Abstract
Objectives
This study evaluated the efficacy of ramelteon for insomnia in adult subjects with ADHD.
Experimental Design
For this randomized, double-blind, placebo-controlled crossover trial, 8 mg of ramelteon was given nightly, within three hours of bedtime, to ADHD-insomnia subjects confirmed by DSM-IV-TR, ADHD-RS, MINI, and clinical interview. All subjects underwent two weeks each of ramelteon and placebo. Objective sleep measures were obtained by actigraphy. Subjective measures included: the Epworth Sleepiness Scale (ESS) and ADHD-RS.
Principal observations
Of 36 subjects entering the study, 58% met criteria for circadian rhythm sleep disorder (CRSD), delayed sleep phase type. During ramelteon period, mid-sleep time, an indicator of circadian phase, occurred significantly earlier, by ~45 minutes compared to placebo period. An association was noted between the magnitude of the sleep phase advance and the timing of ramelteon administration in relationship to sleep start time, but did not reach statistical significance; maximal efficacy was noted 1.5 hours before bedtime. Paradoxically, ramelteon marginally, but significantly increased sleep fragmentation and ESS scores compared to the placebo state.
Conclusions
Ramelteon is efficacious in maintaining an earlier sleep/wake cycle in adults with ADHD and CRSD but can have paradoxical fragmenting effects on sleep and exacerbate daytime sleepiness. In the presence of a circadian rhythm disorder, the usual dosing and timing parameters for ramelteon need to be carefully considered.
Keywords: melatonin agonists, circadian rhythm disorder, circadian rhythm sleep disorder, sleep-wake schedule disorder, attention disorders, childhood behavioral disorder, actigraphy, chronobiologic treatments, delayed sleep phase
Introduction
ADHD is a common neuropsychiatric condition affecting 6–8% of children and 4.4% of adults that causes substantial and pervasive impairment.1,2 Sleep problems occur in over 70% of children and adults with ADHD; the most common complaint is initial insomnia.3,4 In a large controlled study, well-documented adult ADHD subjects self-reported later and more variable bedtimes, prolonged sleep latency often greater than one hour, non-restful sleep and increased daytime sleepiness compared to controls and independent of other psychiatric comorbidities.5 While the subjective perception of sleep disturbance exceeds what is observed by polysomnography or actigraphy,6 the few existing studies in child populations, characterize a significant insomnia in ADHD patients with notable circadian rhythm disruption. Controlled sleep studies of children have demonstrated decreased total sleep times and significant delays in sleep-latency times, sleep-onset times, and salivary dim-light melatonin onset (DLMO).7-9 One study confirmed similar findings in adults; adults with ADHD and sleep onset insomnia showed a later start and end time to sleep periods, later onset of DLMO and attenuated 24-hour amplitude in rest versus activity pattern on actigraphy, suggestive of a weak and delayed circadian rhythm.10
The delay in salivary dim-light melatonin onset and sleep-onset and offset times in subjects with ADHD6,8-10 meet DSM-IV-TR criteria for Circadian Rhythm Sleep Disorders (CRSD), delayed sleep phase type.11 CRSD, delayed phase sleep type is a chronic condition characterized by persistent inability to fall asleep and arise at a conventional time. CRSDs comprise 10% of cases of insomnia in sleep disorder clinics and affects 7.3% of youths 12 to 20 years in the general population.7,11 CRSD, delayed type is relatively rare in older subject ages 40–64, affecting only 3% of the community population in one survey12,13 much lower than percentages seen in the older adult ADHD population.10,14 While the sleep disorder alone causes significant cognitive, medical and psychiatric morbidity, aggravation of ADHD symptoms of impulsivity, distractibility and inattentiveness by fatigue compounds the morbidity, and exacerbates the already increased risk of injury and car accidents.2,15 Increasing evidence highlights the importance of fully treating ADHD and its associated comorbidities.2,9,16 Given the high comorbidity of untreated ADHD with substance abuse, clinicians need appropriate non-addicting treatment options for the insomnia related to ADHD.17 Anecdotal evidence suggests that adolescents and adults with ADHD frequently self-medicate their insomnia with addictive substances increasing their risk for addiction.2,18
Addressing the insomnia with attention to the disrupted circadian mechanism has important treatment implications. Chronobiotic treatments offer new options. This is the first study to examine the efficacy of the only available FDA approved melatonin-agonist, ramelteon, an agent with known capability of phase-shifting the circadian rhythm in healthy adults19,20 for treatment of insomnia due to ADHD. Ramelteon is a potent and highly selective agonist for the melatonin subtype I and II receptors (MT1 and MT2, respectively), located in the suprachiasmatic nucleus (SCN) of the hypothalamus.21,22 The SCN regulates the timing, duration, and organization of daily sleep/wake cycles and physiological rhythms.20,22 Intrinsic circadian rhythms are synchronized to the 24-hour day by external cues, light being the most potent resetting agent. A multi-synaptic pathway from the SCN mediates the dramatic reduction in pineal melatonin that occurs when light is provided at night.23,24 Stimulation of MT1 is believed to cause a hypnotic effect while stimulation of MT2 is believed to have a phase-shifting effect, advancing or delaying the phase of the sleep-wake cycle depending on timing of melatonin release relative to the endogenous clock phase.25-27
Ramelteon is rapidly absorbed and peaks in less that one hour with a rapid elimination half-life of 1.36 hour, an excellent pharmacokinetic profile for a soporific agent.20,28,29 Adults with ADHD and insomnia show delayed onset of evening rise in endogenous melatonin by DLMO compared to ADHD patients without insomnia and controls.10 In twelve, controlled clinical trials of healthy and chronic insomnia subjects, ramelteon demonstrates sleep-promoting effects with significant reduction in latency to persistent sleep, total sleep time and sleep efficiency according to both polysomnography studies and subjective assessments.20,30-34 In one polysomnography study of primary insomnia, patients who were given ramelteon 30 minutes before bedtime showed improvements in prolonged total sleep time and decreased sleep latency equivalent to studies of benzodiazepine-receptor agonists zolpidem and eczopiclone without evidence of residual psychomotor or cognitive effects or addiction.35-37
The American Academy of Sleep Medicine Practice Parameters calls for further research on sleep-promoting agents for treatment of CRSDs, stating that “CRSDs are among the most neglected causes of insomnia in the literature.”38,39 While ramelteon has not been studied in ADHD or in CRSDs, it has been shown to have circadian phase shifting effects in healthy adults.19 Additionally, ramelteon has been shown to be effective in other psychiatric conditions that have evidence of circadian rhythm disruption or low melatonin levels such as schizophrenia.33 Exogenous melatonin, a chronobiotic classified as a food supplement has demonstrated efficacy in studies of ADHD patients with insomnia.14 The use of oral melatonin in insomnia remains controversial; two recent meta-analyses have shown conflicting reports.40,41 The Ingeborg meta-analysis compared the results of controlled trials of melatonin versus placebo in individuals with delayed sleep phase disorder and found that melatonin advanced the timing of mean endogenous melatonin onset and clock hour of sleep in children and adults and reduced sleep latency and total sleep time in the children.40 The meta-analysis by Buscemi showed melatonin treatment to be equivocal.41 Variability in response across studies can be attributed in part to the lack of rigorous production standards for a non-FDA approved food supplement. Melatonin’s safety profile and dosing are not well established.42-44 Analysis of samples of the 0.5 mg melatonin capsule in one study showed that they varied from 0.5–0.8 mg; 3.0 mg capsules varied from 2.4 to 7.8 mg.25 Formulation variations are less problematic with ramelteon. While there are few comparative studies of ramelteon and melatonin, one study in cats demonstrated that both agents increased slow-wave sleep; the effect of exogenous melatonin was noted to be weaker and of duration 2 hours shorter than ramelteon.45
The purpose of this study was to evaluate the efficacy of ramelteon on ADHD-insomnia subjects who lack significant confounding comorbidities. We hypothesized:
Ramelteon will positively impact ADHD-insomnia by objective measures including: advancing sleep-onset time and mid-sleep time, decreasing sleep latency (time between lying down to sleep and initiation of sleep) and increased total sleep time.
