Abstract
In vitiligo, gradual cutaneous depigmentation and cytotoxic T cell activity against melanocytes is accompanied by a paucity of regulatory T cells (Tregs) in vitiligo patient skin, indicating that autoimmune responses are not adequately held in check. Thus we sought a means to repopulate patient skin with Tregs. We hypothesized that enhanced expression of CCL22 can promote Treg skin homing to suppress depigmentation. The mouse Ccl22 gene was cloned into an expression vector and resulting DNA was used for gene gun treatment. Two spontaneous depigmentation models with different kinetics of melanocyte loss were utilized, expressing tyrosinase-reactive and gp100-reactive T cell receptor transgenes. Mice were subjected to 5 gene gun treatments 6 days apart, scanned for depigmentation weekly thereafter and monitored for activation and proliferation of relevant T cells and for Treg infiltration to the skin. Significantly reduced depigmentation 2 weeks after treatment was accompanied by a markedly increased abundance of Tregs in the skin at the expense of melanocyte reactive, TCR transgenic T cells as well as by reduced proliferation and reduced IFN-γ production in response to cognate peptide. Continued treatment may be necessary for sustained, local immunosuppression. These findings suggest that topical CCL22 may be used for the treatment of vitiligo.
Keywords: CCL22, regulatory T cells, vitiligo, immunosuppression, DNA treatment
INTRODUCTION
Vitiligo patients develop gradual skin depigmentation (Le Poole and Boissy, 1997). The disease has a prevalence of approximately 0.5%, with little evidence for gender preference or for an association with particular skin tones (Tour et al, 2014). The velocity of depigmentation varies among patients, and disease progression is thought to accelerate under stress (Jeon et al, 2014). On average however, patients lose approximately 1% of pigmentation each year (Cedercreutz et al, 2010). Though the disease is triggered by stressful events that can directly harm melanocytes, progressive depigmentation involves cytotoxic activity by melanocyte reactive T cells (Le Poole et al, 1996). Once the melanocytes are destroyed, the immune response resolves as well.
To understand why melanocyte reactive T cells mediate depigmentation in vitiligo, while the same triggers applied to control individuals will not initiate disease, regulatory immune mechanisms should be considered. Even desirable immune responses to infectious organisms should dissolve once an infection is cleared (Mittrücker and Kaufmann, 2004). To accomplish such regulatory activity, a subset of T cells (regulatory T cells or Tregs) can secrete IL-10, suppress T cell proliferation and prevent activation of other components of the immune response (Ozdemir et al, 2009).
Tregs are characterized by expression of the FoxP3 transcription factor, high levels of surface CD25 for IL-2 uptake and other surface molecules involved in immunosuppressive functions, including the glucocorticoid-induced tumor necrosis factor receptor related protein or GITR (Taams and Akbar, 2005). In several autoimmune diseases, a reduction in the number of regulatory CD4+CD25+FoxP3+ T cells has been reported (Pellerin et al, 2014). In vitiligo, a limited number of Treg is found in the skin, signifying a reduced ability to hold local immune responses in check (Klarquist et al, 2010); reduced Treg numbers were found not only in lesional, but also in non-lesional and peri-lesional vitiligo skin. Though others have reported limited circulating Treg numbers (Lili et al, 2012), our observations were consistent with a biologically insignificant increase in circulating Treg. Questioning why Treg do not readily migrate towards vitiligo skin, we found a remarkable reduction in the number of CCL22 producing cells (Klarquist et al, 2010). CCL22 is commonly secreted by mast cells, macrophages and inflammatory dendritic cells (Shimura et al, 2010) and is a known ligand of CCR4. This receptor is found on Th2 cells, supportive of humoral responses, and on Treg (Yoshie and Matsushima, 2014).
We thus proposed to develop proof of concept for the idea, that overexpression of Ccl22 in the skin can recruit Treg, and provide a therapeutic platform to treat vitiligo. An advantage to treating vitiligo is, that in this autoimmune disease, the target organ is external and can be directly accessed while simultaneously monitoring disease. Here we have made use of mouse models that develop progressive vitiligo due to a transgenic T cell receptor reactive with melanocytes. In Pmel-1 mice, T cells respond to a gp100-derivative peptide (Hwang et al, 2006); some spontaneous depigmentation of the pelage is observed beyond 20 weeks, and vitiligo will develop slowly. Spontaneous disease will also develop in h3TA2 mice with a T cell receptor transgene reactive with human tyrosinase (Mehrotra et al, 2012), but the disease will develop much more rapidly than in Pmel-1 mice, starting at 4 weeks of age. Finally, we treated wildtype mice to perform immune monitoring experiments; to locally overexpress Ccl22 in mice, we fired Ccl22 encoding plasmid DNA via biolistic gene gun into naired ventral skin to achieve rapid and focal overexpression of the chemokine. The concept is illustrated in Fig. S1. We repeatedly gene gun treated mice using an expression vector encoding mouse Ccl22, and followed depigmentation by flatbed scanning. We also measured chemokine overexpression and followed Treg skin infiltration to treated skin. Immunosuppression was further measured among splenocytes to understand whether Treg can alleviate vitiligo symptoms via an overabundance of the chemokine Ccl22. Immediate and longer-term effects of gene gun-mediated Ccl22 overexpression were measured. Splenocytes from Ccl22-treated mice were incubated with cognate peptide to measure resulting T cell responses by IFN-γ ELISA and by proliferation assays using 3Hthymidine incorporation. Our findings have direct implications for vitiligo treatment, will provide insight into the central role Tregs play in autoimmune disease and can be readily extended to other skin immune disorders.
