Abstract
Indian cuisine is renowned and celebrated throughout the world for its spicy treat to the tongue. The flavor and aroma of the food generated due to the use of spices creates an indelible experience. Among the commonly utilized spices to stimulate the taste buds in Indian food, whole or powdered chilli constitutes an inevitable position. Besides being a vital ingredient of of Indian food, chilli occupy an important position as an economic commodity, a major share in Indian economy. Chilli also has uncountable benefits to human health. Fresh green chilli fruits contain more Vitamin C than found in citrus fruits, while red chilli fruits have more Vitamin A content than as found in carrots. The active component of the spice, Capsaicin possesses the antioxidant, anti-mutagenic, anti-carcinogenic and immunosuppressive activities having ability to inhibit bacterial growth and platelet aggregation. Though introduced by the Portuguese in the Seventeenth century, India has been one of the major producers and exporters of this crop. During 2010–2011, India was the leading exporter and producer of chilli in the world, but recently due to a decline in chilli production, it stands at third position in terms of its production. The decline in chilli production has been attributed to the diseases linked with crop like anthracnose or fruit rot causing the major share of crop loss. The disease causes severe damage to both mature fruits in the field as well as during their storage under favorable conditions, which amplifies the loss in yield and overall production of the crop. This review gives an account of the loss in production and yield procured in chili cultivation due to anthracnose disease in Indian sub-continent, with emphasis given to the sustainable management strategies against the conventionally recommended control for the disease. Also, the review highlights the various pathogenic species of Colletotrichum spp, the causal agent of the disease, associated with the host crop in the country. The information in the review will prove of immense importance for the groups targeting the problem, for giving a collective information on various aspects of the epidemiology and management of the disease.
Keywords: anthracnose, Capsicum spp., Colletotrichum capsici, epidemiology, disease management, biocontrol
Introduction
Chilli (Capsicum annum L.) is one of the most important constituent of the cuisines of tropical and subtropical countries and the fourth major crop cultivated globally. Around 400 different varieties of chilies are cultivated throughout the globe. The hottest variety being “Carolina Reaper” developed by a grower Ed Currie of West Indies having the maximum pungency of about 2.2 million SHU (Scoville Heat Units; PuckerButt Pepper Company, 2013). One of the hot chilli varieties of the world “Naga Jalokia,” is the native of Tezpur in Assam, India. Numerous varieties of chilli are grown for vegetables, spices, condiments, sauces, and pickles occupying an indispensable position in Indian diet. Apart from the explicit importance of the crop in the diet, chilli is also used in other forms like medicines and beverages and also as an ornamental plant in the gardens. Nutrition wise these are enriched with high Vitamin A and C content; high iron, potassium, and magnesium content with the ability to boost the immune system and lower the cholesterol levels (Grubben and Mohamed El, 2004). India has been a leading producer, consumer and exporter of chilli especially in dried form. Various varieties of the crop are found in India and its quality varies among the states of the country.
The host crop-capsicum annum L.
Known for over 9500 years, chilli is the native of Southern America and was first cultivated in Peru at around 7500 BC (MacNeish, 1964). It has been the first ever domesticated crop of America. During the course of evolution, three important species of Capsicum i.e., C. annuum, C. frutescens and C. chinense evolved from a common ancestor that grew wildly in the North of the Amazon basin (NW-Brazil, Columbia) spreading to the other parts of America. Initially, people cultivated them with other crops in order to protect their crop from the damage caused by birds. By the end of eighteenth century five species of chilli i.e., C. annuum L., C. baccatum L., C. chinense Jacq., C. frutescens L., and C. pubescens R. & P. were domesticated in different parts of the America (IBPGR, 1983).
Introduction of chilli to India is being credited to the voyage of Columbus, who brought the seeds from Spain, introducing it to Europe, which subsequently spread to Africa and Asia (Heiser, 1976). However, Columbus confused the pungent fruits of Capsicum with black pepper Piper nigrum L. calling them as red pepper owing to the red colored fruits. Capsicum is not related to the Piper genus. The popularity of the crop extended swiftly across Europe is moving to India, China and then to Japan. It was incorporated into all the Asian and European cuisines almost instantaneously unlike the other spices introduced from the Western Hemisphere. Since its introduction, it has been cultivated aggressively in all the tropical and sub-tropical countries.
In its course of evolution staring from Central America to the whole of Europe, Africa and Asia, copious terminologies have been used to name the pungent fruits. The phraseology in case of Capsicum is however very confusing. Names like pepper, chili, chile, chilli, aji, paprika are used to denote the pungent fruits of Capsicum. The term “Capsicum” is reserved for taxonomic discussion. The vernacular names include pepper, chile, and aji. The common term used to denote chilli in India is “Mirchi” in Hindi. Bell pepper generally refers to the blocky non pungent fruits of Capsicum known as “Shimla Mirch” in Hindi.
Belonging to the Solanaceae family, Capsicum genus consists of approximately 22 wild species and five domesticated species (Bosland, 1994). Having chromosome number 2n = 24, Capsicum species may be herb or sub-shrub of height up to 2.5 m with extensively branched stem having hairy growth with purplish spots near the nodes. The tap root is strong with numerous lateral roots. Flowers are generally solitary, terminal, bisexual and pentamerous with campanulate to rotate corolla. Stamens are adnate at the base of the corolla tube with blue to purplish anthers. The ovary is superior having 2–4 chambers. Filiform style is found with capitate stigma. Chilli fruits are considered vegetable and are botanically berries (Figure 1).
Figure 1.

The botanical characteristics of Capsicum annum L., the chilli plant (A), the flower (B), immature green fruits (C) and the ripe red fruits (D).
Based on the fruit characteristics, i.e., color, pungency, shape, flavor, size, and their use, the types of chilli are classified (Bosland, 1992, 1996). It is a perennial crop and can be grown throughout the year. The major harvest season is between December and March with its supply reaching maximum during February-April (Bosland, 1996).
Uses and importance
Chilli is used in all forms starting from fresh green fruits with ripe fruits along with its dried and powdered form. Fresh green pungent fruits are generally used in salads, stuffing, and as a flavoring agent in cooked meals. The non-pungent varieties are cooked as vegetables or processed with other food items for flavor (Welbaum, 2015). Very pungent varieties are consumed in small quantities generally considered as a condiment or spice for seasoning and for stimulating appetite. Hot peppers are also pickled in salt and vinegar used in ketchups as flavoring agents (Grubben and Mohamed El, 2004). Apart from its extensive use in preparation of processed food, chilli varieties are also used as coloring agents in salad dressings, meat products, cosmetics, and even clothing.
Capsicum possesses various medicinal and nutritional values as well. It is interesting to quote that fresh green chilli fruits contain more Vitamin C than found in citrus fruits, while red chilli fruits have more Vitamin A content than as found in carrots (Osuna-García et al., 1998; Than et al., 2008). The active ingredient of the spice, capsaicin is a complex of capsaicinoid alkaloids found in variable concentration in different chilli varieties. It is found in abundance in the placental tissues and cross walls of the fruits. However, in very pungent fruits, it is distributed in all the fleshy parts of the fruit (Grubben and Mohamed El, 2004). The amount of capsaicin has been the measure of the pungency of the chilli variety and is generally expressed in Scoville Heat Units (SHU) (Scoville, 1912).
Capsaicin possesses the antioxidant, anti-mutagenic, anti-carcinogenic, and immunosuppressive activities having the ability to inhibit bacterial growth and platelet aggregation. It is also used as anti-arthritic and anti-inflammatory agent. Regular consumption of chilli fruit is helpful against hemorrhoids, varicose veins, anorexia, and liver congestion. Pure and processed form of chilli extracts is used externally as rubefacient and analgesic in case of back pain, rheumatism, articular and muscular pains and swollen feet and even as antidote in case of poisoning. The two non-foods and non-pharmacological application of capsaicin are in the preparation of “pepper sprays” as weapons of self-defense and in preparation of “natural and organic pesticide” (Welbaum, 2015).
Global production and indian share
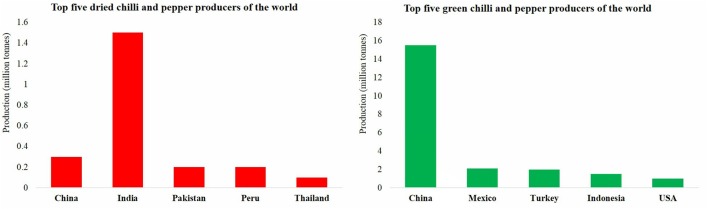
Being an important crop of tropical and sub-tropical countries, cultivated in large areas of Asia, Africa, South, and Central America and southern Europe, the total area under cultivation in the world is about 1.9 million hectares (Vanitha et al., 2013). About 42.2% (801,600 ha) cultivable land of the total area under chilli production at world level lies in India, producing approximately 21.4% (1.4 million t) of world's total chilli production (FAOSTAT, 2012). India has been the major producer as well as exporter of the dried chilli in the world (Figure 2). The other important producers of chilli include China, Mexico, Peru, Turkey, Thailand, Indonesia followed by countries in tropical Africa mainly Ghana and Ethiopia.
Figure 2.
Production of chilli and pepper (dried and green) in top five chilli producing countries in the world (FAOSTAT, 2011).
Chilli is a universal spice crop of India grown in almost all the states of the country. The quality of the chilli varies from state to state. For instance, chilli of Karnataka is known for its oil content, Gujarat quality is majorly known for its sharp color while that of Rajasthan is well known for making pickles. The chilli cultivated in Assam is famous for its pungency and that grown in Andhra Pradesh is mostly used as vegetables and liked by non-vegetarians. Major chilli producing states include Andhra Pradesh, Maharashtra, Karnataka, and Madhya Pradesh (Post harvest profile of chilli, 2009). Apart from being a large consumer and producer of chilli, India is also the largest exporter of the crop. Over 30% of the chilli produced in India is exported to countries of West Asia, East Asia, USA, Sri Lanka, and Bangladesh, most commonly in dried form (FAOSTAT, 2012).
Constraints in chilli production
Though being an important spice crop grown worldwide, many constraints decrease production, causing significant reduction in yield and seed production. Plant diseases have been a major reason for the crop losses worldwide. Diseases caused by fungi, bacteria, viruses or nematodes have adversely affected chilli production in almost all parts of the world. A summary of the various diseases associated with the host crop chilli is outlined in Table 1.
Table 1.
Diseases of chilli reported from different parts of the world.
