Abstract
Background:
Depression is a serious psychiatric disorder that easily causes physical impairments and a high suicide rate. Monosialotetrahexosylganglioside is a crucial ganglioside for the central nervous system and has been reported to affect the function of the brain derived neurotrophic factor system. This study is aimed to evaluate whether monosialotetrahexosylganglioside has antidepressant-like effects.
Methods:
Antidepressant-like effects of monosialotetrahexosylganglioside were assessed in the chronic social defeat stress model of depression, and various behavioral tests were performed. Changes in the brain derived neurotrophic factor signaling pathway after chronic social defeat stress and monosialotetrahexosylganglioside treatment were also investigated. A tryptophan hydroxylase inhibitor and brain derived neurotrophic factor signaling inhibitors were used to determine the antidepressant mechanisms of monosialotetrahexosylganglioside.
Results:
Monosialotetrahexosylganglioside administration significantly reversed the chronic social defeat stress-induced reduction of sucrose preference and social interaction in mice and also prevented the increased immobility time in the forced swim test and tail suspension test. In addition, monosialotetrahexosylganglioside completely ameliorated the stress-induced dysfunction of brain derived neurotrophic factor signaling cascade in the hippocampus and medial prefrontal cortex, 2 regions closely involved in the pathophysiology of depression. Furthermore, the usage of brain derived neurotrophic factor signaling cascade inhibitors, K252a and anti-brain derived neurotrophic factor antibody, each abolished the antidepressant-like effects of monosialotetrahexosylganglioside, while the usage of a serotonin system inhibitor did not.
Conclusions:
Taken together, our findings suggest that monosialotetrahexosylganglioside indeed has antidepressant-like effects, and these effects were mediated through the activation of brain derived neurotrophic factor signaling cascade.
Keywords: depression, chronic social defeat stress, hippocampus, medial prefrontal cortex
Introduction
Depression is a common illness with potentially life-threatening emotional and behavioral symptoms, and this kind of neuropsychiatric disorder will become the second-most burdensome disease in the world according to the World Health Organization (Zonana and Gorman, 2005; Vicente et al., 2006; Olesen et al., 2012). The monoamine hypothesis of depression postulates that insufficient serotonergic and noradrenergic transmission in the brain accounts for many or most symptoms of depression (Chopra et al., 2011; Prins et al., 2011). However, many patients are drug refractory or experience intolerable side effects from antidepressants regulating monoaminergic neurotransmission (McGrath et al., 2006). Moreover, these drugs all show delayed onset of action and unpredictable efficacy in individual patients (McGrath et al., 2006), prolonging the search for more effective agents with fewer side effects.
Brain derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, is distributed throughout the central nervous system (Hofer et al., 1990; Conner et al., 1997). BDNF induces the phosphorylation and activation of cAMP response element-binding protein (CREB) by combining the tyrosine kinase B (TrkB) receptor and then activating the MAPK-ERK and PI3K-AKT signaling pathways, 2 key downstream signaling pathways of BDNF (Shaywitz and Greenberg, 1999; Lim et al., 2008). Previous studies have already demonstrated that depression is associated with decreased BDNF signaling in the hippocampus and medial prefrontal cortex (mPFC), 2 regions closely implicated in the pathophysiology of depression, and this decrease can be normalized by antidepressant treatment (Nibuya et al., 1996; Thome et al., 2000; Blendy, 2006; Gronli et al., 2006; Castren and Rantamaki, 2010; Razzoli et al., 2011; Zhou et al., 2014). Animal studies found that administration of BDNF into the hippocampus produced antidepressant-like effects in models of depression (Shirayama et al., 2002; Hoshaw et al., 2005). Interestingly, depression is also accompanied with enhanced BDNF expression and CREB activation in the nucleus accumbens (NAc), another region involved in the pathophysiology of depression, while blockade of BDNF function in the NAc exhibits an antidepressant-like phenotype (Shirayama and Chaki, 2006).
Gangliosides are sialic acid-containing glycosphingolipids synthesized by neuronal and glial cells,and are abundant in the brain (Svennerholm, 1956; Suzuki, 1965; Derry and Wolfe, 1967). Gangliosides are crucial for brain development and plasticity (Ferrari and Greene, 1998; Hadjiconstantinou and Neff, 1998; Lim et al., 2011). Previous studies have shown that accumulation of gangliosides in the brain leads to neurite outgrowth, while lack of gangliosides causes neurodegeneration (Purpura and Suzuki, 1976; Sparrow et al., 1984; Yamashita et al., 2005). Moreover, exogenously administered gangliosides have been shown to affect the survival of different neuronal types, including glutamatergic, dopaminergic, and cholinergic neurons (Sofroniew et al., 1986; Favaron et al., 1988; Hadjiconstantinou and Neff, 1988; Schneider et al., 1992). Experimental data have shown that one ganglioside, monosialotetrahexosylganglioside (GM1), exhibit properties similar to neurotrophins (Mocchetti, 2005), and several reports found that the neurotrophic activity of GM1 derives from its ability to activate Trks, the family of high-affinity neurotrophin receptors (Mutoh et al., 1995; Ferrari and Greene, 1996; Duchemin et al., 2002; Rabin et al., 2002; Duchemin et al., 2008). GM1 also induces the synthesis and release of BDNF and in turn activates an autocrine loop (Lim et al., 2011; Valdomero et al., 2015).
Since GM1 has important physiological properties such as activating neurotrophin receptors and promoting BDNF release, here we hypothesize that GM1 may have antidepressant-like effects. In this study, we firstly assessed the antidepressant-like effects of GM1 using the chronic social defeat stress (CSDS) model of depression and explored the molecular mechanisms responsible for these effects.
Materials and Methods
Animals
Adult male C57BL/6J mice (6–8 weeks old) were obtained from the Experimental Animal Center of Medical College, Nantong University. Before use, mice were housed 5 per cage under standard conditions (12-h-light/-dark cycle; lights on from 7:00 am to 7:00 pm; 24 ±1°C ambient temperature; 55±10% relative humidity) for 1 week with free access to food and water. Behavioral experiments were carried out during the light phase. The experimental procedures involving animals and their care were conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Materials
Monosialotetrahexosylganglioside (GM1, purity >95%), fluoxetine and p-chlorophenylalanine methyl ester (PCPA) were purchased from Sigma (St. Louis, MO). K252a was purchased from Alomone Laboratories (Jerusalem, Israel). Chicken anti-BDNF neutralizing anti-body (G1641) and chicken IgY control Ig (G1161) were purchased from Promega (Madison, WI).The repeated drug treatment of mice was performed once daily between 9:30 and 10:30 am. The dosages of GM1, fluoxetine, K252a, anti-BDNF neutralizing antibody, and PCPA were chosen based on previous reports (Jiang et al., 2015a; Valdomero et al., 2015). GM1, fluoxetine, K252a, and PCPA were administered i.p. in a volume of 10mL/kg. Chicken anti-BDNF neutralizing antibody and chicken IgY control Ig were intracerebroventricularly infused.
CSDS
Social defeat and avoidance testing were performed according to previously reported methods (Berton et al., 2006; Donahue et al., 2014). C57BL/6 mice were exposed to different CD1 aggressor mice each day for 10 minutes for a total of 10 days. After the 10 minutes of contact, C57BL/6 mice were separated from the aggressors by plastic dividers with holes, where they were exposed to chronic stress in the form of threat for the next 24 hours. Nondefeated control mice were handled daily. Twenty-four hours after the last session, defeated C57BL/6 mice were subjected to the social interaction test and sorted into either susceptible or unsusceptible phenotype based on interaction scores. All the CSDS-unsusceptible mice were removed. Then the CSDS-susceptible mice were housed individually and received daily injections of vehicle or tested compounds for another 14 days. After that, various behavioral tests were performed. All the mice were first anaesthetized using 0.5% pentobarbital sodium (10mL/kg) and then sacrificed by cervical dislocation.