RESULTS
Depigmentation is markedly reduced by Ccl22 DNA treatment
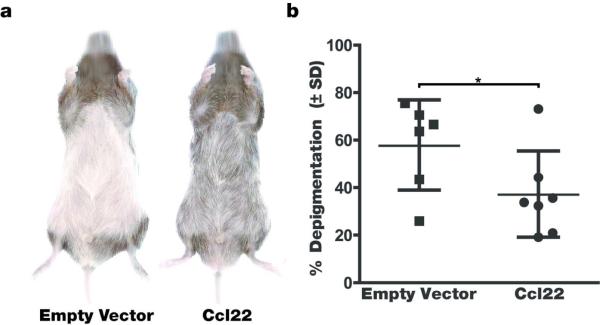
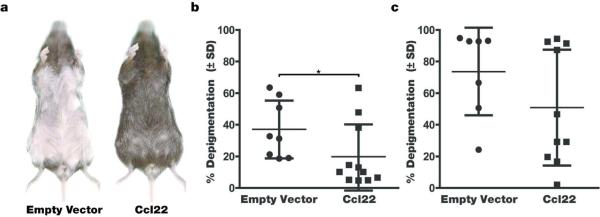
Firstly, we evaluated the effects of Ccl22 DNA treatment on depigmentation observed in vitiligo-prone mice. Mice expressing a melanocyte reactive T cell receptor transgene were repeatedly gene gun treated by shooting DNA coated bullets into naired ventral skin to achieve Ccl22 overexpression. When treating h3TA2 mice that normally develop rapid vitiligo already apparent in mice 5 weeks of age (Mosenson et al, 2013), reduced depigmentation was observed in vitiligo-prone animals after the pelage returned. The example shown in Fig. 1a illustrates the profound effect that Ccl22 encoding DNA has on depigmentation, with animals in the control group almost fully depigmented whereas just over half of pigmentation remained in Ccl22 treated mice. In Fig. 1b, depigmentation was quantified as 57.6 +/− 19.0% in the control group (n=6) and 37.0 +/− 18.1% in the Ccl22-treated group (n=7), thus 64.2% of the original depigmentation was prevented in response to Ccl22-encoding DNA (P=0.036). This experiment was executed 3 times with similar results, including 13 animals in all. Animals from each treatment series are included in the analysis. In Fig. 2, the experiment was repeated in Pmel-1 mice representing a slowly depigmenting model. Representative mice are shown prior to the 4th treatment in Fig. 2a, and depigmentation is quantified in Fig. 2b where treatment restored 46.4% of the original pigment loss when compared to empty vector treated control animals evaluated at 19.9 +/− 20.3% (n=11) and 37.1 +/− 18.3% (n=8) depigmentation of regrowing hair, respectively (P=0.037). Remarkably, upon re-assessment 2 weeks after the 5th and final treatment as shown in Fig. 2c, the differences between the Ccl22 treated (51.0 +/− 36.7%, n=10) and control groups (73.6 +/− 27.7%, n=7) were no longer significant (P=0.09). Taken together, we conclude that Ccl22 DNA treatment can markedly prevent depigmentation, yet continued treatment is required to maintain a therapeutic effect.
Fig. 1. Significantly reduced depigmentation in h3TA2 mice.
Mice were treated with Ccl22 encoding or empty vector DNA 5 times, 6 days apart and depigmentation was evaluated after hair regrowth when animals were 11 weeks of age.(a) Representative mice from each group are shown and (b) Results were quantified. Note markedly (35.8%) reduced depigmentation in Ccl22 treated animals (n=6 (EV), n=7 (Ccl22), P<0.05).
Fig. 2. Ccl22 DNA treatment markedly reduced depigmentation among ageing Pmel1 mice.
Twenty-one week old mice were treated with murine Ccl22-encoding DNA or empty vector alone for 5 weeks just at the onset of depigmentation, and were imaged when the pelage returned, 2 weeks after the final treatment. (a) Depigmentation in representative mice after treatment (b) Quantification in all mice evaluated, showing 46.4% reduced depigmentation among treated animals 3 weeks after the last treatment (n=8 (EV), n=11 (Ccl22), P<0.05) (c ) Continued treatment is required for prolonged inhibition of depigmentation, as shown by re-evaluation of the mice under b. 6 weeks after the last treatment (n=7 (EV), n=10 (Ccl22), P=0.09).The relatively slow depigmentation process in the Pmel1 mice allows for a solid comparison of depigmentation at different post-treatment periods.