Subjective insomnia symptoms such as sleepiness and impaired daytime functioning inattention, would improve with ramelteon treatment.
The majority of patients would suffer from CRSD-delayed type insomnia by clinical and actigraphic data rather than primary initial type insomnia.
More detailed data documenting descriptive elements of the sleep disorder in the ADHD subjects and the effect of stimulant medication on the sleep parameters compared to a control group will be published separately. We expect that a study of adults controlling for parent factors such as enforced bedtimes will address our study questions more clearly. Most studies in adults with ADHD are one or two day trials in sleep labs with polysomnography often with patients with multiple psychiatric and sleep comorbidities, especially sleep apnea; our study is designed to explore the effect of a novel, non-addictive therapeutic agent on the more pure ADHD patient, excluding Generalized Anxiety Disorder, active depression, sleep apnea, and other psychiatric and sleep comorbidities, over a longer period and while in the natural setting.
Materials and Methods
Study Design
This study was a randomized, double blind, placebo-controlled crossover trial of ramelteon, 8 mg daily, in adult subjects who meet DSM-IV-TR criteria for ADHD and for insomnia. Candidate subjects were screened by medical and psychiatric history. The study included subjects with a DSM-IV-TR primary diagnosis of ADHD by historical evaluation by an experienced clinician, ADHD-RS scale and Mini International Neuropsychiatric Interview (MINI), and DSM-IV-TR criteria for initial insomnia, either primary insomnia or Circadian Rhythm Sleep Disorder, delayed sleep phase type. Exclusion criterion included elevated Hamilton Depression Scale (Ham-D) and Hamilton Anxiety Scale (HAM-A); major Axis I psychiatric diagnosis that would better account for insomnia such as bipolar disorder, psychosis, severe anxiety disorder, untreated depression, adjustment disorder; unstable medical conditions; and other more severe active sleep disorders that better explained the insomnia symptoms. Subjects age 19 to 65 were recruited via solicitation with advertisements in local newspapers and from existing clinic populations. Subjects who met study criteria for ADHD-insomnia were enrolled in the study to begin baseline collection procedures. The eight-week trial included a one-week baseline measurement, 2 weeks of ramelteon or placebo, a two-week “washout” phase between treatments, and 2 weeks of the other medication state. Subjects were stratified at randomization into two groups: Group A initially received a maintenance dose of 8 mg of ramelteon, and Group B an identical tablet of placebo. All raters and researchers were blind to treatment state. Following the week 3 and 4 “washout period”, Groups A and B were crossed over to the alternate drug state. The crossover design allowed each subject to serve as his/her own control.
Objective Sleep Estimates and Activity Rhythm
Objective measures of sleep quality were measured by wrist actigraphy (Actiwatch AW-64 Actigraphy System, Phillips Respironics, Murrysville, PA). The Actiwatch device measures continuous sleep-wake cycles in humans by detecting 24-hour movement.46,47
The subjects wore the Actiwatch 24 hours a day and kept daily sleep logs during both drug states and the washout period. Total sleep time, sleep start and end times, sleep onset latency, sleep fragmentation, and sleep efficiency (time in bed actually spent asleep) were calculated with a standard algorithm (Actiware-Sleep version 5.04 Sleep Scoring Software, Phillips Respironics, Murrysville, PA) Mid-sleep time was calculated as the mid-point between sleep start and end time as an indicator of circadian phase. Activity patterns over days and nights were assessed by calculating the periodicity and amplitude of the rhythm with a chi-square periodogram using a standard algorithm with the Clocklab Analysis software (Actimetrics, Wilmette, IL).
Subjective Sleep
Daytime functioning was measured at each visit using the following scales: the Epworth Sleepiness Scale (ESS), a measure of daytime sleepiness, Attention deficit/hyperactivity disorder rating scale (ADHD-RS), Clinical Global Improvement Scale (CGIS) and a clinic designed measure called the UAB questionnaire (see Supplementary Figure 1); a 4-point Likert scale to specifically assess occupational and social dysfunction in the ADHD patient and to assess evening and bedtime behaviors in detail (see Supplementary Materials). Sleep quality was measured with the aid of the Pittsburgh Sleep Quality Index (PSQI) and a Sleep-wake Diary.
Figure 1.