Additional experimental procedures are available online in the supplemental Information.
Data Analysis
All analyses were performed using SPSS 13.0 software (SPSS Inc) and data are presented as the mean ± SEM. Differences between mean values were evaluated using 1-way ANOVA (posthoc LSD test) or 2-way ANOVA (posthoc Bonferroni’s test), as appropriate. P<.05 was considered statistically significant.
Results
Chronic GM1 Treatment Restores the CSDS-Induced Depressive-Like Symptoms
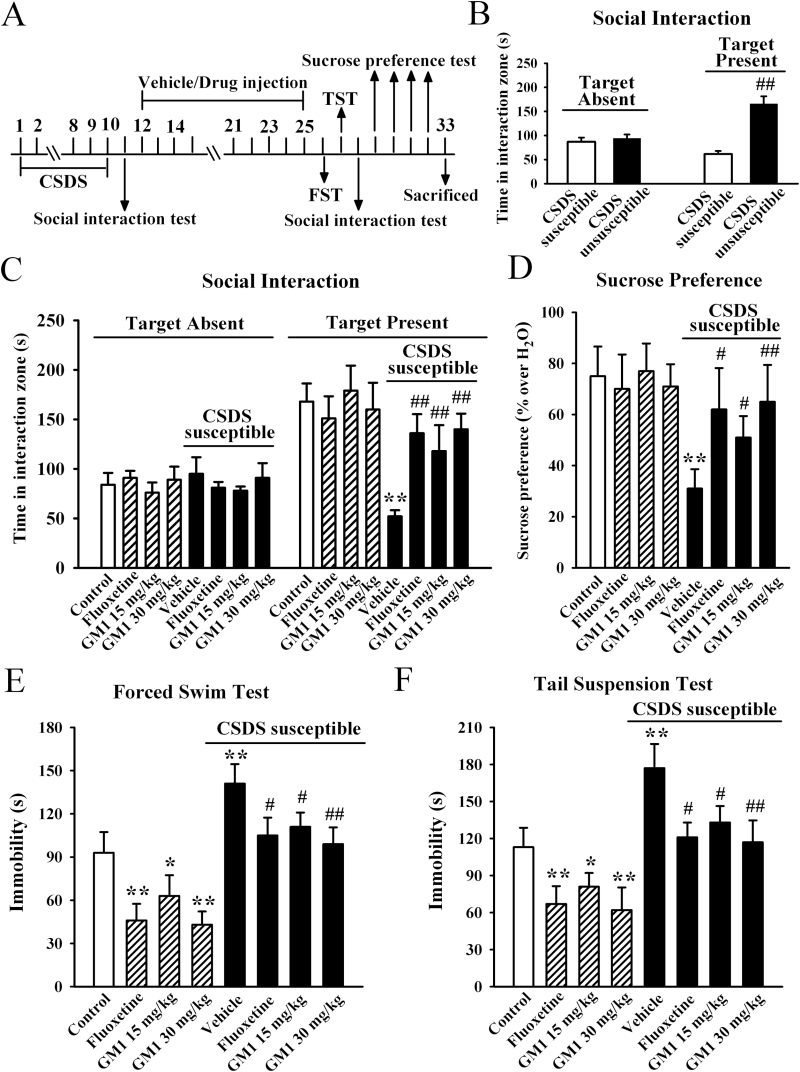
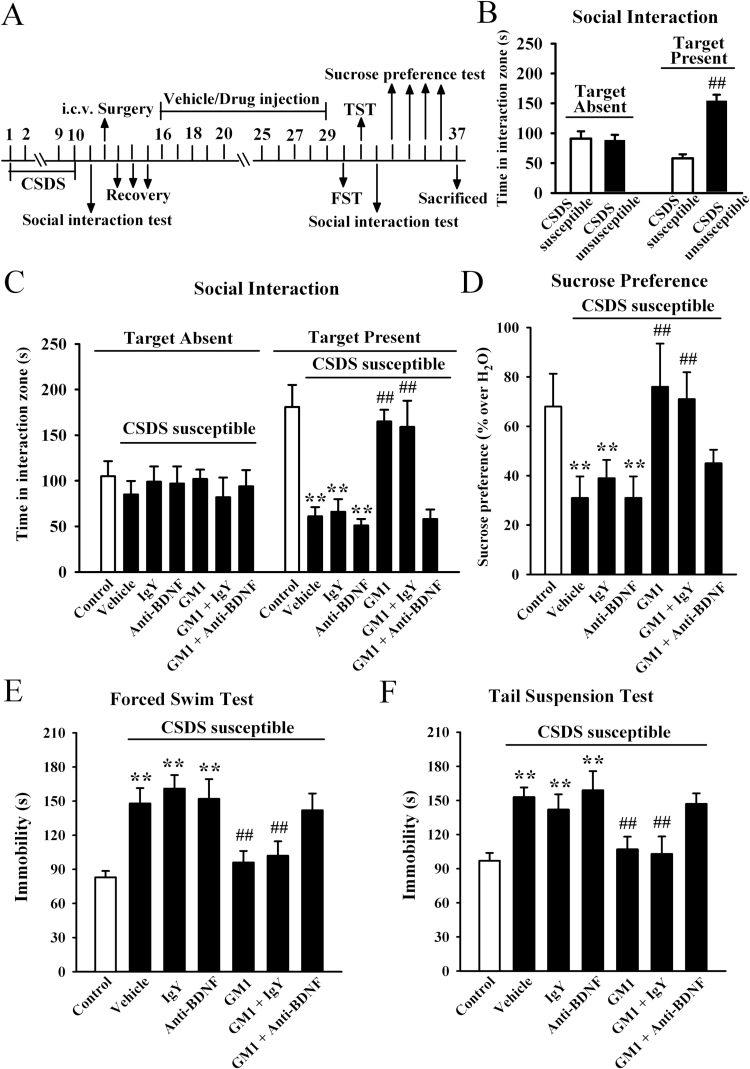
We firstly considered the possibility that GM1 could reverse depression in an animal model of depression using a chronic social defeat stress protocol that mimics many symptoms of depression in humans (Berton et al., 2006). After a brief (10 minutes) daily exposure to a highly aggressive resident mouse for 10 days, CSDS-susceptible mice were selected and received 14-day treatment of GM1/fluoxetine/vehicle, with behavioral tests then performed. For the social interaction test, ANOVA analysis revealed a significant interaction [F(3, 88) = 61.437, P<.01] with significant effects for CSDS [F(1, 88) = 56.319, P<.01] and drug treatment [F(3, 88) = 14.895, P<.01]. In the absence of an aggressor, all mice spent similar amounts of time in the interaction zone. Compared with nonstressed control mice, CSD-susceptible mice spent about 70% less time in the interaction zone when an aggressor was introduced into the cage (n = 12, P<.01 vs control) (Figure 1C). The 14-day treatment of fluoxetine reversed this avoidance behavior, increasing the interaction time such that it was close to that of control mice (n = 12, P<.01 vs CSDS susceptible + vehicle; Figure 1C), in agreement with previous reports (Tsankova et al., 2006). Interestingly, while GM1 treatment had no significant effects in control animals, 14-day treatment of GM1 significantly increased the interaction time of CSDS susceptible mice relative to control mice (n = 12, P<.01 vs CSDS susceptible + vehicle; Figure 1C), similar to fluoxetine. Further analysis revealed that the interaction time was increased by 126.9±14.4% and 169.2±11.3% for 15mg/kg and 30mg/kg GM1, respectively.
Figure 1.