| S. No. | Disease | Causal organism | Symptoms | Plant parts affected | Countries affected | References |
|---|---|---|---|---|---|---|
| FUNGAL DISEASES | ||||||
| 1. | Fruit Rot | Colletotrichum truncatum (capsici) Colletotrichum gleosporoides Colletotrichum acutatum | Water soaked and sunken lesions with characteristic rings of acervuli in concentric rings | Leaf, stem, and fruits | Tropical and sub-tropical countries | Than et al., 2008; Saxena et al., 2014 |
| 2. | Cercospora leaf spot or velvet spot | Cercospora capsici Cercospora unonidicola | Small brown and circular lesions with light gray center and dark margins | Leaf, stem | Worldwide with most severe in warm and moist conditions | Cerkauskas, 2004 |
| 3. | Phytophthora blight | Phytophthora capsici | Affects root and lower portion of the stem leading to wilting | Leaf, stem, and fruits | South Korea and countries with high humidity and summer rainfall | Sanogo and Carpenter, 2006 |
| 4. | Damping off | Pythium spp. Fusarium spp. Sclerotinia spp. | Death of seedlings and subsequent reductions of plant stands | Roots and crown of older plants | Worldwide | Koike et al., 2007 |
| 5. | Wilting | Verticillium dahliae | stunting, defoliation, and wilting, with discoloration of the vascular system | Whole plant | World wide | Sanogo, 2003 |
| 6. | Powdery mildew | Leveillula taurica | Chlorotic blotches and spots on leaves followed by their shedding | leaves | Places with warm and dry climate | Glawe et al., 2010 |
| 7. | Root rot | Rhizoctonia solani | wilting and death | Lower region of stem and root | Worldwide | Sanogo, 2003 |
| 8. | Fusarium wilt | Fusarium solani var. capsici | leaf chlorosis, vascular discoloration, and wilting | leaf | New Mexico | Crawford, 1934 |
| BACTERIAL DISEASES | ||||||
| 1. | Bacterial wilt | Ralstonia solanacearum | Browning of roots and lower part of stem leading to wilting of plant | Root, Stem | Tropical and subtropical countries with high rainfall | Nguyen and Ranamukhaarachchi, 2010 |
| 2. | Bacterial spot | Xanthomonas campestris pv. vesicatoria | Water soaked lesions on leaves that turn brown, patches on fruits and stem | Leaves, stem, and fruit | Tropical and sub-tropical countries | Abbasi et al., 2002 |
| 3. | Bacterial canker | Corynebacterium michiganense | Light brown and raised lesions | Leaves, stem | USA | Ivey and Miller, 2000 |
| 4. | Bacterial soft rot | Erwinia carotovora | Softening of tissues | Fruits | Areas with wet and cold climate conditions | Stommel et al., 1996 |
| VIRAL DISEASES | ||||||
| 1. | Pepper leaf curl virus (PLCV) | Whitefly transmitted Geminivirus | Extensive yellowing of leaves with stunted growth | Leaves, stem | India, United States, Nigeria, South Asian Countries | Chattopadhyay et al., 2008 |
| 2. | Pepper veinal mottle virus (PVMV) | Aphid transmitted Potyvirus | Veinal and intraveinal chlorosis with stunted leaves and fruits | Leaves, Fruits | Afghanistan, Africa, and India | Berger et al., 2005; Arogundade et al., 2012 |
| 3. | Alfalfa mosaic virus (AMV) | Aphid transmitted Bacilliform virus | Distinct white to yellow calico pattern mosaic on leaves | Leaves | New Zealand | Fletcher, 1983 |
| 4. | Pepper mottle virus (PeMV) | Aphid transmitted Potyvirus | Mottled leaves with green vein banding and distortion | leaves | Florida, Arizona, Southern USA, Mexico, Central America, India, Thailand | Kaur et al., 2014 |
| 5. | Beet curly top virus (BCTV) | Leaf hopper transmitted Geminivirus | Stunted and yellowed plants | Whole plant | Western United States, Eastern Mediterranean basin | Stanley, 2008 |
| 6. | Pepper severe mosaic virus (PepSMV) | Aphid transmitted Potyvirus | Leaf shedding, necrotic streaks and spots on fruits, stem, and leaves | Leaf, stem, fruits | Argentina | Ahn et al., 2006 |
| 7. | Chilli veinal mottle virus | Aphid transmitted Potyvirus | Mottled leaves with green vein banding, mottled, and distorted pods | Leaf, fruits | Asian countries | Moury et al., 2005 |
| NEMATODE INFECTIONS | ||||||
| 1. | Root knot | Meloidogyne incognita | Stunted growth, low flowering and yield | Roots, fruits | Different parts of the world | Thiyagarajan and Kuppusamy, 2014 |
| INSECTS AND MITE INFECTION | ||||||
| 1. | Mite feeding injury | Polyphagotarsonemus latus | “Inverted spoon” shaped leaves, Pods with rusty/corky surface | Leaves, fruits | Australia, Asia, Africa, Europe, North and South America, Pacific Islands | Venzon et al., 2008 |
| 2. | Thrips feeding injury | Thrips parvispinus Scirtothrips dorsalis | “Boat shaped” curled leaves, distorted pods | Leaves, fruits | India, Sri Lanka, Orient and Pacific Islands, Continental USA | Maharijaya et al., 2011; Johari et al., 2014 |
| 3. | Aphid feeding injury | Myzus persicae Aphis gossypii | Distorted, mottled young leaves, chlorosis, leaf drop, reduced fruit size | Leaves, fruits | World wide | Tapia et al., 2008; Varghese and Mathew, 2012 |
Other than the losses due to the pests and pathogens, crop loss in post-harvest conditions further add in delimiting the yield and production of the crop (Prusky, 2011). Specifically, in developing countries, the post-harvest losses are more serious owing to poor transportation and storage facilities (Sharma et al., 2009). Moreover, due to the recent strengthening of the food security norms in the international markets, the trade of food contaminated with fungal toxins, mycotoxins has been declared unhealthy for human consumption (WHO, 2002). Species belonging to Aspergillus genus like A. flavus (Bankole et al., 2004) and A. parasiticus (Garcia-Villanova et al., 2004) have been majorly kept responsible for production of harmful aflatoxins B and G, whose contamination in food commodities has led to serious health problems in human, like cancer and aflatoxicosis (Jeffrey and Williams, 2005). Mycotoxin contamination in dried chillies has limited its export to major export destinations like the UK and USA. Post harvest loss due to aflatoxin contamination in chilli has been reported to be about 20% to about 100% of samples obtained from Turkey (Demircioglu and Filazi, 2010) and Malaysia (Reddy et al., 2011).
Among the large number of diseases affecting chilli cultivation, anthracnose disease caused by Colletotrichum species, bacterial wilt by Psuedomonas solanacearum and viral diseases like chilli veinal mottle virus (CVMV) infection and cucumber mosaic virus (CMV) infection have been most detrimental to chilli production (Than et al., 2008).
Anthracnose disease of chilli
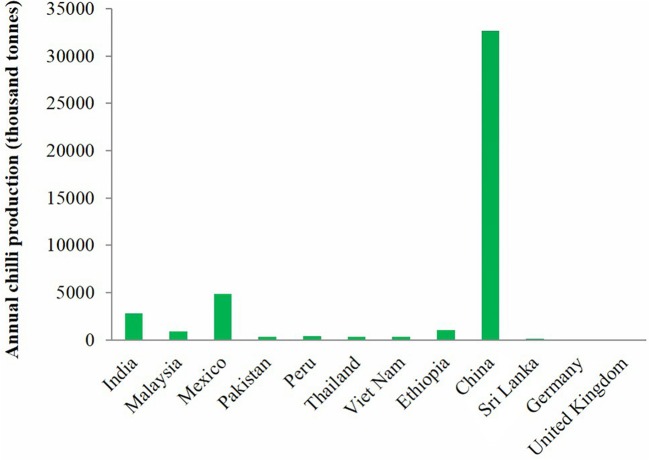
Anthracnose disease has been reported to be a major constraint in chilli production in tropical subtropical countries causing huge losses. India had been the largest producer and exporter of chilli, but since a few years the production has declined significantly and presently, India stands at the third number in terms of chilli production (FAOSTAT, 2012; Figure 3). An estimated annual loss of about 29.5%, amounting whopping figure of US$ 491.67 million has been reported from India alone (Garg et al., 2014). In India, a calculated loss of 10–54% has been reported in yield of the crop due to the anthracnose disease (Lakshmesha et al., 2005; Ramachandran and Rathnamma, 2006). Significant losses have been reported from other parts of the world as well, like a significant amount of 20–80% loss has been accounted from Vietnam (Don et al., 2007) and about 10% from Korea (Byung, 2007). The loss is high owing to the post and pre harvest involvement of the pathogen causing a loss of 10–80% of the marketable yield of chilli fruits (Than et al., 2008).
Figure 3.
Annual chilli production by world top producers (FAOSTAT, 2012).
The fruit lesion is the most economically important aspects of the disease as sometimes, even a small lesion on the fruit is enough to lower its market value thereby affecting the profitable yield of the crop (Manandhar et al., 1995). The disease is reported to affect almost all aerial parts of the plant. Chiefly, it causes fruit rot at both green and red stages primarily attacking ripe fruits, hence is also known by the name ripe fruit rot of chilli (Agrios, 2005). The disease is seed borne, soil borne, water borne and airborne and hence may lead to damage at the seedling stage or on the aerial parts of the plants. Many species of Colletotrichum have been associated with the pepper anthracnose in different countries. Table 2 gives the brief outline of the different Colletotrichum species reported to be associated with the anthracnose of chilli in different parts of the world. However, in India, primarily three important species, namely, C. capsici, C. acutatum and C. gleosporoides have been reported to be linked with the disease, with C. capsici Syd. Butler and Bisby causing major damage at the ripe fruit stage of the plant (Ranathunge et al., 2012; Saxena et al., 2014).
Table 2.
Species of Colletotrichum associated with anthracnose of chilli in different parts of the world.
| S.No. | Country | Species associated | References |
|---|---|---|---|
| 1. | Australia | C. brisbanense | Damm et al., 2009 |
| 2. | Brazil | C. boninense | Tozze and Massola, 2009 |
| 3. | India | C. capsici, C. acutatum | Ranathunge et al., 2012; Saxena et al., 2014 |
| 4. | Indonesia | C. acutatum, C. gloeosporioides, C. nymphaeae, C. capsici | Damm et al., 2009; Voorrips et al., 2004 |
| 5. | Korea | C. acutatum, C. gloeosporioides, C. coccodes, C. dematium | Park and Kim, 1992 |
| 6. | Mexico | C. capsici | Damm et al., 2009 |
| 7. | New Zealand | C. kartsii, C. novae-zelandiae, C. nigrum, C. coccodes | Damm et al., 2012b; Liu et al., 2013 |
| 8. | Papua New Guinea | C. capsici, C. gloeosporioides | Pearson et al., 1984 |
| 9. | Sri Lanka | C. acutatum | Damm et al., 2012a |
| 10. | Taiwan | C. acutatum, C. capsici, C. gloeosporioides | Manandhar et al., 1995 |
| 11. | Thailand | C. acutatum, C. capsici, C. gloeosporioides, C. siamense, C. scovillei, C. asianum | Than et al., 2008; Damm et al., 2009; Phoulivong et al., 2012; Weir et al., 2012 |
| 12. | United States | C. capsici, C. gloeosporioides, C. acutatum, C. coccodes, | Harp et al., 2008 |
| 13. | Vietnam | C. acutatum, C. capsici, C. gloeosporioides, C. nigrum | Don et al., 2007 |
| 14. | Zimbabwe | C. nymphaeae | Damm et al., 2009 |
Epidemiology and disease symptoms
Environmental factors play an important role in deciding the severity and spread of any disease. The favorable host, pathogen and weather conditions lead to establishment of disease (Agrios, 2005). Thus, before proposing the management strategy of the disease, a thorough knowledge regarding the epidemiology of the disease should be studied. Anthracnose disease of chilli is generally most common among the tropical and sub-tropical countries. Hot and humid environmental conditions support the spread of the disease.
Other important environmental factors governing the severity of the disease include rainfall intensity and duration, humidity, leaf surface wetness and light. Amongst them leaf surface wetness has been directly linked with the severity of the disease owing to the better establishment of the pathogen in respect of germination, attachment and penetration into host tissues (Than et al., 2008). The relationship between the environmental factors like rainfall intensity and duration and the prevailing temperature and humidity along with the crop geometry and inoculum spread leads to possible development of disease as well (Dodd et al., 1992). Temperature also affects the development of the disease and presence of surface wetness and competitive microbiota further favors the disease development (Royle and Butler, 1986). Temperature around 27°C with relative humidity of 80% have reported to be the most optimum conditions for successful establishment of the disease in a given area (Roberts et al., 2001). The development of the disease also depends on the host cultivar, along with its resistance against the pathogen.
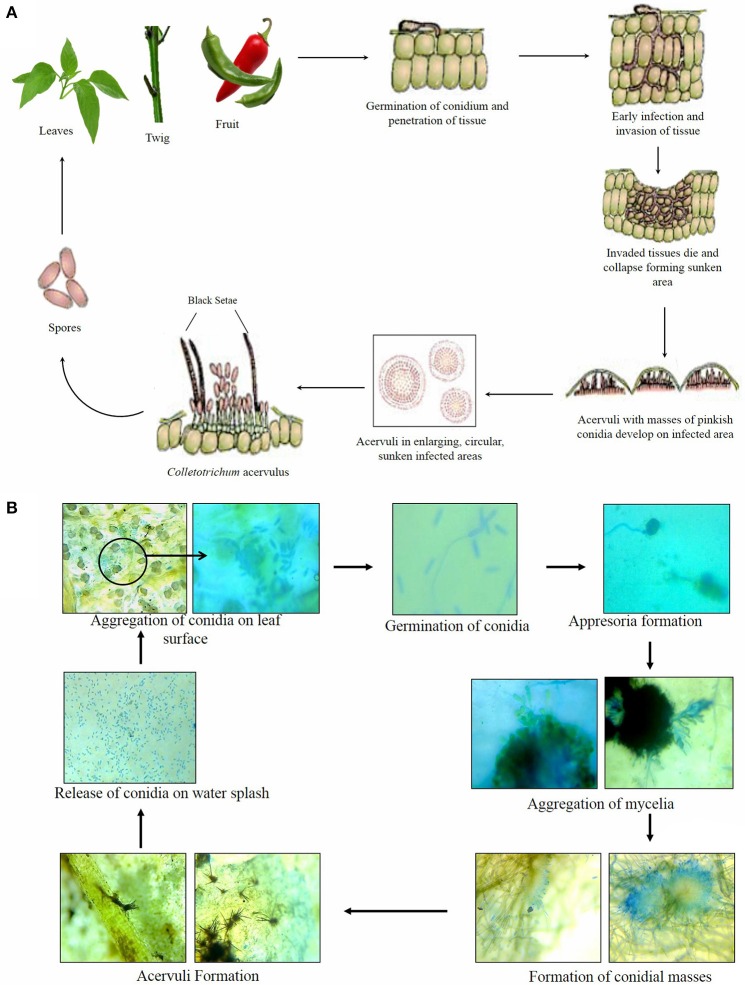
On attainment of favorable conditions, characteristic symptoms appear on the ripe chilli fruit, which appear as sunken circular or angular lesions (Figure 4A). Often multiple lesions coalesce to form severe fruit rot. Generally, the lesions are characterized by the presence of black colored spots in concentric rings at maturity. Initially, orange to pink conidial masses may be visible on the fruit surface. The dark spots when observed under a microscope are the acervuli structures containing setae hairs entrapping the conidia of the pathogen. Further, the pathogen forms micro sclerotia in plant debris or seed, soil, which is the mode of survival under unfavorable conditions. The pathogen infects all parts of the host plant, including stems and leaves (Figures 4B,C). Lesions on stems and leaves appear as small sunken grayish brown spots with dark margins, further on which development of acervuli in concentric rings could be easily seen.
Figure 4.