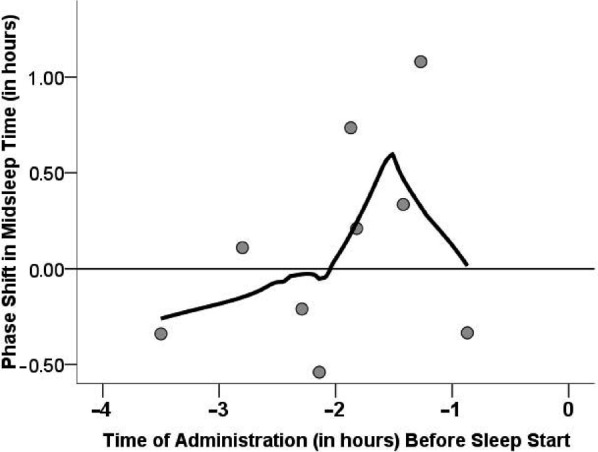
Circadian Phase Shift as a Function of Medication Timing Relative to Bedtime. Phase Shift in Mid-Sleep Time was Calculated from The Difference Between Weeks 4 and 6 in the Placebo-Active Group. Scatter Plot Depicts the Phase Shift (in Minutes) for the Time of Ramelteon Administration per Patient. Data Were Fitted with a Smoothing Function (Using a Loess Epanechnikov Kernel Function for 80% of The Data)
Pharmaceuticals
Takeda Pharmaceuticals North America, Inc. supplied unmarked tablets containing 8 mg of ramelteon and matching placebo tablets. Subjects were instructed to administer study medication once daily between 20:00 and 21:00 hours or 30 minutes prior to desired sleep time. Subjects were allowed to vary their dosage time up to three hours prior to sleep. On average, subjects reported that the medication was taken at 10:05 pm (SD: 1h 22 min). Timing of medication ranged from 6:00 pm to 12:40 am, a broader range than we expected.
Statistical Analysis
Differences in the outcome variables were assessed with a paired samples t-test (two tailed), two-way ANOVA for two-factor designs, or contingency analysis (Fisher’s exact test) for two-level categorical data. For Fragmentation, there was an effect of time across the 8-week trial (independent of treatment condition); therefore, an ANCOVA was used with time included as the covariate. All statistics were acquired with PASW Statistics 18.0 (IBM, Chicago, IL).
Results
Thirty-six patients were enrolled in the trial; forty percent were over age 40. Table 1 displays the socio-demographic characteristics of the study population. Despite a high degree of education (almost 90% of subjects had at least some college training) only 50% of patients were in full time employment, a sign of underachievement typically observed in patients with ADHD. An equal number of men and women participated in the study. A total of 32 patients completed both drug states and were included for analysis. Four subjects dropped out during the study; two due to transportation issues (specifically, high gas prices), one due to discomfort from the actiwatch and poor compliance with visits, and one due to inability to concentrate to complete daily study paperwork. Sleep experts, authors K.G. and R.B., individually examined actigraph readings. After reviewing their results with the two experienced clinicians K.A. and R.F., three subjects were removed from statistical analysis due to marked arrhythmicity or complete phase reversal of sleep-wake cycle, both of which are symptoms of more severe types of CRSD and thus meet the exclusion criteria for the study. One subject displayed such marked fragmentation of sleep that despite an absence of focal neurological signs on physical exam it was determined this subject should be sent for neurologic and medical work-up at the conclusion of the study. Another subject with similar actigraphy readings complained of inability to sleep at all on some nights; this patient was also recommended for neurologic work-up. Both of these subjects met criteria for CRSD, irregular sleep-wake pattern. A third patient who demonstrated a complete reversal of days and nights despite a full-time day job was also excluded from statistical analysis; this subject met criteria for CRSD, complete sleep wake cycle reversal. For the purposes of this paper, further data analysis included only those patients who met inclusion criteria by actigraphy. Table 2 displays the DSM-IV-TR diagnostic categories of the subjects after analysis of all scales and actigraphy measures. 58% of all subjects met criteria for CRSD, delayed sleep phase type, 8.3% met criteria for other, more severe CRSD, as described above and 33% met criteria for Primary Insomnia. These findings are consistent, with Van Veen, et al. (2010) demonstrating that most of insomnia related to ADHD is of the CRSD type.10
Table 1. Socio-Demographic Characteristics in 36 Patients with Adhd and Soi.
| VARIABLE | N = | % | |
|---|---|---|---|
| Age | 19–30 | 12 | 33.3 |
| 30–40 | 10 | 27.8 | |
| 40–50 | 5 | 13.9 | |
| 50–65 | 9 | 25 | |
| Sex | Male | 19 | 52.8 |
| Female | 17 | 47.2 | |
| Marital Status | Never Married | 15 | 41.7 |
| Married Once | 14 | 38.9 | |
| Married More than once | 4 | 11.1 | |
| Divorced | 2 | 5.6 | |
| Cohabitating | 1 | 2.8 | |
| Education (completed) | Less than GED | 1 | 2.8 |
| High School Diploma | 3 | 8.3 | |
| Some Tech or College | 9 | 25 | |
| College Degree | 14 | 38.9 | |
| Some Post Graduate | 4 | 11.1 | |
| Postgraduate Degree | 5 | 13.9 | |
| Employment Status | Unemployed, not looking | 4 | 11.1 |
| Unemployed, looking | 5 | 13.9 | |
| Part-time | 3 | 8.3 | |
| Full-time | 18 | 50 | |
| Retired, not working | 2 | 5.6 | |
| Disabled | 4 | 11.1 |
Table 2. Descriptive Features of 36 Patients with Adhd and Soi.
| INSOMNIA SUBTYPES | N = 36 | % |
|---|---|---|
| Primary Insomnia | 12 | 33.3 |
| Circadian Rhythm Sleep Disorder, delayed sleep phase type* | 21 | 58.3 |
| Irregular Sleep-Wake Pattern or Complete Sleep Wake Cycle Reversal | 3 | 8.3 |
| Poor Sleep Habits**,*** | 5 | N/A |
by DSM-IV-TR criteria and MINI questionnaire.
>2 hr between target bedtime and in bedtime or ≥2 on UABQ items 2b or 2c (i.e., stays up to complete tasks or enjoyable activity).
present in addition to one of other subtypes of insomnia.
Sleep Timing
Changes in sleep timing due to drug were examined for the second trial period, the placebo-then-active group, since baseline actigraphy measures for the first trial period were separated by a month in time from the active drug period in the active-then-placebo group (see limitations section). Sleep phase was operationally defined as mid-sleep time, the mid-time point between sleep start and end time. Phase changes in mid-sleep time provide an accurate measure of advances and delays in circadian timing second only to dim-light melatonin onset DLMO. Change in mid-sleep time was calculated as the difference between the average mid-sleep time during baseline period, and the average mid-sleep time during placebo and drug periods. In order to examine the interaction of ramelteon and timing of drug administration, the variable Medication Time was categorized into two categories: less than or greater than two hours before sleep start time. A two-way ANOVA (Medication Time × Drug) revealed a significant main effect of drug (F1,15 = 4.7, p = 0.046), such that subjects exhibited a mid-sleep time that occurred 46 min earlier (advanced) during ramelteon treated state compared to placebo-treated state. Compared to baseline, ramelteon induced a 7-min phase advance on average (mean ± SD: 7.0 ± 32.3 min) while placebo induced a 39-min phase delay on average (mean ± SD: 39.2 ± 44.6 min). There was no significant main effect of Medication Time (F1,15 = 2.5, p = 0.134) nor a significant Medication Time × Drug interaction (F1,15 = 0.3, p = 0.605).
