Abstract
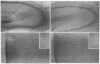
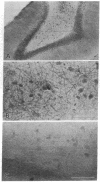
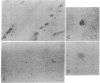
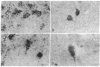
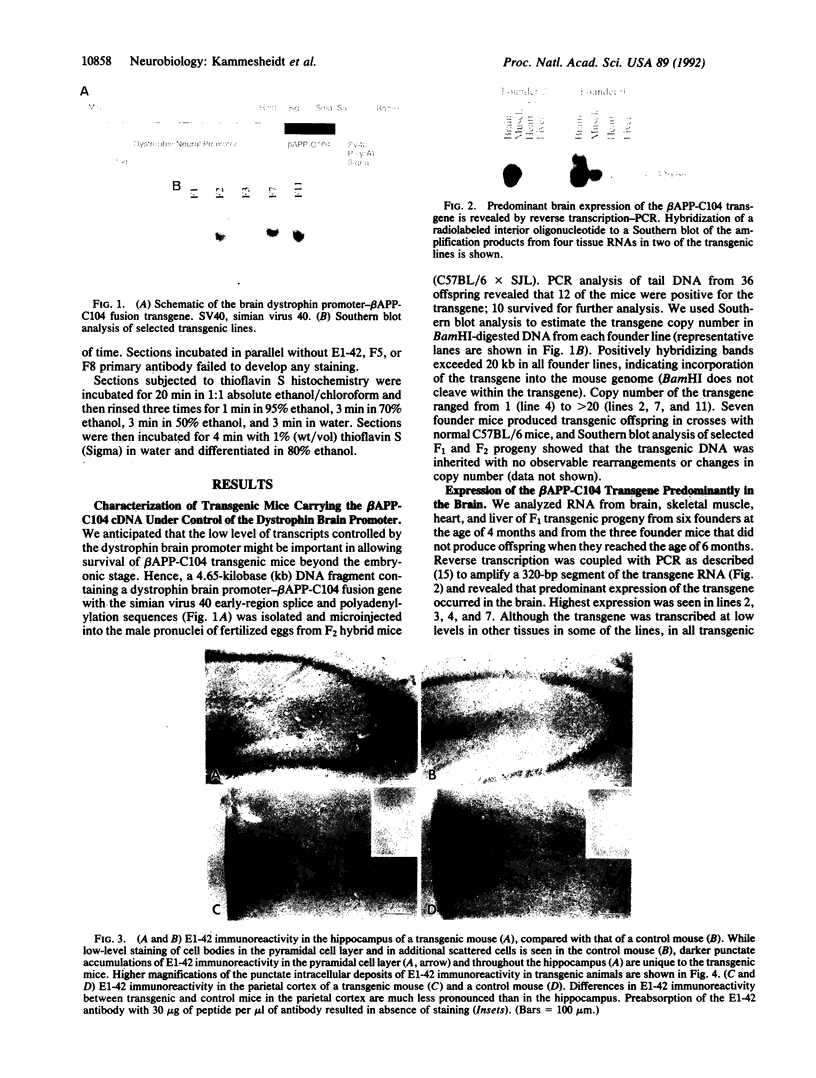
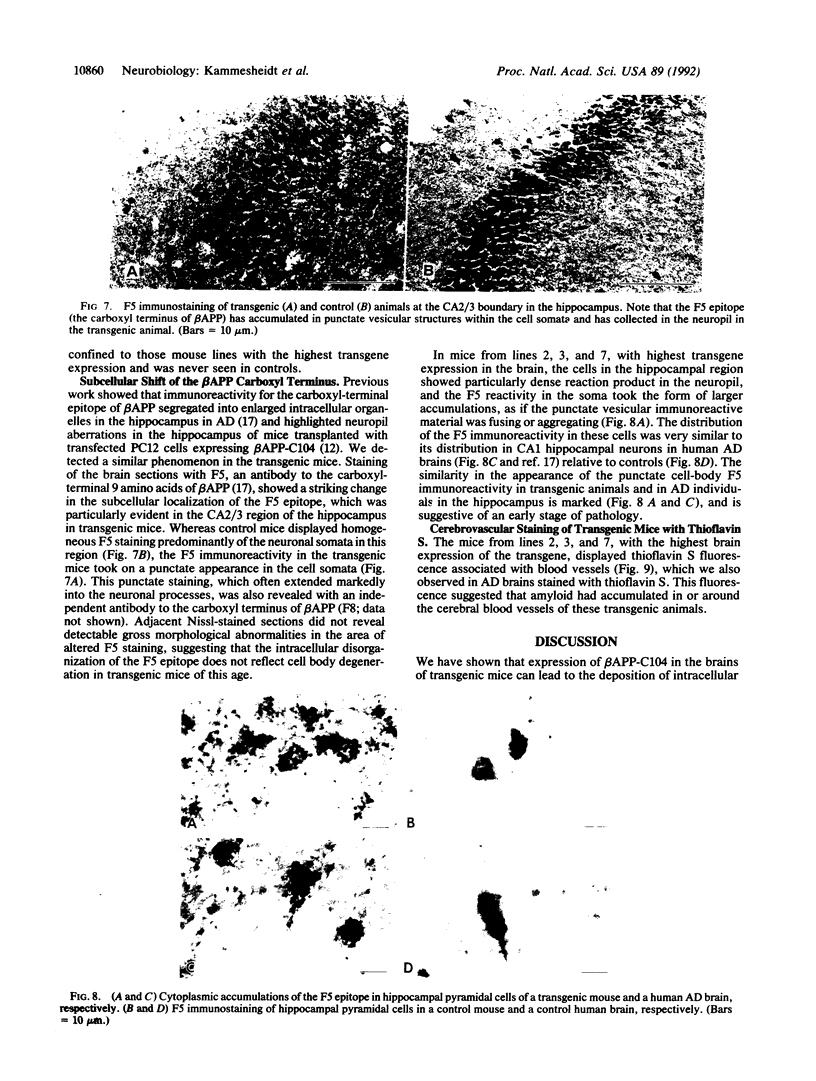
The deposition of amyloid in senile plaques and along the walls of the cerebral vasculature is a characteristic feature of Alzheimer disease. The peptide comprising the carboxyl-terminal 100 amino acids of the beta-amyloid precursor protein (beta APP) has been shown to aggregate into amyloid-like fibrils in vitro and to be neurotoxic, suggesting that this fragment may play a role in the etiology of Alzheimer disease. To address this question, we expressed this carboxyl-terminal 100-amino acid peptide of beta APP in transgenic mice under the control of the brain dystrophin promoter. We used an antibody to the principal component of amyloid, beta/A4, to demonstrate cell-body and neuropil accumulation of beta/A4 immunoreactivity in the brains of 4- and 6-month-old transgenic mice. Only light cytoplasmic staining with this antibody was visible in control mice. In addition, immunocytochemical analysis of the brains with an antibody to the carboxyl terminus of beta APP revealed abnormal aggregation of this epitope of beta APP within vesicular structures in the cytoplasm and in abnormal-appearing neurites in the CA2/3 region of the hippocampus in transgenic mice, similar to its aggregation in the cells of Alzheimer disease brains. Thioflavin S histochemistry suggested accumulations of amyloid in the cerebrovasculature of transgenic mice with the highest expression of the beta APP-C100 transgene. These observations suggest that expression of abnormal carboxyl-terminal subfragments of beta APP in vivo may cause amyloidogenesis and specific neuropathology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Esch F. S., Keim P. S., Sambamurti K., Lieberburg I., Robakis N. K. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci Lett. 1991 Jul 8;128(1):126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- Barrow C. J., Zagorski M. G. Solution structures of beta peptide and its constituent fragments: relation to amyloid deposition. Science. 1991 Jul 12;253(5016):179–182. doi: 10.1126/science.1853202. [DOI] [PubMed] [Google Scholar]
- Benowitz L. I., Rodriguez W., Paskevich P., Mufson E. J., Schenk D., Neve R. L. The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer disease subjects. Exp Neurol. 1989 Dec;106(3):237–250. doi: 10.1016/0014-4886(89)90156-8. [DOI] [PubMed] [Google Scholar]
- Boyce F. M., Beggs A. H., Feener C., Kunkel L. M. Dystrophin is transcribed in brain from a distant upstream promoter. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1276–1280. doi: 10.1073/pnas.88.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P., Neve R. L., Kammesheidt A., Rhoads R. E., Vanaman T. C. Analysis of the tissue-specific distribution of mRNAs encoding the plasma membrane calcium-pumping ATPases and characterization of an alternately spliced form of PMCA4 at the cDNA and genomic levels. J Biol Chem. 1992 Mar 5;267(7):4376–4385. [PubMed] [Google Scholar]