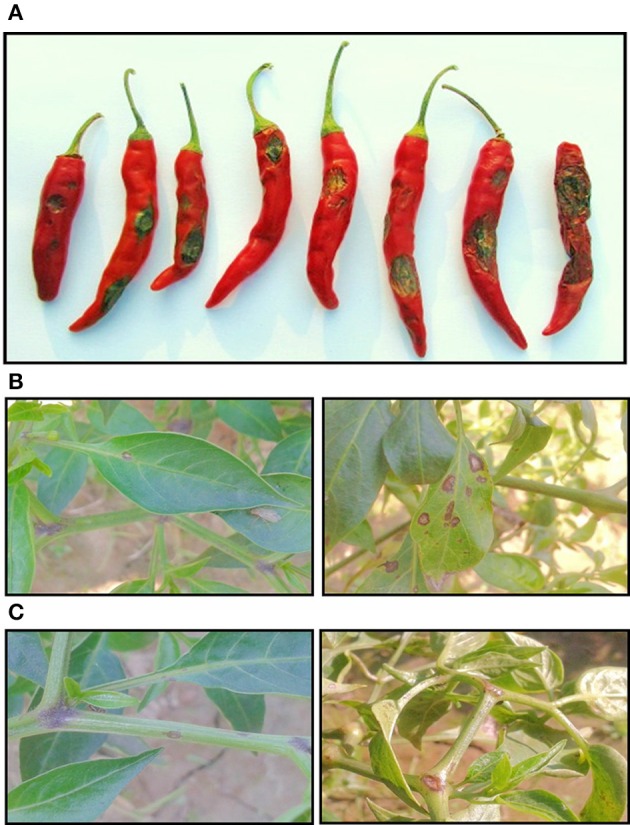
Characteristic symptoms of anthracnose on chilli fruits (A), leaves (B) and stems (C).
The pathogen-colletotrichum capsici
Colletotrichum spp. has been rated as among the ten most notorious pathogens in the world, causing heavy crop losses worldwide (Dean et al., 2012; Figure 5). Specifically, Colletotrichum is an asexual genus belonging to phylum Ascomycete and Coeleomycetes class of Fungi imperfectii (Dean et al., 2012). Despite significant developments in studies related to this plant-patho system, the taxonomic position of the pathogen remains unclear. The systematics of the fungal pathogens from this genus still exist in ambiguity with the number of species ranging from 29 to over 700 depending upon the criteria selected for separation (Sutton, 1992).
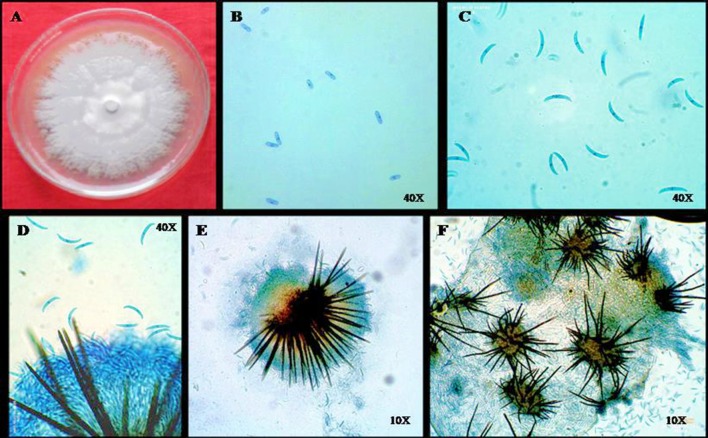
Figure 5.
The morphological appearance of Colletotrichum isolates on PDA growth medium (A) and the microscopic appearance of the characteristic structures, i.e., conidia (B,C), setae (D), acervuli (E), and the acervuli as seen on surface of the chilli fruits (F).
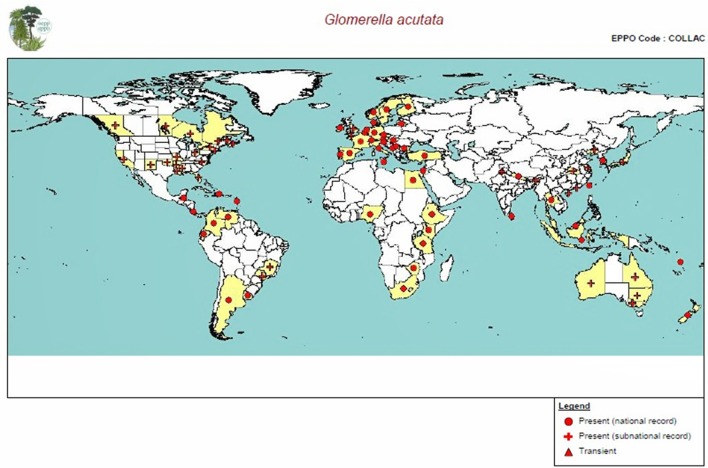
Being economically important pathogen, its host range varies from fruits, vegetables, ornamental plants to important staple food crops. The species of this genus are reported to cause anthracnose disease in more than 121 plant genera from 45 different plant families (Farr et al., 2016). It also causes blights of aerial plant parts and post-harvest rots. The damage may extend to severe economic loss in tropical and sub-tropical countries, causing infection to staple foods like bananas, sorghum, cassavas, legumes, and cereals (Bailey and Jeger, 1992). Particularly, this pathogen exhibit efficient infection in post-harvest conditions owing to its ability to cause latent infection, where the symptoms appear on the fruits only after the harvest or during storage or at the market shelf. Losses up to 100% have been recorded due to Colletotrichum spp. (Prusky, 1996). Another important parameter for its successful colonization and severe disease spread may be credited to its cosmopolitan nature. Many species of Colletotrichum may be found on a single host or single species may be able to infect different hosts (Sander and Korsten, 2003). Broad, imprecise and often overlapping fungal plant relationships exist in Colletotrichum plant-patho system (Freeman and Shabi, 1996). With its ability to infect many hosts along with adapting to new environments, the pathogen poses serious threat to the different crop production system through cross infection problems (Photita et al., 2005). Figure 6 gives the distribution of the sexual teleomorph of the Colletotrichum in different parts of the world.
Figure 6.
Distribution map of Glomerella acutata (sexual teleomorph of Colletotrichum acutatum) (Source: EPPO-PQR).
Impact on global economic loss posed by the pathogen has triggered extensive studies on diverse aspects of the biology of the pathogen for better understanding of its infection process and host interaction mechanisms. In light of this, host specificity of the pathogen (Freeman, 2000; Correll et al., 2007) along with the biology involved in the infection mechanisms used by the pathogen (Perfect et al., 1999; O′Connell et al., 2000) and the various fungal-host interactions (Prusky et al., 2000) studies have been carried out. Studies related to genetic diversity and the epidemiology have also been reported (Freeman, 2000; Timmer and Brown, 2000). The genus has been used as a model for studying the genetic basis of symbiotic life styles (Rodriguez, 2000) leading to the development of infection and disease forecasting systems (Uddin et al., 2002). The use of molecular markers like DNA fragments analysis [e.g., Randomly amplified polymorphic DNA (RAPD) and Arbitrarily primed (AP)-PCR] has improved the speed and accuracy in identification and characterization of Colletotrichum spp. (Lewis et al., 2004; Photita et al., 2005). Further the the nucleotide sequenceof the 5.8S gene and internal transcribed spacer (ITS) region has facilitated the construction of Colletotrichum species specific primers providing a rapid and accurate method for diagnostic purpose and phylogenetic analysis (Torres-Calzada et al., 2011). Many studies have been carried out to resolve the issue of species complex in Colletotrichum spp. in different areas of the world on various hosts like mango (Kamle et al., 2013), guava (Mohd Anuar et al., 2014), herbaceous plants (Photita et al., 2005), soursop (Álvarez et al., 2014), and also in medicinal plants as endophytes (Lima et al., 2012) using molecular marker analysis.
The anthracnose causing pathogen in chilli varieties have been reported to be Colletotrichum capsici (Sydow), Butler and Bisby (Than et al., 2008). Three pathotypes have been linked with the infection on ripe fruits, while two have been reported causing infection at mature green fruit stages (Mongkolporn et al., 2004; Montri et al., 2009). The convoluted relationships of the fungi with the host have left certain gaps in the knowledge of the infection process and the interactions of different species with chilli plant. Different species have been reported to be linked causing infection in different parts of the chilli plant, for instance, C. acutatum and C. gloeosporioides infect the fruits at all stages of development without causing much harm to leaves and stems of the plant, while C. coccodes and C. dematium mostly cause high infestation on leaves and aerial parts of the plant (Kim et al., 2004). Differential rates of infestation has also been reported by the species of the genus infecting different fruits of the plant. For instance, in Korea, Hong and Hwang (1998) reported C. gloeosporioides as the most prevalent species causing an infestation in chilli fruits at both ripe stages and unripe stage; while existence of C. capsici and C. acutatum as the prevalent species infectionsnfection on ripe and unripe chilli fruits respectively, in the North eastern region of India have been reported (Saxena et al., 2014).
Infection stages and disease cycle
Colletotrichum employs different strategies for causing infection to the host plant which initiate from the intracellular hemibiotrophic mode to the intramural necrotrophic mode of nutrition (Bailey and Jeger, 1992). Liao et al. (2012) has reported an intermediate stage showing partial endophytic life style of the pathogen before adapting to the necrotrophic mode of nutrition in the host plant. Different species of this genus exhibit different mechanism of infection depending on the host infected. For instance, Peres et al. (2005) reported the epiphytic or endophytic mode of survival of C. acutatum in an orchard infected with the bitter rot of apple. Also, intramural necrotrophy by C. capsici was reported by Pring et al. (1995) while infecting cowpea leading to subsequent necrosis caused due to dissolution of cell wall structures. The biotrophic phase of infection by C. capsici is also well studied in the infection caused to broad bean or lentil characterized by the presence of large multilobed, multi septate, vesicular primary hyphae (Latunde-Dada and Lucas, 2007).
Initial infection starts with the attachment of the conidia to the host surface preceded with its germination and production of adhesive appresoria followed with its penetration into the host epidermis. This is further accompanied by the growth and colonization of plant tissue by the fungus, resulting in the formation of specific symptom structures that is acervuli containing the spores of the fungus for further spread (Prusky et al., 2000). The pathogen sometimes remains in quiescent state in the form of appresorial structures in tissues of unripe fruits and cause infection after the fruits ripe or mature (Than et al., 2008). A dendroid structure composed of multiple, thick-walled hyphal branches with swollen or sharp ends from the penetration pore of the appresorium has been recently reported to be an intermediate structure which penetrates the host cuticle layer and infect the epidermal cells during C. acutatum infestation in chilli (Liao et al., 2012).
Though the pre-penetration mechanisms exhibited by Colletotrichum species appear somewhat related to each other, the post penetration events such as spore adhesion, melanization and cutinization hold certain disparity. Based on the previous studies, four kind of infection strategies with varied hosts have been studied in C. acutatum plant patho system (Peres et al., 2005). First is the biotrophic growth of the pathogen, where the formation of appresoria from the conidia is followed by the formation of secondary conidia which further infects and spreads the pathogen inside the host leaves (e.g., The biotrophic disease cycle in citrus leaves). The second is the subcuticular intramural necrotrophy with the development of wide and swollen hyphae in the anticlinal and periclinal walls of host epidermal cells (e.g., The necrotrophic disease cycle on strawberry). The third strategy is the hemibiotrophic mode of infection where the pathogenic hyphae interact with the infection vesicles within the host cells (e.g., The biotrophic disease cycle on blueberry fruits). The fourth type of interaction is the combination of hypertrophic and subcuticular intra and intracellular development of the pathogen generally observed during infestation of almond leaves and fruits.
As far as studies related to infection and colonization by Colletotrichum species i.e., C. gloeosporioides on susceptible chilli variety is considered, no biotrophic stage in the form of infection vesicle has been found during the infection (Kim et al., 2004). An increased number of small vacuole with the condensed cytoplasm in the epidermal cells followed with cell destruction extending to the sub epidermal cells of the plant due to the action of pathogen enzyme has been noticed during the early stages of infection in chilli plant. During the later stages, inter and intra cellular colonization of the pathogen occurs indicating the governance of necrotrophic mode of fungal growth. Figures 7A,B shows the diagrammatic representation and the microscopic representation of different stages of infection of Colletotrichum spp.
Figure 7.
(A) Disease cycle of anthracnose disease of chilli (Capsicum annum L.) caused by Colletotrichum spp. [Source: modified from Agrios (2005)], (B) Different stages of infection by the Colletotrichum spp. on chilli leaf as seen under microscope.
Disease management
Management of chilli anthracnose has been a burning issue for the agriculturists and the farmers as till date, no effective control measures has been proposed. The fall in the chilli production and the drop in fruit quality have further intensified the need for developing a sustainable approach for controlling the spread of the disease. No single management technique has been found to efficiently control the disease. Generally, using a combination of the different strategies like chemical control, biological control, physical control and intrinsic resistance has been recommended for managing the disease (Agrios, 2005). The management strategies for controlling Colletotrichum spp. from spreading and establishing a disease can be discussed under four broad categories: Use of cultural Practices, use of chemical control, use of resistant varieties and finally the use of biological control. Table 3, gives a summarized information on the strategies used for controlling the anthracnose disease in chilli from different parts of the world.
Table 3.