Although low statistical power prevented a significant Drug × Medication Time interaction, we descriptively examined the optimal time of administration of ramelteon since this factor is clinically relevant. As shown in Figure 1, the size of the phase advance in the ramelteon-treated group depended on the time of administration of drug in relationship to sleep start time according to a predictable curve. Specifically, mid-sleep time was maximally advanced by approximately 40 min when medication was administered approximately 1.5 h before sleep start time.
Table 3 displays objective measures of sleep timing; treatment groups are shown separately due drug order effects. Due in part to wide inter-subject variability and small sample size, no group differences reached statistical significance, however, several trends are worth discussion. For group A, ramelteon treatment trended toward an earlier sleep start time (40 min on average) compared to placebo treatment; sleep end time was unchanged. Circadian amplitude, obtained from a chi-square periodogram analysis,48 is a measure of the circadian rhythm strength; ramelteon treatment trended toward increased amplitude compared to placebo.
Table 3. Sleep Times and Circadian Parameters of 36 Patients with Adhd and Soi.
| PLACEBO/ACTIVE (GROUP A) | ACTIVE/PLACEBO (GROUP B) | |||||||
|---|---|---|---|---|---|---|---|---|
| PLACEBO | ACTIVE | PLACEBO | ACTIVE | P | ||||
| Sleep Start Time | 12:55 ± 0:24 | 12:15 ± 0:13 | 1:17 ± 0:43 | 1:08 ± 0:29 | 0.196 | |||
| Sleep End Time | 7:11 ± 0:15 | 7:12 ± 0:26 | 7:28 ± 0:37 | 7:05 ± 0:28 | 0.217 | |||
| Amplitude | 638.2 ± 47.1 | 654.4 ± 44.0 | 632.0 ± 65.8 | 599.8 ± 44.6 | 0.508 | |||
For Amplitude, n = 15.
Other Sleep Parameters
Table 4 displays the other sleep parameters determined by actigraphy. Ramelteon treatment reduced sleep latency of Group B (duration to fall asleep) by an average of 17 min and lengthened sleep latency of Group A by an average of 11 min, although the total sleep time for this group increased by 34 min. None of these comparisons reached statistical significance; however, the extremely high placebo effect in insomnia trials complicates studies.30,49 Zammit notes that even in large trials of eszapidone and zolpidem, 15–29 minute improvents in sleep latency were clinically significant.35,37 As expected, there were no significant effects of ramelteon on other sleep parameters, such as sleep efficiency, wake after sleep onset, percentage of time awake during sleep session, number of sleep bouts, and immobile time (data not shown).
Table 4. Actigraphic Sleep Parameters During Placebo and Drug periods.
| PLACEBO/ACTIVE (GROUP A) | ACTIVE/PLACEBO (GROUP B) | ||||||
|---|---|---|---|---|---|---|---|
| PLACEBO | ACTIVE | PLACEBO | ACTIVE | P | |||
| Sleep Latency (in min) | 32.3 ± 11.7 | 43.5 ± 9.9 | 39.5 ± 11.0 | 22.5 ± 6.0 | 0.928 | ||
| Sleep efficiency | 74.4 ± 3.6 | 73.5 ± 2.7 | 74.7 ± 3.6 | 75.6 ± 2.4 | 0.198 | ||
| Total sleep time (in min) | 332.7 ± 16.6 | 356.6 ± 12.9 | 321.0 ± 20.7 | 310.0 ± 14.1 | 0.681 | ||
Sleep Fragmentation
Several paradoxical negative results were noted. The fragmentation index (see Methods section) indicated that subject’s sleep became more fragmented throughout the course of the 8-week study irrespective of drug state (ANCOVA, Time: F1,20 = 9.5, p = 0.006). Ramelteon marginally, but significantly increased the sleep fragmentation score even when the time effect was accounted for (ANCOVA, F1,20 = 6.9, p = 0.016). Fragmentation scores for placebo and ramelteon conditions were 19.9 ± 9.6 and 22.1 ± 12.1 (mean ± SD), respectively.
Subjective Sleep Measures
Figure 2 demonstrates the paradoxical response of the drug state on sleepiness. Contrary to our predictions subjects had significantly higher Epworth Sleepiness Scale (ESS) scores during ramelteon state compared to placebo state (mean ± SD; ramelteon: 8.9 ± 6.0; placebo: 6.1 ± 6.5; paired samples t-test, two-tailed, t24 = –2.56, p < 0.017). Patients with an ESS score of over “10” are generally referred for treatment. Contingency analysis indicated that a higher percentage of subjects scored greater than “10” during ramelteon state compared to placebo state. (Fisher’s exact test, two-sided, p = 0.047). Figure 2 demonstrates that the percentage of subjects in the clinical range for excessive sleepiness more than doubled while on ramelteon compared to placebo. No evidence of difference between medication, baseline and placebo state was noted for any other subjective sleep assessment tools or ADHD measures.
Figure 2.
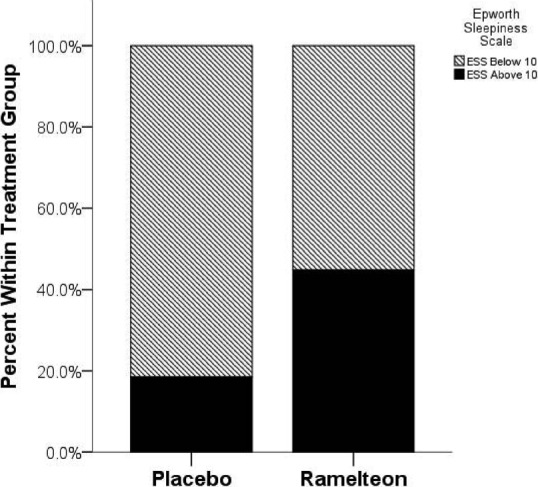
Epworth Sleepiness Scale Scores. Bar Graph Depicting the Percentage of Subjects Within Each Treatment Group that had an Ess Score Greater than 10, A Criterion which is Commonly Used to Diagnose Excessive Daytime Sleepiness
All adverse events are recorded in Table 5. No serious adverse events occurred during the study. No subjects dropped out of the study due to adverse events; all reported events were mild. Verbalized complaints of drowsiness were the most frequent event but occurred with equal or higher frequency in the placebo and washout periods. One subject developed hives during the medication period, but this was mild and transient and did not require medication discontinuation. Overall, the medication state was well tolerated.
