Abstract
Background:
Our previous study demonstrated that metabolic inflammation exacerbates dopaminergic neuronal degeneration in type 2 diabetes mice. Metformin, a typical oral hypoglycemic agent for diabetes, has been regarded as an activator of AMP-activated protein kinase and a regulator of systemic energy metabolism. Although metformin plays potential protective effects in many disorders, it is unclear whether metformin has a therapeutic role in dopaminergic neuron degeneration in Parkinson’s disease.
Methods:
In the present study, a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine plus probenecid-induced mouse model of Parkinson’s disease was established to explore the neuroprotective effect of metformin on dopaminergic neurons in substania nigra compacta. We next cultured SH-SY5Y cells to investigate the mechanisms for the neuroprotective effect of metformin.
Results:
We showed that treatment with metformin (5mg/mL in drinking water) for 5 weeks significantly ameliorated the degeneration of substania nigra compacta dopaminergic neurons, increased striatal dopaminergic levels, and improved motor impairment induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine plus probenecid. We further found that metformin inhibited microglia overactivation-induced neuroinflammation in substania nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine plus probenecid Parkinson’s disease mice, which might contribute to the protective effect of metformin on neurodegeneration. Furthermore, metformin (2mM) activated AMP-activated protein kinase in SH-SY5Y cells, in turn inducing microtubule-associated protein 1 light chain 3-II-mediated autophagy and eliminating mitochondrial reactive oxygen species. Consequently, metformin alleviated MPP+-induced cytotoxicity and attenuated neuronal apoptosis.
Conclusions:
Our findings demonstrate that metformin may be a pluripotent and promising drug for dopaminergic neuron degeneration, which will give us insight into the potential of metformin in terms of opening up novel therapeutic avenues for Parkinson’s disease.
Keywords: AMPK, autophagy, metformin, mitochondrial ROS, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases characterized the progressive loss of dopaminergic (DA) neurons in the substantia nigra compacta (SNc) and aggregation of Lewy bodies in neurons (Savitt et al., 2006). Although aging, oxidative damage, and neuroinflammation have been recognized to play crucial roles in the pathogenesis of PD, the precise etiology remains obscure (van der Brug et al., 2015; Volta et al., 2015). Emerging evidence suggests PD is a systemic metabolic disease, and metabolic abnormality correlates with functional alternations in PD (Lu and Hu, 2012; Poewe and Djamshidian-Tehrani, 2015). Notably, our previous study demonstrated that metabolic inflammation exacerbates DA neuronal degeneration in response to acute 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) challenge in type 2 diabetes mice (Wang et al., 2014). Furthermore, neurodegeneration in a rat model of PD is exacerbated by feeding a high-fat diet, which results in insulin resistance and oxidative stress in both peripheral organ and striatum (Morris et al., 2010; Khang et al., 2015). It was also found that inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, increase in both the brain and peripheral system in PD patients and animal models (Cartier et al., 2005; Chao et al., 2014). These results indicate that metabolic abnormality and systemic inflammation exert important roles in the pathogenesis of PD.
Energy metabolism disturbance and mitochondrial dysfunction, which cause ATP deletion, have been implicated in the pathogenesis of PD (Cho et al., 2010). As a serine-threonine heterotrimeric kinase, AMP-activated protein kinase (AMPK) acts as a sensor of cellular energy status and has been recognized to play a central role in modulating intracellular metabolic cascade signals (Woods et al., 2005; Hardie, 2008). AMPK is switched on by an increase in the AMP/ATP ratio, which leads to the phosphorylation of AMPK at Thr 172 by AMPK kinases (Woods et al., 2005). AMPK activation regulates many pathways, generally causing conservation and generation of ATP (Hardie, 2007). AMPK plays a key role in cell survival during metabolic stress by maintaining metabolic homeostasis. Recent studies indicate that AMPK plays a critical role in regulating autophagy in neurons. AMPK is upstream of mechanistic target of rapamycin complex 1 (MTORC1), and the activation of AMPK inhibits MTORC1 activity through the phosphorylation of the MTORC1-associated proteins RPTOR and tuberous sclerosis 2, thereby activating autophagy (Egan et al., 2011). Recent reports further showed that AMPK activation attenuated amyloid β-induced cytotoxicity in a model of Alzheimer’s disease (AD) and prevented cell death via ceramide accumulation in astrocytes (Kickstein et al., 2010). Furthermore, it has been reported that AMPK activation inhibited rotenone-induced apoptosis in SH-SY5Y cell, a typical cell line of DA neuron, indicating that AMPK signaling functions as a potential target for PD therapy (Choi et al., 2010; Wu et al., 2011).
Metformin, a biguanide family member commonly used in treatment for type 2 diabetes, appears to increase liver and peripheral tissue sensitivity to insulin as well as reduce hepatic glucose production (Hardie, 2008; Rojas and Gomes, 2013). Currently, metformin has been regarded as a widely accepted AMPK activator, which can accelerate AMPK phosphorylation at Thr172 site and induce macroautophagy and mitophagy (Woods et al., 2005; Hardie, 2008). Therefore, metformin may be used for the therapy of multiple disorders, such as diabetes, cardiovascular diseases, and cancers (Del Barco et al., 2011; Panicker et al., 2012; Jara and Lopez-Munoz, 2015). Notably, several studies demonstrate that metformin is beneficial for AD and Huntington’s disease (Ma et al., 2007; Li et al., 2012). Recently, Patil et al. (2014) reported the neuroprotective effect of metformin in PD model. They found that metformin treatment significantly attenuated DA neuronal loss and improved the antioxidant activity,, but did not explain the potential mechanism and drug target for the neuroprotective effect of metformin. In the present study, we prepared a MPTP plus probenecid (MPTP/p) mouse model of PD to explore the therapeutic effect of metformin on DA neuronal degeneration and to clarify the exact mechanism in SH-SY5Y cells treated with MPP+. Our study demonstrates that metformin protects DA neurons in SNc of PD mice via enhancement of AMPK-mediated autophagy and mitochondrial ROS clearance, suggesting that metformin may be a pluripotent and promising drug for PD therapy.
Materials and Methods
The study protocol was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Animals and Treatment
Ten-week-old male C57BL/6 mice were randomly divided into saline-treated group, MPTP/p-treated group, MPTP/p+metformin (MET)-treated group, and MET alone treated group. Mice received 20mg/kg MPTP and 250mg/kg probenecid, which blocks the rapid clearance of the MPTP neurotoxin, every 3.5 days for 5 weeks.
Mice received metformin (Pacific Ocean, Tianjin, China) 5mg/mL in drinking water starting on the third day of experiment. Water bottles were replaced with refresh metformin solution or water every day.