DNA treatment elevates Ccl22 expression in treated skin
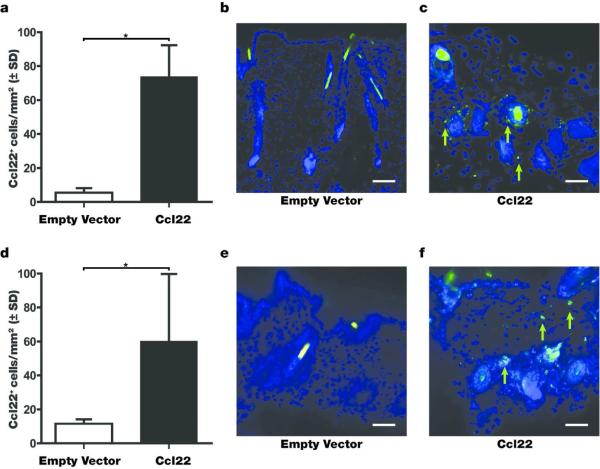
DNA treatment with an expression plasmid encoding Ccl22 cDNA should result in markedly enhanced Ccl22 expression within the skin; here we assessed the lasting effects of Ccl22-encoding DNA treatment on Ccl22 expression. Initially, wild type C57BL/6 mice were gene gun treated 5 times, 5 days apart with 4.8 μg of Ccl22-encoding expression vector DNA prior to harvesting skin tissue in order to establish chemokine expression levels resulting from treatment. As shown in Fig. 3a, a remarkable difference between empty vector DNA treated and Ccl22 treated mice was observed, with chemokine expression virtually absent from untreated mouse skin whereas an increase from 5.4 +/− 4.6, n=3 to 73.7 +/− 32.1, n=3) cells per mm2, representing a 13.9 fold increase was observed after Ccl22 treatment. These differences are further illustrated in Fig. 3b-c, showing empty vector DNA-treated skin compared to skin from a Ccl22 treated mouse. Prompted by these results, we likewise probed the skin of Pmel-1 mice harvested 14 days after the final treatment. In Fig. 3d-f, a 5.2 fold increase from 11.6 +/− 2.4 (n=6) to 60.4 +/− 39.16 (n=6) cells per mm2 in Ccl22 expressing cells is maintained (P=0.014), although differences in depigmentation were no longer significant by then (Fig. 2). These findings indicate that a prolonged period of highly elevated Ccl22 expression is required to keep depigmentation at bay.
Fig. 3. Ccl22 treatment increases Ccl22 expression in treated skin.
C57BL/6 mice were treated with Ccl22 encoding expression vector DNA 5 times before harvesting skin tissue 14 days after the final treatment. (a) The number of Ccl22 expressing cells was 13.9 fold increased in treated skin samples (n=3 (EV and Ccl22), P<0.05). (b) Ccl22 staining before and (c) after treatment. In (d), Ccl22 expressing cells were quantified in the skin of treated Pmel-1 mice, again showing an increase, of 5.2 fold (n=6 (EV), n=6 (Ccl22), P<0.05), with (e) empty vector treated skin and (f) Ccl22 treated skin examples. Scale bar represents 48μm.
Ccl22 treatment enhances Treg recruitment
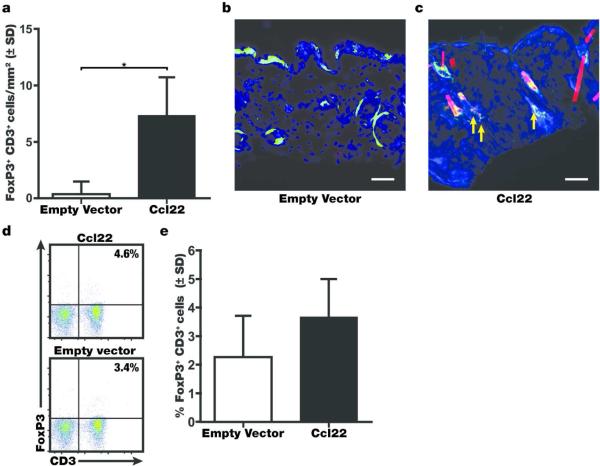
Overexpression of Ccl22 in the skin will generate a chemokine gradient that can support Treg recruitment to the treatment site. Treg abundance in the skin of treated mice was initially evaluated in skin samples of the h3TA2 strain. Recruitment of Treg was markedly increased following Ccl22 treatment, significantly raising the abundance of FoxP3+, CD3+ Treg as measured by immunoenzymatic double staining, and single stainings performed with an antibody to CD25 instead of FoxP3 further supported these findings (not shown). Immunofluorescence was also used to locate CD3+FoxP3+ cells in the skin of ageing Pmel-1 mice treated for 5 weeks, and harvested 2 weeks after the final treatment. Double stained cells appear as yellow in the images (arrows: examples), and these cells were 14.9-fold more abundant after treatment with Ccl22-encoding DNA at 7.34 +/− 3.39 cells/mm2 compared to 0.49+/− 0.86 cells/mm2 in response to empty vector DNA (Fig 4a, n=3, P=0.033). Finally, Treg abundance was measured among splenocytes of treated Pmel1 mice 2 weeks after the final treatment. Of note, peripheral Treg percentages evaluated by fluorometric measurement of FoxP3/CD3 expression analysis of splenocytes shown in Fig. 4e were not significantly increased (P=0.08) by Ccl22 treatment, at average Treg percentages among total T cells of 2.2 +/− 1.5% in the empty vector group (n=4) compared to 3.7 +/− 1.3% in the Ccl22 group (n=5) in the spleens of treated mice. These results suggest that Ccl22 treatment results in enhanced Treg recruitment and slightly increased systemic Treg levels, associated with enhanced abundance of Treg in the skin.
Fig. 4. Treg are increasingly abundant following Ccl22 treatment.
Mice were treated with Ccl22 encoding or empty vector DNA and skin tissue was harvested 2 weeks after the final treatment. (a) The number of FoxP3+CD3+ Treg was quantified in the skin of C57BL/6 mice (n=3 (EV and Ccl22), P<0.05) and representative stainings are shown for (b) empty vector and (c) Ccl22 treated skin of Pmel-1 mice, again showing (15 fold) enhanced Treg infiltration to the skin among treated animals (n=6 (EV and Ccl22), P<0.05). (d) representative FACS histograms show (e) a trend towards a minor increase in Treg abundance among T cells was also observed in the circulation of h3TA2 mice, as measured among splenocytes (n=4 (EV), n=5 (Ccl22), P=0.08). Scale bar represents 48μm.