Monosialotetrahexosylganglioside (GM1) produces robust antidepressant-like effects in the chronic social defeat stress (CSDS) model of depression. (A) Schematic timeline of the experimental procedure. A total of 109 C57BL/6J mice were used in this experiment with 61 CSDS-stressed mice and 48 nonstressed mice. After 10 days of CSDS, nonstressed mice and CSDS-susceptible mice received daily injection of vehicle (0.9% saline), fluoxetine (20mg/kg), or GM1 (15, 30mg/kg) for another 14 days, after which behavioral tests were conducted. (B) The social interaction results for CSDS-susceptible mice (n = 48) and unsusceptible mice (n = 13) in this experiment. (C) The antidepressant-like effects of GM1 in the social interaction test. CSDS-susceptible + GM1 mice spent significantly more time engaged in social interaction than CSDS-susceptible + vehicle mice (n = 12). (D) GM1 treatment reversed the decrease in sucrose preference induced by CSDS. CSDS susceptible + GM1 mice displayed higher sucrose preference than CSDS susceptible+vehicle mice (n=12). (E) The antidepressant-like effects of GM1 in the forced swim test (FST). CSDS-susceptible + GM1 mice displayed significantly lower immobility time than CSDS-susceptible + vehicle mice in the FST (n = 12). (F) The antidepressant-like effects of GM1 in the tail suspension test (TST). CSDS-susceptible+GM1 mice displayed significantly lower immobility time than CSDS-susceptible + vehicle mice in the TST (n=12). Data are expressed as the mean ± SEM; * P<.05, ** P<.01 vs control; # P<.05, ## P<.01 vs CSDS-susceptible/CSDS-susceptible + vehicle. Comparison was made by 2-way ANOVA followed by posthoc Bonferroni’s test.
We then performed the sucrose preference test. Data are summarized in Figure 1D, and 2-way ANOVA also indicated a significant interaction [F(3, 88) = 22.034, P<.01] with significant effects for CSDS [F(1, 88) = 34.509, P<.01] and drug treatment [F(3, 88) = 11.163, P<.01]. CSDS induced a 58.7±6.2% decrease in sucrose preference, compared with control group (n = 12, P<.01 vs control). While GM1 produced no significant effects in naive mice, 14-day treatment with GM1 in CSDS-susceptible mice caused a robust increase of sucrose intake in animals also resulting in an antidepressant-like effect (n = 12, P<.01 vs CSDS susceptible + vehicle) (Figure 1D). Further analysis showed that sucrose intake was increased by 64.5±7.1% and 109.7±9.6% with administration of 15mg/kg and 30mg/kg GM1, respectively.
Additionally, the forced swim test (FST) and tail suspension test (TST), 2 widely used behavioral tests for assessing potential antidepressant-like effects (Cryan and Holmes, 2005; Cryan and Slattery, 2007), were performed. The FST and TST data are summarized in Figures 1E and F, respectively. For the FST data, ANOVA analysis indicated a significant interaction [F(3, 88) = 70.664, P<.01] with significant effects for CSDS [F(1, 88) = 56.841, P<.01] and drug treatment [F(3, 88) = 21.828, P<.01]. For the TST data, ANOVA also revealed a significant interaction [F(3, 88) = 64.266, P<.01] with significant effects for CSDS [F(1, 88) = 54.772, P<.01] and drug treatment [F(3, 88) = 18.856, P<.01]. CSDS stress robustly increased the immobility time of susceptible mice in the FST and TST compared with control group (n = 12, P<.01 vs control), while these changes were fully reversed by 30mg/kg GM1 administration (n = 12, P<.01 vs CSDS susceptible + vehicle), similar to fluoxetine. Moreover, it was found that GM1 treatment also reduced the immobility duration of naive mice in the FST and TST tests (n = 12, P<.01 vs control).
To exclude the possible effects of GM1 on spontaneous locomotor activity which may contribute to immobility in the FST and TST (Bourin et al., 2001), naive mice and CSDS susceptible mice were administered GM1, and then exposed to the open-field test. ANOVA revealed no significant effects for either CSDS [F(1, 88) = 1.074, P=.397] or drug treatment [F(3, 88) = 0.816, P=.397], and there was no significant differences found in the number of squares an animal crossed in either the peripheral area or central area between all groups (n = 12) (supplemental Figure 1). Together, these results suggest that GM1 is able to reverse the CSDS-induced behavioral symptoms.
GM1 Treatment Reverses the CSDS-Induced Decrease of BDNF Signaling Pathway in the Hippocampus and mPFC
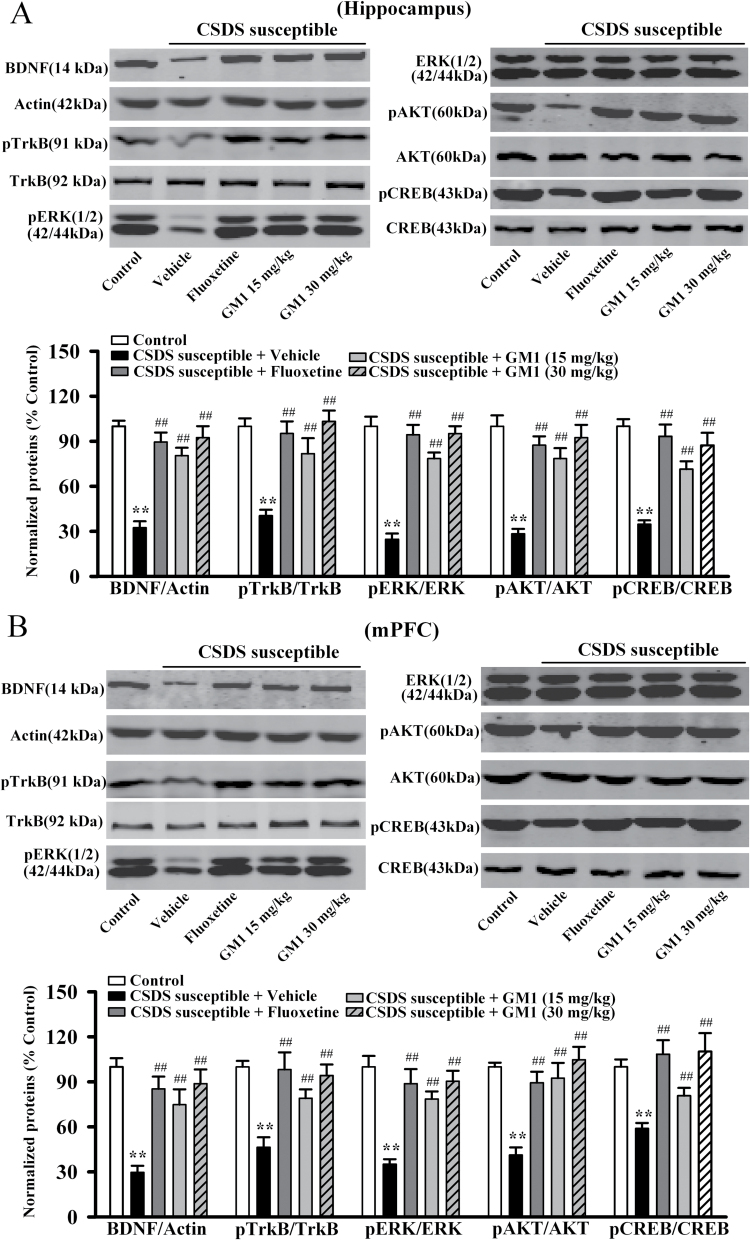
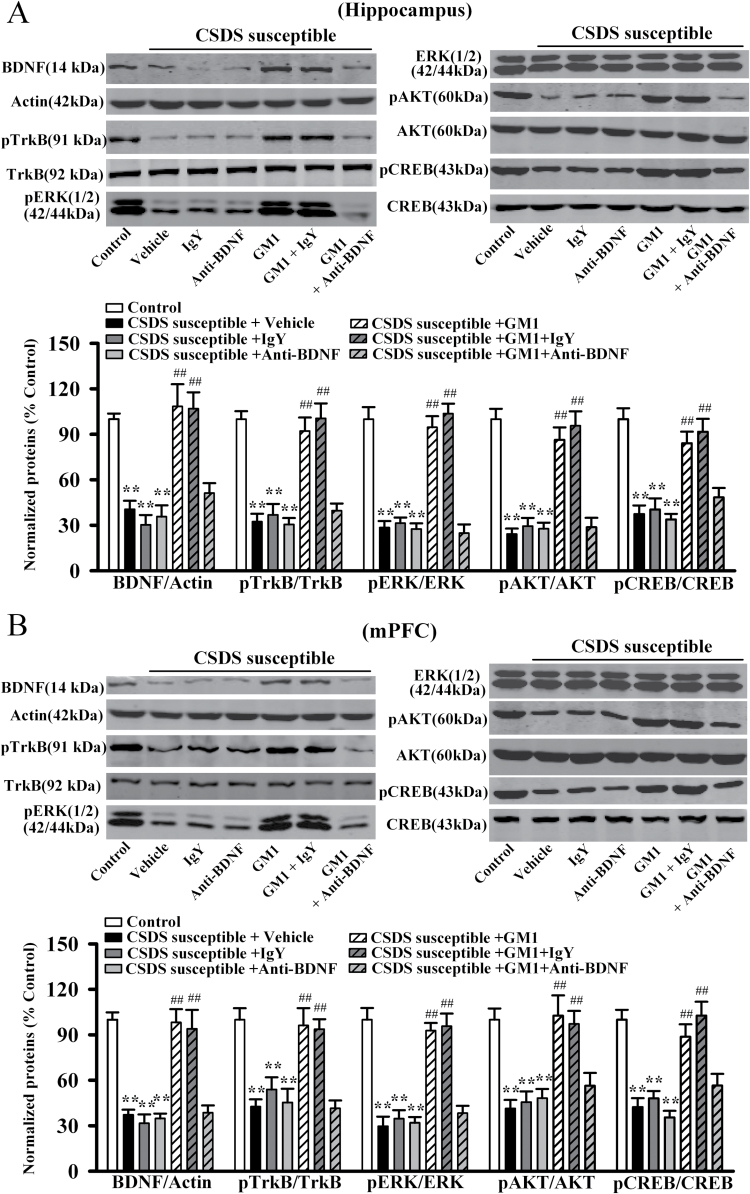