Table 5. Adverse Events Associated with Ramelteon Administration.
| ACTIVE | PLACEBO | WASHOUT | |
|---|---|---|---|
| Drowsiness | 2 | 3 | 4 |
| Headache | 0 | 0 | 2 |
| Hives | 1 | 0 | 0 |
| Dizziness | 0 | 1 | 0 |
| Jitteriness | 0 | 0 | 1 |
Numbers indicate the frequency of each complaint.
Discussion
This study is the first controlled trial to examine the effects of ramelteon, on subjects with ADHD and insomnia. Ramelteon treatment resulted in a significantly earlier mid-sleep time, an indicator of circadian phase, compared to placebo in a population with delayed circadian clock function. Ramelteon induced a 7-min phase advance on average compared to baseline state while placebo group had on average a 39-min phase delay compared to baseline state, a 46-min difference. It is improbable that placebo actually induced a delay in sleep onset; our interpretation of the above results is that ramelteon enabled subjects to stick more consistently to a desirable bedtime than placebo. The magnitude of the advance was maximal, 40 minutes, when the medication was taken approximately 1.5 hours before bedtime. Our results, though less robust, agree with the one similar study by Richardson demonstrating ramelteon improved circadian parameters in healthy adult subjects.19
Ramelteon has shown positive results in many, varying trials in non-human animals, healthy adults, insomnia patients and the elderly.20,30,34,50-53 While ramelteon demonstrated efficacy over placebo in maintaining an earlier circadian cycle over a two-week time span as measured by mid-sleep time, drug state simultaneously exacerbated sleep fragmentation and worsened daytime sleepiness in this study population. The percentage of patients scoring over 10 on the ESS, the clinically significant range for this scale, more than doubled during ramelteon versus placebo state. Of note, study subjects experienced these changes without an increase in complaints of adverse events or sleepiness. ADHD patients are known to be poor at self observation and possibly did not notice a significant difference from their chronically sleep deprived state. One recent study also showed paradoxical findings with ramelteon; based on ramelteon’s high tolerability, Cohen et al. gave healthy subjects 8 mg of ramelteon, 30 minutes prior to a 2 hour nap before a simulated 8 hour night shift.54 Results showed that ramelteon state demonstrated little benefit in altering sleep efficiency but significantly worsened neurobehavioral performance on EEG and psychometric assessments during the 12 hours following the nap.54 The authors hypothesize that the high homeostatic sleep pressure of nighttime, which already predisposes to poor performance coincided with active ramelteon metabolite presence in the circulation, resulting in intensification of performance deficits.54 In a similar fashion, in our study we speculate that the presence of ramelteon and/or its metabolites during the daytime in the circadian phase delayed subject who is already fighting high sleep pressure in the morning and even early afternoon, significantly worsened daytime alertness and function as measured by ESS. While the half-life of ramelteon is only 1.36 hours; its one active metabolite, monohydroxylated ramelteon has a half-life of 2.62 hours.28,29 Monohydroxylated ramelteon is less potent and has less binding affinity for MT receptors; however exposure to receptor sites is much longer than to the parent drug.28,29 Elimination response curves demonstrate that some ramelteon and more monohydroxylated ramelteon are present in early morning hours.28,29 Previous studies suggest that healthy and primary insomnia subjects who lack circadian rhythm abnormalities and are on a normal sleep-wake schedule will lack high sleep pressure during daytime and may have better tolerance small daytime levels of ramelteon.19,30,33,34
Similar conflicting results are paralleled in the much larger melatonin literature and demonstrate the extremely complicated nature of pharmacodynamic action at the melatonin receptor. A very high inter-subject variability in melatonin receptor sensitivity explains variations in-group studies of melatonin agonists regardless of dose.28,29 Subjects in this study responded with wide inter-subject and intra-subject variability. In addition, some evidence exists that melatonin agonists more effectively alter sleep parameters in melatonin-depleted individuals such as the elderly.27,44,52,55 In summary, mostly unknown differences operating in the neuropsychiatrically and circadian rhythm challenged ADHD patient may account for differences in responsivity to ramelteon.
A significant increase in sleep fragmentation, irrespective of drug state, was noted over the course of the study. While sleep hygiene often improves initially during sleep studies as a result of keeping daily sleep logs, it may not have been sustainable over the course of 8 weeks, particularly for ADHD patients known for enthusiastic beginnings but poor follow through. In addition, subjects had significantly worsened sleep fragmentation accompanying increased sleepiness during ramelteon period, indicative of restless sleep. One explanation is that the presence of even physiological doses of melatonin at the MT receptor in the morning is known to delay rather than advance circadian rhythms, and ramelteon has a 3–16 fold greater affinity than melatonin for the receptor.28,56,57 Two studies by Burgess, et al. have carefully examined the timing of two different doses of melatonin and how they altered the circadian cycle by either advancing or delaying sleep in a predictable fashion according to a phase response curve.25,26 Melatonin at 0.5 mg was shown to phase advance the sleep maximally (1.5 hours) when it was taken 9–11 hour before sleep midpoint and maximally delay sleep 1.3 hours when given 13.6 hours after dim light melatonin onset (DLMO) in the early morning.25 A similar curve was produced for the 3 mg dose but the maximal advances occurred when it was taken even earlier, 11–12 hours before sleep midpoint.25 As 3 mg is already a supra-therapeutic dose, it is not known if higher doses or more potent melatonergic agents such as ramelteon would require an even earlier time of administration. In addition, the 3 mg dose on the phase-response curve shows a “dead zone” of no response from about the time of the baseline DLMO (about 3 hours before bedtime) to the mid-sleep point.25 Dosing after this point in time will delay sleep onset.26,56 Clinical trials with ramelteon determined that the high tolerability and flattened dose response curve seen in very large groups of subjects allowed all patients to be given the 8 mg dose without a need for adjustment.32,58 For this reason we elected to administer the medication according to standard Physician Desk Reference (PDR) recommendations for our study subjects; 8 mg Ramelteon 30 minutes prior to sleep in the “dead zone” for phase response. (Unfortunately, the important Burgess et al phase response paper describing this phenomenon was only recently published after our trial was complete).25 Figure 2 shows a similar phase response curve in our subjects when the change in mid-sleep time from baseline is plotted against time before bed at which drug was taken. Optimal advance occurred when medication was taken about 1.5 hours before bedtime but low power, lack of statistical significance and the limited time span for which drug was taken (within 3 hours of bed) limits the conclusions that can be drawn from this result. However, most positive studies for use of melatonin in CRSDs show timing of melatonin 1.5–6 hours before bedtime, consistent with these results.22,32,38,40 Dosing ramelteon too close to bedtime may have had initial salutary hypnotic effects58; however, active metabolite lingering from the phase advance zone, past the dead zone, into the delay zone of the phase response curve25 may have disrupted the circadian cycle, resulting in fragmented sleep and next day fatigue.