Enhanced abundance of Treg corresponds to a decreased presence of melanocyte-reactive T cells in treated skin
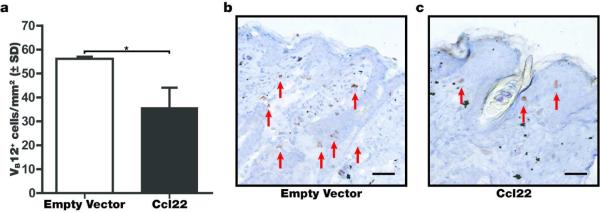
As an initial measure of Treg function, we evaluated the indirect effects on effector T cell abundance in the skin of rapidly depigmenting mice. In vitiligo-prone, h3TA2 mice, melanocyte-reactive T cells that eliminate melanocytes from the skin are readily detectable by immunostaining for the TCR. Reduced abundance of Vβ12 expressing T cells in Ccl22 treated mice is illustrated in Fig. 5, and the number of melanocyte-reactive T cells is quantified among 3 mice per group using skin samples of h3TA2 mice available 3 weeks after the final treatment, when mice were 8 weeks of age. Though Tregs also express the transgenic T cell receptor (Fig. S2), the Treg cohort constitutes a negligible subset of the total Vβ12 expressing T cell numbers observed. The abundance of effector T cells is 36.4% reduced in the skin of Ccl22 treated mice at 35.7 +/− 8.2 cells/mm2 compared to empty vector treated mice at 56.2 +/− 1.0 cells/mm2 (n=3, P=0.024). As CCR4 expression is generally lower among effector T cells when compared to Treg, these data are best explained as an indirect effect of Ccl22 treatment on increased Treg abundance, suppressing effector T cell recruitment to and proliferation within the skin.
Fig. 5. Ccl22 reduces the abundance of melanocyte-reactive T cells in treated skin.
(a) The abundance of melanocyte-reactive T cells in the skin was measured by immunostaining using antibodies to the transgenic TCR-β subunit of h3TA2 mice. Such effector T cells were much more abundant in (b) empty vector treated and (c) 36.4% reduced in Ccl22 treated mice (n=3 (EV and Ccl22), P<0.05). These data suggest that enhanced Treg infiltration can lead to suppression of effector T cell activity and diminished depigmentation in situ. Scale bar represents 48μm.
Enhanced regulatory function is observed in Ccl22 treated mice
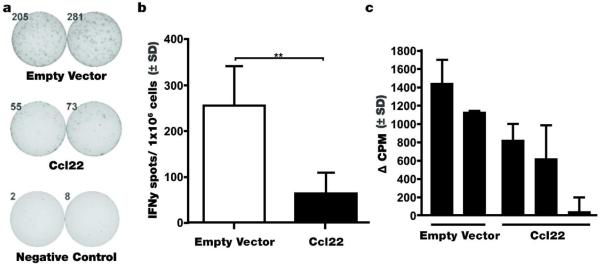
Given the favorable Treg to effector T cell ratio in the skin of Ccl22 DNA treated mice as implied by the abundance of Treg as a percentage of total T cells, associated with reduced depigmentation, we performed functional assays to measure effector functions in treated animals. Splenocytes were harvested from mice treated with Ccl22-encoding or empty vector DNA, and cells were incubated with cognate peptide prior to quantification of IFN-γ secreting T cells by ELISPOT. Representative examples of cytokine-releasing T cells among samples derived from mice either Ccl22 DNA treated, or empty vector DNA treated h3TA2 mice incubated with cognate epitope peptide, hTyr368-376, are shown in Fig. 6a next to quantified ELISPOTS to compare effector functions among both populations, harvested 3 weeks after the last DNA treatment, showing 75.2% reduced T cell activation among the Ccl22-treated group at 63.4 +/− 45.7 spots/well compared to 255.5 +/− 86.2 spots/well in the empty vector control group (n=4, P=0.007). A sample of splenocytes was likewise subjected to proliferation measurements as shown in Fig. 6b. With measurements ranked in order of increasing proliferation, the latter data correspond directly to the ranking of samples in order of an increasing number of IFN-γ releasing T cells in Fig. 6a. These data support a markedly decreased effector function among vitiligo-prone, h3TA2 mice treated with Ccl22.
Fig. 6. Suppressed immune function observed in Ccl22 treated mice in response to Ccl22 DNA treatment.
Splenocytes harvested from h3TA2 mice treated with Ccl22 encoding or empty vector DNA 3 weeks after the last treatment were incubated with and without cognate peptide for 24 hrs (a) the proportion of IFN-gamma secreting cells was measured in presence of 30 μg/ml peptide as illustrated and (b) was 75.2% reduced in response to Ccl22 (n=4 (EV and Ccl22), p<0.01) and (c) proliferation measured by 3H-Thymidine incorporation at 1:4 EGFP Foxp3+Treg: effector h3TA2 cells in response to 10μg/ml hTyr368-376. Decreased proliferation aligned with decreased IFN-γ secretion in the same samples in b.