The BDNF-ERK/AKT-CREB signaling cascade plays an important role in the pathophysiology of depression (Gourley et al., 2008; Lee and Son, 2009; Castren and Rantamaki, 2010), and GM1 has been reported to possess neurotrophic activity (Mutoh et al., 1995; Rabin et al., 2002; Lim et al., 2011). Therefore, we performed western-blot analysis to measure the levels of BDNF signaling pathway proteins in the hippocampus and mPFC following CSDS and GM1 treatment. The mature BDNF protein level is expressed as a ratio of the expression of mature BDNF to β-actin. As shown in Figure 2A, ANOVA indicated a significant interaction [F(3, 22) = 35.659, P<.01] with significant effects for CSDS [F(1, 22)= 97.548, P<.01] and drug treatment [F(3, 22) = 25.155, P<.01], CSDS robustly reduced the mature BDNF expression in the hippocampus (n = 6, P<.01 vs control), and 14-day treatment of GM1 increased the mature BDNF protein level by 148.1±13.5% and 185.2±9.8% at a dosage of 15mg/kg and 30mg/kg (n = 6, P<.01 vs CSDS susceptible + vehicle), respectively. These results are similar to those of 20mg/kg fluoxetine. Similarly, as shown in Figure 2B, ANOVA revealed a significant interaction [F(3, 22) = 28.769, P<.01] with significant effects for CSDS [F(1, 22) = 92.445, P<.01] and drug treatment [F(3, 22) = 19.618, P<.01], CSDS also decreased the mature BDNF expression in the mPFC (n = 6, P<.01 vs control; Figure 2B), and chronic GM1 treatment enhanced the mature BDNF protein level by 152.7±10.9% and 200±14.6% at a dosage of 15mg/kg and 30mg/kg (n = 6, P<.01 vs CSDS susceptible + vehicle; Figure 2B), respectively. In addition, GM1 administration promoted the mature BDNF expression in the hippocampus [ANOVA: F(3, 20) = 29.569, P<.01] and mPFC [ANOVA: F(3, 20) = 34.206, P<.01] of naive mice (n = 6, P<.01 vs control) (supplemental Figure 2), consistent with previous findings (Pitto et al., 1998; Duchemin et al., 2002; Lim et al., 2011).
Figure 2.
Monosialotetrahexosylganglioside (GM1) treatment reverses the chronic social defeat stress (CSDS)-induced decrease of brain derived neurotrophic factor (BDNF) signaling pathway in the hippocampus and medial prefrontal cortex (mPFC). (A) Western blot results showed that CSDS-susceptible + GM1 mice displayed significantly higher BDNF, pTrkB, pERK1/2, pAKT, and pCREB expression in the hippocampus than CSDS-susceptible + vehicle mice (n=6). (B) Parallel to the hippocampus data, CSDS-susceptible + GM1 mice also had significantly more BDNF, pTrkB, pERK1/2, pAKT, and pCREB expression in the mPFC compared with CSDS-susceptible + vehicle mice (n = 6). Data are expressed as the mean ± SEM; **P<.01 vs control; ## P<.01 vs CSDS susceptible + vehicle. Comparison was made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Furthermore, the activated (and phosphorylated) forms of TrkB (pTrkB), ERK1/2 (pERK1/2), AKT (pAKT), and CREB (pCREB) that have been linked to the activation of BDNF signaling cascade were examined in the hippocampus and mPFC (Lim et al., 2008). As shown in Figure 2A, chronic GM1 treatment significantly reversed the CSDS-induced decrease in hippocampal pTrkB [ANOVA: CSDS, F(1, 22) = 103.187, P<.01; drug treatment, F(3, 22) = 26.348, P<.01; interaction, F(3, 22) = 35.971, P<.01], pERK1/2 [ANOVA: CSDS, F(1, 22) = 110.764, P<.01; drug treatment, F(3, 22) = 28.894, P<.01; interaction, F(3, 22) = 39.361, P<.01], pAKT [ANOVA: CSDS, F(1, 22) = 138.711, P<.01; drug treatment, F(3, 22) = 33.605, P<.01; interaction, F(3, 22) = 44.472, P<.01], and pCREB [ANOVA: CSDS, F(1, 22) = 99.759, P<.01; drug treatment, F(3, 22) = 23.373, P<.01; interaction, F(3, 22) = 32.841, P<.01] expression (n = 6, P<.01 vs CSDS susceptible + vehicle). Similarly, the decreased pTrkB [ANOVA: CSDS, F(1, 22) = 118.655, P<.01; drug treatment, F(3, 22) = 30.226, P<.01; interaction, F(3, 22) = 41.583, P<.01], pERK1/2 [ANOVA: CSDS, F(1, 22) = 143.422, P<.01; drug treatment, F(3, 22) = 34.597, P<.01; interaction, F(3, 22) = 46.472, P<.01], pAKT [ANOVA: CSDS, F(1, 22) = 104.433, P<.01; drug treatment, F(3, 22) = 26.166, P<.01; interaction, F(3, 22) = 35.659, P<.01], and pCREB [ANOVA: CSDS, F(1, 22) = 97.228, P<.01; drug treatment, F(3, 22) = 22.863, P<.01; interaction, F(3, 22) = 31.779, P<.01] levels in the mPFC of CSDS-susceptible mice were also restored by GM1 exposure (n = 6, P<.01 vs CSDS susceptible + vehicle) (Figure 2B). Moreover, GM1 administration also enhanced the pTrkB, pERK1/2, pAKT, and pCREB expression in the hippocampus [ANOVA: pTrkB, F(3, 20) = 39.344, P<.01; pERK1/2, F(3, 20) = 42.507, P<.01; pAKT, F(3, 20) = 26.488, P<.01; pCREB, F(3, 20) = 33.637, P<.01] and mPFC [ANOVA: pTrkB, F(3, 20) = 20.491, P<.01; pERK1/2, F(3, 20) = 16.524, P<.01; pAKT, F(3, 20) = 15.884, P<.01; pCREB, F(3, 20) = 19.775, P<.01] of naive mice (n=6, P<.01 vs control) (supplemental Figure 2). By contrast, the total ERK1/2, AKT, and CREB levels were unchanged among all experimental groups.