- Cataldo A. M., Nixon R. A. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci U S A. 1990 May;87(10):3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings B. J., Su J. H., Geddes J. W., Van Nostrand W. E., Wagner S. L., Cunningham D. D., Cotman C. W. Aggregation of the amyloid precursor protein within degenerating neurons and dystrophic neurites in Alzheimer's disease. Neuroscience. 1992 Jun;48(4):763–777. doi: 10.1016/0306-4522(92)90265-4. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Weidemann A., Multhaup G., Salbaum J. M., Lemaire H. G., Kang J., Müller-Hill B., Masters C. L., Beyreuther K. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer's disease. EMBO J. 1988 Apr;7(4):949–957. doi: 10.1002/j.1460-2075.1988.tb02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Joachim C., Games D., Morris J., Ward P., Frenkel D., Selkoe D. Antibodies to non-beta regions of the beta-amyloid precursor protein detect a subset of senile plaques. Am J Pathol. 1991 Feb;138(2):373–384. [PMC free article] [PubMed] [Google Scholar]
- Jucker M., Walker L. C., Martin L. J., Kitt C. A., Kleinman H. K., Ingram D. K., Price D. L. Age-associated inclusions in normal and transgenic mouse brain. Science. 1992 Mar 13;255(5050):1443–1445. doi: 10.1126/science.1542796. [DOI] [PubMed] [Google Scholar]
- Kozlowski M. R., Spanoyannis A., Manly S. P., Fidel S. A., Neve R. L. The neurotoxic carboxy-terminal fragment of the Alzheimer amyloid precursor binds specifically to a neuronal cell surface molecule: pH dependence of the neurotoxicity and the binding. J Neurosci. 1992 May;12(5):1679–1687. doi: 10.1523/JNEUROSCI.12-05-01679.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Terakado K., Usami M., Yoshikawa K. Formation of amyloid-like fibrils in COS cells overexpressing part of the Alzheimer amyloid protein precursor. Nature. 1990 Oct 11;347(6293):566–569. doi: 10.1038/347566a0. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve R. L., Kammesheidt A., Hohmann C. F. Brain transplants of cells expressing the carboxyl-terminal fragment of the Alzheimer amyloid protein precursor cause specific neuropathology in vivo. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3448–3452. doi: 10.1073/pnas.89.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., Cordell B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature. 1991 Jul 18;352(6332):239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wolf D., Quon D., Wang Y., Cordell B. Identification and characterization of C-terminal fragments of the beta-amyloid precursor produced in cell culture. EMBO J. 1990 Jul;9(7):2079–2084. doi: 10.1002/j.1460-2075.1990.tb07375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]