DISCUSSION
The role of CCL22 in disease pathology has been under investigation. We recently demonstrated that a quantitative increase in Treg can control vitiligo (Chatterjee et al, 2014). In follow-up to our original report of reduced CCL22 expression in vitiligo skin (Klarquist et al, 2010), we proposed that overexpression of CCL22 in depigmenting skin might halt disease progression. This would render patients eligible for repigmentation treatment by autologous transplantation and other means currently available (Wassef et al, 2013). For proof of principle, we overexpressed murine Ccl22 in mouse models of vitiligo. Our data convincingly show that continued expression of the chemokine interferes with disease progression by recruiting Treg to suppress ongoing T cell mediated cytolytic anti-melanocyte responses. To understand the implications of our findings, findings related to CCL22 expression, disease development and treatment should be considered.
Originally known as MDC for ‘macrophage derived cytokine’ (Landi et al., 2014), high MDC expression was shown to discriminate lymphocyte dense Hodgkins lymphoma from classic Hodgkins disease, and chemokine overexpression was held responsible for attracting eosinophils (Hedvat et al., 2001). Meanwhile in solid tumors, PGE2 elevates the production of CCL22, leading to undesirable Treg recruitment (Karavitis et al., 2012). We and others have reported increased CCR4 expression by Treg in melanoma (Klarquist et al., (2009), Pigment Cell Melanoma Res 22: pp 495 (abstract)); here also, therapeutic opportunities exist, as Abs to CCR4/CCR10 prevent Treg migration into inflamed skin (Wang et al., 2010; Sugiyama et al., 2013).
By contrast, for a number of autoimmune diseases, investigators consider reduced CCL22 as a factor in disease pathology. Newly diagnosed multiple sclerosis patients, for example, display lowered serum levels of CCL22 (Jafarzadeh et al., 2014). Yet no such difference was found in Addison's disease (Ekman et al., 2014). Such discrepancies may relate to the predominant immune mechanism driving either disease, namely T cell mediated versus humoral immunity, involving different regulatory mechanisms. In this respect, CCL22 is also a Th2 attractant, and its expression correlates with lymphocytic infiltration in Sjögrens syndrome (Moriyama et al., 2012).
For diseases involving reduced Treg recruitment to affected tissues, new developments include the use of random amino acid copolymers thought to stimulate CCL22 expression by APCs, which are currently under development for the treatment of MS (Koenig et al., 2013). Increased CCL22 secretion can be assigned to DCs, possibly of the CD11b+CD11c+ subtype more so than from macrophages in response to copolymer administration (Kawamoto et al., 2013). For the current study, the significance of this is that CD11b+CD11c+ DCs are readily available in vitiligo (Mosenson et al., 2013). Another strategy advocated to date is adenoviral overexpression of CCL22 for diabetes treatment, showing successful Treg recruitment and protection from diabetes development in NOD mice (Montane et al., 2011). A limitation to its application for diabetes is the loss of insulin producing cells from the pancreas before symptoms arise (La Torre, 2012).
The suggested involvement of CCL22 in autoimmune disease, including multiple sclerosis, remains controversial, and the opposite reasoning has also been advocated. As CCL22 recruits macrophages, anti-CCL22 treatment was used in a mouse model of MS to delay onset and dampen disease severity (Dogan et al., 2011). Again, this holds relevance for vitiligo, as macrophages are likewise abundant in the perimeter of advancing skin lesions (Le Poole et al., 1996). What this inhibitory strategy has in common with our DNA treatment strategy, is that both require continued application to remain efficacious (Dogan et al., 2011; Forde et al., 2011).
Another means of manipulating CCL22 expression can be administration of vitamin D. Expression of CCL22 is elevated in response to 1α,25-(OH)(2)D(3), which again, can be therapeutic for T cell mediated autoimmune disease but not humoral autoimmune disease (Kuo et al., 2010). In fact, type 2 cytokines (i.e. IL-4) increase CCL22 secretion, suggesting that Treg responses are not affected in antibody-mediated autoimmune disease (Butti et al., 2008). The use of topical vitamin D3 analogs for the treatment of vitiligo is however unlikely to become mainstream until vitiligo disease activity is better defined, because responses to such treatment tend to be slow and thus, underappreciated (Parsad and Kanwar, 2009). The same concept of supporting CCL22 responses to drive Th2 responses (in combination with antigen administration) has shown promise in Alzheimers (Movsesyan et al., 2008).
Here, we demonstrate that overexpression of Ccl22 in the skin can help to recruit Tregs and leads to suppression of ongoing depigmentation for the duration of treatment in TCR transgenic animal models of vitiligo. This holds true despite somewhat reduced abundance of Tregs in one of the models engaged in our studies. As reported previously, the 1383i receptor expressed in h3TA2 mice functions irrespective of CD4 or CD8 coreceptor expression (Mehrotra et al, 2012), and can likewise function in Treg (Brusko et al, 2010).
Taken together, we conclude that inhibition of the chemokine can be of value for diseases where enhancement of T cell mediated immunity is desirable, whereas forced overexpression of CCL22 has potential for vitiligo and other conditions involving hyperactive T cell mediated immunity. The chemokine is best overexpressed within or in close proximity to affected tissues, but responses can be measured away from the application site as well. In this regard, we measured enhanced Treg recruitment to the skin, reduced abundance of effector Tells, and suppression of T cell effector functions. Whereas DNA-based treatments are generally met with some hesitation, several applications exist that show promise for disease treatment. The Treg chemo-attractant cytokine CCL22 is thus of great interest as a potential therapeutic for vitiligo in active phases of the disease.