We also examined the BDNF system in NAc following CSDS and GM1 treatment. As shown in supplemental Figure 3, ANOVA indicated significant effects for CSDS [F(1, 30) = 80.223, P<.01] and GM1 [F(2, 30) = 19.856, P<.01] but with no interaction [F(2, 30) = 0.935, P=.0274]. While GM1 administration promoted the levels of BDNF and pCREB in the NAc of naive mice, consistent with Valdomero et al. (2015), GM1 had no effects on the enhanced expression of accumbal BDNF and pCREB in CSDS susceptible mice (n = 6). Collectively, the GM1-induced effects on the BDNF signaling cascade in hippocampus and mPFC may be involved in its antidepressant-like effects.
GM1 Produced Antidepressant-Like Effects through the BDNF Signaling Pathway
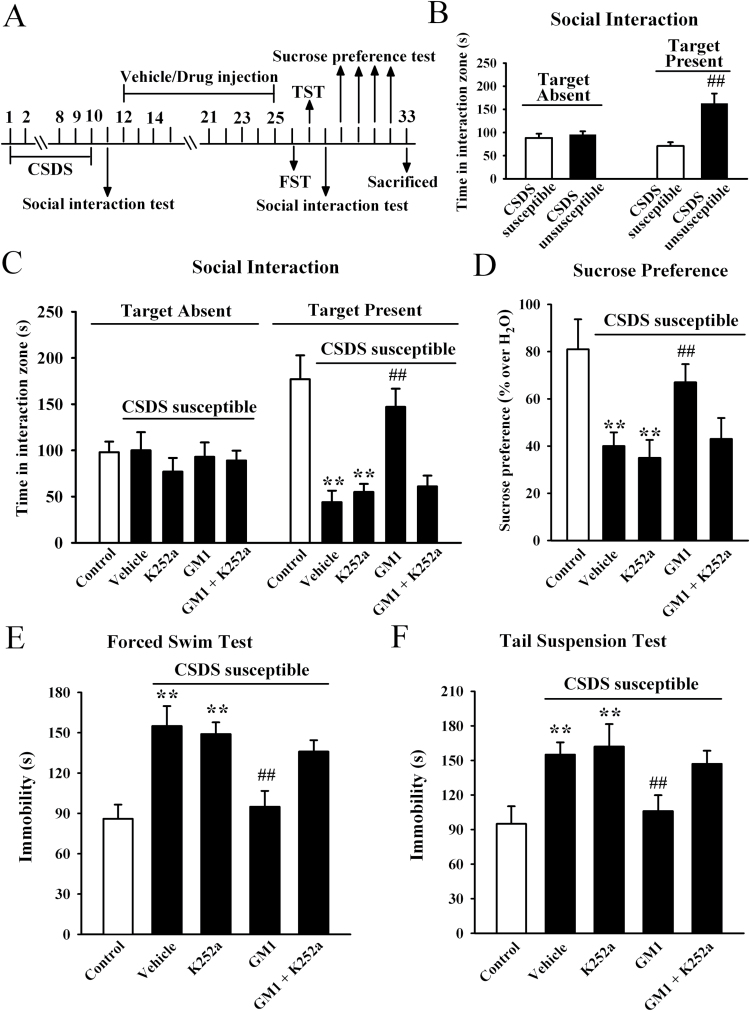
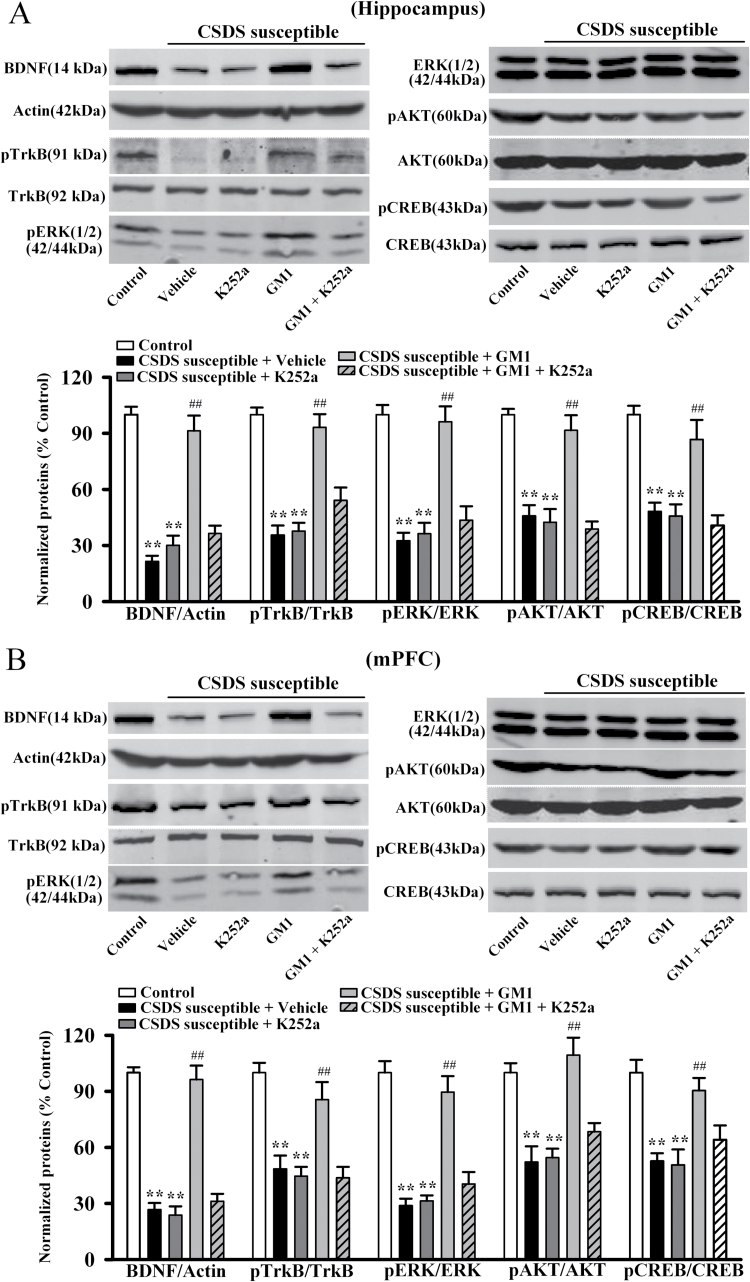
To further determine whether BDNF system is necessary for the effects of GM1, K252a was applied as a potent pharmacological inhibitor of the BDNF receptor TrkB (Tapley et al., 1992; Yan et al., 2010). CSDS-susceptible mice were co-treated with GM1 (30mg/kg) and K252a (25 μg/kg) for 14 days, and behavioral tests were then performed. Data are summarized in Figure 3. Co-treatment of K252a with GM1 significantly blocked the antidepressant-like effects of GM1 in the social interaction test [ANOVA: GM1, F(1, 40) = 55.421, P<.01; K252a, F(1, 40) = 81.879, P<.01; interaction, F(1, 40) = 47.438, P<.01; n = 11, Figure 3C], sucrose preference test [ANOVA: GM1, F(1, 40) = 46.406, P<.01; K252a, F(1, 40) = 70.182, P<.01; interaction, F(1, 40) = 44.636, P<.01; n = 11, Figure 3D], FST test [ANOVA: GM1, F(1, 40) = 48.707, P<.01; K252a, F(1, 40) = 71.588, P<.01; interaction, F(1, 40) = 42.273, P<.01; n=11, Figure 3E], and TST test [ANOVA: GM1, F(1, 40) = 59.476, P<.01; K252a, F(1, 40) = 86.101, P<.01; interaction, F(1, 40) = 52.772, P<.01; n = 11] (Figure 3F). Similarly, K252a injection prevented the effects of GM1 on BDNF signaling pathway in depressed mice, as the mature BDNF, pTrkB, pERK1/2, pAKT, and pCREB levels in the hippocampus and mPFC of CSDS-susceptible + GM1 + K252a mice were significantly lower than that of CSDS-susceptible + GM1 mice, respectively (Figure 4). Moreover, naive mice were also co-treated with GM1 (30mg/kg) and K252a (25 μg/kg) for 14 days, and behavioral tests showed that while K252a treatment produced no effects on the social interaction (n=11, supplemental Figure 4A) and sucrose preference (n=11, supplemental Figure 4B) of animals, it blocked the GM1-reduced immobility of naive mice in the FST [ANOVA: GM1, F(1, 40) = 37.346, P<.01; K252a, F(1, 40) = 72.851, P<.01; interaction, F(1, 40) = 32.906, P<.01; n = 11, Figure S4C] and TST [ANOVA: GM1, F(1, 39) = 47.362, P<.01; K252a, F(1, 39) = 84.723, P<.01; interaction, F(1, 39) = 42.477, P<.01; n = 10–11] (Figure supplemental 4D). Further western blotting results showed that K252a also prevented the effects of GM1 on BDNF signaling pathway in naive mice (n = 6) (supplemental Figure 5).