Control measures for managing chilli anthracnose reported from different parts of the world.
| Species targeted | Active Constituent | References |
|---|---|---|
| CHEMICAL CONTROL | ||
| Colletotrichum capsici | Carbendazim | Than et al., 2008 |
| Colletotrichum spp. | Dithiocarbamates, benzimidazole and trizole compounds | Waller, 1992 |
| C. capsici | Bavistine (carbendazim) | Ngullie et al., 2010 |
| C. capsici | Maneb (Mancozeb) | Smith, 2000 |
| C. capsici | Carbendazim, Mancozeb, Trinidazole, Propiconazole | Hegde and Anahosur, 2001 |
| C. capsici | Carbendazim, Propiconazole | De los Santos and Romero, 2002 |
| C. capsici | Strobilurin fungicides | Lewis and Miller, 2003 |
| C. gloeosporioides, C. capsici | Azoxystrobin | Saxena et al., 2016b |
| C. capsici | Thiophanate methyl | Ushakiran et al., 2006 |
| C. capsici | Propiconazole and Difenoconazole | Gopinath et al., 2006 |
| Colletotrichum spp. | Carbendazim, Mancozeb | Park, 2007 |
| C. capsici | Benzimidazole | Kim et al., 2007 |
| C. capsici | Azoxystrobin | Chen et al., 2009 |
| C. gloeosporioides | Bavistin (carbendazim) | Ngullie et al., 2010 |
| C. gleosporoides, C. capsici | Carbendazim | Suwan and Na-Lampang, 2013 |
| C. capsici | Difenoconazole | Soytong et al., 2014 |
| PHYSICAL CONTROL | ||
| C. gloeosporioides | Temperature (25°–30°C) | Denner et al., 1986 |
| C. capsici | Rice Straw and Plastic Mulches | Vos et al., 1995 |
| C. capsici | Crop rotation | Roberts et al., 2001 |
| C. capsici, C. acutatum | Crop rotation, using disease free seeds, fallowing | Agrios, 2005 |
| C. acutatum, C. capsici | Good drainage | Than et al., 2008 |
| C. capsici, C. acutatum, C. gloeosporioides | Use of resistant plant genotypes | Pollegioni et al., 2012 |
| C. acutatum | Light intensity (Green light) | Yu et al., 2013 |
| BIOLOGICAL CONTROL | ||
| C. capsici | Trichoderma viride | Naglot et al., 2015 |
| C. capsici | Psuedomonas fluorescens | Ramamoorthy and Samiyappan, 2001 |
| C. gloeosporioides | Mixture of PGPRs | Jetiyanon and Kloepper, 2002 |
| C. capsici | P. fluorescens, B. subtilis | Bharathi et al., 2004 |
| C. capsici | P. fluorescens | Ekbote, 2005 |
| C. capsici | T. harzianum and P. fluorescens | Srinivas et al., 2006 |
| C. capsici | T. harzianum | Oanh et al., 2006 |
| C. gloeosporioides | T. harzianum | Jebessa and Ranamukhaarachchi, 2006 |
| C. capsici | Pichia guilliermondi | Chanchaichaovivat et al., 2007 |
| C. gloeosporioides | T. harzianum | Boonratkwang et al., 2007 |
| C. capsici | P. fluorescens | Intanoo and Chamswarng, 2007 |
| C. acutatum | Bacillus subtilis | Wharton and Diéguez-Uribeondo, 2004 |
| C. acutatum | Candida oleophila | |
| C. capsici | P. fluorescens | Anand et al., 2009 |
| C. capsici | Bacillus subtilis | Sutarya et al., 2009 |
| C. capsici | Pichia guilliermondii | Nantawanit et al., 2010 |
| C. capsici | T. viride, P. fluorescens | Ngullie et al., 2010 |
| C. gloeosporioides, C. capsici | Actinomycetes | Intra et al., 2011 |
| C. acutatum | B. vallismortis strain BS07 | Park et al., 2013 |
| C. capsici | Colletotrichum globusum | Vasanthakumari and Shivanna, 2013 |
| BOTANICALS AND BIOLOGICAL ELICITORS | ||
| C. capsici | Fungal glucan, Polytran L | Bhandel and Paxton, 1991 |
| C. capsici | Neem extract, Rhinocanthus nasuta extract, Garlic extract | Singh and Korpraditskul, 1999 |
| C. capsici | Extracts of plucao and sabsua | Puttawong and Wongroung, 2009 |
| C. capsici | Neem extract, Garlic extract | Ngullie et al., 2010 |
| C. capsici | Crude Extract of Piper betle L. | Johnny et al., 2011 |
| C. capsici | Extract of Coleus aromaticus | Ajith et al., 2012 |
| INTEGRATED MANAGEMENT | ||
| C. capsici | T. harzianum+Captan+Neem Cake | Sharma et al., 2004 |
| C. capsici | P. fluroescens+Azoxystrobin | Anand et al., 2010 |
Use of cultural practices
The pathogen being seed borne, wind borne and water borne apart from being soil borne, the practices to control its spread should target three main areas of disease free crop production in the field: proper drainage, crop rotation and removal of any infected plant parts of the field. Water splashes may easily spread the conidia of the pathogen from infected to uninfected plant parts. Also, relative humidity aids successful colonization of the pathogen. So, the field should have proper drainage and irrigation to prevent the outbreak of the disease. Also, proper distance between the plants should be maintained so as to reduce dense canopy, which gives way for creating moisture (Than et al., 2008). Another important practice is the use of transplants raised from disease free seeds of the chilli variety. The transplants should be kept weed free and away from other solanaceous crops. Ideally, the crop should be rotated after every 2–3 years with crops those are not the host of Colletotrichum (Roberts et al., 2001). The use of rice straw and plastic mulches has also been reported for effective control of the disease (Vos et al., 1995).
Use of chemical fungicides
In the absence of any accurate method of controlling the disease, chemical control has been sought as the most effective measure to control the spread of the disease. The longer time required for developing the resistant cultivar and the short span result of the use of fungicides further popularize this method of controlling the disease specifically for anthracnose disease (Wharton and Diéguez-Uribeondo, 2004). However, the remnant toxic residues of the chemicals in the fruits create hindrance to the expected export of the chilli products to other countries, in turn affecting the economy of the country. Also, relying on a single chemical component result in the development of resistance in the pathogenic isolates, which further augments the difficulty in the management of the disease (Staub, 1991; Than et al., 2008). Traditionally, recommended fungicide for the control of the disease is manganese ethylenebisdithiocarbamate (Maneb) (Smith, 2000) and carbendazim, though the use of both fungicides has been found ineffective under severe disease outbreak. The chemical fungicides generally recommended for controlling anthracnose disease are based on copper compounds, dithiocarbamates, benzimidazole and triazole compounds (Waller, 1992). Newer chemicals like strobilurins based fungicides (e.g., azoxystrobin, pyraclostrobin) have also been used for its management. However, only a few reports are available using this class of fungicide controlling chilli anthracnose under large field trials (Schilder et al., 2001; Lewis and Miller, 2003; Chen et al., 2009).
The effective control through the use of chemical fungicides is possible by the timely application during the critical period favorable for the onset of the disease. Generally, fungicides should be applied at young expanding tissues, including fruits, leaves and flowers to restrict the entry of the pathogen to the plant system (Wharton and Diéguez-Uribeondo, 2004). However, numerous reports on the destructive effects of the use of fungicides on farmers' health, economic status, and toxic contamination of the environment, particularly in developing countries cannot be ignored (Voorrips et al., 2004; Garg et al., 2014). Different classes of fungicides have specific mode of action along with their duration of effect on disease control. So, wise choice of fungicides by the farmers in a particular area, according to prevailing environmental conditions, should be taken into consideration. Rotation of two or more different classes of fungicides is highly recommended for increasing the chance of better protection against the disease in the fields (Förster et al., 2007).
Use of resistant varieties
Developing resistance against the pathogen in the host is seeking to be the most important and sustainable approach for managing the disease. This strategy not only eliminates the losses caused due to the disease, but also remove the chemical and mechanical expense of the disease control (Agrios, 2005). The principle behind the use of resistant cultivars is to trigger the host defense response that in turn would inhibit or retard the growth of the pathogen involving the use of a single gene pair: a host resistance gene and the pathogen avirulence gene (Flor, 1971). In lieu of the existing biotechnological approach to manage diseases, certain successful resistant varieties of chilli against C. capsici have been reported from different parts of the world (Yoon, 2003; Voorrips et al., 2004; Garg et al., 2014). Though, not much success has been sought in developing resistant chilli varieties in the species Capsicum annum L., which is the only species grown worldwide (Park, 2007). The two major requirements before proceeding for developing the cultivar is the knowledge of the resistant varieties of Capsicum occurring wildly in the region and the different pathotypes of the pathogen found in that region. Many varieties resistant to Colletotrichum spp. and information regarding the pathotypes of the pathogen has been reported and is available AVRDC, 2003; Babu et al., 2011). However, the challenging task of resistant breeding is exceptionally difficult in Colletotrichum-chilli pathosystem due to the association of more than one species of the pathogen with the disease (Sharma et al., 2005; Saxena et al., 2014) along with the differential ability of the pathogenic virulence (Montri et al., 2009).
Recently, a study carried by Garg et al. (2014) reported the existence of nine resistant varieties (BS-35, BS-20, BS-28, Punjab Lal, Bhut Jolokia, Taiwan-2, IC-383072, Pant C-1, and Lankamura Collection) of Capsicum spp. out of the 42 varieties existing in use in the area which could be employed for developing successful resistant cultivars through breeding programs. The information on the resistance varieties against Colletotrichum may also be utilized for studying the inheritance of the resistance from one generation to another (Kim et al., 2008) and also to locate and study the quantitative trait loci (QTLs) maps for resistance (Lee et al., 2010).
Use of botanicals and biological control agents
Disease control through the use of botanicals and crude extracts of medicinal plants have been explored in recent years for their effective antifungal and antimicrobial properties. Their easy decomposition, non-residual activity and non phytotoxic properties further popularize their use for controlling phytopathogens. Several studies using crude plant extracts have also been conducted to access the control of Colletotrichum spp. on chilli (Ngullie et al., 2010; Johnny et al., 2011). They have shown different degree of effectiveness of extracts of sweetflag (Acorus calamus L.), palmrosa (Cymbopogon martini) oil, Ocimum sanctum leaf extract, neem (Azadirachta indica) oil, garlic, Piper betle L., Coleus aromaticus, plucao, and sabsua against the pathogen growth and spore germination.
Biocontrol strategy for disease management has stood up as a sustainable approach required for restoring the lost homeostasis of the environment. Though, for managing chilli anthracnose this particular strategy has not gained much momentum yet, the potential of using biocontrol agents (BCAs) for controlling the pathogen was elucidated way back by Lenné and Parbery (1976). The possibilities of using BCAs for controlling the post harvest loss of fruits has been illustrated by Jeger and Jeffries (1988) and Korsten and Jeffries (2000). Till date, the BCAs used for studying the antagonistic potential against Colletotrichum spp. affecting chilli crop include Psuedomonas fluorescens, Trichoderma spp., Bacillus subtilis, Candida oleophila, and Pichia guilliermondi (Table 3).
Trichoderma as a biocontrol agent against colletotrichum spp.
Belonging to class Ascomycete, Trichoderma is a well-studied ubiquitous genus. Well known as a saprophytic fungus, it has high adaptive potential as evident from its ability to colonize wood, bark, agricultural wastes and other substrates apart from its omnipresence in a variety of soil types (Singh et al., 2012; Mukherjee et al., 2014). Its biocontrol potential has been well established against numerous important phytopathogens like Alternaria, Colletotrichum, Phytophthora, Pythium, Rhizoctonia, Sclerotinia, Verticillium etc. (Begum et al., 2008; Imtiaj and Lee, 2008; Jain et al., 2012; Singh et al., 2013). The mechanisms involved have been attributed to be mycoparasitim, antibiosis, competition for nutrients and space along with its ability to induce systemic resistance in the plants against the pathogens (Harman, 2006; Shoresh et al., 2010; Hermosa et al., 2012). Also, the efficient enhancement in plant growth with significant increase in biomass has also been attributed to the application of Trichoderma species. (Yedidia et al., 2001; Jain et al., 2012). Recently, the ability of the fungus to induce biotic tolerance in plants by enhancing the mechanical strength of the plant system has been studied against phtytopathogenic infestation (Singh et al., 2013; Saxena et al., 2015).
Specifically, in the Colletotrichum plant pathosystem, its potential has been elucidated owing to its fast colonizing ability and mycoparasitic nature, which results in coiling and parallel growth of the pathogen (Begum et al., 2008; Živkovic et al., 2010). This property has been further attributed due to the secretion of extracellular enzymes, including glucanases, chitinases etc. that degrade the pathogenic mycelia thereby restricting its growth and further colonization in the host tissue (Harman, 2006; Vinale et al., 2008; Singh et al., 2012). The various studies reporting the application of Trichoderma species for the biological control of Colletotrichum in different host have been summarized in Table 4.
Table 4.