Rotarod Test
This test is used to evaluate mouse forelimb and hindlimb motor balance and coordination. The equipment was performed according to a previous publication (Chung et al., 2010). On the 37th day, mice were placed in a separate compartment on the rod and tested at 20rpm. The latency to fall (time on rod) was recorded.
High Performance Liquid Chromatography Analysis
Dissected striatal tissues were homogenized with 0.1M HClO4 and 0.1mM EDTA buffer and centrifuged at 20000rpm for 25 minutes. The supernatant was injected into autosampler at 4°C (UltiMate 3000, ESA) and eluted through a C18 column (2.2 μm, 120 Å, 2.1×100mm, DIONEX) with catecholamine analysis mobile phase and was detected by ESA Coulochem III electrochemical detector. The mobile phase consisted 90mM NaH2PO4, 50mM citrate, 1.7mM 1-octanesulfonic acid, 50 μM EDTA, and 10% acetonitrile.
Western Blotting
The method was described in a previous publication (Hu et al., 2010). Different primary antibodies (rabit anti-pAMPK/AMPK [1:1000; Cell Signaling], rabbit anti- microtubule-associated protein 1 light chain 3 (LC3) [1:500; SAB], rabbit anti-p62 [1:500; SAB], mouse anti-α-synuclein [1:1000; Millipore], β-actin [1:1000, Boster], and H3 [1:800, Millipore]) and the secondary antibodies (1:800, Boster) were used in the present study.
Immunohistochemistry
The immunostaining method was described in a previous publication (Hu et al., 2010). For cell quantification in in vivo studies, the numbers of TH-, α-synuclein-, and IBA-1-immunoreactive cells in the SNc of the midbrain were assessed using the optical fractionator (Stereo Investigator 7, MBF Bioscience, Williston, VT). Briefly, the regions of SNc in the midbrain sections were outlined at low magnification (40×). For TH+, IBA-1+, and α-synuclein+ cells, the counting frame size was 50 μm × 50 μm and the sampling grid size was 100 μm × 100 μm. All stereological analyses were performed under the 200× magnification of an Olympus BX52 microscope (Olympus America Inc., Melville, NY). Within 1 counting frame, positive cells counted must show both immunostaining in the cell body and blue staining in the nuclei, and the nuclei does not touch or cross the red avoidance lines of the counting frame. The sampling scheme was designed to have a coefficient of error <10% in order to get reliable results. The total numbers of immunoreactive cells in the entire extent of SNc were counted from 4 mouse brains per group. Each brain contained 12 serial sections at 3 intervals. The stereologer was blinded to the person analyzing the histology and treatment groups for each experiment.
Quantitative Real-Time PCR (qRT-PCR)
The method for qRT-PCR of TNF-α, IL-6, TGF-β, IL-4, and IL-10 was performed according to a previous publication (Sun et al., 2011). Briefly, total RNA was extracted from midbrain tissues using TriPure reagent (Roche Diagnostics Ltd., Indianapolis, IN) followed by treatment with RNase-free DNaseI (Invitrogen Life Technologies). Total RNA (2 µg) of each sample was reverse-transcribed into cDNA and amplified using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara, TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s directions. RT-PCR was performed with Fast Start Universal SYBR Green Master (Rox) (Roche Diagnostics). Reactions were amplified and analyzed by mean of an ABI 7300 Real Time PCR System (Applied Biosystems Japan, Co. Ltd.). PCR primers were as follows: GAPDH was used as housekeeping gene (forward 5’-TGGTGCCAAAAGGGTCATCTCC-3’ and reverse 5’-GCCAGCCCCAGCATCAAAGGTG-3’), TNF-α (forward 5’-CATCTTCTCAAAATTCGAGTGACAA-3’ and reverse 5’-TGGGAG TAGACAAGGTACAACCC-3’), IL-6 (forward 5’-ATCCAGTTGCCTTC TTGGGACTGA-3’ and reverse 5’-TAAGCCTCCGACTTGTGAAG TGGT-3’), TGF-β (forward 5’-ACCGCAACAACGCCATCTAT-3’ and reverse 5’-GTAACGCCAGGAATTGTTGC-3’), IL-4 (forward 5’-TC GGCATTTTGAACGAGGTC-3’ and reverse 5’-GAAAAGCCCGAAAG AGTCTC-3’), and IL-10 (forward 5’-GGACTTTAAGGGTTACTTGG GTTGCC-3’and reverse 5’-CATTTTGATCATCATGTATGCTTCT-3’).
Cell Culture and Treatments
SH-SY5Y cells were routinely grown in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivation fetal bovine serum and cultured at 37°C under humidified 5% CO2 atmosphere. To activate AMPK, SHSY5Y cells were preincubated with 2mM MET for 1 hour and 200 μM MPP+ (Sigma) was added for 48 hours. To block AMPK or autophagy, SHSY5Y cells were preincubated with 10 μM Compound C (CC) (Sigma) for 30 minutes or with 5mM 3MA (Sigma) for 3 hours before MET addition.
Assay of MTT Conversion
SH-SY5Y Cell viability was evaluated with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) conversion method in 96-well plates. After treatment, the media was replaced with Dulbecco’s modified Eagle’s medium to which MTT reagent was added. Then the plates were placed at 37°c and 5% CO2 for 3 hours. At the end of the experiment, cells were lysed in 200 μL dimethyl sulfoxide for 10 minutes. Absorbance at the wavelength of 490nm was read on a multimode reader (Vario Skan Flash, 3001, Thermo Scientific). The obtained values were presented as folds of the controls.
Assay of Lactate Dehydrogenase (LDH) Release
After incubation with MPP+ for 48 hours, LDH release was measured in the culture medium with an LDH diagnostic kit (Jiancheng Bioengineering) in accordance with the manufacturer’s instructions. LDH activity was calculated by data measured from absorbance at 490nm. The obtained values were presented as folds of the controls.
Flow Cytometric Analyses
This method was performed according to a previous publication (Zhou et al., 2011). Mitotracker green (Beyotime, C1048) and Mitotracker deep red (Invitrogen, M22426) were used in the present study. Fluorescence levels were detected by flow cytometer (Guava Easycyte 8, Millipore).
Detection of Intracellular ROS
The cell-permeant 2’,7’-dichlorodihydrofluorescein diacetate was used to detect intracellular generation of ROS by modification (Li et al., 2010). After drugs treatment, SH-SY5Y cells were incubated with the fluorescent probe 2’,7’-dichlorodihydrofluorescein diacetate (25 μM) for 30 minutes. After washing 3 times with cold phosphate buffered saline, the intensity of fluorescence was determined by a multimode reader (Vario Skan Flash, 3001, Thermo Scientific) under an emission wavelength at 530nm and excitation wavelength at 485nm. The obtained values were presented as folds of the controls.