MATERIALS AND METHODS
Murine Treg in vitro assays
Splenocytes (1 × 106 cells/well) from FoxP3-eGFP/B6 mice (The Jackson Laboratory, Bar Harbor, ME) were plated in 5 Nm pore size transwells (Cell Biolab, Inc., San Diego, CA, USA). RPMI 1640 with 10% FBS and antibiotics (Life Technologies, Grand Island, NY, USA) was added to the outer well in the presence or absence of 100-500ng/ml mouse Ccl22 (R&D Systems, Minneapolis, MN, USA). Cells were allowed to migrate for 2 hrs before harvesting cells from the bottom well (Klarquist et al, 2010; Justus et al, 2014). Cells from top and bottom wells and a splenocyte sample not included in the migration assay were subjected to immunofluorescent staining to detect Treg, using 145-2C11, RM4-5, and PC61 antibodies to CD3, CD4, and CD25, respectively (BD Biosciences, San Jose, CA, USA). Splenocytes were sorted using a BD FACS Aria III sorter (Becton Dickinson, Franklin Lakes, NJ, USA). Sorted Treg from reporter mice were combined with effector cells from TCR transgenic mice recognizing gp10022-35 (Pmel1 mice) or human tyrosinase368-376 (h3TA2 mice) in IFN-gamma ELISPOT plates in the presence of cognate peptide. The resulting suppression by Treg cells was quantified by comparing to the spot numbers produced by the same number of effector cells without Treg cells.
DNA bullet preparation
Full length primers to amplify the mouse Ccl22 encoding gene were 5’ATGGCTACCCTGCGTGTCCCACTC3’ and 5’ CTAGGACAGTTTATGGAGTAGCTTCTTCACCCAG3’. The PCR product was isolated from gel after amplification of cDNA, prepared using RNA extracted from cultured B16 melanoma cells CRL-6475 (ATCC, Manassas, VA). Resulting DNA was ligated into the TA expression vector CT-GFP TOPO (Invitrogen, Grand Island, NY) and sequenced using a T7 primer (Invitrogen). This DNA and empty vector control DNA was used to coat 1μm gold particles (Alfa Aesar, Ward Hill, MA) in presence of spermidine (Sigma Aldrich, St. Louis, MO). Expression vector-gold complexes were used to coat Teflon bullets (BioRad, Hercules, CA), containing 1 μg of expression plasmid DNA each. Bullets were stored under vacuum desiccation and used within 14 days.
Mouse experiments
Experiments involving mice were conducted as approved by the Institutional Animal Care and Use Committees of Loyola University Chicago or the Medical University of South Carolina. C57BL/6 mice and Pmel-1 mice, transgenic for a gp100-reactive T cell receptor (Overwijk et al., 2003), were obtained from Jackson Labs (Bar Harbor, ME, USA). In Pmel-1 mice, the transgenic TCR which is encoded on chromosome 2 expresses an H2-Db restricted T cell receptor consisting of Tcrα-V1 and Tcrβ-V13 recognizing pmel-17 amino acids 25-33 by >95% of CD8 T cells. These mice express FoxP3 among an increased percentage of CD4 T cells (Quatromoni et al, 2011). A third strain of mice, h3TA2, is transgenic for a tyrosinase-reactive T cell receptor and for human HLA-A2 (Mehrotra et al., 2012) and was bred in house. In these mice, a transgenic TCR of human origin consisting of TCRα-V4 and TCRβ-V12 expressed by >80% of CD3+ T cells effectively recognizes the human HLA-A*0201 restricted, tyrosinase-derived peptide 368-376 and displays cross reactivity towards its mouse homologue. The Treg population occupies about 2% of the splenocyte population and their abundance is approximately 3-fold enriched in the absence of IFN-γ (Chatterjee et al, 2014). The Pmel-1 strain shows spontaneous depigmentation starting at 6 months of age; h3TA2 mice exhibit rapid spontaneous depigmentation initiating as early as 4 weeks of age, and these mice continue to rapidly depigment. All mice were used in experiments at 3-6 mice per group as indicated. Most animals were treated starting at 5 weeks of age; some groups of Pmel-1 mice, however, were treated starting at 21 weeks of age. At least 3 repeat experiments were performed for each mouse strain. Mice were ventrally naired (Church & Dwight Co Inc., Ewing, NJ, USA) as needed and treated five times using empty vector coated bullets or Ccl22 encoding bullets prepared as described above, 4.8 μg DNA every 5 days. To test the role of Ccl22 in an inducible vitiligo model (Denman et al, 2008), C57BL/6 mice were treated with bullets containing both epitope-optimized TRP-1ee/ng encoding DNA (Guevara-Patino et al, 2006) as an immunogen, and Ccl22 encoding DNA while the control group received the same TRP-1 encoding DNA combined with empty vector DNA. Wild type, C57BL/6 mice were included solely to test, whether Ccl22 overexpression and Treg migration can likewise be impacted in animals not naturally prone to develop depigmentation.
Treg proliferation assays
Splenocytes (5 × 105) from h3TA mice treated with Ccl22-encoding or empty vector DNA were cultured in 96-well microculture plates in RPMI 1640 containing 0.5% syngeneic mouse serum and 5 × 10−5 M 2-mercaptoethanol. Triplicate cultures were stimulated with 10μg/ml of cognate peptide (tyrosinase368-376) for 72 h. Cultures were then pulsed with 1μCi of 3H-thymidine (ICN Biochemicals, Costa Mesa, CA) and harvested 18 h later. Measurements of 3H-thymidine incorporation into DNA were expressed as Δ cpm ( = counts per minute in response to cognate peptide stimulation – counts per minute of unstimulated control samples).