Studies of exogenous melatonin suggest that for the MT-1 action, the soporific effect of melatonin is such that the higher the dose, the more profound the effect,59 therefore, when the dose is too high, chronobiological effects may be lost and only the hypnotic actions remain.16,29,57 Ramelteon’s stronger potency and much greater affinity for MT-1 receptors than that of melatonin make it an extremely potent initial soporific.28,50 However, improper timing and excessive dosing can be problematic for MT-2 receptor activation involved in circadian entrainment.28,29 While ramelteon has not been studied in ADHD or in CRSDs, one study demonstrated significant circadian phase shifting effects in healthy adults but only at the lower doses of 1.0, 2.0, or 4.0 mg and not at the 8.0 and 16.0 mg doses.19 In blind individuals, for whom lack of light signals can lead to free-running circadian rhythms, physiological doses of melatonin such as 0.3 mg may be more effective for entrainment than doses greater than 2.0 mg.57 From a clinical standpoint the more neuropsychiatrically challenged ADHD patient with primary insomnia (a third of our study population), a dose 8 mg or higher just before bed may be required to activate MT-1 receptors and induce somnolence. For the other two-thirds of ADHD patients with a circadian rhythm disorder, careful timing of 1, 2 and 4 mg doses for the MT-2 receptor activation might be more efficacious for entraining an earlier rhythm. Differentiating dosing and timing of ramelteon based on the typing of the insomnia should be explored in future studies.
Consistent with other studies of ADHD and insomnia, ADHD parameters for this study did not change during drug state over placebo.4,7,8,42 Previous studies with standard hypnotics have also shown improved sleep but not ADHD symptoms.13,60 The time at which subjects took their medication ranged from 6:00 pm to 12:40 am, but averaged 10:00 pm, a wide range which demonstrates the irregular habits and inconsistency ADHD patients show in following directions. This factor can affect clinical and study outcomes. Another incidental finding was that clinical history from an ADHD patient does not always reveal the true sleep disorder or the level of sleepiness and daytime function. ADHD patients were better at quantifying their sleepiness by functional scales and actigraphy than subjective spontaneous history. Three of our 36 patients who initially met criteria for a CRSD or primary insomnia were found to have other diagnosis by actigraphy. Clinicians should have a low threshold to refer ADHD-insomnia patients for a sleep study when faced with a poor treatment responder.
Limitations of Study
One important factor that affected analysis was the large placebo effect for most measures over baseline, a phenomenon common in insomnia studies.49 No clear paradigm to define a true response in the face of extremely high placebo effect has been agreed upon by sleep experts.
Actigraphy has limitations in validity for determining sleep onset latency as it only detects movement, not sleep state. Removal of the Actiwatch when swimming or bathing may not be accurately reported. Other complications were the loss and destruction of the study Actiwatches due to the impulsive and forgetful nature of patients with ADHD. The advantage of examining naturalistic behavior by actigraphy was also a disadvantage; all subjects’ sleep became more fragmented as the study progressed over time, complicating the evaluation of change.
A shortcoming of the study design affected the collection of baseline actigraphy data needed for the sleep timing (but not sleep quality) measurements. Baseline data was collected for all study parameters during the baseline week with the exception of actigraphy data which started simultaneously with week 1 drug period and continued through week 8. Actigraphy data obtained during washout weeks 3 and 4 served as baseline data for the placebo-active group as no active medication had been given previously, but could not be used for the active-placebo group due to the potential lingering circadian effects of drug. For the active-placebo group, week 7 and 8 washout period data was available as baseline data, but the separation of the active drug and baseline by four weeks render timing comparisons unreliable due to potential changes in season, light, daylight savings and personal schedules Hence, changes in sleep timing due to drug were only examined for the second trial period, the placebo-then-active group, reducing the statistical power for that measure and excluding half of participants from these measurements.
Adults with ADHD have very limited tolerance for sustained activity on repetitive tasks such as paperwork. A sleep study, which requires nightly logs over a period of weeks, is inherently challenging for ADHD patients. It is likely the patients may have completed some of the logs from memory after missing a night or two, decreasing their accuracy. Imprecision in sleep log times can significantly affect latency estimates. One motivated patient sat for 3 hours in the waiting room trying to complete paperwork; at least one other was less motivated. Rybak had poor compliance with ongoing diaries in a 4-week study, a problem noted in other studies.61 The length of the study was selected to allow the Ramelteon two weeks to entrain the circadian rhythm; unfortunately the extended length of the study allowed enough time to go by for the subjects’ sleep to fragment over time. Also the chaotic behavioral patterns that can translate into poor sleep habits and odd insomnia medication dosing times complicate the assessment and treatment of the ADHD insomnia subject. Five of our subjects demonstrated that they regularly stayed up either to complete tasks or perform an enjoyable activity on the UAB insomnia questionnaire even when they felt tired enough from “study medication” to sleep. A behavioral program would have to precede pharmacologic management for a positive response in these types of patients.
Conclusion
This study confirms that the majority of adults with insomnia related to ADHD have a CRSD and that ramelteon is effective in maintaining an earlier mid-sleep time in this population but can also fragment sleep and exacerbate daytime sleepiness. Initial positive and later negative effects of ramelteon on sleep in this study population suggests that the CRSD population may be particularly vulnerable to the residual presence of the potent melatonin agonist, leaving subjects with restless, fragmented sleep at night (as if it was day-time) and intensifying the already strong daytime sleepiness and impairment due to the existing delay in sleep pressure into daytime hours (as if it were night-time).
