Abstract
Background
The Hamilton Depression Rating Scale (HAMD17) is an outcome measure widely used in major depressive disorder (MDD) clinical trials. The objective of this analysis was to assess the validity of the anxiety/somatisation factor of the HAMD17 as a measure of anxiety in patients with MDD.
Methods
We pooled data from 1466 outpatients with MDD from four 8-week controlled studies of duloxetine. We performed a factor analysis of the HAMD17 to investigate the anxiety/somatisation factor.
Results
The HAMD17 factor analysis yielded 6 factors, but did not yield the pre-specified anxiety/somatisation factor. This latter factor showed weak correlation with the Hamilton Anxiety Scale total and subscale scores at baseline (0.46), but higher correlation coefficients over the trials up to 0.81. We identified another anxiety factor that included the hypochondriasis item in this sample.
Conclusion
Findings from this large sample suggest that the factor structure of the HAMD17 is unstable in MDD and that the anxiety/somatisation subscale should not be routinely used for anxiety assessment in depressed patients.
Keywords: Depression, Anxiety, Hamilton, Factor structure, Duloxetine
Introduction
The Hamilton Depression Rating Scale (HAMD17) 17 items is a widely used rating scale to assess the severity of depression.1 The scale has been the standard for the assessment of antidepressants’ efficacy in clinical trials for forty years. The total score of the 17 items (range 0-52) reflects the severity of depression. The scores on the individual items reflect the severity of individual symptoms.
In an initial evaluation of the factor’s structure, Hamilton2 investigated the factor structure of the HAMD17 and identified 4 factors/subsets of items in a population of 272 depressed patients. The first factor mapped to the core symptoms of depression (depressed mood, guilt, suicide, and psychomotor retardation), the second factor mapped to bipolarity (anxiety, agitation versus retardation), and the third factor mainly mapped to somatic symptoms while the fourth factor mapped to a group of heterogeneous items. The factor structure of the HAMD17 was later evaluated by Cleary and Guy on 480 patients with “neurotic” depression.3 Their analysis identified 6 different factors; among them, the first factor they labelled “Anxiety/somatisation”. It included 6 items (no. 10, 11, 12, 13, 15, 17), namely, 10- anxiety psychic, 11- anxiety somatic, 12- gastrointestinal and 13- general somatic symptoms, 15- hypochondriasis, and 17- insight. The score of the anxiety/somatisation factor/subscale items was used in many studies to investigate the specific effect of antidepressants on anxiety-related symptoms4-10 in major depressive disorder (MDD). However, several authors reported that the factor structure of the HAMD17 is unstable across samples of depressed patients.11-12 This finding was replicated by Bagby et al. (2004) in their extensive review of the HAMD psychometric properties.13 This factor structure instability limits the use of the HAMD subfactors as dimensional assessment tools in depression.13
The purpose of this report is to investigate the factor structure of the HAMD17 in a large population (N = 1466) of patients with major depressive disorder from four placebo-controlled duloxetine studies. It also assesses the validity of the Cleary and Guy’s HAMD17 anxiety/ somatisation factor as an anxiety severity measure compared with the Hamilton Anxiety Scale (HAMA).14
Materials and Methods
Population
Data from four multisite, randomised, double-blind, placebo- and active comparator-controlled registration studies of duloxetine in adult outpatients with MDD were pooled for this analysis. These four studies are the duloxetine-controlled studies that assessed anxiety symptoms in depressed patients using both the Cleary and Guy’s HAMD17 anxiety/somatisation factor and the HAMA at each visit. The choice of these anxiety measures was done a priori.
Materials and Methods
All four studies shared the same design except for study drug doses (Table 1) and have been previously published.15-17 They included adult patients with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, (DSM IV) MDD, assessed by the structured interview, the MINI (Mini-International Neuropsychiatric Interview, MINI18), with a baseline HAMD17 total score of at least 15. After a double-blind, 1-week placebo lead-in period all the patients were randomised to duloxetine, paroxetine, or placebo for the 8-week acute treatment phase.