Statistical Analysis
Data are presented as the mean±SEM. The significance of the difference with different treatments was determined by 1-way or 2-way ANOVA, followed by Tukey’s posthoc test. Differences were considered significant at P<.05.
Results
Metformin Improves Motor Impairment and Increases Dopamine Level in the Striatum of MPTP/ p PD Mice
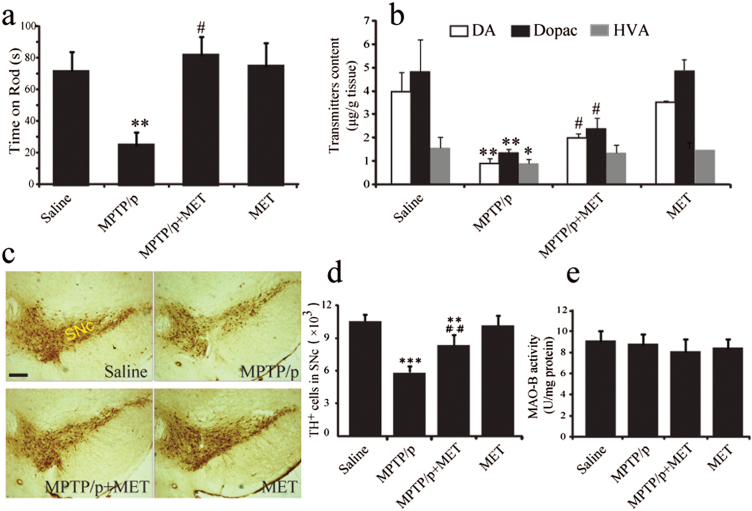
To explore the neuroprotective effects of metformin in PD, we determined whether metformin improved MPTP-induced motor deficits by testing performance on a rotarod apparatus at first. Mice were evaluated 7 days after the last MPTP injection by measuring time on the rod. MPTP/p injections decreased the latency to falling to 24.7±8.0 minutes, a 65.4% decrease compared with those in the saline treatment group (Figure 1a). Metformin administration recovered MPTP-induced motor impairment, which also increased sustained rotarod time to 81.7±11.4 minutes (2-way ANOVA, metformin: F1,28=5.760, P=.023; MPTP: F1,28=8.839, P=.006; interaction: F1,28=6.894, P=.014).
Figure 1.
Metformin improved motor behavior and increased catecholamine levels in 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) mice. Time on rod was measured by rotarod test (a). Dopamine, dihydroxyphenylacetic acid, and homovanillic acid were analyzed by High Performance Liquid Chromatography (b). Data are presented as the mean ± SEM, n=8 for each group. Metformin prevented dopaminergic (DA) cells degeneration induced by MPTP plus probenecid (MPTP/p) in the midbrain. Immunohistochemical staining of TH-positive neurons in substania nigra compacta (SNc) of mice with ×40 magnifications (c). Stereological counts of Tyrosine hydroxylase-positive cells in mouse SNc (d). Data are presented as the mean±SEM, n=4 for each group. Scale bar, 200 μm. Evaluation of monoamine oxidase B activity of midbrain tissues (e), n=5 for each group. *P<.05, **P<.01, and ***P<.001 vs saline-treated mice. #P<.05 and ##P<.01 vs MPTP/p-treated mice. VTA, ventral tegmental area.
Furthermore, HPLC analysis showed that the levels of DA, dihydroxyphenylacetic acid, and homovanillic acid in the striatum of MPTP/p PD mice were significantly decreased by 73.3%, 71.9%, and 42.4%, respectively, compared with those in saline-treated mice (Figure 1b). However, treatment of MPTP/p PD mice with metformin for 5 weeks significantly increased DA and dihydroxyphenylacetic acid levels by 115.5% (2-way ANOVA, metformin: F1,28=5.422, P=.031; MPTP: F1,28=47.625, P<.001; interaction: F1,28=4.885, P=.040) and 77% (2-way ANOVA, metformin: F1,28=6.844, P=.036; MPTP: F1,28=52.479, P<.001; interaction: F1,28=7.815, P=.019) compared with untreated MPTP/p mice (Figure 1b). Metformin alone had no effect on DA levels. These results suggest that metformin improves motor function, resulting from the elevation of DA level in the striatum of MPTP/p PD mice.
Metformin Prevents DA Neuron Degeneration and Attenuates α-Synuclein Accumulation in SNc of MPTP/p PD Mice
Next, we examined the effect of metformin on DA neuron impairment induced by MPTP. Chronic MPTP/p administration induced a 48% loss of TH-positive cells in SNc (Figure 1c-d) compared with those in saline-treated mice. Interestingly, metformin significantly increased the number of TH-positive neurons by 25% in SNc of MPTP/p PD mice (2-way ANOVA, metformin: F1,12=5.841, P=.033; MPTP: F1,12=134.053, P<.001; interaction: F1,12=11.055, P=.006). In addition, we evaluated monoamine oxidase B activity of midbrain tissues, and the results showed that metformin had no influence on monoamine oxidase B activity (2-way ANOVA, metformin: F1,17=2.964, P=.103; MPTP: F1,17=0.247, P=.626) (Figure 1e). These findings indicate that metformin prevents DA neuron degeneration in the SNc of MPTP/p PD model mice without influencing MPTP metabolism.
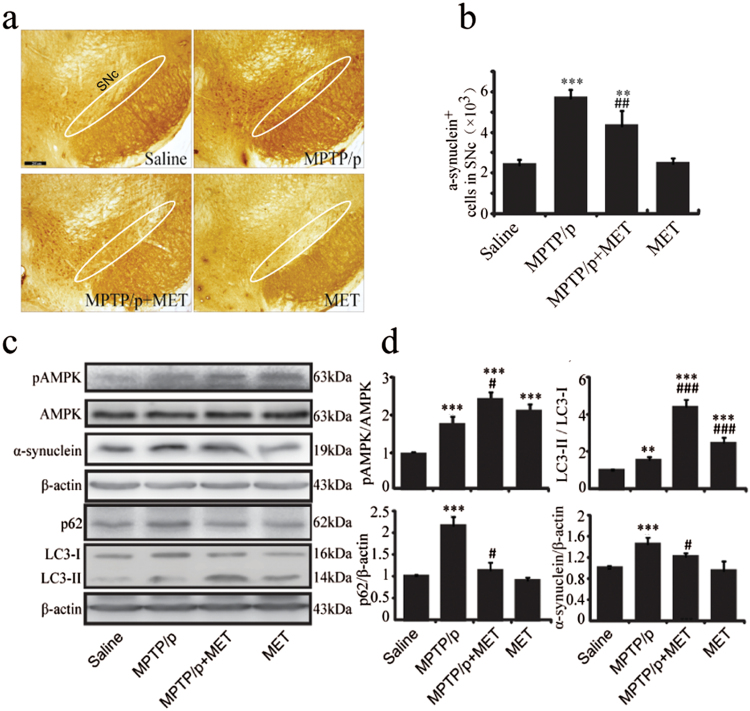
α-Synuclein, the primary component of Lewy’s body, has been considered as a critical hallmark of PD. Immunohistochemistry staining exhibited that α-synuclein immunoreactivity was significantly increased in the SNc of MPTP/p PD mice (Figure 2a), and α-synuclein-positive cells increased 1.4-fold compared with those in the saline group (Figure 2b). Consistent with immunostaining results, western-blotting analysis showed that α-synuclein expression was significantly increased in the midbrain of MPTP/p PD mice (Figure 2c-d). Metformin administration induced a 47.3% decrease of α-synuclein positive cells (2-way ANOVA, metformin: F1,12=7.359, P=.019; MPTP: F1,12=29.748, P<.001; interaction: F1,12=10.689, P=.007) (Figure 2b) and significantly reduced α-synuclein expression (2-way ANOVA, Metformin: F1,12=5.340, P=.041; MPTP: F1,12=29.430, P<.001; interaction: F1,12=7.459, P=.045) (Figure 2c-d).