Figure 3.
Blockade of brain derived neurotrophic factor (BDNF) signaling cascade by K252a abolishes the antidepressant-like actions of monosialotetrahexosylganglioside (GM1). (A) Schematic timeline of the experimental procedure. Total 73 C57BL/6J mice were used in this experiment with 62 chronic social defeat stress (CSDS)-stressed mice and 11 nonstressed mice. CSDS-susceptible mice were co-injected with GM1 and K252a for 14 days, and behavioral tests were then performed. The vehicle refers to 0.1% DMSO in 0.9% saline. (B) The social interaction results for CSDS-susceptible mice (n = 44) and unsusceptible mice (n = 18) in this experiment. (C) Co-treatment GM1 with K252a blocked the antidepressant-like effects of GM1 in the social interaction test. CSDS-susceptible + GM1 + K252a mice displayed significantly lower social interaction than CSDS-susceptible + GM1 mice (n = 11). (D) CSDS susceptible + GM1 + K252a mice displayed significantly lower sucrose preference than CSDS susceptible + GM1 mice (n=11). (E) CSDS susceptible+GM1+K252a mice displayed significantly higher immobility time than CSDS susceptible+GM1 mice in the forced swim test (FST) (n = 11). (F) CSDS-susceptible+GM1+K252a mice also displayed significantly higher immobility time than CSDS-susceptible + GM1 mice in the tail suspension test (TST) (n = 11). Data are expressed as the mean ± SEM; ** P<.01 vs control; ## P<.01 vs CSDS-susceptible/CSDS-susceptible + vehicle. Comparison was made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Figure 4.
K252a treatment antagonizes the effects of monosialotetrahexosylganglioside (GM1) on brain derived neurotrophic factor (BDNF) signaling cascade in the chronic social defeat stress (CSDS) model. (A) Western blot data revealed that CSDS-susceptible + GM1 + K252a mice displayed significantly lower BDNF, pTrkB, pERK1/2, pAKT, and pCREB expression in the hippocampus than CSDS-susceptible + GM1 mice (n = 6). (B) Similarly, western blot data showed that CSDS-susceptible + GM1 + K252a mice also had significantly lower BDNF, pTrkB, pERK1/2, pAKT, and pCREB expression in the medial prefrontal cortex (mPFC) than CSDS susceptible + GM1 mice (n = 6). Data are expressed as the mean ± SEM; ** P<.01 vs control; ## P<.01 vs CSDS-susceptible + vehicle. Comparison was made by 2-way ANOVA followed by posthoc Bonferroni’s test.
In a parallel series, we used an anti-BDNF antibody to specifically block the BDNF system, as utilized previously (Jiang et al., 2015a; Jiang et al., 2015b). CSDS-susceptible mice were co-treated with GM1 (30mg/kg) and anti-BDNF antibody (20 μg/mL) for 14 days, and behavioral tests were then performed. Data are summarized in Figure 5. Anti-BDNF infusion fully abolished the antidepressant-like effects of GM1 in the social interaction test [ANOVA: GM1, F(1, 38) = 79.366, P<.01; Anti-BDNF, F(1, 38) = 97.451, P<.01; interaction, F(1, 38) = 70.115, P<.01; n = 10–11, Figure 5C], sucrose preference test [ANOVA: GM1, F(1, 38) = 60.268, P<.01; Anti-BDNF, F(1, 38) =81.603, P<.01; interaction, F(1, 38) = 56.121, P<.01; n = 10–11, Figure 5D], FST test [ANOVA: GM1, F(1, 38) = 60.448, P<.01; Anti-BDNF, F(1, 38) = 78.927, P<.01; interaction, F(1, 38) = 54.879, P<.01; n = 10–11, Figure 5E] and TST test [ANOVA: GM1, F(1, 38) = 64.629, P<.01; Anti-BDNF, F(1, 38) = 85.025, P<.01; interaction, F(1, 38) = 58.864, P<.01; n = 10–11] (Figure 5F). Furthermore, parallel to the behavioral data, anti-BDNF infusion also blocked the GM1-induced effects on BDNF signaling pathway. We found that the mature BDNF, pTrkB, pERK1/2, pAKT, and pCREB levels in both the hippocampus and mPFC regions of CSDS-susceptible + GM1 + anti-BDNF mice were significantly lower than that of CSDS-susceptible + GM1 mice, respectively (n=5) (Figure 6). Thus, these results indicate that the antidepressant-like effects of GM1 require BDNF system.
Figure 5.
Blockade of brain derived neurotrophic factor (BDNF) signaling cascade by anti-BDNF infusion abolishes the antidepressant-like effects of monosialotetrahexosylganglioside (GM1). (A) Schematic timeline of the experimental procedure. Total 103 C57BL/6J mice were used in this experiment with 92 chronic social defeat stress (CSDS)-stressed mice and 11 nonstressed mice. CSDS-susceptible mice were co-treated with GM1 and anti-BDNF antibody for 14 days, with behavioral tests then performed. The vehicle refers to 0.9% saline. (B) The social interaction results for CSDS-susceptible mice (n = 63) and unsusceptible mice (n = 29) in this experiment. (C) Co-treatment with GM1 and anti-BDNF blocked the antidepressant-like effects of GM1 in the social interaction test. CSDS-susceptible +GM1+anti-BDNF mice displayed significantly lower social interaction than CSDS-susceptible+GM1 mice (n=10–11). (D) SDS-susceptible+GM1+anti-BDNF mice displayed significantly lower sucrose preference than CSDS-susceptible+GM1 mice (n=10–11). (E) CSDS-susceptible + GM1+anti-BDNF mice displayed significantly higher immobility time than CSDS-susceptible + GM1 mice in the forced swim test (FST) (n = 10–11). (F) CSDS-susceptible + GM1 + anti-BDNF mice also displayed significantly higher immobility time than CSDS susceptible + GM1 mice in the tail suspension test (TST) (n = 10–11). Results are expressed as the mean ± SEM; ** P<.01 vs control; ## P<.01 vs CSDS-susceptible/CSDS-susceptible + vehicle. Comparison was made by 2-way ANOVA followed by posthoc Bonferroni’s test
Figure 6.
