149 | 39±11.8 (49)39±12.4 (54) | DI=8±8.3DI=9±9.4 | Time to recurrence: RIS-LAI >PBO |
| Vieta 2012, MC, industry |
398 | DB | 18 m | BDI (DSM-IV-TR, MINI-confirmed). Age=18–65. Acute pt (manic or mixed episode: YMRS>20 and CGI-S ≥4), or nonacute pt (between mood episodes: YMRS <12 and CGI-S ≤3), and ≥1 manic episode within 4 m of enrollment). Pt had ≥2 mood episodes in the previous y. Nonacute pt: unchanged BD medication for ≥4wk before screening. Pt who did not experience a recurrence in open-label RIS-LAI for 12wk were randomly allocated to PBO-injections or to continue RIS-LAI for up to 18 m. | RIS-LAI: 25, 37.5, or 50mg/2 wk | 132 | 35.6±11.1 (40) | NR | Time to recurrence: RIS- LAI=PBO, OLA>PBO |
| OLA: 10mg/dPBO | 131135 | 36.5±11.0 (55)36.6±11.0 (48) | |||||||
| Yatham 2007, Canada, industry |
49 | OP | 6 m | BDI and BDII (DSM-IV-TR). Age=18–65. Stable pt (CGI-S ≥3 and YMRS or MADRS ≥13): receiving one oral SGAs (OLA, QUE, RIS) in combination with a maximum of 2 of LAM, Li, or VAL and 1 AD. TAU: LAM, Li, VAL, AD or any combination of these agents (unchanged during the study). | RIS-LAI (26.1±3.6mg/2wk)+TAU | 23 | 41.8±13.1 (52) | NR | CGI-S, YMRS and MADRS: RIS- LAI+TAU=SGA+TAU |
| SGA (OLA=8±6.5mg/d, QUE=352.3±309.5mg/d, RIS=1.4±0.5mg/d)+TAU | 26 | 40.1±12.6 (46) |
Abbreviations: AAD, age at diagnosis; AD, antidepressant; AP, antipsychotic; ARI, aripiprazole; BD, bipolar disorder; BMI (kg/m2), body mass index; CAR, carbamazepine; CGI-S, Clinical Global Impressions-Severity; DB, double blind; DI (years), duration of illness; DSM-(IV)-(TR), Diagnostic and Statistical Manual of Mental Disorders, (4th Edition) (Text Revision); Flup-DEC, flupenthixol decanoate; HAMD, Hamilton Depression Rating Scale; LAI, long-acting injectable; LAM, lamotrigine; Li, lithium; m, month; MADRS, Montgomery Åsberg Depression Rating Scale; MC, multiple countries; MD, mood stabilizer; MINI, Mini International Neuropsychiatric Interview; MS, mood stabilizer; n, number of patients; NR, not reported; OLA, olanzapine; OP, open; PBO, placebo; Pt, patient; QUE, quetiapine; RIS, risperidone; SGA, second generation antipsychotic; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TAU, treatment-as-usual; TCA, tricyclic antidepressant; TOP, topiramate; VAL, valproate; wk, week; y, year; YMRS, Young Mania Rating Scale; ZIP, ziprasidone.