Depigmentation analysis
Depigmentation was measured in mice receiving Ccl22 encoding DNA or vector only, starting 2 weeks after the final treatment as hair returned. Depigmentation was followed for 6 weeks. Mice were placed on a flatbed scanner (Hewlett-Packard Company, Palo Alto, CA) and the ventral and dorsal sides of the mice were imaged. The images were captured in Adobe Photoshop Software (Adobe Systems, Inc, San Jose, CA). Histograms were prepared essentially as described, displaying mice before treatment to capture a cutoff point incorporating 95% of pixels to reflect pigmented pelage (to allow for small areas not covered by pigmented hair). The increase in the proportion of pixels crossing the cutoff value reflects the % depigmentation of mice after treatment (Denman et al., 2008).
ELISPOT analysis, fluorocytometry and immunohistochemistry
Cytokine production was measured directly from cultured splenocytes of treated mice. We quantified peptide reactive T cells by ELISPOT kits (MABTECH, Mariemont, OH). Briefly, plates were coated with capture anti-IFN-γ antibodies. Splenocytes were cultured in RPMI1640 plus 10%FBS in presence of up to 30μg/ml cognate peptides (Pi Proteomic, LLC, Huntsville, AL) or PBS. After 24h, cytokine secreting cells were detected using biotinylated anti-cytokine antibodies, followed by horseradish peroxidase-conjugated streptavidin (MABTECH) and 3-amino-9-ethylcarbazole substrate (Sigma-Aldrich, St Louis, MO). Cytokine secreting cells were quantified using ELISPOT equipment (Cellular Technology Ltd, Cleveland, OH). Results were compared for animals treated with and without Ccl22 encoding DNA for the treated mice.
Meanwhile, skin harvested from treated animals was originally snap frozen and stained for Ccl22. The number of Ccl22 expressing cells per area was compared for mice that were or were not treated with Ccl22 encoding DNA. For comparison, spleens were snap frozen and 8 μm cryosections were fixed in 4% paraformaldehyde and subjected to Ccl22 immunostaining using FITC-labeled primary antibodies (ABCD1, Bioss, Woburn, MA). We also stained skin with antibodies to CD3 (FITC-labeled 145-2C11; BD-Pharmingen), to human Vβ12 binding to the tyrosinase-reactive T cell receptor (FITC-labeled 511; ThermoScientific, Hanover Park, IL, USA ) and FoxP3 (Biotin-labeled FJK-16s; eBioscience) with subsequent detection using streptavidin-PE (Southern Biotech, Birmingham, AL) to quantify total T cell infiltration and Treg populations in the skin. Fluorescent cells were captured using an Olympus AX80T Microscope (Melville, NY, USA) and images were analyzed in Adobe Photoshop to quantify the number of stained cells and the tissue area >9 images per staining.
Cell surface markers were evaluated among red blood cell-depleted splenocytes from a 6-week-old h3T, TCR transgenic mouse using antibodies CD3-APC-Cy7 (145-2C11; BioLegend, San Diego, CA), CD25-PerCp-Cy5.5 (PC61; BD Biosciences, San Jose, CA), CCR4-PE-Cy7 (2G12; BioLegend), and Vβ12-FITC (511; Thermo Scientific, Hanover Park, IL). For intranuclear detection of transcription factor FoxP3, paraformaldehyde-fixed splenocytes were incubated in the presence of 0.3% saponin and FoxP3-APC (FJK-16s; eBioscience, Sand Diego, CA) antibody before being detected on a LSR-II flow cytometer (BD Biosciences).
Statistics
Data were presented as mean± SD throughout the manuscript and analyzed for statistical significance of differences among two groups using Student's t-tests accounting for unequal variance.