Anti-brain derived neurotrophic factor (BDNF) infusion blocks the effects of monosialotetrahexosylganglioside (GM1) on BDNF signaling pathway in the chronic social defeat stress (CSDS) model. (A) Western blot results showed that CSDS-susceptible + GM1 + anti-BDNF mice displayed significantly less BDNF, pTrkB, pERK1/2, pAKT, and pCREB expression in the hippocampus than CSDS-susceptible + GM1 mice (n = 5). (B) As in the hippocampus, western blot results indicated that the GM1-induced promotion of BDNF, pTrkB, pERK1/2, pAKT, and pCREB levels in the medial prefrontal cortex (mPFC) was also blocked by anti-BDNF antibody (n = 5). Data are expressed as the mean ± SEM; ** P<.01 vs control; ## P<.01 vs CSDS-susceptible + vehicle. Comparison was made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Serotonin Depletion Does Not Alter the Antidepressant-Like Effects of GM1
Selective serotonin reuptake inhibitors are the most widely used antidepressants; therefore we wanted to test whether the GM1-induced antidepressant-like effects depend on serotonin system. The tryptophan hydroxylase inhibitor PCPA was used to deplete serotonin (Heurteaux et al., 2006; Coryell et al., 2009). CSDS-susceptible mice were co-treated with GM1 (30mg/kg) and PCPA (300mg/kg) for 14 days, and behavioral tests were then performed. Data are summarized in supplemental Figure 6. It was found that PCPA co-treatment did not block the antidepressant-like effects of GM1 in the social interaction test [ANOVA: GM1, F(1, 39) = 67.213, P<.01; PCPA, F(1, 39) = 0.956, P=.0257; n=10–11, supplemental Figure 6C], sucrose preference test [ANOVA: GM1, F(1, 39) = 49.677, P<.01; PCPA, F(1, 39) = 1.348, P=.141; n=10–11, supplemental Figure 6D], FST test [ANOVA: GM1, F(1, 39) = 53.014, P<.01; PCPA, F(1, 39) = 1.088, P=.179; n = 10–11, supplemental Figure 6E] and TST test [ANOVA: GM1, F(1, 38) = 64.296, P<.01; PCPA, F(1, 38) = 0.951, P=.261; n=10–11, supplemental Figure 6F], while PCPA co-treatment significantly prevented the effects of fluoxetine.
Discussion
The major findings of this study are as follows. First, GM1 produces significant antidepressant-like effects in the CSDS model of depression, confirmed by different behavioral tests, including the social interaction, sucrose preference, FST, and TST tests. Second, the antidepressant-like effects of GM1 require BDNF signaling pathway, since these effects were blocked by selective inhibition of BDNF system in the brain. Together, these data indicate that GM1 could be developed as a novel antidepressant.
GM1 has several important physiological properties such as impacting neuronal plasticity, releasing neurotrophins, and repairing damaged neurons in the brain (Mocchetti, 2005; Lim et al., 2011; Valdomero et al., 2015; Zhang et al., 2015). In this study, the most important reason for assuming that GM1 may possess antidepressant-like effects came from several reports demonstrating that GM1 could affect the function of BDNF signaling pathway in the brain, since BDNF is critical for the pathophysiology of depression. For example, Valdomero et al. (2015) reported that GM1 administration induced a significant increase in BDNF protein levels in the NAc. Similarly, Lim et al. (2011) found that exogenous GM1 could evoke the release of mature BDNF from hippocampal neurons. Furthermore, Duchemin et al. (2002) reported that GM1 can induce the phosphorylation and activation of tyrosine kinase receptors for neurotrophins, TrkA, TrkB, and TrkC in the striatum, hippocampus, and frontal cortex. Zakharova et al. (2014) also reported that GM1 has protective effects on PC12 cells exposed to hydrogen peroxide depending on the activation of Trk receptor tyrosine kinase and downstream activation of AKT and ERK1/2 signaling. Here, we found that GM1 indeed produced significant antidepressant-like activities in the CSDS model of depression, and more importantly, GM1 blocked the CSDS-induced changes on BDNF signaling pathway in the hippocampus and mPFC, in accordance with these previous reports.
GM1 has multiple pharmacological effects on the central nervous system. Previous reports have shown that GM1 produced improvements in several neurological disorders, including Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and cerebral ischemia-reperfusion (Oikawa et al., 2009; Maglione et al., 2010; Schneider et al., 2010, 2013; Di Pardo et al., 2012; Yang et al., 2013; Zhang et al., 2015). To our knowledge, our study is the first instance of experimental evidence showing that GM1 produces beneficial effects against depression, a most burdensome neuropsychiatric disease worldwide. This finding is very interesting and exciting in providing a new potential antidepressant. In support of this result, there is other evidence implying the antidepressant-like effects of GM1, such as: (1) gangliosides enhance the antidepressant-like and neurochemical effects induced by chronic desipramine treatment (Molina et al., 1989); (2) ganglioside pretreatment enhanced the antiimmobility effect induced in the FST after a chronic treatment with mianserin, clomipramine, nialamide, or repeated electroconvulsive shock in mice (Cordoba et al., 1990); and (3) gangliosides attenuate the stress-induced changes in body weight and motor activity, and attenuate the behavioral response to 5-methoxy-N,N-dimethyltryptamine (Cancela et al., 1996).
Since GM1 produces antidepressant-like effects similar to those of fluoxetine, we must consider the possibility that these effects may be mediated through monoaminergic systems, particularly the serotonergic system. However, we found that depleting serotonin by PCPA did nothing to lessen the antidepressant-like action of GM1, indicating that the molecular mechanisms of GM1 are distinct from the conventional antidepressants.