LAI-APs vs Placebo
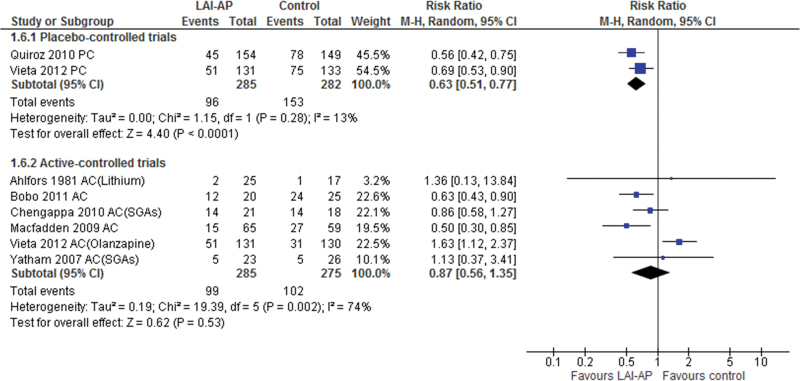
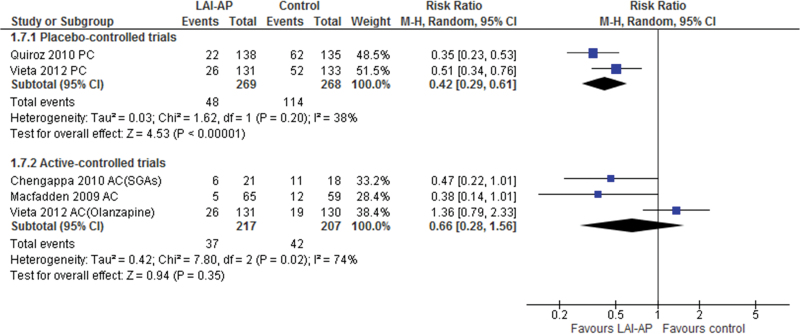
Risperidone-LAI outperformed the placebo regarding the primary outcome, study-defined relapse rate of any mood symptom (RR=0.63, 95% CI=0.51 to 0.77, P<.0001, I2=13%, NNT=−5, P<.00001, N=2, n=567) (Figure 1). Risperidone-LAI was also superior to the placebo in study-defined relapse rate for manic, hypomanic, or mixed symptoms (RR= 0.42, 95% CI= 0.29 to 0.61, P<.00001, I2=38%, NNT=−4, P<.00001, N =2, n =537, Figure 2), and improved scores on the Young Mania Rating Scale (WMD=−5.80, 95% CI=−7.57 to −4.04, P<.00001, I2=0%, N= 2, n=532), MADRS (WMD=−1.76, 95% CI=−3.23 to −0.28, P=.02, I2=0%, N=2, n=532), and CGI-S scale (WMD=−0.76, 95% CI = −1.03 to −0.50, P<.00001, I2=0%, N =2, n=532). In contrast, the risperidone-LAI arm did not differ from the placebo in study-defined relapse rate of depressive symptoms (Table 2). While the meta-analyses of all-cause discontinuation revealed a statistically significant superiority of risperidone-LAI over the placebo (RR=0.75, P=.007, NNT= −5), there was no significant difference in discontinuation rate due to adverse events between groups (RR=1.99, P=.33). Risperidone-LAI was associated with a lower incidence of mania (RR=0.31, P=.001, NNH =−14) and use of benzodiazepines (RR =0.54, p =0.02, NNH=−17) compared with the placebo (Table 2). However, risperidone-LAI was associated with a higher incidence of potential prolactin-related adverse events (RR=4.82, P=.001, NNH=not significant) and weight gain (≥7% increased) (RR =3.80, P<.0001, NNH =10) compared with the placebo (Table 2).
Figure 1.
Study-defined Relapse Rate of Any Mood Symptoms. AC, active-controlled trial; LAI-AP, long-acting injectable antipsychotics; PC, placebo-controlled trial; SGA, second generation antipsychotic; 95% CI, 95% confidence interval.
Figure 2.
Study-defined Relapse Rate of Manic/Hypomanic/Mixed Symptoms. AC, active-controlled trial; LAI-AP, long-acting injectable antipsychotics; PC, placebo-controlled trial; SGA, second generation antipsychotic; 95% CI, 95% confidence interval.
Table 2.