Ramelteon has a novel mechanism of action as a hypnotic agent; its circadian rhythm entraining properties, make it worthy of further research in ADHD patients. More controlled studies furthering an understanding of how best to work with melatonergic agents, specifically with attention to dosing, timing, typing of the insomnia and daytime blood levels of ramelteon and its metabolites will increase the utility of these agents. DLMO levels may eventually be available for clinical use; these would provide useful information to assist in more individualized timing of medication in relation to bedtime, allowing the medication to be given at the appropriate point on each persons phase response curve. Actigraphy is useful in this study population in typing the different subtypes of insomnia and even identifying a small group of more impaired subjects who did not separate out by clinical history and standardized scales. ADHD patients form a large clinical population sorely in need of a well-tolerated, non-addictive medication,31 which can be safely given on a chronic basis without alteration of the sleep architecture. From a clinical standpoint, the authors recommend careful sub-typing of the insomnia. When a CRSD is present, ramelteon should be prescribed initially at doses of 1 to 4 mg per day and worked up as necessary at a time 1.5–3 hours before sleep time and with careful attention to functional factors indicating daytime sleepiness. Compliance with timing recommendations for insomnia medication should be explored regularly. Pharmacologic treatment should be combined with behavioral treatments to strengthen the circadian rhythm and heighten synchronizing cues, such as sleep hygiene principles, exercise and exposure to early morning bright light.
UAB Insomnia Questionnaire.

Circle each items for the last week on a scale of 0-3, 0 being absent or poor, 3 being present to maximum extent.
What time would you like to go to bed on workdays? (i.e. bedtime at which you go to sleep easier and perform best the next day if going to sleep at this time?)___________________________________________________________________________
What time do you typically get in bed with the intent to sleep?___________________________________________________________________________
Acknowledgments
Takeda Pharmaceuticals North America, Inc. funded this study as an investigator-initiated trial. The authors would like to thank Samantha W. White, Pam Lucas, Rakesha Garner, Kimberly H. Lindbergh, and Amelia Tankersly for their assistance in research evaluation. The other authors have no financial affiliations relevant to the subject of this article.
References
- 1.Kessler RC, Adler L, Barkley R et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006 Apr;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkley RAM K.R., Fischer M. ADHD in Adults: What the Science Says. New York: Guilford Press; 2008. [Google Scholar]
- 3.Sobanski E. Psychiatric comorbidity in adults with attention-deficit/hyperactivity disorder (ADHD) Eur Arch Psychiatry Clin Neurosci. 2006 Sep;256(1 Suppl.):i26–31. doi: 10.1007/s00406-006-1004-4. [DOI] [PubMed] [Google Scholar]
- 4.Sadeh A, Pergamin L, Bar-Haim Y. Sleep in children with attention-deficit hyperactivity disorder: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2006 Dec;10(6):381–398. doi: 10.1016/j.smrv.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Surman CB, Adamson JJ, Petty C et al. Association between attention-deficit/hyperactivity disorder and sleep impairment in adulthood: evidence from a large controlled study. J Clin Psychiatry. 2009 Nov;70(11):1523–1529. doi: 10.4088/JCP.08m04514. [DOI] [PubMed] [Google Scholar]
- 6.Philipsen A, Feige B, Hesslinger B et al. Sleep in adults with attention-deficit/hyperactivity disorder: a controlled polysomnographic study including spectral analysis of the sleep EEG. Sleep. 2005 Jul 1;28(7):877–884. doi: 10.1093/sleep/28.7.877. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijden KB, Smits MG, Gunning WB. Sleep-related disorders in ADHD: a review. Clin Pediatr (Phila) 2005 Apr;44(3):201–210. doi: 10.1177/000992280504400303. [DOI] [PubMed] [Google Scholar]
- 8.van der Heijden KB, Smits MG, Gunning WB. Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J Sleep Res. 2006 Mar;15(1):55–62. doi: 10.1111/j.1365-2869.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 9.Van der Heijden KB, Smits MG, Van Someren EJ, Gunning WB. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22(3):559–570. doi: 10.1081/CBI-200062410. [DOI] [PubMed] [Google Scholar]
- 10.Van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, Van Someren EJ. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010 Jun 1;67(11):1091–1096. doi: 10.1016/j.biopsych.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C.: American Psychiatric Association; [Google Scholar]
- 12.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res. 2006 Jun;15(2):162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 13.Owens JA, Rosen CL, Mindell JA. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003 May;111(5 Pt 1):e628–635. doi: 10.1542/peds.111.5.e628. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age-related sleep-maintenance insomnia: assessment in a clinical trial of melatonin replacement. Sleep. 1998;21(1):52–68. [PubMed] [Google Scholar]
- 15.Biederman J, Fried R, Monuteaux MC et al. A laboratory driving simulation for assessment of driving behavior in adults with ADHD: a controlled study. Ann Gen Psychiatry. 2007;6:4. doi: 10.1186/1744-859X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss MD, Wasdell MB, Bomben MM, Rea KJ, Freeman RD. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry. 2006 May;45(5):512–519. [PubMed] [Google Scholar]
- 17.Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3–8. [PubMed] [Google Scholar]
- 18.Pereira RR, van de Wetering BJ. [Caffeine, cannabis and cocaine: from automedication to ‘automutilation’ in adults with ADHD] Ned Tijdschr Geneeskd. 2004 Dec 25;148(52):2573–2576. [PubMed] [Google Scholar]
- 19.Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med. 2008 Oct 15;4(5):456–461. [PMC free article] [PubMed] [Google Scholar]
- 20.Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008 Feb;4(1):69–79. doi: 10.2147/ndt.s483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vachharajani NN, Yeleswaram K, Boulton DW. Preclinical pharmacokinetics and metabolism of BMS-214778, a novel melatonin receptor agonist. J Pharm Sci. 2003 Apr;92(4):760–772. doi: 10.1002/jps.10348. [DOI] [PubMed] [Google Scholar]
- 22.Kayumov L, Brown G, Jindal R, Buttoo K, Shapiro CM. A randomized, double-blind, placebo-controlled crossover study of the effect of exogenous melatonin on delayed sleep phase syndrome. Psychosom Med. 2001 Jan-Feb;63(1):40–48. doi: 10.1097/00006842-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Tamarkin L, Reppert SM, Klein DC. Regulation of pineal melatonin in the Syrian hamster. Endocrinology. 1979 Feb;104(2):385–389. doi: 10.1210/endo-104-2-385. [DOI] [PubMed] [Google Scholar]