Figure 2.
Metformin accelerated AMP-activated protein kinase (AMPK) phosphorylation, induced autophagy in 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) mice and decreased α-synuclein accumulation. (a) Immunohistochemical staining of α-synuclein positive cells in substania nigra compacta (SNc) of mice with ×40 magnifications. (b) Stereological counts of α-synuclein positive cells in mouse SNc. (c-d) Western blotting analysis of pAMPK, AMPK, α-synuclein, p62, and light chain 3 (LC3). Data are presented as the mean±SEM, n=4 for each group. ** P<.01 and *** P<.001 vs saline-treated mice. # P<.05, ## P<.01, and ### P<.001 vs MPTP plus probenecid (MPTP/p)-treated mice. Scale bar, 200 μm. MET, metformin.
Metformin Enhances AMPK Phosphorylation and Autophagy in the Midbrain of MPTP/p PD Model Mice
AMPK is a major regulator of cellular and organism energy homeostasis. In this study, we intended to explore the effect of metformin administration on AMPK activation. As determined by western blotting, we found that the phosphorylation level of Thr172 in the active site of AMPK was increased in the midbrain following chronic MPTP/p injections and cotreatment with metformin. The phosphorylation level of AMPK was increased 1.8-fold in MPTP/p mice compared with that in saline-treated mice (Figure 2d). In addition, AMPK phosphorylation was increased 2.3-fold in metformin-treated MPTP/p mice and was higher than untreated MPTP/p mice (2-way ANOVA, metformin: F1,12=68.997, P<.001; MPTP: F1,12=131.140, P<.001; interaction: F1,12=40.013, P=.021) (Figure 2d). Given that autophagy is an important function of AMPK activation, we next detected LC3 and p62, which are key components of autophagy. Our data showed that LC3-II level in the midbrain of MPTP/p PD mice was significantly elevated compared with saline-treated mice (Figure 2d), but LC3-II upregulation failed to decrease p62 (an autophagy substrate) expression (Figure 2d). This result indicates that autophagic dysfunction is involved in DA neuron degeneration induced by MPTP/p treatment. In contrast, metformin administration not only increased LC3-II expression (2-way ANOVA, metformin: F1,12=530.348, P<.001; MPTP: F1,12=74.853, P<.001; interaction: F1,12=12.480, P=.004), but also reduced p62 expression (2-way ANOVA, metformin: F1,12=30.830, P=.003; MPTP: F1,12=62.409, P<.001; interaction: F1,12=19.662, P=.010) (Figure 2d). These results indicate that the enhancement of neuronal autophagy by metformin administration contributes to its neuroprotective effects on DA neurons.
Metformin Inhibits Microglial Activation and Neuroinflammation in the Midbrain of MPTP/ p PD Mice
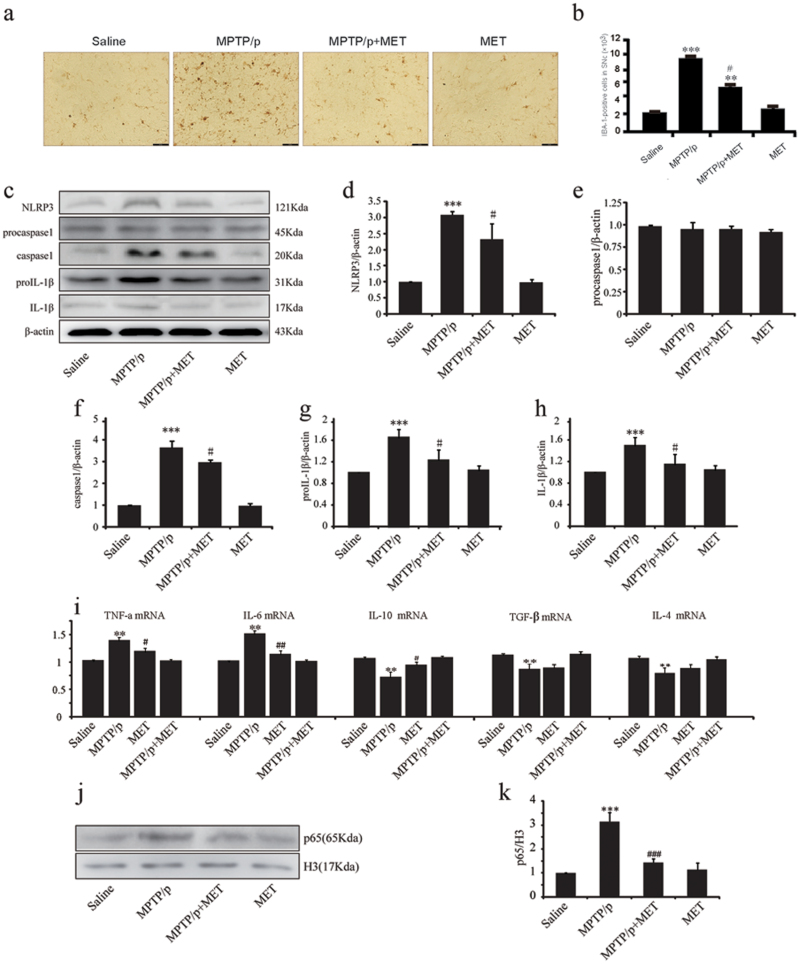
It has been known that activated microglia play a critical role in DA neuron death in MPTP/p model. Thus, we examined whether metformin could inhibit microglia overactivation in SNc of MPTP/p model mice. In saline-treated mice, a few IBA-1 positive cells were detected in SNc. In contrast, MPTP/p injections resulted in a significant increase of IBA-1-positive cells in SNc of MPTP/p PD mice, while metformin administration dramatically suppressed the activation of microglia in SNc (2-way ANOVA, metformin: F1,12=12.036, P=.012; MPTP: F1,12=50.730, P<.001; interaction: F1,12=28.007, P=.038) (Figure 3a-b). This result indicates that metformin can suppress microglial activation induced by MPTP.