The Results of Meta-Analysis of Randomized, Placebo-Controlled Trials
| Efficacy | ||||||
|---|---|---|---|---|---|---|
| Outcome | Number of Studies | Number of Patients | I2 | Effect Size | 95% Confidence Interval | P |
| Study-defined relapse rate of depressive symptoms | 2 | 537 | 0% | Risk ratio = 1.21 | 0.81 to 1.81 | .35 |
| Young Mania Rating Scale total scores | 2 | 532 | 0% | Weighted mean differences = -5.80 | -7.57 to -4.04 | <.00001 |
| Montgomery-Asberg Depression Scale total scores | 2 | 532 | 0% | Weighted mean differences = -1.76 | -3.23 to -0.28 | .02 |
| Clinical Global Impressions- Severity scores | 2 | 532 | 0% | Weighted mean differences = -0.76 | -1.03 to -0.50 | <.00001 |
| Safety | ||||||
| Discontinuation due to all-cause* | 2 | 570 | 66% | Risk ratio = 0.75 | 0.61 to 0.92 | .007 |
| Discontinuation due to adverse events | 2 | 570 | 0% | Risk ratio = 1.99 | 0.49 to 8.07 | .33 |
| Discontinuation due to death | 2 | 570 | No deaths were reported. | |||
| Discontinuation due to withdrawal consent | 2 | 570 | 0% | Risk ratio = 1.07 | 0.65 to 1.76 | .80 |
| At least one adverse event | 2 | 570 | 21% | Risk ratio = 1.10 | 0.94 to 1.28 | .23 |
| Potential prolactin-related adverse events**, a | 2 | 570 | 0% | Risk ratio = 4.82 | 1.88 to 12.40 | .001 |
| Somnolence | 2 | 570 | 0% | Risk ratio = 1.82 | 0.62 to 5.38 | .28 |
| Insomnia | 2 | 570 | 0% | Risk ratio = 1.03 | 0.66 to 1.60 | .91 |
| Mania*** | 2 | 570 | 0% | Risk ratio = 0.31 | 0.16 to 0.63 | .001 |
| Depression | 2 | 570 | 39% | Risk ratio = 1.57 | 0.57 to 4.31 | .38 |
| Use of benzodiazepines**** | 2 | 570 | 0% | Risk ratio = 0.54 | 0.32 to 0.91 | .02 |
| Anxiety | 2 | 570 | 0% | Risk ratio = 0.85 | 0.39 to 1.87 | .69 |
| Headache | 2 | 570 | 73% | Risk ratio = 0.53 | 0.10 to 2.67 | .44 |
| Diabetes mellitus | 2 | 570 | 0% | Risk ratio = 3.91 | 0.43 to 35.18 | .22 |
| Weight gain (≥7% increased) ***** | 2 | 570 | 0% | Risk ratio = 3.80 | 2.00 to 7.21 | <.0001 |
a Adverse events considered to be potentially prolactin-related (such as galactorrhea or libido decreased), as reported by the investigator.
*Number need to harm = −5, P = .006.
**Number need to harm = not significant.
***Number need to harm = −14, P = .03.
****Number need to harm = −17, P = .02.
*****Number need to harm = 10, P < .00001.
LAI-APs vs Oral Medications
Neither pooled LAI-APs nor any single individual LAI-AP (flupenthixol or risperidone) differed from oral medications regarding the primary outcome and secondary efficacy outcomes (Figures 1 and 2; Table 3). There were also significant differences in discontinuation rate between the treatment groups (Table 3). Risperidone-LAI was associated with a higher incidence of potential prolactin-related adverse events (RR=2.66, P =.03, NNH=20) compared with oral medications, but there were no significant differences in other individual adverse events between the groups (Table 3).
Table 3.