Trichoderma mediated control of Colletotrichum species.
| S.No. | Trichoderma species used | Targeted Colletotrichum species | Host plant | References |
|---|---|---|---|---|
| 1. | T. viride | C. dematium | Pigeon Pea | Kumar et al., 2000 |
| 2. | T. viride | C. lindemuthium | Cow Pea | Adebanjo and Bankole, 2004 |
| 3. | T. harzianum | C. acutatum | Strawberry | Freeman et al., 2004 |
| 4. | T. harzianum | C. graminicola | Maize | Harman et al., 2004 |
| 5. | T. harzianum | C. gloeosporioides | Grape | Soytong et al., 2005 |
| 6. | T. viride | C. capsici | Chilli | Kaur et al., 2006 |
| 7. | T. harzianum | C. acutatum | Blueberry | Verma et al., 2006 |
| 8. | T. harzianum | C. dematium | Soybean | Shovan et al., 2008 |
| 9. | T. harzianum | C. acutatum, C. gloeosporioides | Fruits | Živkovic et al., 2010 |
| 10. | T. harzianum, T. psuedokoningii | C. destructivum | Cow pea | Akinbode and Ikotun, 2011 |
| 11. | T. viride, T. harzianum, T. hamatum | C. lindemuthium | Bean | Padder and Sharma, 2011 |
| 12. | T. viride | C. gloeosporioides | Sarpagandha | Ghosh and Chakraborty, 2012 |
| 13. | T. harzianum | C. capsici | Chilli | Rahman et al., 2013; Saxena et al., 2016a |
| 14. | Trichoderma spp. | C. gloeosporioides | Mango | Admasu et al., 2014 |
| 15. | T. viride and T. harzianum | C. lindemuthium | Haricot Bean | Amin et al., 2014 |
The mode of use of this fungus has been restricted to seed biopriming or root treatment of the plants. The effect of the foliar sprays of Trichoderma species to prevent spread of foliar disease has not been studied extensively. However, effective results have been reported by the foliar sprays of other antagonistic microbes like yeast, Psuedomonas, Bacillus etc. that managed to control the growth and colonization of the pathogenic fungus (Chanchaichaovivat et al., 2007; Anand et al., 2009; Sutarya et al., 2009). The reports have very well elucidated the enhanced efficiency of the microbes to combat the growth of pathogen at leaf and fruit surface acting as a first line of defense for the protection of the host plants. Similar approach for efficient control of foliar disease of Capsicum i.e., anthracnose could be proposed by using Trichoderma strains as well. There have been reports showing effective potential of Trichoderma species in controlling Colletotrichum infestation in other hosts like cowpea (Adebanjo and Bankole, 2004). Also report on effective colonization of Trichoderma species in the phylloplane of plants is well studied (Bae et al., 2011). In order to control chilli anthracnose very few attempts have been made to use Trichoderma isolates obtained from the phylloplane of healthy host plant. The need for an effective all around protection of the plant triggered our group to study the effectiveness of Trichoderma isolates dwelling at the phylloplane of the healthy plants to concur the pathogenic ingression. Recently, Trichoderma isolates from the phylloplane of healthy leaves were found equally successful in controlling disease incidence as evident from the elevated induction of defense related enzymes and reduced disease incidence on host plants (Saxena et al., 2016a).
Future prospects
Though the epidemic nature of the disease has been studied for ages, many areas are still unexplored in terms of host-pathogen interaction, its spread and effective management strategies. There lies an urgent need to develop an efficient integrated management strategy keeping in concern the different environmental factors and pathogenic resistance, driving the successful colonization of the pathogen in the host tissues. An insight into the pathogen's lifestyle would provide valuable information required to develop targets for developing resistant varieties of chilli against the pathogen. Also, modifications in conventionally recommended cultural practices suiting to a particular agro-climatic region will prove helpful in better management of the disease. More studies are required for acquiring in-depth information regarding various modes of infection by the pathogen and the pathogenic variability associated within a region with the post-harvest as well as pre-harvest loss in the crop production. The overall knowledge about the key aspects of a disease triangle will enable better management of the disease keeping track of the quality and quantity of the crop produced thereby contributing efficiently to the country's economy.
Author contributions
AS has drafted the Manuscript; RR, HS, and VG have critically reviewed the draft for important intellectual content and provided substantial contribution for the concept and design of the Manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AS is grateful to Department of Science and Technology, Govt. Of India for providing INSPIRE Fellowship under the AORC Scheme.
References
- Abbasi P. A., Soltani N., Cuppels D. A., Lozarovits G. (2002). Reduction of bacterial spot disease severity on tomato and pepper plants with foliar application of ammonium lignosulfonate and potassium phosphate. Plant Dis. 86, 1232–1236. 10.1094/PDIS.2002.86.11.1232 [DOI] [PubMed] [Google Scholar]
- Adebanjo A., Bankole S. A. (2004). Evaluation of some fungi and bacteria for biocontrol of anthracnose disease of cowpea. J. Basic Microb. 44, 3–9. 10.1002/jobm.200310310 [DOI] [PubMed] [Google Scholar]
- Admasu W., Sahile S., Kibret M. (2014). Assessment of potential antagonists for anthracnose (Colletotrichum gloeosporioides) disease of mango (Mangifera indica L.) in North Western Ethiopia (Pawe). Arch. Phytopathol. 47, 2176–2186. 10.1080/03235408.2013.870110 [DOI] [Google Scholar]
- Agrios G. N. (2005) Plant Pathology. St. Louis, MO: Academic Press. [Google Scholar]
- Ahn H. I., Yoon J. Y., Hong J. S., Yoon H. I., Kim M. J., Ha J. H., et al. (2006). The complete genome sequence of pepper severe mosaic virus and comparison with other potyviruses. Arch. Virol. 151, 2037–2045. 10.1007/s00705-006-0776-1 [DOI] [PubMed] [Google Scholar]
- Ajith P. S., Lakshmesha K. K., Murthy S. M., Lakshmidevi N. (2012). Botanicals for control of anthracnose of bell peppers. J. Plant Protect. Sci. 4, 13–19. [Google Scholar]
- Akinbode O. A., Ikotun T. (2011). Potentials of two Trichoderma species as antagonistic agents against Colletotrichum destructivum of cowpea. Afr. J. Microbiol. Res. 5, 551–554. 10.5897/AJMR10.151 [DOI] [Google Scholar]
- Álvarez E., Gañán L., Rojas-Triviño A., Mejía J. F., Llano G. A., González A. (2014). Diversity and pathogenicity of Colletotrichum species isolated from soursop in Colombia. Eur. J. Plant Pathol. 139, 325–338. 10.1007/s10658-014-0388-7 [DOI] [Google Scholar]
- Amin M., Teshele J., Tesfay A. (2014). Evaluation of bioagents seed treatment against Colletotrichum lindemuthianum in haricot bean anthracnose under field condition. Res. Plant Sci. 2, 22–26. 10.12691/plant-2-1-5 [DOI] [Google Scholar]
- Anand T., Chandrasekaran A., Kuttalam S., Senthilraja G., Samiyappan R. (2010). Integrated control of fruit rot and powdery mildew of chilli using the biocontrol agent Pseudomonas fluorescens and a chemical fungicide. Biol. Control. 52, 1–7. 10.1016/j.biocontrol.2009.09.010 [DOI] [Google Scholar]
- Anand T., Chandrasekaran A., Raguchander T., Prakasam T., Samiyappan R. (2009). Chemical and biological treatments for enhancing resistance in chili against Collectotrichum capsici and Leveillula taurica. Arch. Phytopathol. PFL 1, 1–19. 10.1080/03235400701191721 [DOI] [Google Scholar]
- Arogundade O., Balogun O. S., Kareem K. T. (2012). Occurrence and distribution of pepper veinal mottle virus and cucumber mosaic virus in pepper in Ibadan, Nigeria. Virol J. 9, 79. 10.1186/1743-422X-9-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVRDC (2003). AVRDC Progress. Report for 2002, Asian Vegetable Research and Development Centre, AVRDC publication 03-563. [Google Scholar]
- Babu B. S., Pandravada S. R., Rao R. D. V. J. P., Anitha K., Chakrabarty S. K., Varaprasad K. S. (2011). Global sources of pepper genetic resources against arthropods, nematodes and pathogens. Crop Prot. 30, 389–400. 10.1016/j.cropro.2010.12.011 [DOI] [Google Scholar]
- Bae H., Roberts D. P., Lim H. S., Strem M. D., Park S. C., Ryu C. M., et al. (2011). Endophytic Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol. Plant Microbe Interact. 24, 336–351. 10.1094/MPMI-09-10-0221 [DOI] [PubMed] [Google Scholar]
- Bailey J. A., Jeger M. J. (1992). Colletotrichum: Biology, Pathology and Control. Vol. 388 Wallingford, CT: Commonwealth Mycological Institute. [Google Scholar]
- Bankole S. A., Ogunsanwo B. M., Mabekoje O. O. (2004). Natural occurrence of moulds and afltoxins in melon seeds from markets in Nigeria. Food Chem. Toxicol. 42, 1309–1324. 10.1016/j.fct.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Begum M. M., Sariah M., Abidin Z. M. A., Puteh B. A., Rahman A. M. (2008). Antagonistic potential of selected fungal and bacterial biocontrol agents against Colletotrichum truncatum of soybean seeds. Pertanica J. Trop. Agric. Sci. 31, 45–53. [Google Scholar]
- Berger P. H., Adams M. J., Barnett O. W., Brunt A. A., Hammond J., Hill J. H., et al. (2005). Virus taxonomy, in Eighth Report of the International Committee on Taxonomy of Viruses, eds Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A.(San Diego, CA: Elsevier Academic Press; ), 819–841. [Google Scholar]
- Bhandel I. S., Paxton J. D. (1991). Phytoalexin biosynthesis induced by the fungal glucan Polytran L in soybean, pea and sweet pepper tissues. J. Agric. Food Chem. 39, 2156–2157. 10.1021/jf00012a010 [DOI] [Google Scholar]
- Bharathi R., Vivekananthan R., Harish S., Ramanathan A., Samiyappan R. (2004). Rhizobacteria-based bio-formulations for the management of fruit rot infection in chillies. Crop Prot. 23, 835–884. 10.1016/j.cropro.2004.01.007 [DOI] [Google Scholar]
- Boonratkwang C., Chamswarng C., Intanoo W., Juntharasri V. (2007). Effect of Secondary Metabolites from Trichoderma Harzianum Strain Pm9 on Growth Inhibition of Colletotrichum gloeosporioides and Chilli Anthracnose Control, in Proceeding of the 8th National Plant Protection Conference (Phisanulok: Naresuan University; ), 323–336. [Google Scholar]
- Bosland P. W. (1992). Chiles: a diverse crop. Hort. Technol. 2, 6–10. [Google Scholar]
- Bosland P. W. (1994). Chiles: history, cultivation, and uses, in Spices, Herbs, and Edible Fungi, ed. Charalambous G.(New York, NY: Elsevier Publishing; ), 347–366. [Google Scholar]
- Bosland P. W. (1996). Capsicums: innovative uses of an ancient crop, in Progress in New Crops, ed Janick J. (Arlington, VA: ASHS Press; ), 479–487. [Google Scholar]
- Byung S. K. (2007). Country report of Anthracnose research in Korea, in First International Symposium on Chili Anthracnose, Hoam Faculty House, Seoul National University (Seoul: ), 24. [Google Scholar]
- Cerkauskas R. (2004). Cercospora leaf spot, in AVRDC Fact Sheet: Pepper Diseases, ed Tom K.(Taiwan: AVRDC–The World Vegetable Center; AVRDC Publication; ), 4–575. [Google Scholar]
- Chanchaichaovivat A., Ruenwongsa P., Panijpan B. (2007). Screening and identification of yeast strains from fruit and vegetables: potential for biological control of postharvest chilli anthracnose (Colletotrichum capscii). Biol. Control 42, 326–335. 10.1016/j.biocontrol.2007.05.016 [DOI] [Google Scholar]
- Chattopadhyay B., Singh A. K., Yadav T., Fauquet C. M., Sarin N. B., Chakraborty S. (2008). Infectivity of the cloned components of a begomovirus: DNA beta complex causing chilli leaf curl disease in India. Arch. Virol. 153, 533–539. 10.1007/s00705-007-0017-2 [DOI] [PubMed] [Google Scholar]
- Chen Y., Li-hua J., Ming-guo Z. (2009). Effect of Azoxystrobin on Oxygen Consumption and cyt b gene expression of Colletotrichum capsici from Chilli Fruits. Agr. Sci. China 8, 628–631. 10.1016/S1671-2927(08)60255-2 [DOI] [Google Scholar]
- Correll C. J., Cornelius K., Feng C., Ware S. B., Gabor B., Harp T. L. (2007). Overview of the phylogenetics species concept in Colletotrichum as it relates to chilli anthracnose, in Abstracts of the First International Symposium on Chilli Anthracnose National Horticultural Research Institute, eds Oh D. G., Kim K. T.(Rural Development of Administration; ), 20. [Google Scholar]
- Crawford R. F. (1934). The etiology and control of chile wilt, produced by Fusarium annuum. NM Agric. Exp. Sta. Tech. Bull. 223:20. [Google Scholar]
- Damm U., Cannon P. F., Woudenberg J. H. C., Crous P. W. (2012a). The Colletotrichum acutatum species complex. Stud. Mycol. 73, 37–113. 10.3114/sim0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U., Cannon P. F., Woudenberg J. H. C., Johnston P. R., Weir B. S., Tan Y. P., et al. (2012b). The Colletotrichum boninense species complex. Stud. Mycol. 73, 1–36. 10.3114/sim0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U., Woudenberg J. H. C., Cannon P. F., Crous P. W. (2009). Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 39, 45–87. [Google Scholar]