In summary, GM1 has wide-ranging pharmacologic effects and many reveal positive therapeutic indexes. Our results show that GM1 possesses antidepressant-like activities through the promotion of BDNF signaling pathway proteins, providing a new insight in understanding the pharmacological effects of GM1 and shedding light on the development of new antidepressants with higher efficacy and fewer side effects.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China for Dr. Bo Jiang (no. 81401116) and by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Blendy JA. (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59:1144–1150. [DOI] [PubMed] [Google Scholar]
- Bourin M, Fiocco AJ, Clenet F. (2001) How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16:9–21. [DOI] [PubMed] [Google Scholar]
- Cancela LM, Volosin M, Molina VA. (1996) Gangliosides attenuate stress-induced changes on body weight, motor activity and on the behavioral response to 5-methoxy-N,N-dimethyltryptamine. Brain Res Bull 40:105–110. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70:289–297. [DOI] [PubMed] [Google Scholar]
- Chopra K, Kumar B, Kuhad A. (2011) Pathobiological targets of depression. Expert Opin Ther Targets 15:379–400. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17:2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba NE, Basso AM, Molina VA, Orsingher OA. (1990) Gangliosides enhance the anti-immobility response elicited by several antidepressant treatments in mice. Psychopharmacology (Berl) 100:555–557. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. (2009) Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci 29:5381–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. (2007) Animal models of mood disorders: recent developments. Curr Opin Psychiatry 20:1–7. [DOI] [PubMed] [Google Scholar]
- Derry DM, Wolfe LS. (1967) Gangliosides in isolated neurons and glial cells. Science 158:1450–1452. [DOI] [PubMed] [Google Scholar]
- Di Pardo A, Maglione V, Alpaugh M, Horkey M, Atwal RS, Sassone J, Ciammola A, Steffan JS, Fouad K, Truant R, Sipione S. (2012) Ganglioside GM1 induces phosphorylation of mutant huntingtin and restores normal motor behavior in Huntington disease mice. Proc Natl Acad Sci U S A 109:3528–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr (2014) Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchemin AM, Ren Q, Mo L, Neff NH, Hadjiconstantinou M. (2002) GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J Neurochem 81:696–707. [DOI] [PubMed] [Google Scholar]
- Duchemin AM, Ren Q, Neff NH, Hadjiconstantinou M. (2008) GM1-induced activation of phosphatidylinositol 3-kinase: involvement of Trk receptors. J Neurochem 104:1466–1477. [DOI] [PubMed] [Google Scholar]
- Favaron M, Manev H, Alho H, Bertolino M, Ferret B, Guidotti A, Costa E. (1988) Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc Natl Acad Sci U S A 85:7351–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Greene LA. (1996) Prevention of neuronal apoptotic death by neurotrophic agents and ganglioside GM1: insights and speculations regarding a common mechanism. Perspect Dev Neurobiol 3:93–100. [PubMed] [Google Scholar]
- Ferrari G, Greene LA. (1998) Promotion of neuronal survival by GM1 ganglioside. Phenomenology and mechanism of action. Ann N Y Acad Sci 845:263–273. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. (2008) Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 63:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. (2006) Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav 85:842–849. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Neff NH. (1988) Treatment with GM1 ganglioside restores striatal dopamine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mouse. J Neurochem 51:1190–1196. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Neff NH. (1998) GM1 ganglioside: in vivo and in vitro trophic actions on central neurotransmitter systems. J Neurochem 70:1335–1345. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. (2006) Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci 9:1134–1141. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. (1990) Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J 9:2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. (2005) Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res 1037:204–208. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang C, Chen XF, Tong LJ, Zhang W. (2015. a) Tetramethylpyrazine produces antidepressant-like effects in mice through promotion of BDNF signaling pathway. Int J Neuropsychopharmacol 18 In press. pii:pyv010.doi:10.1093/ijnp/pyv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang C, Zhu Q, Tong LJ, Zhang W. (2015. b) WY14643 produces anti-depressant-like effects in mice via the BDNF signaling pathway. Psychopharmacology (Berl) 232:1629–1642. [DOI] [PubMed] [Google Scholar]
- Lee E, Son H. (2009) Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 42:239–244. [DOI] [PubMed] [Google Scholar]
- Lim JY, Park SI, Oh JH, Kim SM, Jeong CH, Jun JA, Lee KS, Oh W, Lee JK, Jeun SS. (2008) Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res 86:2168–2178. [DOI] [PubMed] [Google Scholar]
- Lim ST, Esfahani K, Avdoshina V, Mocchetti I. (2011) Exogenous gangliosides increase the release of brain-derived neurotrophic factor. Neuropharmacology 60:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione V, Marchi P, Di Pardo A, Lingrell S, Horkey M, Tidmarsh E, Sipione S. (2010) Impaired ganglioside metabolism in Huntington’s disease and neuroprotective role of GM1. J Neurosci 30:4072–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, Thase ME, Davis L, Biggs MM, Shores-Wilson K, Luther JF, Niederehe G, Warden D, Rush AJ. (2006) Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 163:1531–1541; quiz 1666. [DOI] [PubMed] [Google Scholar]
- Mocchetti I. (2005) Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol Life Sci 62:2283–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina VA, Keller EA, Orsingher OA. (1989) Gangliosides enhance behavioral and neurochemical effects induced by chronic desipramine (DMI) treatment. Eur J Pharmacol 160:247–252. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. (1995) Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci U S A 92:5087–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. (1996) Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16:2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa N, Yamaguchi H, Ogino K, Taki T, Yuyama K, Yamamoto N, Shin RW, Furukawa K, Yanagisawa K. (2009) Gangliosides determine the amyloid pathology of Alzheimer’s disease. Neuroreport 20:1043–1046. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B, CDBE2010 study group, European Brain Council (2012) The economic cost of brain disorders in Europe. Eur J Neurol 19:155–162. [DOI] [PubMed] [Google Scholar]
- Pitto M, Mutoh T, Kuriyama M, Ferraretto A, Palestini P, Masserini M. (1998) Influence of endogenous GM1 ganglioside on TrkB activity, in cultured neurons. FEBS letters 439:93–96. [DOI] [PubMed] [Google Scholar]
- Prins J, Olivier B, Korte SM. (2011) Triple reuptake inhibitors for treating subtypes of major depressive disorder: the monoamine hypothesis revisited. Expert Opin Investig Drugs 20:1107–1130. [DOI] [PubMed] [Google Scholar]
- Purpura DP, Suzuki K. (1976) Distortion of neuronal geometry and formation of aberrant synapses in neuronal storage disease. Brain Res 116:1–21. [DOI] [PubMed] [Google Scholar]
- Rabin SJ, Bachis A, Mocchetti I. (2002) Gangliosides activate Trk receptors by inducing the release of neurotrophins. J Biol Chem 277:49466–49472. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Domenici E, Carboni L, Rantamaki T, Lindholm J, Castren E, Arban R. (2011) A role for BDNF/TrkB signaling in behavioral and physiological consequences of social defeat stress. Genes Brain Behav 10:424–433. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Pope A, Simpson K, Taggart J, Smith MG, DiStefano L. (1992) Recovery from experimental parkinsonism in primates with GM1 ganglioside treatment. Science 256:843–846. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Sendek S, Daskalakis C, Cambi F. (2010) GM1 ganglioside in Parkinson’s disease: results of a five year open study. J Neurol Sci 292:45–51. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Gollomp SM, Sendek S, Colcher A, Cambi F, Du W. (2013) A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J Neurol Sci 324:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S. (2006) Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Pearson RC, Cuello AC, Tagari PC, Stephens PH. (1986) Parenterally administered GM1 ganglioside prevents retrograde degeneration of cholinergic cells of the rat basal forebrain. Brain Res 398:393–396. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, McGuinness C, Schwartz M, Grafstein B. (1984) Antibodies to gangliosides inhibit goldfish optic nerve regeneration in vivo. J Neurosci Res 12:233–243. [DOI] [PubMed] [Google Scholar]
- Suzuki K. (1965) The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem 12:629–638. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. (1956) Composition of gangliosides from human brain. Nature 177:524–525. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. (1992) K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 7:371–381. [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. (2000) cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci 20:4030–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525. [DOI] [PubMed] [Google Scholar]
- Valdomero A, Perondi MC, Orsingher OA, Cuadra GR. (2015) Exogenous GM1 ganglioside increases accumbal BDNF levels in rats. Behav Brain Res 278:303–306. [DOI] [PubMed] [Google Scholar]
- Vicente B, Kohn R, Rioseco P, Saldivia S, Levav I, Torres S. (2006) Lifetime and 12-month prevalence of DSM-III-R disorders in the Chile psychiatric prevalence study. Am J Psychiatry 163:1362–1370. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Wu YP, Sandhoff R, Werth N, Mizukami H, Ellis JM, Dupree JL, Geyer R, Sandhoff K, Proia RL. (2005) Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci U S A 102:2725–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Qu HD, Sun LR, Li SJ, Cao X, Fang YY, Jie W, Bean JC, Wu WK, Zhu XH, Gao TM. (2010) Fuzi polysaccharide-1 produces antidepressant-like effects in mice. Int J Neuropsychopharmacol 13:623–633. [DOI] [PubMed] [Google Scholar]
- Yang R, Wang Q, Min L, Sui R, Li J, Liu X. (2013) Monosialoanglioside improves memory deficits and relieves oxidative stress in the hippocampus of rat model of Alzheimer’s disease. Neurol Sci 34:1447–1451. [DOI] [PubMed] [Google Scholar]
- Zakharova IO, Sokolova TV, Vlasova YA, Furaev VV, Rychkova MP, Avrova NF. (2014) GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cells against hydrogen peroxide toxicity. Neurochem Res 39;2262–2275. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fang X, Zhou Y, Deng X, Lu Y, Li J, Li S, Wang B, Xu R. (2015) The possible damaged mechanism and the preventive effect of monosialotetrahexosylganglioside in a rat model of cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc Dis 24:1471–1478. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–423. [DOI] [PubMed] [Google Scholar]
- Zonana J, Gorman JM. (2005) The neurobiology of postpartum depression. CNS Spectr 10:792–799, 805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.