Table 1. Summary of the Four Double-Blind, Placebo-Controlled Duloxetine Studies in Patients with MDD Included in the Pooled Analysis.
| STUDY | TREATMENT DURATION | DRUG | PATIENTS | DRUG DOSE AND DESIGN |
|---|---|---|---|---|
| 1 | 8 weeks | Placebo | 90 | – |
| (Nemeroff 2002) | Duloxetine | 91 | 40 mg/day (20 mg b.i.d.) | |
| Duloxetine | 84 | 80 mg/day (40 mg b.i.d.) | ||
| Paroxetine | 89 | 20 mg/day (q.d.) | ||
| 2 | 8 weeks | Placebo | 89 | – |
| (Goldstein 2004) | Duloxetine | 86 | 40 mg/day (20 mg b.i.d.) | |
| Duloxetine | 91 | 80 mg/day (40 mg b.i.d.) | ||
| Paroxetine | 87 | 20 mg/day (q.d.) | ||
| 3 | 8 weeks | Placebo | 93 | – |
| (Detke 2004) | Duloxetine | 93 | 80 mg/day (40 mg b.i.d.) | |
| Duloxetine | 93 | 120 mg/day (60 mg b.i.d.) | ||
| Paroxetine | 85 | 20 mg/day (q.d.) | ||
| 4 | 8 weeks | Placebo | 99 | – |
| (Perahia 2006) | Duloxetine | 93 | 80 mg/day (40 mg b.i.d.) | |
| Duloxetine | 102 | 120 mg/day (60 mg b.i.d.) | ||
| Paroxetine | 97 | 20 mg/day (q.d.) |
b.i.d., twice daily; MDD, major depressive disorder; q.d., once daily.
Protocols were reviewed and approved by the ethical review board at each centre in accordance with the principles of the Declaration of Helsinki, and all patients provided written informed consent prior to any study procedures or the administration of study drug.
The primary objective of these studies was to investigate the efficacy of duloxetine vs. placebo in MDD using the HAMD17 total score mean change (baseline to endpoint) as the primary outcome measure. The protocols identified a priori several secondary outcome measures including the HAMD17 anxiety/somatisation factor described previously, the HAMA total score, and the Clinical Global Impression-Severity Scale CGI-S.19 The HAMD17 anxiety/somatisation factor, as described by Cleary and Guy, is the sum of items 10 (anxiety psychic), 11 (anxiety somatic), 12 (somatic symptoms gastrointestinal), 13 (general somatic symptoms), 15 (hypochondriasis), and 17 (insight). The HAMA has 2 widely used subscales: the HAMA somatic anxiety factor (sum of items 7 to 13) and the HAMA psychic anxiety factor (sum of items 1 to 6 and 14).
Analyses
We analysed the pooled data on all patients in the three treatment groups from studies 1 to 4 using SAS software, version 8.02 for Windows. We analysed the scores at each visit on the primary and secondary outcome measures above, as well as the baseline to endpoint change scores on each measure. The HAMD17 and HAMA data from the studies were analyzed using a restricted maximum, likelihood-based, mixed-effects repeated measures (MMRM) method. This method was used for imputing missing data.
Analysis Plan
The analysis plan involved several steps: 1- descriptive analysis of the above outcome measures at each visit and at endpoint; 2- attempt to replicate the presence of the pre-specified Cleary and Guy’s anxiety/somatisation factor; 3- assess the psychometric properties of the anxiety/somatisation factor using correlation analyses with the validated HAMA. The details of these analyses are described below.
Correlations between HAMD17 and HAMA scores (total score and 2 dimensions) were analysed using Spearman-rank correlation. When both the HAMD and HAMA were not available at the same endpoint, the last visit when the patient completed both scales was used as the endpoint. This ensured that both scales were completed at the same visit.
To assess the clinical validity of HAMD17 anxiety/somatisation subscale, comparisons between subgroups were made using Mann-Whitney-Wilcoxon rank test. Quantitative variables are described by their mean, standard deviation (SD), and minimum, maximum. Qualitative variables are described by frequencies and percentages.
Factor Analysis: HAMD17 Factors Identification in Our Sample
We used the same methodology as Cleary and Guy to try to replicate their findings and particularly to identify an anxiety factor in MDD.3 This factor analysis was done with baseline values from all treatment groups pooled. We used an eigenvalue ≥1 criterion (Kaiser’s criterion) to determine the number of factors, and then a Varimax rotation to determine the optimal configuration of items on factors.20 To group the items we looked at the best contribution (highest absolute value) of each item to each factor, but balanced this criterion with the clinical relevance of such grouping in the event of its concurrent association with another factor. The factor analysis was also performed in each of the 4 studies to identify the presence of the anxiety/somatisation factor at a study level.