Figure. 3.
Metformin inhibits glia activation and proinflammation factor expression. (a) Immunohistochemical staining of ionized calcium binding adapter molecule 1 (IBA-1) positive cells in substania nigra compacta (SNc) of mice with ×200 magnifications. (b) Stereological counts of IBA-1 positive cells in mouse SNc. (c-h, j,k) Western-blotting analysis of Nod-like receptor protein 3 (NLRP3), procaspase1, capase1, prointerleukin-1β (proIL-1β), interleukin-1β (IL-1β), and p65. (i) Real-time PCR (RT-PCR) analysis of Tumor necrosis factor-alpha (TNF-α), IL-6, IL-10, Transforming growth factor-β (TGF-β), and IL-4. Data are presented as the mean ± SEM, n=4 for each group. ** P<.01 and *** P<.001 vs saline-treated mice. # P<.05, ## P<.01, and ### P<.001 vs MPTP-treated mice. Scale bar, 40 μm. MET, metformin; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Since activated microglia can produce a large of cytokines, including proinflammatory and antiinflammatory factors, we next evaluated the effect of metformin on neuroinflammation by qRT-PCR and western-blotting analysis. We showed that NLRP3 inflammasome was significantly activated by MPTP/p treatment, evidenced by the upregulation of caspase-1 and IL-1β. Notably, metformin administration dramatically inhibited NLRP3 expression (2-way ANOVA, metformin: F1,12=8.550, P=.013; MPTP: F1,12=165.845, P<.001; interaction: F1,12=5.092, P=.043), caspase-1 activation (2-way ANOVA, metformin: F1,12=11.542, P=.005; MPTP: F1,12=642.585, P<.001; interaction: F1,12=9.150, P=.011), and IL-1β production (2-way ANOVA, metformin: F1,12=5.519, P=.037; MPTP: F1,12=21.544, P=.001; interaction: F1,12=8.988, P=.011) (Figure 3d-h). Additionally, the mRNA levels of proinflammatory genes, including TNF-α and IL-6 were markedly increased in the midbrain of MPTP/p PD mice compared with those in saline-treated mice (Figure 3i). Metformin administration inhibited the expressions of TNF-α and IL-6 mRNA. We also found that antiinflammatory cytokines IL-4, IL-10, and TGF-β were decreased in midbrain of MPTP/p PD mice (Figure 3i). Metformin administration for 5 weeks significantly increased IL-10 production, but failed to affect IL-4 and TGF-β levels (Figure 3i). Furthermore, expression of p65 in nuclear of MPTP/p PD mice increased about 3-fold. However, p65 nuclear translocation was significantly inhibited by metformin compared with those in untreated MPTP/p mice (2-way ANOVA, metformin: F1,12=40.702, P<.001; MPTP: F1,12=101.281, P<.001; interaction: F1,12=57.091, P<.001) (Figure 3j-k). These results indicate that metformin inhibits microglia-mediated neuroinflammation in the midbrain of MPTP/p PD mice.
Metformin Protects SH-SY5Y Cells against MPP+-Induced Apoptosis via AMPK Activation and Subsequent Autophagy
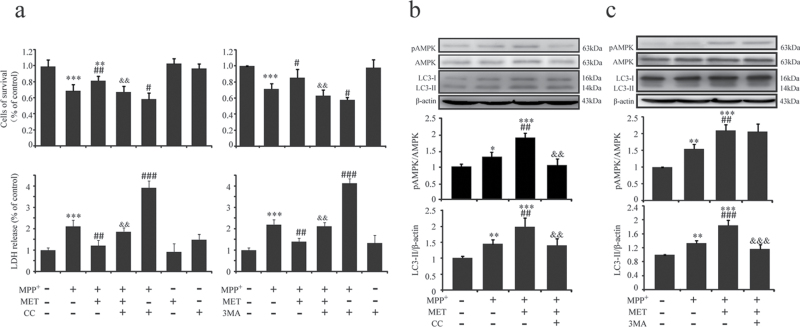
To investigate the mechanism for the neuroprotective effects of metformin, we explored its role in AMPK and subsequent autophagy in SH-SY5Y cells. It was found that metformin increased SH-SY5Y cell survival and inhibited LDH release compared with that in MPP+-treated cells (Figure 4a). As determined by western blotting, we found the phosphorylation levels of AMPK Thr172 site were increased in SH-SY5Y cells treated with or without metformin in the presence of MPP+ incubation (Figure 4b). Phosphorylation levels of AMPK in metformin/MPP+ co-treated cells were higher than that in MPP+ alone treated cells (Figure 4b). Furthermore, pretreatment of metformin induced a significant upregulation of LC3-II (Figure 4b). CC is a potent AMPK inhibitor that has been widely used for studying AMPK signaling (Jung et al., 2008). Our results showed that CC blocked the cytoprotective role of metformin (Figure 4a) and prevented metformin-induced activation of AMPK (Figure 4b), accompanied by the downregulation of LC3-II (Figure4b). 3MA is an inhibitor widely used to block autophagy. Our data showed that 3MA also blocked the cytoprotective role of metformin (Figure 4a) and decreased LC3-II expression (Figure 4c), while it did not influence phosphorylation level of AMPK (Figure 4c). These results indicate that AMPK activation and subsequent autophagy enhancement are required for the neuroprotective effect of metformin.
Figure 4.
Metformin attenuated SHSY5Y cells injury induced by MPP+. To activate AMP-activated protein kinase (AMPK), SHSY5Y cells were preincubated with MET (2mM) for 1 hour, and MPP+ (200 μM) was added for 48 hours. To block AMPK or autophagy, SHSY5Y cells were preincubated with Compound C (CC) (10 μM) for 30 minutes or with 3-Methyladenine (3MA) (5mM) for 3 hours before MET addition. Cytotoxicity was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) conversion and lactate dehydrogenase (LDH) release (a). Levels of pAMPK, AMPK, and light chain 3 (LC3) were determined by western blotting (b-c). Data are presented as the mean±SEM of 3 independent experiments. * P<.05, ** P<.01, and *** P<.001 vs untreated cells. # P<.05, ## P<.01, and ### P<.001 vs MPP+-treated cells. && P<.01 and &&& P<.001 vs MET/MPP+ co-treated cells. MET, metformin.