- Dean R., Van Kan J. A. L., Pretorius Z. A., Hammond-Kosack K. E., Di Pietro A., Spanu P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. 10.1111/j.1364-3703.2011.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De los Santos G., Romero M. F. (2002). Effect of different fungicides in the control of Colletotrichum acutatum, causal agent of anthracnose crown rot in strawberry plants. Crop Prot. 21, 17–23. 10.1016/S0261-2194(01)00054-0 [DOI] [Google Scholar]
- Demircioglu S., Filazi A. (2010). Detection of aflatoxin levels in red pepper produced in Turkey. J. Veter. Med. Assoc. 81, 63–66. 10.1016/j.foodcont.2006.03.013 [DOI] [Google Scholar]
- Denner F. D. N., Kotze J. M., Putterill J. F. (1986). The effect of temperature on spore germination, growth and appressorium formation of Colletotrichum gloeosporiodes and Dothiorella aromatic. S. Afr. Avocado Growers' Assoc. Yearb. 9, 19–22. [Google Scholar]
- Dodd J. C., Estrada A., Jeger M. J. (1992). Epidemiology of Colletotrichum gloeosporioides in the Tropics, in Colletotrichum: Biology, Pathology and Control, eds Bailey J. A., Jeger M. J.(Wallingford, CT: CAB International; ), 308–325. [Google Scholar]
- Don L. D., Van T. T., Phuong Vy T. T., Kieu P. T. M. (2007). Colletotrichum spp. attacking on chilli pepper growing in Vietnam, Country Report, in Abstracts of the First International Symposium on Chilli Anthracnose, eds Oh D. G., Kim K. T. (Seoul: Seoul National University; ), 24. [Google Scholar]
- Ekbote S. K. (2005). Effect of Pseudomonas fIuorescens on anthracnose of chilli caused by Colletotrichum capsici. Karnataka, J. Agric. Sci. 18, 162–165. [Google Scholar]
- Farr D. F., Rossman A. Y., Palm M. E., McCray E. B. (2016). Fungal Databases, Systematic Botany and Mycology Laboratory, ARS, USDA. Available online at: http://nt.ars-grin.gov/fungaldatabases/
- FAOSTAT (2011). Production Data. Available online at: http://faostat.fao.org/
- FAOSTAT (2012). Production Data. Available online at: http://faostat.fao.org/
- Fletcher J. D. (1983). New plant disease records in New Zealand: additional host of alfafa mosaic virus. N. Z. J. Agr. Res. 26, 403–404. 10.1080/00288233.1987.10417964 [DOI] [Google Scholar]
- Flor H. H. (1971). Current status for the gene-for-gene concept. Annu. Rev. Phytopathol. 9, 275–296. 10.1146/annurev.py.09.090171.001423 [DOI] [Google Scholar]
- Förster H., Driever G. F., Thompson D. C., Adaskaveg J. E. (2007). Postharvest decay management for stone fruit crops in California using the “reduced-risk” fungicides fludioxonil and fenhexamid. Plant Dis. 91, 209–215. 10.1094/PDIS-91-2-0209 [DOI] [PubMed] [Google Scholar]
- Freeman S. (2000). Genetic diversity and host specificity of Colletotrichum species on various fruits, in Colletotrichum: Host Specificity, Pathology, and Host-Pathogen Interaction, eds Prusky D., Freeman S., Dickman M. B. (St. Paul, MN: The American Phytopathological Society; ), 131–144 [Google Scholar]
- Freeman S., Minz D., Kolesnik I., Barbul O., Zveibil A., Maymon M., et al. (2004). Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. Eur. J. Plant Pathol. 110, 361–370. 10.1023/B:EJPP.0000021057.93305.d9 [DOI] [Google Scholar]
- Freeman S., Shabi E. (1996). Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts. Physiol. Mol. Plant Pathol. 49, 395–404. 10.1006/pmpp.1996.0062 [DOI] [Google Scholar]
- Garcia-Villanova R. J., Cordon C., González Paramás A. M., Aparicio P., Garcia Rosales M. E.. (2004). Simultaneous immune affinity column cleanup and HPLC analysis of aflatoxins and ochratoxin A in Spanish bee pollen. J. Agric. Food Chem. 52, 7235– 7239. 10.1021/jf048882z [DOI] [PubMed] [Google Scholar]
- Garg R., Loganathan M., Saha S., Roy B. K. (2014). Chilli Anthracnose: a review of causal organism, resistance source and mapping of gene, in Microbial Diversity and Biotechnology in Food Security, eds Kharwar R. N., Upadhyay R., Dubey N., Raguwanshi R. (Springer; ), 589–610. [Google Scholar]
- Ghosh S. K., Chakraborty N. (2012). In vitro biological control of Colletotrichum gloeosporioides, causal organism of anthracnose of sarpagandha (Roulvolfia serpentina). Agric. Biol. J.N. Am. 3, 306–310. 10.5251/abjna.2012.3.8.306.310 [DOI] [Google Scholar]
- Glawe D. A., Barlow T., Eggers J. E., Hamm P. B. (2010). First report of powdery mildew caused by Leveillula taurica of field-grown sweet pepper in the Pacific Northwest. [Online]. Plant Health Progr. 10.1094/PHP-2007-0708-01-BR [DOI] [Google Scholar]
- Gopinath K., Radhakrishnana N. V., Jayaraj J. (2006). Effect of propiconazole and difenoconazole on the control of anthracnose of chilli fruits caused by Colletotrichum capsici. Crop Prot. 25, 1024–1031. 10.1016/j.cropro.2006.02.001 [DOI] [Google Scholar]
- Grubben G. J. H., Mohamed El T. I. (2004). Capsicum annuum L., in PROTA 2: Vegetables/Légumes, eds Grubben G. J. H., Denton O. A. (Wageningen: PROTA; ), 154–163. [Google Scholar]
- Harman G. E. (2006). Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96, 190–194. 10.1094/PHYTO-96-0190 [DOI] [PubMed] [Google Scholar]
- Harman G. E., Petzoldt R., Comis A., Chen J. (2004). Interactions between Trichoderma harzianum strain T22 and maize inbred line Mo17 and effects of these interactions on diseases caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 94, 147–153. 10.1094/PHYTO.2004.94.2.147 [DOI] [PubMed] [Google Scholar]
- Harp T. L., Pernezny K., Ivey M. L. L., Miller S. A., Kuhn P. J., Datnoff L. (2008). The etiology of recent pepper anthracnose outbreaks in Florida. Crop Prot. 27, 1380–1384. 10.1016/j.cropro.2008.05.006 [DOI] [Google Scholar]
- Hegde G. M., Anahosur K. H. (2001). Evaluation of fungitoxicants against fruit rot of chilli and their effect on biochemical constituents. Karnataka J. Agric. Sci. 14, 836–838. [Google Scholar]
- Heiser C. B. (1976). Peppers Capsicum (Solanaceae), in The Evolution of Crops Plants, ed Simmonds S. W.(London: Longman Press; ), 265–268. [Google Scholar]
- Hermosa R., Viterbo A., Chet I., Monte E. (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158, 17–25. 10.1099/mic.0.052274-0 [DOI] [PubMed] [Google Scholar]
- Hong J. K., Hwang B. K. (1998). Influence of inoculum density, wetness duration, plant age, inoculation method, and cultivar resistance on infection of pepper plants by Colletotrichum coccodes. Plant Dis. 82, 1079–1083. 10.1094/PDIS.1998.82.10.1079 [DOI] [PubMed] [Google Scholar]
- IBPGR (1983). Genetic resources of Capsicum. Rome: International Board for Plant Genetic Resources. [Google Scholar]
- Imtiaj A., Lee S. T. (2008). Antagonistic effect of three Trichoderma species on the Alternaria porri pathogen of onion blotch. World J. Agric. Sci. 4, 13–17. [Google Scholar]
- Intanoo W., Chamswarng C. (2007). Effect of Antagonistic Bacterial Formulations for Control of Anthracnose on Chilli Fruits, in Proceeding of the 8th National Plant Protection Conference, Naresuan University (Phisanulok: ), 309–322. [Google Scholar]
- Intra B., Mungsuntisuk I., Nihira T., Igarashi Y., Panbangred W. (2011). Identification of actinomycetes from plant rhizospheric soils with inhibitory activity against Colletotrichum spp., the causative agent of anthracnose disease. BMC Res. Notes 4:98. 10.1186/1756-0500-4-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey M. L. L., Miller S. A. (2000). First report of Bacterial canker of pepper in Ohio. Plant Dis. 84:810 10.1094/PDIS.2000.84.7.810C [DOI] [PubMed] [Google Scholar]
- Jain A., Singh S., Sarma B. K., Singh H. B. (2012). Microbial consortium mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J. Appl. Microbiol. 112, 537–550. 10.1111/j.1365-2672.2011.05220.x [DOI] [PubMed] [Google Scholar]
- Jebessa M. T., Ranamukhaarachchi S. L. (2006). Attempts to biologically control anthracnose disease in chilli peppers. Trop. Sci. 46, 74–77. 10.1002/ts.78 [DOI] [Google Scholar]
- Jeffrey A. M., Williams G. M. (2005). Risk assessment of DNA-reactive Carcinogens in Food. Toxicol. Appl. Pharm. 207, 628–635. 10.1016/j.taap.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Jeger M. J., Jeffries P. (1988). Alternative to chemical usage for disease management in the post-harvest environment. Aspects Appl. Biol. 17, 47–57. [Google Scholar]
- Jetiyanon K., Kloepper J. W. (2002). Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol. Control 24, 285–291. 10.1016/S1049-9644(02)00022-1 [DOI] [Google Scholar]
- Johari A., Herlinda S., Pujiastuti Y., Irsan C., Sartiami D. (2014). Morphological and genetic variation of Thrips parvispinus (Thysanoptera: Thripidae) in chili plantation (Capsicum annuum L.) in the lowland and highland of Jambi province, Indonesia. Am. J. BioSci. 2, 17–21. 10.11648/j.ajbio.s.2014020601.14 [DOI] [Google Scholar]
- Johnny L., Yusuf U. K., Nulit R. (2011). Antifungal activity of selected plant leaves crude extracts against a pepper anthracnose fungus, Colletotrichum capsici (Sydow) butler and bisby (Ascomycota: Phyllachorales). Afr. J. Biotechnol. 10, 4157–4165. 10.5897/AJB10.2085 [DOI] [Google Scholar]
- Kamle M., Pandey B. K., Kumar P., Kumar M. (2013). A Species- Specific PCR based Assay for rapid detection of mango anthracnose pathogen Colletotrichum gloeosporioides Penz and Sacc. J. Plant Pathol. Microbiol. 4:184 10.4172/2157-7471.1000184 [DOI] [Google Scholar]
- Kaur M., Sharma O. P., Sharma P. (2006). In vitro effect of Trichoderma species on Colletotrichum capsici causing fruit rot of chilli (Capsicum annuum L.). Indian Phytopathol. 59, 243–245. [Google Scholar]
- Kaur S., Kang S. S., Sharma A., Sharma S. (2014). First report of Pepper mottle virus infecting chilli pepper in India. New Dis. Rep. 30:14 10.5197/j.2044-0588.2014.030.014 [DOI] [Google Scholar]
- Kim K. K., Yoon J. B., Park H. G., Park E. W., Kim Y. H. (2004). Structural modifications and programmed cell death of chilli pepper fruits related to resistance responses to Colletotrichum gloeosporioides infection. Phytopathology 94, 1295–1304. 10.1094/PHYTO.2004.94.12.1295 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Yoon J. B., Park H. G. (2008). Inheritance of anthracnose resistance in a new genetic resource, Capsicum baccatum PI594137. J. Crop Sci. Biotech. 11, 13–16. [Google Scholar]
- Kim Y. S., Min J. Y., Kang B. K., Bach N. V., Choi W. B., Park E. W., et al. (2007). Analyses of the less benzimidazole-sensitivity of the isolates of Colletotrichum spp. causing the anthracnose in pepper and strawberry. Plant Pathol. J. 23, 187–192. 10.5423/PPJ.2007.23.3.187 [DOI] [Google Scholar]
- Koike S. T., Gladders P., Paulus A. (2007). Capsicum: pepper, in Vegetable Diseases: A color Handbook, ed Northcott J. (London: Manson Publishing Ltd; ), 208–209. [Google Scholar]
- Korsten L., Jeffries P. (2000). Potential for biological control of diseases caused by Colletotrichum, in Colletotrichum Host Specificity–Pathology and Host-Pathogen Interaction, eds Prusky D., Freeman S., Dickman M. B.(St. Paul, MN: APS Press; ), 266–295. [Google Scholar]
- Kumar P., Kumar A., Kumar K. (2000). Bio-control of seed-borne fungal pathogens of pigeon pea (Cajanus cajan (L.) Millsp.). Ann. Plant Protect. Sci. 8, 30–32. [Google Scholar]
- Lakshmesha K., Lakshmidevi K., Aradhya N., Mallikarjuna S. (2005). Changes in pectinase and cellulase activity of Colletotrichum capsici mutants and their effect on anthracnose disease on Capsicum fruit. Arch. Phytopathol. Plant Prot. 38, 267–279. 10.1080/03235400500094100 [DOI] [Google Scholar]
- Latunde-Dada A. O., Lucas J. A. (2007). Localized hemibiotrophy in Colletotrichum: cytological and molecular taxonomic similarities among C. destructivum, C. linicola and C. truncatum. Plant Pathol. 56, 437–447. 10.1111/j.1365-3059.2007.01576.x [DOI] [Google Scholar]
- Lee J., Jee-Hwa H., Jae W. D., Jae B. (2010). Identification of QTLs for resistance to anthracnose to two Colletotrichum species in pepper. J. Crop Sci. Biotech. 13, 227–233. 10.1007/s12892-010-0081-0 [DOI] [Google Scholar]
- Lenné J. M., Parbery D. G. (1976). Phyllosphere antagonists and appressoria formation in Colletotrichum gloeosporioides. T. Brit. Mycol. Soc. 66, 334–336. 10.1016/S0007-1536(76)80065-4 [DOI] [Google Scholar]
- Lewis I. M. L., Miller S. A. (2003). Evaluation of Fungicides and a Biocontrol Agent for the Control of Anthracnose on Green Pepper Fruit. Nematicide Test Report [Online], New Fungicide and Nematicide Data Committee of the American Phytopathological Society.