HAMD17 Anxiety/Somatisation Factor Psychometric Properties Assessment
We investigated the psychometric properties of the HAMD17 anxiety/somatisation factor to validate its use as an anxiety outcome measure in clinical trials. This psychometric analysis plan included: 1) construct validity by examining the item convergent validity and the item discriminant validity; 2) concurrent validity by examining the correlation between its score and the HAMA total and factors scores, and the correlation of its score with the HAMD17 remaining items score when the anxiety/somatisation factor items are excluded; 3) clinical validity assessed by its ability to discriminate between patients with and without anxiety-related adverse events, and between patients with and without anxiety at baseline based on HAMA total score threshold (<15 vs. ≥15).
Construct Validation
We performed a multi-trait analysis of the Cleary and Guy’s HAMD17 anxiety/somatisation factor structure as follows:
– Analysis of the correlation between each item’s baseline score with the anxiety/somatisation total score using the Cronbach’s coefficient alpha.
– Analysis of the correlation between each item’s baseline score with the anxiety/somatisation total score excluding this item using the Cronbach’s coefficient alpha.
Concurrent Validity
The concurrent validity assessment plan included the following analyses:
– Correlation between the HAMD17 anxiety/somatisation factor and the HAMA total score, HAMA psychic and somatic anxiety factor scores at each visit, and between the HAMD17 total score excluding the anxiety/somatisation factor items and the HAMA total and factor scores.
– Correlation between the change from baseline to endpoint in the HAMD17 anxiety/somatisation subscore and the change from baseline to endpoint in the HAMA total and factor scores.
– Correlation between the HAMD17 anxiety/somatisation factor and the HAMD17 total score excluding the anxiety/somatisation items at each visit.
Clinical Validity
We assessed the clinical validity of the anxiety/somatisation factor/subscale by its ability to discriminate anxious from non-anxious patients. We compared the baseline subscale score in patients with treatment-emergent anxiety-related adverse events vs. the score in patients without anxiety-related adverse events during the course of the trials, and compared the subscale score in patients with baseline HAMA total scores <15 (non-anxious patients) vs. patients with baseline HAMA total scores ≥15 (anxious patients) using a Mann-Whitney-Wilcoxon rank test.
Results
Population
This pooled analysis included a total of 1466 patients: 975 females (66.5%) and 491 males (33.5%) with an age range from 18 to 82 years (mean = 43.2 yrs). The basic design of the 4 pooled studies is described in Table 1. For the majority of the analyses, data from the three treatment groups were pooled.
The mean (SD) baseline scores on the HAMD17 total and HAMD anxiety/somatisation factor were 21.9 (3.7) and 7.2 (2.0), respectively, while the mean (SD) baseline score on the HAMA total was 18.6 (5.7) (Table 2). HAMD and HAMA scores were slightly higher in women. The majority of patients (62.8%) had depression of moderate intensity on the CGI-S. The HAMD17 total score and HAMD anxiety/somatisation scores progressively decreased over the 8 weeks of treatment. At endpoint, the mean HAMD17 and anxiety/somatisation scores were 11.4 and 4.2, respectively, and 70% of patients were rated as normal to borderline-mildly depressed on the CGI-S (mean score = 2.8).
Table 2. HAMD17 and HAMA Scores at Baseline and Endpoint.