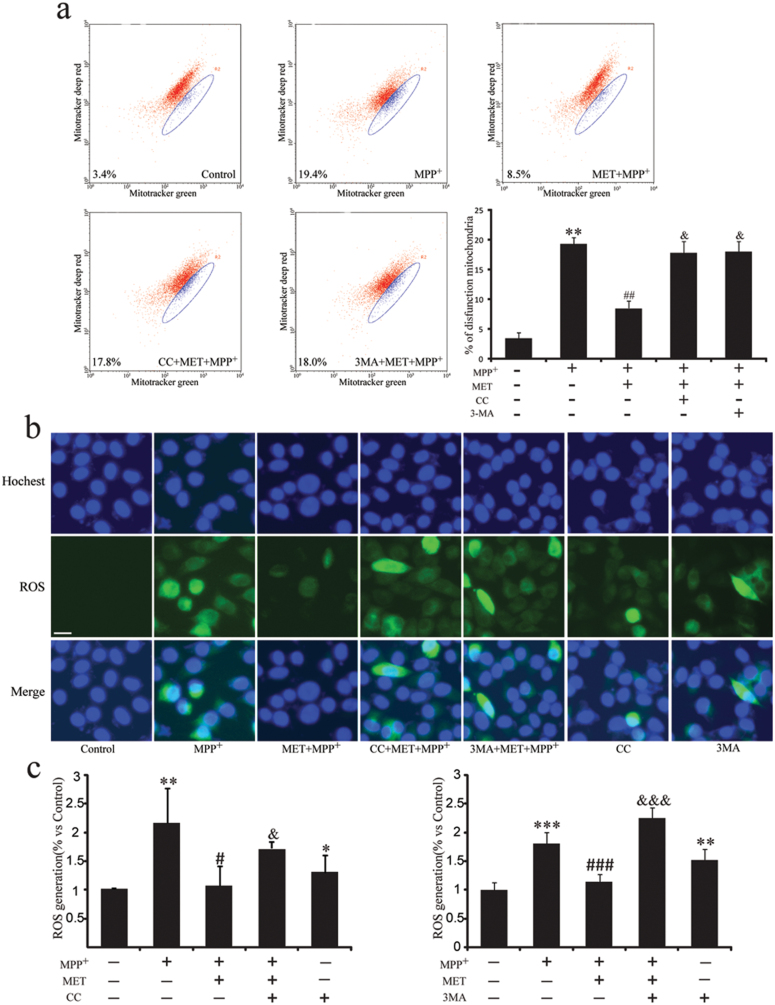
Metformin Promotes Dysfunctional Mitochondria Clearance and Suppresses ROS Generation
Various reports suggest mitochondria dysfunction is involved in PD. As determined by flow cytometric analyses, in agreement with previous reports (Li et al., 2003), complex I inhibitor MPP+ resulted in the loss of mitochondrial membrane potential. The dysfunctional mitochondria proportion increased to 19.4%, while it was 3.4% in untreated cells (Figure 5a). Pretreatment with metformin significantly recovered mitochondrial membrane potential, and the dysfunctional mitochondria proportion was reversed to 8.5% (Figure 5a). Mitochondria are the main source of intracellular ROS. We found ROS production was increased about 2-fold induced by MPP+, while it was suppressed by the pretreatment with metformin (Figure 5b-c). Either AMPK inhibitor CC or autophagy inhibitor 3MA blocked the effects of metformin on clearance of dysfunctional mitochondria and suppression of ROS production (Figure 5b-c). These results indicate that AMPK activation by metformin is beneficial for clearance of dysfunctional mitochondria and inhibition of ROS generation.
Figure 5.
Metformin increases dysfunctional mitochondria clearance and inhibits ROS degeneration. To activate AMP-activated protein kinase (AMPK), SHSY5Y cells were preincubated with MET (2mM) for 1 hour, and MPP+ (200 μM) was added for 48 hours. To block AMPK or autophagy, SHSY5Y cells were preincubated with Compound C (CC) (10 μM) for 30 minutes or with 3-Methyladenine (3MA) (5mM) for 3 hours before MET addition. (a) After drug treatment, cells were stained with Mitotracker green and Mitotracker deep red for 30 minutes and analyzed by flow cytometry. Photos of ROS fluorescence (b) and intensity of intracellular ROS fluorescence (c). Blue color represents nuclear and green color represents ROS. Scale bar, um. Data are presented as the mean ± SEM of 3 independent experiments. Scale bar, 10 μm. * P<.05, ** P<.01, and *** P<.001 vs untreated cells. # P<.05, ## P<.01, and ### P<.001 vs MPP+-treated cells. && P<.01 and &&&P<.001 vs MPP+/MET co-treated cells. MET, metformin.
Discussion
Metformin, a classic oral hypoglycemic agent, has been recognized to exert the potential effects in degenerative diseases, including diabetes and AD (Li et al., 2012). Patil et al. (2014) reported the neuroprotective effect of metformin in MPTP-induced PD model. They prepared a subacute PD model by MPTP administration for 5 consecutive days and treated mice with metformin (500mg/kg) orally for 21 days. They found that metformin treatment for 20 days resulted in an improvement of the locomotor and muscular activities in MPTP-treated mice than the acute treatment (4 days). Metformin treatment significantly attenuated TH+ neuronal loss and improved the antioxidant activity, evidenced by enhanced activity of superoxide dismutase, L-Glutathione, and catalase CAT and reduced lipid peroxidation compared with the MPTP-treated group. But they did not explain the potential mechanism and drug target for the neuroprotective effect of metformin. On the other hand, our previous study has demonstrated that metabolic inflammation exacerbates DA neuronal degeneration in type 2 diabetes mice (Wang et al., 2014). The present work is a further exploration based on that publication. We showed that treatment with metformin for 5 weeks significantly ameliorated the degeneration of substantia nigra DA neurons, increased striatal DA levels, and improved motor impairment in MPTP/p-induced PD chronic model, which administrated MPTP for 1 month and induced classic PD-like neuron injury. We further found that metformin alleviated MPP+-induced cytotoxicity and attenuated neuronal apoptosis via inducing LC3-II-mediated autophagy and in turn eliminating mitochondrial ROS. 3MA, the inhibitor of autophay, could abolish the role of metformin in DA neurons. Furthermore, we clarified that the neuroprotective effect of metformin was dependent on AMPK activation. Together, our present study demonstrates that metformin may be a pluripotent and promising drug for treatment of DA neuron degeneration in PD.