- 24.Klein DC, Weller J. Input and output signals in a model neural system: the regulation of melatonin production in the pineal gland. In Vitro. 1970 Nov-Dec;6(3):197–204. doi: 10.1007/BF02617764. [DOI] [PubMed] [Google Scholar]
- 25.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010 Jul;95(7):3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008 Jan 15;586(2):639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JN, Liu RY, van Heerikhuize J, Hofman MA, Swaab DF. Alterations in the circadian rhythm of salivary melatonin begin during middle-age. J Pineal Res. 2003 Jan;34(1):11–16. doi: 10.1034/j.1600-079x.2003.01897.x. [DOI] [PubMed] [Google Scholar]
- 28.Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006 Feb;46(2):140–148. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: a novel therapeutic drug for sleep disorders. CNS Neurosci Ther. 2009 Winter;15(1):32–51. doi: 10.1111/j.1755-5949.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009 Mar 1;32(3):351–360. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006 Oct;63(10):1149–1157. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]
- 32.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007 Aug 15;3(5):495–504. [PMC free article] [PubMed] [Google Scholar]
- 33.Borja NL, Daniel KL. Ramelteon for the treatment of insomnia. Clin Ther. 2006 Oct;28(10):1540–1555. doi: 10.1016/j.clinthera.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006 Jan;7(1):17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Scharf MB, Roth T, Vogel G, Walsh J. A multicenter placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192–199. [PubMed] [Google Scholar]
- 36.Zammit GK. Ramelteon: a novel hypnotic indicated for the treatment of insomnia. Psychiatry (Edgmont) 2007 Sep;4(9):36–42. [PMC free article] [PubMed] [Google Scholar]
- 37.Zammit GK, McNabb LS. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979–1991. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]
- 38.Sack RL, Auckley D, Auger RR et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007 Nov 1;30(11):1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgenthaler TI, Lee-Chiong T, Alessi C et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007 Nov 1;30(11):1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingeborg M, van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010 Dec 1;33(12):1605–1614. doi: 10.1093/sleep/33.12.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buscemi N, Vandermeer B, Hooton N et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006 Feb 18;332(7538):385–393. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res. 2009 Aug;47(1):1–7. doi: 10.1111/j.1600-079X.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 43.Kunz D, Mahlberg R, Muller C, Tilmann A, Bes F. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials. J Clin Endocrinol Metab. 2004 Jan;89(1):128–134. doi: 10.1210/jc.2002-021057. [DOI] [PubMed] [Google Scholar]
- 44.Arendt J. Does melatonin improve sleep? Efficacy of melatonin. BMJ. 2006 Mar 4;332(7540):550. doi: 10.1136/bmj.332.7540.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyamoto M, Nishikawa H, Doken Y, Hirai K, Uchikawa O, Ohkawa S. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. 2004 Nov 1;27(7):1319–1325. doi: 10.1093/sleep/27.7.1319. [DOI] [PubMed] [Google Scholar]
- 46.E VS. Improving actigraphic sleep estimates in insomnia and dementia: How many nights? J Sleep Res. 2007;16:269–275. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 47.Littner M, C. K, Anderson W, Bailey D, R. B, Davila D. ea. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep Med. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 48.Ciarleglio CM, Gamble KL, Axley JC et al. Population encoding by circadian clock neurons organizes circadian behavior. J Neurosci. 2009 Feb 11;29(6):1670–1676. doi: 10.1523/JNEUROSCI.3801-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCall W, D’Agostine R, Dunn AS. A meta-analysis of sleep changes associated with placeo in hypnotic clinical trials. Sleep Med. 2003;4:57–62. doi: 10.1016/s1389-9457(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 50.Rawashdeh O, Hudson R. Circadian Periods of Sensitivity for Ramelteon on the onset of Running-wheel Activity and the Peak of Suprachiasmatic Nucleus Neuronal Firing Rhythms in C3H/HeN Mice. Chronobiology International. 2011;(28):31–38. doi: 10.3109/07420528.2010.532894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson GS, Zammit G, Wang-Weigand S, Zhang J. Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry. 2009 Apr;70(4):467–476. doi: 10.4088/jcp.07m03834. [DOI] [PubMed] [Google Scholar]
- 52.Gooneratne NS, Gehrman P, Gurubhagavatula I, Al-Shehabi E, Marie E, Schwab R. Effectiveness of ramelteon for insomnia symptoms in older adults with obstructive sleep apnea: a randomized placebo-controlled pilot study. J Clin Sleep Med. 2010 Dec 15;6(6):572–580. [PMC free article] [PubMed] [Google Scholar]
- 53.Roth T, Seiden D, Wang-Weigand S, Zhang J. A 2-night, 3-period, crossover study of ramelteon’s efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin. 2007 May;23(5):1005–1014. doi: 10.1185/030079907x178874. [DOI] [PubMed] [Google Scholar]
- 54.Cohen DA, Wang W, Klerman EB, Rajaratnam SM. Ramelteon prior to a short evening nap impairs neurobehavioral performance for up to 12 hours after awakening. J Clin Sleep Med. 2010 Dec 15;6(6):565–571. [PMC free article] [PubMed] [Google Scholar]
- 55.Zammit G, Wang-Weigand S, Rosenthal M, Peng X. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009 Feb 15;5(1):34–40. [PMC free article] [PubMed] [Google Scholar]
- 56.Lewy AJ, Bauer VK, Ahmed S et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998 Jan;15(1):71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 57.Lewy AJ, Emens J, Sack RL, Hasler BP, Bernert RA. Zeitgeber hierarchy in humans: resetting the circadian phase positions of blind people using melatonin. Chronobiol Int. 2003 Sep;20(5):837–852. doi: 10.1081/cbi-120024215. [DOI] [PubMed] [Google Scholar]
- 58.Zammit G, Schwartz H, Roth T, Wang-Weigand S, Sainati S, Zhang J. The effects of ramelteon in a first-night model of transient insomnia. Sleep Med. 2009 Jan;10(1):55–59. doi: 10.1016/j.sleep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Arendt J. Melatonin and its Agonists. British Journal of Psychiatry. 2008 [Google Scholar]
- 60.Brown TE, McMullen WJ., Jr Attention deficit disorders and sleep/arousal disturbance. Ann N Y Acad Sci. 2001 Jun;931:271–286. doi: 10.1111/j.1749-6632.2001.tb05784.x. [DOI] [PubMed] [Google Scholar]
- 61.Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006 Oct;67(10):1527–1535. doi: 10.4088/jcp.v67n1006. [DOI] [PubMed] [Google Scholar]