- Lewis M. L., Nava-Diaz C., Miller S. (2004). Identification and management of Colletotrichum acutatum on immature Bell Peppers. Plant Dis. 88, 1198–1204. 10.1094/PDIS.2004.88.11.1198 [DOI] [PubMed] [Google Scholar]
- Liao C. Y., Chen M. Y., Chen Y. K., Kuo K. C., Chung K. R., Lee M. H. (2012). Formation of highly branched hyphae by Colletotrichum acutatum within the fruit cuticles of Capsicum spp. Plant Pathol. 61, 262–270. 10.1111/j.1365-3059.2011.02523.x [DOI] [Google Scholar]
- Lima J. S., Figueiredo J. G., Gomes R. G., Stringari D., Goulin E. H., Adamoski D., et al. (2012). Genetic diversity of Colletotrichum spp. an endophytic fungus in a Medicinal Plant, Brazilian Pepper Tree. ISRN Microbiol. 2012:215716. 10.5402/2012/215716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Cai L., Crous P. W., Damm U. (2013). Circumscription of the anthracnose pathogens Colletotrichum lindemuthianum and C. nigrum. Mycologia 105, 844–860. 10.3852/12-315 [DOI] [PubMed] [Google Scholar]
- MacNeish R. S. (1964). Ancient Mesoamerican civilization. Science 143, 531–537. 10.1126/science.143.3606.531 [DOI] [PubMed] [Google Scholar]
- Maharijaya A., Vosman B., Steenhuis-Broers G., Harpenas A., Purwito A., Visser R. G. F., et al. (2011). Screening of pepper accessions for resistance against two thrips species (Frankliniella occidentalis and Thrips parvispinus). Euphytica 177, 401–410. 10.1007/s10681-010-0277-x [DOI] [Google Scholar]
- Manandhar J. B., Hartman G. L., Wang T. C. (1995). Anthracnose development on pepper fruits inoculated with Colletotrichum gloeosporioides. Plant Dis. 79, 380–383. 10.1094/PD-79-0380 [DOI] [Google Scholar]
- Mohd Anuar I. S., Vijaya S. I., Zakaria L. (2014). Molecular characterization and pathogenicity of Colletotrichum sp, from guava. Arch. Phytopathol. PFL 47, 1549–1556. 10.1080/03235408.2013.850198 [DOI] [Google Scholar]
- Mongkolporn O., Thierry J., Kanchana-udomkarn C., Lin Q. (2004). Genetic analysis of resistance to pepper anthracnose caused by Colletotrichum capsici. Thai J. Agri. Sci. 35, 259–264. [Google Scholar]
- Montri P., Taylor P. W. J., Mongkolporn O. (2009). Pathotypes of Colletotrichum capsici, the causal agent of chili anthracnose, in Thailand. Plant Dis. 93, 17–20. 10.1094/PDIS-93-1-0017 [DOI] [PubMed] [Google Scholar]
- Moury B., Palloix A., Caranta C., Gognalons P., Souche S., Selassie K. G., et al. (2005). Serological, molecular, and pathotype diversity of Pepper veinal mottle virus and Chili veinal mottle virus. Phytopathology 95, 227–232. 10.1094/PHYTO-95-0227 [DOI] [PubMed] [Google Scholar]
- Mukherjee A. K., Kumar A. S., Kranthi S., Mukherjee P. K. (2014). Biocontrol potential of three novel Trichoderma strains: isolation, evaluation and formulation. 3 Biotech 4, 275–281. 10.1007/s13205-013-0150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglot A., Goswami S., Rahman I., Shrimali D. D., Yadav K. K., Gupta V. K., et al. (2015). Antagonistic Potential of Native Trichoderma viride Strain against Potent Tea Fungal Pathogens in North East India. Plant Pathol. J. 31, 278–289. 10.5423/PPJ.OA.01.2015.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantawanit N., Chanchaichaovivat A., Panijpan B., Ruenwongsa P. (2010). Induction of defense response against Colletotrichum capsici in chilli fruit by the yeast Pichia guilliermondii strain R13. Biol. Control 52, 145–152. 10.1016/j.biocontrol.2009.10.011 [DOI] [Google Scholar]
- Ngullie M., Daiho L., Upadhyay D. N. (2010). Biological management of fruit rot in the world's hottest chilli (Capsicum chinense jacq.). J. Plant Prot. Res. 50, 269–273. 10.2478/v10045-010-0047-8 [DOI] [Google Scholar]
- Nguyen M. T., Ranamukhaarachchi S. L. (2010). Soil-Borne antagonists for biological control of bacterial wilt disease caused by Ralstonia solanacearum in tomato and pepper. J. Plant Pathol. 92, 395–405. 10.4454/jpp.v92i2.183 [DOI] [Google Scholar]
- O′Connell R. J., Perfect S., Hughes B., Carzaniga R., Bailey J. A., Green J. (2000). Dissecting the cell biology of Colletotrichum infection processes, in Colletotrichum: Biology, Pathology, and Control, eds Bailey J. A., Jegar M. J.(Wallingfords: CAB International; ), 57–77. [Google Scholar]
- Oanh L. T. K., Korpraditskul V., Rattanakreetakul C., Wasee S. (2006). Influence of biotic and chemical plant inducers on resistance of chili to anthracnose. Kasetsart J. (Nat. Sci.) 40, 39–48. [Google Scholar]
- Osuna-García J. A., Wall M. W., Waddell C. A. (1998). Endogenous levels of tocopherols and ascorbic acid during fruits ripening of New Mexican-type chilli Capsicum annuum L. cultivars. J. Agr. Food Chem. 46, 5093–5096. 10.1021/jf980588h [DOI] [Google Scholar]
- Padder B. A., Sharma P. N. (2011). In vitro and in vivo antagonism of biocontrol agents against Colletotrichum lindemuthianum causing bean anthracnose. Arch. Phytopathol. 44, 961–969. 10.1080/03235400903460619 [DOI] [Google Scholar]
- Park H. G. (2007). Problems of anthracnose in pepper and prospects for its management, in Abstracts of the First International Symposium on Chilli Anthracnose, eds Oh D. G., Kim K. T. (Seoul: National Horticultural Research Institute, Rural Development of Administration; ), 19. [Google Scholar]
- Park J. W., Balaraju K., Kim J. W., Lee S. W., Park K. (2013). Systemic resistance and growth promotion of chili pepper induced by an antibiotic producing Bacillus vallismortis strain BS07. Biol. Control 65, 246–257. 10.1016/j.biocontrol.2013.02.002 [DOI] [Google Scholar]
- Park K. S., Kim C. (1992). Identification, distribution, and etiological characteristics of anthracnose fungi of red pepper in Korea. Korean J. Plant Pathol. 8, 61–69. [Google Scholar]
- Pearson M. N., Bull P. B., Speke H. (1984). Anthracnose of Capsicum in Papua, New Guinea; varietal reaction and associated fungi. Trop. Pest Manag. 30, 230–233. 10.1080/09670878409370888 [DOI] [Google Scholar]
- PuckerButt Pepper Company (2013). Smokin' Ed's Carolina Reaper® wins Guinness World Record–Smokin' Ed's Carolina Reaper® Officially the Hottest Chili Pepper in the World! Available online at: http://www.guinnessworldrecords.com/news/2013/11/confirmed-smokin-eds-carolina-reaper-sets-new-record-for-hottest-chilli-53033
- Peres N. A., Timmer L. W., Adaskaveg J. E., Correll J. C. (2005). Life cycles of Colletotrichum acutatum. Plant Dis. 89, 784–796. 10.1094/PD-89-0784 [DOI] [PubMed] [Google Scholar]
- Perfect S. E., Hughes H. B., O'Connell R. J., Green J. R. (1999). Colletotrichum: a model genus for studies on pathology and fungal-plant interactions. Fungal Genet. Biol. 27, 186–198. 10.1006/fgbi.1999.1143 [DOI] [PubMed] [Google Scholar]
- Photita W., Taylor P. W. J., Ford R., Hyde K. D., Lumyong S. (2005). Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Divers. 18, 117–133. [Google Scholar]
- Phoulivong S., McKenzie E. H. C., Hyde K. D. (2012). Cross infection of Colletotrichum species; a case study with tropical fruits. Curr. Res. Environ. Appl. Mycol. 2, 99–111. 10.5943/cream/2/2/2 [DOI] [Google Scholar]
- Pollegioni P., Van der Linden G., Belisario A., Gras M., Anselmi N., Olimpieri I., et al. (2012). Mechanisms governing the response to anthracnose pathogen in Juglans spp. J. Biotechnol. 159, 251–264. 10.1016/j.jbiotec.2011.08.020 [DOI] [PubMed] [Google Scholar]
- Post harvest profile of chilli (2009). Directorate of Marketing and Inspection. Nagpur: Ministry of Agriculture, Government of India. [Google Scholar]
- Pring R. J., Nash C., Zakaria M., Bailey J. A. (1995). Infection process and host range of Colletotrichum capsici. Physiol. Mol. Plant Pathol. 46, 137–152. 10.1006/pmpp.1995.1011 [DOI] [Google Scholar]
- Prusky D. (1996). Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol. 34, 413–434. 10.1146/annurev.phyto.34.1.413 [DOI] [PubMed] [Google Scholar]
- Prusky D. (2011). Reduction of the incidence of postharvest quality losses, and future Prospects. Food Secur. 3, 463–474. 10.1007/s12571-011-0147-y [DOI] [Google Scholar]
- Prusky D., Koblier I., Aridi R., Beno-Moalem D., Yakoby N., Keen N. T. (2000). Resistance mechanisms of subtropical fruits to Colletotrichum gloeosporioides, in Colletotrichum: Biology, Pathology, and Control, eds Bailey J. A., Jeger M. J.(Wallingford: CAB International; ), 232–244 [Google Scholar]
- Puttawong S., Wongroung S. (2009). Plucao (Houttuynia cordata) Thunb and sabsua (Eupatorium odoratum L.) extracts suppress Colletotrichum capsici and Fusarium oxysporum. J. Agric Food Ind. Organ. 2009, S381–S386. [Google Scholar]
- Rahman M. A., Razvy M. A., Alam M. F. (2013). Antagonistic activities of Trichoderma strains against chili anthracnose pathogen. Int. J. Microbiol. Mycol. 1, 7–22. [Google Scholar]
- Ramachandran N., Rathnamma K. (2006). Colletotrichum acutatum—a new addition to the species of chilli anthracnose pathogen in India, in Paper presented at the Annual Meeting and Symposium of Indian Phytopathological Society, Central Plantation Crops Research Institute (Kasarago: ). [Google Scholar]
- Ramamoorthy V., Samiyappan R. (2001). Induction of defense-related genes in Pseudomonas fluorescens treated chilli plants in response to infection by Colletotrichum capsici. J. Mycol. Plant Pathol. 31, 146–155. 10.1016/j.biocontrol.2009.10.011 [DOI] [Google Scholar]
- Ranathunge N. P., Mongkolporn O., Ford R., Taylor P. W. J. (2012). Colletotrichum truncatum pathosystem on Capsicum spp.: infection, colonization and defence mechanisms. Australas. Plant Path. 41, 463–473. 10.1007/s13313-012-0156-0 [DOI] [Google Scholar]
- Reddy K. R. N., Raghavender C. R., Salleh B., Reddy C. S., Reddy B. N. (2011). Potential of aflatoxin B1 production by Aspergillus flavus strains on commercially important food grains. Int. J. Food Sci. Tech. 161–165. 10.1111/j.1365-2621.2010.02468.x [DOI] [Google Scholar]
- Roberts P. D., Pernezny K., Kucharek T. A. (2001). Anthracnose Caused by Colletotrichum sp. on Pepper. University of Florida/Institute of Food and Agricultural Sciences, Available online at: http://edis.ifas.ufl.edu/PP104
- Rodriguez R. J. (2000). US Geological Survey, WA, Redman, RS, Colletotrichum as model system for defining the genetic basis of fungal symbiotic life styles, in Colletotrichum: Host Specificity, Pathology, and Host-Pathogen Interaction, eds Prusky D., Freeman S., Dickman M. B. (St. Paul, MN: The American Phytopathological Society; ), 232–244. [Google Scholar]
- Royle D. J., Butler D. R. (1986). Epidemiological significance of liquid water in crop canopies and its role in disease forecasting, in Water, Fungi and Plants, eds Ayres P. G., Boddy L.(Cambridge: Cambridge University Press; ), 139–156. [Google Scholar]
- Sander G. M., Korsten L. (2003). Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides. Microbiol. Res. 158, 143–150. 10.1078/0944-5013-00186 [DOI] [PubMed] [Google Scholar]
- Sanogo S. (2003). Chile pepper and the threat of wilt diseases. [Online]. Plant Health Progr. 10.1094/PHP-2003-0430-01-RV [DOI] [Google Scholar]
- Sanogo S., Carpenter J. (2006). Incidence of Phytophthora blight and Verticillium wilt within chile pepper fields in New Mexico. Plant Dis. 90, 291–296. 10.1094/PD-90-0291 [DOI] [PubMed] [Google Scholar]
- Saxena A., Raghuvanshi R., Singh H. B. (2014). Molecular, phenotypic and pathogenic variability in Colletotrichum isolates of subtropical region in North Eastern India, causing fruit rot of chillies. J. Appl. Microbiol. 117, 1422–1434. 10.1111/jam.12607 [DOI] [PubMed] [Google Scholar]
- Saxena A., Raghuwanshi R., Singh H. B. (2015). Trichoderma species mediated differential tolerance against biotic stress of phytopathogens in Cicer arietinum L. J. Basic Microbiol. 55, 195–206. 10.1002/jobm.201400317 [DOI] [PubMed] [Google Scholar]
- Saxena A., Raghuwanshi R., Singh H. B. (2016a). Elevation of defense network in chilli against Colletotrichum capsici by phyllospheric Trichoderma strain. J. Plant Growth Regul. 35, 377–389. 10.10007/s00344-015-9542-5 [DOI] [Google Scholar]
- Saxena A., Sarma B. K., Singh H. B. (2016b). Effect of azoxystrobin based fungicides in management of chilli and tomato diseases. Proc. Natl. Acad. Sci. India B Biol. Sci. 86, 283–289. 10.1007/s40011-014-0422-8 [DOI] [Google Scholar]
- Schilder A. M. C., Gillett J. M., Sysak R. W. (2001). Evaluation of Fungicides for Control of Anthracnose Fruit Rot of Blueberries. Fungicide and Infanticide Tests SMF5.