It has been demonstrated that AMPK is involved in MPTP-induced PD model and that activation of AMPK may prevent neuronal cell death (Choi et al., 2010). Since metformin is a well-accepted activator of AMPK and regulates systemic energy metabolism, we explored whether metformin protected DA neuron against MPTP/p-induced neurodegeneration via activating AMPK. Our data showed that metformin led to AMPK phosphorylation both in midbrain of MPTP/p PD mice and in MPP+-injured SH-SY5Y cells. Furthermore, AMPK inhibitor CC could abolish the protective effects of metformin in SH-SY5Y cells, indicating that AMPK activation was required for the protective effect of metformin on DA neurons. On the other hand, we found that MPTP or MPP+ could also activate AMPK by increasing the phosphorylation level of AMPK and this could be explained by a compensative autoregulation mechanism when the mitochondrial respiratory chain was inhibited and the ATP supply was deficient caused by MPTP or MPP+. So the pAMPK level was elevated moderately in response to MPTP challenge. As MPTP or MPP+ was present persistently, finally, cells failed to maintain the autoregulation and subsequently underwent death. In contrast, metformin directly activated AMPK itself and initiated downstream protective signaling to attenuate DA neuron apoptosis. This surmise was supported by the result that AMPK inhibitor CC could abolish the protective effects of metformin in SH-SY5Y cells. It suggests that AMPK activation was required for the protective effect of metformin on DA neurons.
AMPK phosphorylation causes the activation of unc-51 like autophagy activating kinase 1, which initiates autophagosome formation, linking cellular nutrient status to downstream events in autophagy (Egan et al., 2011). Autophagy is an important function of an organism, which mediates the degradation of misfolded/aggregated proteins and dysfunctional organells in cells (Rubinsztein et al., 2015). As a primary regulatory mechanism that maintains nutrient and energy homeostasis in response to stress, autophagy underlies the pathophysiological process in many diseases (Menzies et al., 2015). Increasing evidence suggests that dysregulation of autophagy results in the accumulation of abnormal proteins and/or damaged organelles, which is commonly observed in neurodegenerative diseases, such as AD, Huntington’s disease, and PD (Banerjee et al., 2010). α-Synuclein, a major constituent of Lewy bodies found in PD, is degraded by macrophagy and chaperone mediated autophagy (Xilouri and Stefanis, 2015). Normally, soluble α-synuclein is degraded by chaperone mediated autophagy, but misfolded/aggegration α-synuclein and mutant α-synuclein (as A53T, A30P) are mainly degraded by macrophagy (Lynch-Day et al., 2012). We further found that α-synuclein expression and immune positive cells in SNc were elevated in MPTP/p PD mice. In contrast, metformin administration eliminated α-synuclein accumulation and induced the upregulation of LC3-II, a partner of autophagy. In turn, metformin facilitated the degradation of autophagic substrate, p62. These indicate that metformin can induce autophagy and reduce α-synuclein accumulation. So we speculate the effect on α-synuclein is a follow-up result of metformin alleviating neuronal damage via inducing autophagy and eliminating accumulated α-synuclein. Furthermore, metformin co-treated with MPP+ also induced LC3-II production. However, pretreatment of autophagy inhibitor 3MA blocked LC3-II production and abolished the cytoprotective effect of metformin. We also found although AMPK phosphorylation level evaluated and LC3-II production increased in MPTP/p mice, the substrate of autophagy p62 failed to decrease, indicating that the autophagic flux was impaired in PD mice. Autophagosomes eventually fuse with lysosomes to form autolysosomes, in which the autophagic cargo, such as proteins and organelles, is degraded. It has been reported that autophagic vacuoles occur in the early stage of mouse PD model induced by MPTP (Pan et al., 2008). However, in a progressive mouse model of PD, lysosomal function was impaired. ROS production from dysfunctional mitochondria causes abnormal permeabilization of lysosomal membranes and lysosomal breakdown as well as contributes to the defective autophagy (Dehay et al., 2010). So the defective autophagic flux in PD may contribute to the aggregation of α-synuclein. Our findings demonstrate that AMPK and subsequent autophagy are involved in PD, indicating that metformin prevents neuronal death via the induction of AMPK-autophagy pathway.
Recent evidence suggests that mitochondrial dysfunction plays an important role in the pathogenesis of PD (Moon and Paek, 2015). Autophagic degradation of mitochondria, termed mitophagy, is necessary for the removal of damaged mitochondria (Perier and Vila, 2012). We showed that MPP+ significantly increased the proportion of dysfunctional mitochondria, which was suppressed by the pretreatment of metformin. As mitochondria are the major sources of intracellular ROS, our results exhibited MPP+ notably promoted ROS production accompanied by the accumulation of damaged mitochondria, while metformin significantly suppressed ROS production induced by MPP+. Yet these effects were blocked by either AMPK inhibitor CC or autophagy blocker 3MA, indicating that AMPK-autophagy enhancement contributes to the clearance of dysfunctional mitochondria and suppression of ROS production. Furthermore, activated microglia cells produce several neurotoxic substances, including ROS and proinflammatory cytokines (Cao et al., 2011). It has been shown that proinflammatory cytokines, such as IL-1β and TNF-α, are increased in the brains of PD patients and animal models. We further found that NLRP3 inflammasome activation was involved in PD model and IL-1β production was increased as well as the expression of p65 in the midbrain of MPTP/p mice was significantly increased. However, metformin administration inhibited the activation of inflammasome and nuclear translocation of p65. Together, metformin can suppress ROS generation by enhancement of AMPK-autophagy pathway and in turn inhibits inflammasome activation and attenuates neuroinflammation.
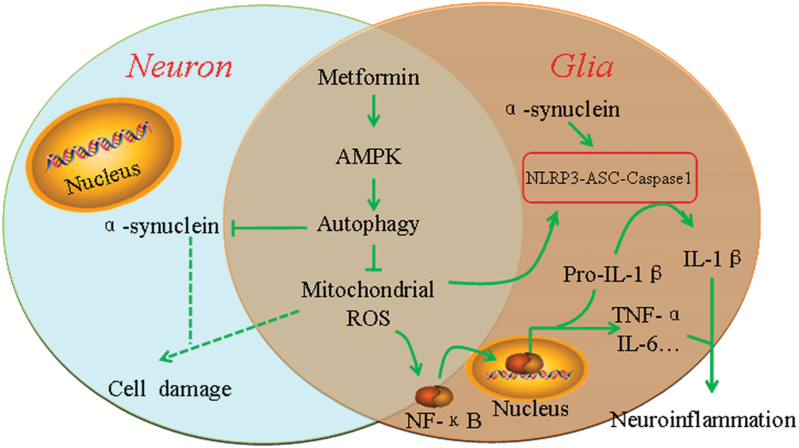
In conclusion, our findings show that metformin exerts the neuroprotective effects on DA neuron degeneration in MPTP/p PD mice. Moreover, we demonstrate that metformin protects DA neurons via the activation of AMPK-autophagy pathway and the inhibition of neuroinflammation (Figure 6). Our study indicates that metformin may be a pluripotent and promising drug for DA neuron degeneration, which will give us an insight into the potential of metformin in terms of opening up novel therapeutic avenues for PD. But it should be noted that metformin is a relatively weak drug with a wide range of pharmacological effects, some of which may affect elderly patients adversely. A few studies have shown that metformin may increase the risk of developing AD (Imfeld et al., 2012). Therefore, whether it benefits or disadvantages the therapy of PD, there is still a long way to discovering the clinical significance of metformin on neurodegenerative diseases.