The Results of Meta-Analysis of Randomized, Active-Controlled Trials
| Efficacy | ||||||
|---|---|---|---|---|---|---|
| Outcome | Number of Studies | Number of Patients | I2 | Effect Size | 95% Confidence Interval | P |
| Study-defined relapse rate of depressive symptoms | 3 | 424 | 55% | Risk ratio = 1.25 | 0.60 to 2.59 | .55 |
| Young Mania Rating Scale total scores | 5 | 507 | 63% | Weighted mean differences = -1.03 | -3.24 to -1.18 | .36 |
| Montgomery-Asberg Depression Scale total scores | 4 | 478 | 37% | Weighted mean differences = 1.27 | -0.59 to 3.12 | .18 |
| Clinical Global Impressions-Severity scores | 5 | 507 | 77% | Weighted mean differences = -0.15 | -0.68 to 0.38 | .57 |
| Number of episodes (any symptoms) | 4 | 378 | 85% | Standardized mean difference = -0.05 | -0.73 to 0.62 | .87 |
| Number of episodes (manic/ hypomanic/ mixed symptoms) | 3 | 345 | 91% | Standardized mean difference = -0.34 | -1.28 to 0.60 | .48 |
| Number of episodes (depressive symptoms) | 3 | 345 | 88% | Standardized mean difference = 0.28 | -0.51 to 1.07 | .49 |
| Safety | ||||||
| Discontinuation due to all-cause | 6 | 576 | 70% | Risk ratio = 0.99 | 0.66 to 1.48 | .97 |
| Discontinuation due to adverse events | 6 | 576 | 0% | Risk ratio = 1.59 | 0.67 to 3.77 | .30 |
| Discontinuation due to death | 2 | 387 | na | Risk ratio = 0.45 | 0.04 to 4.88 | .51 |
| Discontinuation due to withdrawal consent | 4 | 484 | 22% | Risk ratio = 1.31 | 0.53 to 3.24 | .56 |
| At least one adverse event | 3 | 360 | 0% | Risk ratio = 0.99 | 0.92 to 1.05 | .67 |
| Serious adverse event | 3 | 221 | 4% | Risk ratio = 0.71 | 0.30 to 1.70 | .44 |
| Potential prolactin-related adverse events*, a | 4 | 480 | 0% | Risk ratio = 2.66 | 1.12 to 6.33 | .03 |
| Suicide attempts | 2 | 169 | na | Risk ratio = 2.73 | 0.11 to 65.68 | .54 |
| Sedation/somnolence | 4 | 480 | 56% | Risk ratio = 0.90 | 0.28 to 2.90 | .86 |
| Insomnia | 2 | 387 | 0% | Risk ratio = 1.38 | 0.85 to 2.23 | .19 |
| Asthenia/depression | 3 | 435 | 62% | Risk ratio = 0.77 | 0.14 to 4.42 | .77 |
| Use of benzodiazepines | 4 | 480 | 0% | Risk ratio = 0.99 | 0.67 to 1.47 | .96 |
| Fatigue | 2 | 387 | 0% | Risk ratio = 0.38 | 0.14 to 1.01 | .05 |
| Use of anticholinergic drugs | 2 | 93 | 68% | Risk ratio = 1.56 | 0.16 to 15.29 | .70 |
| Extrapyramidal symptoms/tremor | 3 | 217 | 0% | Risk ratio = 1.54 | 0.90 to 2.66 | .12 |
| Akathisia | 2 | 172 | 0% | Risk ratio = 0.99 | 0.33 to 2.97 | .98 |
| Migraine/headache | 3 | 435 | 68% | Risk ratio = 0.33 | 0.05 to 2.04 | .23 |
| Dizziness | 2 | 172 | 0% | Risk ratio = 0.64 | 0.19 to 2.20 | .48 |
| Upper respiratory infection | 2 | 172 | 0% | Risk ratio = 1.82 | 0.44 to 7.53 | .41 |
| Increased appetite | 2 | 311 | 0% | Risk ratio = 0.59 | 0.27 to 1.31 | .19 |
| Weight gain (≥7% increased) | 3 | 426 | 0% | Risk ratio = 0.74 | 0.54 to 1.02 | .07 |
| Body mass index/body weight | 2 | 163 | 47% | Standardized mean difference = 0.36 | -0.12 to 0.84 | .15 |
Abbreviations: na, not applicable.
Adverse events considered to be potentially prolactin-related (such as galactorrhea, menstrual changes or libido decreased), as reported by the investigator.
*Number need to harm = 20, P = .01.