- Scoville W. L. (1912). Note on Capsicum. J. Am. Pharm. Assoc. 1, 453 10.1002/jps.3080010520 [DOI] [Google Scholar]
- Sharma P., Kulshrestha G., Gopal M., Kadu L. N. (2004). Integrated management of chilli die back and anthracnose in Delhi region. Indian Phytopathol. 57, 427–434. [Google Scholar]
- Sharma P. N., Kaur M., Sharma O. P., Sharma P., Pathania A. (2005). Morphological, pathological and molecular variability in Colletotrichum capsici, the cause of fruit rot of chillies in the subtropical region of north western India. J. Phytopathol. 153, 232–237. 10.1111/j.1439-0434.2005.00959.x [DOI] [Google Scholar]
- Sharma R. R., Singh D., Singh R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol. Control 50, 205–221. 10.1016/j.biocontrol.2009.05.001 [DOI] [Google Scholar]
- Shoresh M., Harman G. E., Mastouri F. (2010). Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 48, 21–43. 10.1146/annurev-phyto-073009-114450 [DOI] [PubMed] [Google Scholar]
- Shovan L. R., Bhuiyan M. K. A., Begum J. A., Pervez Z. (2008). In vitro control of Colletotrichum dematium causing anthracnose of soybean by fungicides, plant extracts and T. harzianum. Int. J. Sustain. Crop Prod. 3, 10–17. [Google Scholar]
- Singh A., Jain A., Sarma B. K., Upadhyay R. S., Singh H. B. (2013). Rhizosphere microbes facilitate redox homeostasis in Cicer arietinum against biotic stress. Ann. Appl. Biol. 163, 33–46. 10.1111/aab.12030 [DOI] [Google Scholar]
- Singh H., Korpraditskul V. (1999). Evaluation of some plant extracts for the control of Colletotrichum capsici (Syd.) Butler and Bisby, the causal agent of chilli anthracnose, in Azadirchta indica A. Juss, eds Singh R. P., Saxena R. C.(Enfield, NH: Science Publishers, Inc.), 131–138. [Google Scholar]
- Singh H. B., Singh B. N., Singh S. P., Sarma B. K. (2012). Exploring different avenues of Trichoderma as a potent biofungicidal and plant growth promoting candidate–an overview. Rev. Plant Pathol. 5, 315–426. [Google Scholar]
- Smith K. L. (2000). Peppers, in Ohio Vegetable Production Guide, ed Precheur R. J.(Columbus, Ohio, OH: Ohio State University Extension; ), 166–173. Available online at: https://ag.purdue.edu/btny/midwest-vegetable-guide/Pages/default.aspx [Google Scholar]
- Soytong K., Kanokmedhakul S., Rattanacherdchai K., Charoenporn C. (2014). Microbial elicitors to induce immunity for plant disease control in chilli and tomato, in Basics and Applied Aspects of Biopesticides, ed Sayaraj K.(Springer; ), 99–125. [Google Scholar]
- Soytong K., Srinon W., Rattanacherdchai K., Kanokmedhakul S., Kanokmedhakul K. (2005). Application of antagonistic fungi to control anthracnose disease of grape. J. Agric. Biotechnol. 1, 33–41. [Google Scholar]
- Srinivas C., Niranjana S. R., Praveen K. L., Chandra N. S., Shetty H. S. (2006). Effect of fungicides and bioagents against Collectotrichum capsici on seed quality of chilli. Indian Phytopathol. 59, 62–67. [Google Scholar]
- Stanley J. (2008). Beet curly top virus, in Encyclopedia of Virology, 3rd Edn, eds Mahy B. W. J., van Regenmortel M. H. V. (Academic Press Elsevier; ), 301–307. [Google Scholar]
- Staub T. (1991). Fungicide resistance: practical experience and antiresistance strategies and the role of integrated use. Annu. Rev. Phytopathol. 29, 421–442. 10.1146/annurev.py.29.090191.002225 [DOI] [PubMed] [Google Scholar]
- Stommel J. R., Goth R. W., Haynes K. G., Kim S. H. (1996). Pepper (Capsicum annuum) soft rot caused by Erwinia carotovore subsp. Atroseptica. Plant Dis. 80, 1109–1112. [Google Scholar]
- Sutarya R., Dibiyantoro A., Gniffke P. A., Palada M. C., Hardjo T. (2009). Integrated chilli management to control some major diseases in Brebes district, in Kumpulan Makalah Seminar Ilmiah Perhorti, 61–70. Available online at: http://aciar.gov.au/files/node/13885/app_13_sutarya_et_al_icm_in_brebes_central_java_pd_28038.pdf
- Sutton B. L. (1992). The genus Glomerella and its Colletotrichum anamorph, in Colletotrichum: Biology, Pathology and Control, eds Bailey J. A., Jeger M. J. (Wallingford: CAB Internationa; ), 1–28. [Google Scholar]
- Suwan N., Na-Lampang S. (2013). Characterization and evaluation of carbendazim resistance response of Colletotrichum species. J. Agric. Technol. 9, 1883–1894. [Google Scholar]
- Tapia D., Troncoso A., Vargas R., Ares-Donoso R., Niemeyer H. (2008). Experimental evidence for competitive exclusion of Myzus persicae nicotianae by Myzus persicae s.s. (Hemiptera: Aphididae) on sweet pepper, Capsicum annuum (Solanaceae). Eur. J. Entomol. 105, 643–648. 10.14411/eje.2008.088 [DOI] [Google Scholar]
- Than P. P., Jeewon R., Hyde K. D., Pongsupasamit S., Mongkolporn O., Taylor P. W. J. (2008). Characterization and pathogenicity of Colletotrichum species associated with anthracnose disease on chilli (Capsicum spp.) in Thailand. Plant Pathol. 57, 562–572. 10.1111/j.1365-3059.2007.01782.x [DOI] [Google Scholar]
- Thiyagarajan S. S., Kuppusamy H. (2014). Biological control of root knot nematodes in chillies through Pseudomonas fluorescens's antagonistic mechanism, J. Plant Sci. 2, 152–158. 10.11648/j.jps.20140205.12 [DOI] [Google Scholar]
- Timmer L. W., Brown G. E. (2000). Biology and control of anthracnose diseases of citrus, in Colletotrichum: Host Specificity, Pathology, and Host-Pathogen Interaction, eds Prusky D., Freeman S., Dickman M. B.(St. Paul MN: The American Phytopathological Society; ), 300–316. [Google Scholar]
- Torres-Calzada C., Tapia-Tussell R., Quijano-Ramayo A., Martin-Mex R., Perez-Brito D. (2011). A Species-Specific polymerase chain reaction assay for rapid and sensitive detection of Colletotrichum capsici. Mol. Biotechnol. 49, 48–55. 10.1007/s12033-011-9377-7 [DOI] [PubMed] [Google Scholar]
- Tozze H. J., Massola N. M. (2009). First Report of Colletotrichum boninense causing anthracnose on pepper in Brazil. Dis. Notes 93:106 10.1094/PDIS-93-1-0106A [DOI] [PubMed] [Google Scholar]
- Uddin W., Serlemitsos K., Viji G. A. (2002). Temperature and leaf wetness duration-based model for prediction of gray leaf spot of perennial ryegrass turf. Phytopathology 93, 336–343. 10.1094/PHYTO.2003.93.3.336 [DOI] [PubMed] [Google Scholar]
- Ushakiran L., Chetry G. K. N., Singh N. I. (2006). Fruit rot disease of chilli and their management in agroclimatic conditions of Manipur. J. Mycopathol. Res. 44, 257–262. [Google Scholar]
- Vanitha S. M., Chaurasia S. N. S., Singh P. M., Naik P. S. (2013). Vegetable statistics. Tech. Bull. IIVR 51, 250. [Google Scholar]
- Varghese T. S., Mathew B. T. (2012). Evaluation of newer insecticides against chilli aphids and their effect on natural enemies. Pest Manag. Hort. Ecosyst. 18, 114–117. [Google Scholar]
- Vasanthakumari M. M., Shivanna M. B. (2013). Biological control of anthracnose of chilli with rhizosphere and rhizoplane fungal isolates from grasses. Arch. Phytopathol. 46, 1641–1666. 10.1080/03235408.2013.771901 [DOI] [Google Scholar]
- Venzon M., Rosado M. C., Molina-Rugamab A. J., Duarte V. S., Dias R., Pallini A. (2008). Acaricidal efficacy of neem against Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Crop Prot. 27, 869–872. 10.1016/j.cropro.2007.10.001 [DOI] [Google Scholar]
- Verma N., MacDonald L., Punja Z. K. (2006). Inoculum prevalence, host infection and biological control of Colletotrichum acutatum: causal agent of blueberry anthracnose in British Columbia. Plant Pathol. 55, 442–450. 10.1111/j.1365-3059.2006.01401.x [DOI] [Google Scholar]
- Vinale F., Sivasithamparam K., Ghisalberti E. L., Marra R., Barbetti M. J., Li H. (2008). A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 72, 80–86. 10.1016/j.pmpp.2008.05.005 [DOI] [Google Scholar]
- Voorrips R. E., Finkers R., Sanjaya L., Groenwold R. (2004). QTL mapping of anthracnose (Colletotrichum spp.) resistance in a cross between Capsicum annuum and C. chinense. Theor. Appl. Genet. 109, 1275–1282. 10.1007/s00122-004-1738-1 [DOI] [PubMed] [Google Scholar]
- Vos J. G. M., Uhan T. S., Sutatya R. (1995). Integrated crop management of hot pepper (Capsicum spp.) under tropical lowland conditions: effects of rice straw and plastic mulches on crop health. Crop Prot. 14, 445–452. 10.1016/0261-2194(94)00012-W [DOI] [Google Scholar]
- Waller J. M. (1992). Colletotrichum diseases of perennial and other cash crops, in Colletotrichum: Biology, Pathology and Control, eds Bailey J. A., Jeger M. J.(Wallingford: CAB International; ), 167–186. [Google Scholar]
- Weir B. S., Johnston P. R., Damm U. (2012). The Colletotrichum gloeosporioides species complex. Stud. Mycol. 73, 115–180. 10.3114/sim0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum G. E. (ed.). (2015). Family Solanaceae in Vegetable Production and Practices. CAB International Publisher. [Google Scholar]
- Wharton P. S., Diéguez-Uribeondo J. (2004). The biology of Colletotrichum acutatum. Anal. Jardin Botan. Madrid 61, 3–22. 10.3989/ajbm.2004.v61.i1 [DOI] [Google Scholar]
- WHO (2002). Evaluation of Certain Mycotoxins in Food, Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 906, Geneva. Available online at: http://www.who.int/iris/handle/10665/42448 [PubMed]
- Yedidia I., Srivastva A. K., Kapulnik Y., Chet I. (2001). Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 235, 235–242. 10.1023/A:1011990013955 [DOI] [Google Scholar]
- Yoon J. B. (2003). Identification of Genetic Resource, Interspecific Hybridization and Inheritance Analysis for Breeding Pepper (Capsicum annuum) Resistant to Anthracnose, Ph.D. Thesis, Seoul National University. [Google Scholar]
- Yu S. M., Ramkumar G., Lee Y. H. (2013). Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plant. J. Appl. Microbiol. 115, 509–516. 10.1111/jam.12252 [DOI] [PubMed] [Google Scholar]
- Živković S., Stojanović S., Ivanović Ž., Gavrilovic V., Popovic T., Balaž J. (2010). Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Arch. Biol. Sci. Belgrade 62, 611–623. 10.2298/ABS1003611Z [DOI] [Google Scholar]