Supplementary Material
ACKNOWLEDGEMENTS
These studies were supported by NIH R01AR057643 to CLP.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Brusko TM, Koya RC, Zhu S, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One. 2010;5:e11726. doi: 10.1371/journal.pone.0011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butti E, Bergami A, Recchia A, et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 2008;15:504–15. doi: 10.1038/gt.2008.10. [DOI] [PubMed] [Google Scholar]
- Cedercreutz K, Denman CJ, Klarquist J, et al. Vitiligo etiology and treatment: Parameters derived from a patient survey. J Dermatol Nurses Assoc. 2010;2:265–72. [Google Scholar]
- Chatterjee S, Eby JM, Al-Khami AA, et al. A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol. 2014;134:1285–94. doi: 10.1038/jid.2013.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman CJ, McCracken J, Hariharan V, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–8. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan RN, Long N, Forde E, et al. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J Leukoc Biol. 2011;89:93–104. doi: 10.1189/jlb.0810442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman B, Alstrand N, Bachrach-Lindström M, et al. Altered chemokine Th1/Th2 balance in Addison's disease: relationship with hydrocortisone dosing and quality of life. Horm Metab Res. 2014;46:48–53. doi: 10.1055/s-0033-1351291. [DOI] [PubMed] [Google Scholar]
- Forde EA, Dogan RN, Karpus WJ. CCR4 contributes to the pathogenesis of experimental autoimmune encephalomyelitis by regulating inflammatory macrophage function. J Neuroimmunol. 2011;236:17–26. doi: 10.1016/j.jneuroim.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Patiño JA, Engelhorn ME, Turk MJ, et al. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116:1382–90. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat CV, Jaffe ES, Qin J, et al. Macrophage-derived chemokine expression in classical Hodgkin's lymphoma: application of tissue microarrays. Mod Pathol. 2001;14:1270–6. doi: 10.1038/modpathol.3880473. [DOI] [PubMed] [Google Scholar]
- Hwang LN, Yu Z, Palmer DC, et al. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–8. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh A, Ebrahimi HA, Bagherzadeh S, et al. Lower serum levels of Th2-related chemokine CCL22 in women patients with multiple sclerosis: a comparison between patients and healthy women. Inflammation. 2014;37:604–10. doi: 10.1007/s10753-013-9775-z. [DOI] [PubMed] [Google Scholar]
- Jeon IK, Park CJ, Lee MH, et al. A Multicenter Collaborative Study by the Korean Society of Vitiligo about Patients’ Occupations and the Provoking Factors of Vitiligo. Ann Dermatol. 2014;26:349–56. doi: 10.5021/ad.2014.26.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014;88:e51046. doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Hix LM, Shi YH, et al. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto N, Ohnishi H, Kondo N, et al. The role of dendritic cells in the generation of CD4(+) CD25(HI) Foxp3(+) T cells induced by amino acid copolymers. Int Immunol. 2013;25:53–65. doi: 10.1093/intimm/dxs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarquist J, Denman CJ, Hernandez C, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–86. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig PA, Spooner E, Kawamoto N, et al. Amino acid copolymers that alleviate experimental autoimmune encephalomyelitis in vivo interact with heparan sulfates and glycoprotein 96 in APCs. J Immunol. 2013;191:208–16. doi: 10.4049/jimmunol.1300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YT, Kuo CH, Lam KP, et al. Effects of vitamin D3 on expression of tumor necrosis factor-alpha and chemokines by monocytes. J Food Sci. 2010;75:H200–4. doi: 10.1111/j.1750-3841.2010.01704.x. [DOI] [PubMed] [Google Scholar]
- La Torre D. Immunobiology of beta-cell destruction. Adv Exp Med Biol. 2012;771:194–218. doi: 10.1007/978-1-4614-5441-0_16. [DOI] [PubMed] [Google Scholar]
- Landi A, Weismuller TJ, Lankisch TO, et al. Differential serum levels of eosinophilic eotaxins in primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis. J Interferon Cytokine Res. 2014;34:204–14. doi: 10.1089/jir.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poole C, Boissy RE. Vitiligo. Semin Cutan Med Surg. 1997 Mar;16(1):3–14. doi: 10.1016/s1085-5629(97)80030-2. 1997. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, van den Wijngaard RM, Westerhof W, et al. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–28. [PMC free article] [PubMed] [Google Scholar]
- Lili Y, Yi W, Ji Y, et al. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One. 2012;7:e37513. doi: 10.1371/journal.pone.0037513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S, Al-Khami AA, Klarquist J, et al. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J Immunol. 2012;189:1627–38. doi: 10.4049/jimmunol.1103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrücker HW, Kaufmann SH. Mini-review: regulatory T cells and infection: suppression revisited. Eur J Immunol. 2004;34:306–12. doi: 10.1002/eji.200324578. [DOI] [PubMed] [Google Scholar]
- Montane J, Bischoff L, Soukhatcheva G, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest. 2011;121:3024–8. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Hayashida JN, Toyoshima T, et al. Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjögren's syndrome. Clin Exp Immunol. 2012;169:17–26. doi: 10.1111/j.1365-2249.2012.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosenson JA, Zloza A, Nieland JD, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5:174ra28. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan N, Ghochikyan A, Mkrtichyan M, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS One. 2008;3:e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstain SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir C, Akdis M, Akdis CA. T regulatory cells and their counterparts: masters of immune regulation. Clin Exp Allergy. 2009;39:626–39. doi: 10.1111/j.1365-2222.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- Parsad D, Kanwar AJ. Topical vitamin D3 analogs in the treatment of vitiligo. Pigment Cell Melanoma Res. 2009;22:487–88. doi: 10.1111/j.1755-148X.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Jenks JA, Bégin P, et al. Regulatory T cells and their roles in immune dysregulation and allergy. Immunol Res. 2014;58:358–68. doi: 10.1007/s12026-014-8512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatromoni JG, Morris LF, Donahue TR, et al. T cell receptor transgenic lymphocytes infiltrating murine tumors are not induced to express foxp3. J Hematol Oncol. 2011;4:48. doi: 10.1186/1756-8722-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura C, Satoh T, Igawa K, et al. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. Am J Pathol. 2010;176:227–37. doi: 10.2353/ajpath.2010.090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945–50. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taams LS, Akbar AN. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr Top Microbiol Immunol. 2005;293:115–31. doi: 10.1007/3-540-27702-1_6. [DOI] [PubMed] [Google Scholar]
- Tour SK, Thomas KS, Walker DM, et al. Survey and online discussion groups to develop a patient-rated outcome measure on acceptability of treatment response in vitiligo. BMC Dermatol. 2014;14:10. doi: 10.1186/1471-5945-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef C, Lombardi A, Khokher S, et al. Vitiligo surgical, laser, and alternative therapies: a review and case series. J Drugs Dermatol. 2013;12:685–91. [PubMed] [Google Scholar]
- Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2014 Aug 2; doi: 10.1093/intimm/dxu079. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.