Sensitivity Analyses of LAI-APs vs Oral Medications
Since we found significant heterogeneity in the primary outcome between treatment groups (I2=74%) (Figure 1), we conducted sensitivity analyses in RCT subgroups divided by study duration (≥15 or <15 months), blinding (double blind or open), comparator (SGA monotherapy or other oral medications), type of bipolar disorder (rapid cycling or high frequency of relapse patients vs others), the LAI-AP tested (flupenthixol decanoate or risperidone-LAI), and sample size (total n > 100 or <100) (Table 4). Short duration studies (<15 months), open studies, studies with oral medications other than SGA monotherapy as the comparator, rapid cycling or high frequency of relapse patient studies, and small sample size studies (total n < 100) retained significant heterogeneity, but LAI-AP was superior to the placebo for prevention of study-defined relapse rate of any mood symptom (Table 4).
Table 4.
The Results of Sensitivity Analysis for Relapse Prevention
| Number of Studies | Number of Patients | I2 | Risk Ratio | 95% Confidence Interval | P | I2 | Number Needed to Treat | 95% Confidence Interval | P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study duration | Long (≥ 15 months) | 3 | 342 | 69% | 1.20 | 0.65 to 2.19 | .56 | ||||
| Short (< 15 months) | 3 | 218 | 0% | 0.61 | 0.46 to 0.81 | .0008 | ns | ||||
| Blinding | Double blind | 2 | 385 | 92% | 0.92 | 0.29 to 2.91 | .89 | ||||
| Open | 4 | 175 | 0% | 0.75 | 0.58 to 0.97 | .03 | ns | ||||
| Comparator | Second generation antipsychotic monotherapy | 3 | 349 | 69% | 1.18 | 0.69 to 2.02 | .55 | ||||
| Other oral medications * | 3 | 211 | 0% | 0.59 | 0.44 to 0.80 | .0005 | ns | ||||
| Type of bipolar disorder | Rapid cycling or high frequency of relapse patients | 2 | 169 | 0% | 0.58 | 0.43 to 0.79 | .0004 | 0% | -4 | -7 to -3 | <.0001 |
| Others | 4 | 391 | 31% | 1.19 | 0.74 to 1.91 | .48 | |||||
| Long-acting injectable antipsychotics | Risperidone long-acting injectable | 5 | 518 | 79% | 0.86 | 0.54 to 1.36 | .51 | ||||
| Flupenthixol decanoate | 1 | 42 | na | 1.36 | 0.13 to 13.84 | .80 | |||||
| Sample size | Large (total number of patients > 100) | 2 | 385 | 92% | 0.92 | 0.29 to 2.91 | .89 | ||||
| Small (total number of patients < 100) | 4 | 175 | 0% | 0.75 | 0.58 to 0.97 | .03 | ns |
Abbreviations: na, not applicable; ns, not significant.
*Others: Mood stabilizers, antidepressants, antipsychotic or any combination of these agents
Discussion
This is the first meta-analysis of RCTs (7 studies, 1016 patients in total) examining the efficacy and safety of LAI-APs for bipolar disorder compared with placebo or oral medications. We found that risperidone-LAI was superior to the placebo in preventing relapse of any mood symptom (primary outcome) as well as for preventing manic symptoms, while relapse rate of depressive symptoms was similar to the placebo. Risperidone-LAI also improved MADRS score compared with the placebo, but the effect size was small (WMD=−1.76). Thus, results of this meta-analysis reveal a significant benefit of risperidone-LAI on symptom relapse, especially manic symptoms, compared with the placebo. Although relapse rate of any mood symptom pooled LAI-APs was similar to that on oral medications, LAI-APs was superior to oral medications in sensitivity analysis considering only studies of rapid cycling or high frequency of relapse patients. This result appears consistent with that of risperidone-LAI vs the placebo. Several studies reported that factors that have been associated with poor adherence include history of rapid cycling, bipolar type I disorder, and greater illness severity (Martinez-Aran et al., 2009; Perlis et al., 2010). Other studies reported that poor adherence to medication has been associated with more manic symptoms (Sylvia et al., 2014) and a higher rate of recurrence and hospitalization (Hassan and Lage, 2009; Gutierrez-Rojas et al., 2010) but not with depressive symptoms (Sylvia et al., 2014). We considered that LAI-APs might improve medication adherence and possibly reduce relapse in patients with these subtypes of bipolar disorder. However, the total number of patients in the sensitivity analysis was small (169 subjects from 2 RCTs) (Macfadden et al., 2009; Bobo et al., 2011) and one (Bobo et al., 2011) of the 2 RCTs was an open study. Moreover, the comparator of these 2 RCTs was not SGA monotherapy, and LAI-APs did not outperform SGA monotherapy regarding relapse rate in sensitivity analysis that included only studies that used SGA monotherapy as the comparator. Therefore, a RCT of LAI-AP vs SGA monotherapy for the treatment of rapid cycling or high frequency of relapse bipolar disorder patients is required. In addition, clinicians need to be aware of potential adverse events induced by risperidone-LAI, such as potential prolactin-related adverse events and weight gain.