Figure 6.
Schematic model for the protective mechanism of metformin in the treatment for Parkinson’s disease (PD). Metformin causes activation of AMP-activated protein kinase (AMPK), followed by the induction of autophagy. Induced autophagy enhanced the clearance of injured mitochondria and reduced ROS production and α-synuclein accumulation. Decreased production of ROS reduced Nuclear factor-kappaB (NF-κB) nuclear translocation, and inhibited proinflammatory cytokines production. Furthermore, reduction of ROS production and α-synuclein accumulation attenuated Nod-like receptor protein 3 (NLRP3) inflammasome activation.
Statement of Interest
None.
Acknowledgments
The work reported herein was supported by grants from the National Natural Science Foundation of China (nos. 81473196 and 81573403), the National Science & Technology Major Project (no. 2012ZX09304-001), the Natural Science Foundation of Jiangsu Province (BK20130039), and the key project of the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (no. 15KJA310002).
References
- Banerjee R, Beal MF, Thomas B. (2010) Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci 33(12):541549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ, Li KS, Shen YQ. (2011) Activated immune cells in Parkinson’s disease. J Neuroimmune Pharmacol 6(3):323–329. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. (2005) Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev 48(1):16–42. [DOI] [PubMed] [Google Scholar]
- Chao Y, Wong SC, Tan EK. (2014) Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int 2014:308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Lipton SA. (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci 67(20):3435–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Park C, Jeong JW. (2010) AMP-activated protein kinase is activated in Parkinson’s disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem Biophys Res Commun 391(1):147–151. [DOI] [PubMed] [Google Scholar]
- Chung YC, Kim SR, Jin BK. (2010) Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson’s disease. J Immunol 185(2):1230–1237. [DOI] [PubMed] [Google Scholar]
- Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. (2010) Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 30(37):12535–12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. (2011) Metformin: multi-faceted protection against cancer. Oncotarget 2(12):896–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331(6016):456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8(10):774–785. [DOI] [PubMed] [Google Scholar]
- Hardie DG. (2008) AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 32 Suppl 4:S7–12. [DOI] [PubMed] [Google Scholar]
- Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS. (2010) Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 9(2):135–146. [DOI] [PubMed] [Google Scholar]
- Imfeld P, Bodmer M, Jick SS, Meier CR. (2012) Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc. 60:916–921. [DOI] [PubMed] [Google Scholar]
- Jara JA, Lopez-Munoz R. (2015) Metformin and cancer: between the bioenergetic disturbances and the antifolate activity. Pharmacol Res. [DOI] [PubMed] [Google Scholar]
- Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, Park H, Kim SS, Choe W, Kang I, Ha J. (2008) Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis 29(4):713–721. [DOI] [PubMed] [Google Scholar]
- Khang R, Park C, Shin JH. (2015) Dysregulation of parkin in the substantia nigra of db/db and high-fat diet mice. Neuroscience 294:182–192. [DOI] [PubMed] [Google Scholar]
- Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Kohler A, Glossmann H, Schneider R, Sutherland C, Schweiger S. (2010) Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A 107(50):21830–21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Deng J, Sheng W, Zuo Z. (2012) Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav 101(4):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278(10):8516–8525. [DOI] [PubMed] [Google Scholar]
- Li X, Zhen D, Lu X, Xu H, Shao Y, Xue Q, Hu Y, Liu B, Sun W. (2010) Enhanced cytotoxicity and activation of ROS-dependent c-Jun NH2-terminal kinase and caspase-3 by low doses of tetrandrine-loaded nanoparticles in Lovo cells--a possible Trojan strategy against cancer. Eur J Pharm Biopharm 75(3):334–340. [DOI] [PubMed] [Google Scholar]
- Lu M, Hu G. (2012) Targeting metabolic inflammation in Parkinson’s disease: implications for prospective therapeutic strategies. Clin Exp Pharmacol Physiol 39(6):577–585. [DOI] [PubMed] [Google Scholar]
- Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2(4):a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. (2007) Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett 411(2):98–103. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Rubinsztein DC. (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16(6):345–357. [DOI] [PubMed] [Google Scholar]
- Moon HE, Paek SH. (2015) Mitochondrial Dysfunction in Parkinson’s Disease. Exp Neurobiol 24(2):103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Bomhoff GL, Stanford JA, Geiger PC. (2010) Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 299(4):R1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, Jankovic J. (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131(Pt 8):1969–1978. [DOI] [PubMed] [Google Scholar]
- Panicker GK, Karnad DR, Salvi V, Kothari S. (2012) Cardiovascular risk of oral antidiabetic drugs: current evidence and regulatory requirements for new drugs. J Assoc Physicians India 60:56–61. [PubMed] [Google Scholar]
- Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. (2014) Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277:747–754. [DOI] [PubMed] [Google Scholar]
- Perier C, Vila M. (2012) Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med 2(2):a009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Djamshidian-Tehrani A. (2015) Movement disorders in systemic diseases. Neurol Clin 33(1):269–297. [DOI] [PubMed] [Google Scholar]
- Rojas LB, Gomes MB. (2013) Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Bento CF, Deretic V. (2015) Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J Exp Med 212(7):979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116(7):1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XL, Liu Y, Dai T, Ding JH, Hu G. (2011) Uncoupling protein 2 knockout exacerbates depression-like behaviors in mice via enhancing inflammatory response. Neuroscience 192:507–514. [DOI] [PubMed] [Google Scholar]
- van der Brug MP, Singleton A, Gasser T, Lewis PA. (2015) Parkinson’s disease: from human genetics to clinical trials. Sci Transl Med 7(305):205ps220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volta M, Milnerwood AJ, Farrer MJ. (2015) Insights from late-onset familial parkinsonism on the pathogenesis of idiopathic Parkinson’s disease. Lancet Neurol 14(10):1054–1064. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhai YQ, Xu LL, Qiao C, Sun XL, Ding JH, Lu M, Hu G. (2014) Metabolic inflammation exacerbates dopaminergic neuronal degeneration in response to acute MPTP challenge in type 2 diabetes mice. Exp Neurol 251:22–29. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP–activated protein kinase in mammalian cells. Cell Metab 2(1):21–33. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 19(3):163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Stefanis L. (2015) Chaperone mediated autophagy to the rescue: a new-fangled target for the treatment of neurodegenerative diseases. Mol Cell Neurosci 66(Pt A):29–36. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. [DOI] [PubMed] [Google Scholar]