There are several limitations to the present analysis. First, we detected significant heterogeneity with respect to the primary outcome in the meta-analysis of LAI-APs vs oral medications, and sensitivity analyses did not identify the source. Additionally, although there were substantial differences in sample size among the studies included, the weighting of each was similar. Second, the total numbers of studies and patients included were relatively small. Since significant heterogeneity was detected, the limited sample size indicates that more research is needed to evaluate both the efficacy and tolerability of LAI-APs. Third, because a funnel plot is generally used only if 10 or more studies are included in the meta-analysis, we did not utilize this plot for exploring potential publication bias.
In conclusion, LAI-APs appear effective for relapse prevention in patients with rapid cycling. However, further RCTs of LAI-AP vs oral SGAs using larger sample sizes of rapid cycling patients are needed to definitively assess this potential benefit.
Statement of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study. Dr. Kishi has received speaker’s honoraria from Abbvie, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Mochida, Shionogi, Tanabe-Mitsubishi, Tsumura, Novartis, and Pfizer and has a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists. Dr. Oya has received speaker’s honoraria from Janssen, Eli Lilly, Meiji, and Otsuka and has a Fujita Health University School of Medicine research grant. Dr. Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer and has research grants from Dainippon Sumitomo, GlaxoSmithKline, Tanabe-Mitsubishi, and Otsuka.
Supplementary Material
References
- Ahlfors UG, Baastrup PC, Dencker SJ, Elgen K, Lingjaerde O, Pedersen V, Schou M, Aaskoven O. (1981) Flupenthixol decanoate in recurrent manic-depressive illness. A comparison with lithium. Acta Psychiatr Scand 64:226–237. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Haddad PM, Scott J. (2012) Bipolar disorder. BMJ 345:e8508. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Epstein RA, Lynch A, Patton TD, Bossaller NA, Shelton RC. (2011) A randomized open comparison of long-acting injectable risperidone and treatment as usual for prevention of relapse, rehospitalization, and urgent care referral in community-treated patients with rapid cycling bipolar disorder. Clin Neuropharmacol 34:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengappa KN, Turkin SR, Schlicht PJ, Murphy SL, Brar JS, Fagiolini A, Houck PR, Garbutt RG, Fredrick N. (2010) A pilot, 15-month, randomised effectiveness trial of risperidone long-acting injection (RLAI) vs oral atypical antipsychotic agents (AAP) in persons with bipolar disorder. Acta Neuropsychiatr 22:68–80. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Miklowitz DJ. (2013) Treatment of bipolar disorder. Lancet 381:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante AD, Lafer B, Yatham LN. (2012) Long-acting injectable antipsychotics for the maintenance treatment of bipolar disorder. CNS Drugs 26:403–420. [DOI] [PubMed] [Google Scholar]
- Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Moller HJ, Kasper S, Disorders WTFoTGfB (2013) The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J Biol Psychiatry 14:154–219.23480132 [Google Scholar]
- Gutierrez-Rojas L, Jurado D, Martinez-Ortega JM, Gurpegui M. (2010) Poor adherence to treatment associated with a high recurrence in a bipolar disorder outpatient sample. J Affect Disord 127:77–83. [DOI] [PubMed] [Google Scholar]
- Guy W, Bonato RR. (1970) Manual for the ECDEU assessment battery, 2nd ed Chevy Chase (MD): National Institute of Mental Health. [Google Scholar]
- Hassan M, Lage MJ. (2009) Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge. Am J Health Syst Pharm 66:358–365. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. (2011) Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration www.cochrane-handbook.org.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison KR. (2000) Suicide and bipolar disorder. J Clin Psychiatry 61 Suppl 9:47–51. [PubMed] [Google Scholar]
- Kendall T, Morriss R, Mayo-Wilson E, Marcus E, Guideline Development Group of the National Institute for Health and Care Excellence (2014) Assessment and management of bipolar disorder: summary of updated NICE guidance. BMJ 349:g5673. [DOI] [PubMed] [Google Scholar]
- Macfadden W, Alphs L, Haskins JT, Turner N, Turkoz I, Bossie C, Kujawa M, Mahmoud R. (2009) A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord 11:827–839. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Scott J, Colom F, Torrent C, Tabares-Seisdedos R, Daban C, Leboyer M, Henry C, Goodwin GM, Gonzalez-Pinto A, Cruz N, Sanchez-Moreno J, Vieta E. (2009) Treatment nonadherence and neurocognitive impairment in bipolar disorder. J Clin Psychiatry 70:1017–1023. [DOI] [PubMed] [Google Scholar]
- Miura T, Noma H, Furukawa TA, Mitsuyasu H, Tanaka S, Stockton S, Salanti G, Motomura K, Shimano-Katsuki S, Leucht S, Cipriani A, Geddes JR, Kanba S. (2014) Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry 1:351–359. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- NICE (2014) In: Psychosis and schizophrenia in adults: treatment and management: updated edition 2014. London: National Institute for Health and Care Excellence (NICE). [PubMed] [Google Scholar]
- Perlis RH, Ostacher MJ, Miklowitz DJ, Hay A, Nierenberg AA, Thase ME, Sachs GS. (2010) Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry 71:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe S, Yeeles K, Bremner S, Lauber C, Eldridge S, Ashby D, David AS, O’Connell N, Forrest A, Burns T. (2013) Effectiveness of financial incentives to improve adherence to maintenance treatment with antipsychotics: cluster randomised controlled trial. BMJ 347:f5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V. (2010) Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry 68:156–162. [DOI] [PubMed] [Google Scholar]
- Samalin L, Nourry A, Charpeaud T, Llorca PM. (2014) What is the evidence for the use of second-generation antipsychotic long-acting injectables as maintenance treatment in bipolar disorder? Nord J Psychiatry 68:227–235. [DOI] [PubMed] [Google Scholar]
- Spanarello S, La Ferla T. (2014) The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol 9:310–317. [DOI] [PubMed] [Google Scholar]
- Sylvia LG, Reilly-Harrington NA, Leon AC, Kansky CI, Calabrese JR, Bowden CL, Ketter TA, Friedman ES, Iosifescu DV, Thase ME, Ostacher MJ, Keyes M, Rabideau D, Nierenberg AA. (2014) Medication adherence in a comparative effectiveness trial for bipolar disorder. Acta Psychiatr Scand 129:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Montgomery S, Sulaiman AH, Cordoba R, Huberlant B, Martinez L, Schreiner A. (2012) A randomized, double-blind, placebo-controlled trial to assess prevention of mood episodes with risperidone long-acting injectable in patients with bipolar I disorder. Eur Neuropsychopharmacol 22:825–835. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Fallu A, Binder CE. (2007) A 6-month randomized open-label comparison of continuation of oral atypical antipsychotic therapy or switch to long acting injectable risperidone in patients with bipolar disorder. Acta Psychiatr Scand:50–56. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O’Donovan C, Macqueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Milev R, Bond DJ, Frey BN, Goldstein BI, Lafer B, Birmaher B, Ha K, Nolen WA, Berk M. (2013) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 15:1–44. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. (1978) A rating scale for mania: reliability, validity and sensitivity. The Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
- Zullig LL, Peterson ED, Bosworth HB. (2013) Ingredients of successful interventions to improve medication adherence. JAMA 310:2611–2612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.