| MEAN ± SD (MIN; MAX) TOTAL = 1466 | CGI-S (0;7) | HAMD17 TOTAL (0;52) | HAMD17 ANXIETY/SOMATISATION (ITEMS 10-13, 15, 17) (0;18) | HAMA TOTAL 14 ITEMS (0;56) | HAMA SOMATIC ANXIETY (ITEMS 1-6, 14) (0;28) | HAMA PSYCHIC ANXIETY (ITEMS 7-13) (0;28) |
|---|---|---|---|---|---|---|
| Baseline | 4.4 ± 0.6 (3;7) | 21.9 ± 3.7 (14;37) F 22.1 ± 3.7 M 21.5 ± 3.7 | 7.2 ± 2.0 (1;14) F 7.3 ± 1.9 M 7.0 ± 2.0 | 18.6 ± 5.7 (3;41) F 19.2 ± 5.8 M 17.4 ± 5.3 | 11.4 ± 2.9 (2;24) F 11.6 ± 2.9 M 11.0 ± 2.9 | 7.2 ± 3.7 (0;23) F 7.6 ± 3.8 M 6.4 ± 3.3 |
| Endpoint | 2.8 ± 1.3 (1;7) | 11.4 ± 7.1 (0;40) F 11.5 ± 7.2 M 11.3 ± 6.9 | 4.2 ± 2.6 (0;15) F 4.3 ± 2.6 M 4.1 ± 2.6 | 10.5 ± 6.8 (0;50) F 10.7 ± 7.1 M 10.1 ± 6.4 | 6.2 ± 4.2 (0;27) F 6.2 ± 4.3 M 6.1 ± 4.0 | 4.3 ± 3.4 (0;23) F 4.5 ± 3.5 M 4.0 ± 3.1 |
| Change from baseline to endpoint | −10.5 ± 7.1 (−32;21) F −10.6 ± 7.1 M −10.2 ± 7.2 | −3.0 ± 2.9 (−12;11) F −3.0 ± 2.8 M −2.8 ± 3.0 | −8.0 ± 7.1 (−31;15) F −8.4 ± 7.2 M −7.2 ± 6.7 | −5.2 ± 4.3 (−17;9) F −5.3 ± 4.3 M −4.9 ± 4.1 | −2.9 ± 3.7 (−15;11) F −3.1 ± 3.8 M −2.4 ± 3.4 |
CGI-S, Clinical Global Impression-Severity scale; F, female; HAMA, Hamilton Anxiety scale; HAMD17, 17-item Hamilton Depression Rating scale; M, male; SD, standard deviation.
HAMD17 Factor Analysis
The factor analysis in the total sample yielded six factors that explain 51% of the variance (Table 3). The first factor consisted of insomnia symptoms: items 4 (early insomnia), 5 (middle insomnia), and 6 (late insomnia). Factor 2 includes items labelled as a severity factor: items 2 (feelings of guilt), 3 (suicide), and 17 (insight). Factor 3 includes core depressive symptoms: items 1 (depressed mood), 7 (work and activities), and 8 (retardation). The fourth factor explained 8.41% of the variance and includes the “anxiety” items, i.e., items 10 (anxiety psychic), 11 (anxiety somatic), and 15 (hypochondriasis). We named this factor “Anxiety/hypochondriasis”. Factor 5 includes items 12 (gastrointestinal symptoms) and 16 (loss of weight). Factor 6 is composed of miscellaneous items: 9, agitation; 13, general somatic symptoms; 14, genital symptoms.
Table 3. HAMD17 Factor Analysis (Varimax Rotation).
| VARIMAX ROTATION | ||||||
|---|---|---|---|---|---|---|
| HAMD17 ITEM | FACTOR 1 | FACTOR 2 | FACTOR 3 | FACTOR 4 | FACTOR 5 | FACTOR 6 |
| 05—Insomnia (middle) | 0.757 | |||||
| 06—Insomnia (late) | 0.695 | |||||
| 04—Insomnia (early) | 0.57 | |||||
| 02—Feelings of guilt | 0.654 | |||||
| 03—Suicide | 0.648 | |||||
| 17—Insight | −0.331 | |||||
| 01—Depressed mood | 0.737 | |||||
| 07—Work and activities | 0.634 | |||||
| 08—Retardation | 0.633 | |||||
| 11—Anxiety somatic | 0.681 | |||||
| 15—Hypochondriasis | 0.655 | |||||
| 10—Anxiety psychic | 0.508 | |||||
| 12—Somatic symptoms gastrointestinal | 0.813 | |||||
| 16—Loss of weight | 0.784 | |||||
| 09—Agitation | 0.73 | |||||
| 14—Genital symptoms | −0.400 | |||||
| 13—General somatic symptoms | −0.667 | |||||
| Variance explained by each factor | 9.04% | 8.82% | 8.63% | 8.41% | 8.40% | 7.73% |
HAMD17, 17-item Hamilton Depression Rating scale.
On the factor analysis we failed to find the Cleary and Guy’s anxiety/somatisation factor in our sample. Instead, we found an anxiety/ hypochondriasis factor that included only 3 of the 6 Cleary and Guy’s items.3
The factor analysis was also performed on each of the four studies separately. It yielded different factors in each study sample, confirming the unstable nature of the HAMD17 structure and its variability across studies. The analysis yielded 8 factors in study 1, 7 factors in study 2, 7 factors in study 3, and 5 factors in study 4. The items included in every factor vary dramatically in comparison to the pooled sample analysis. The anxiety/somatisation factor was not found in any individual study.
HAMD17 Anxiety/Somatisation Factor Psychometric Properties Assessment
Construct Validation
The HAMD17 anxiety/somatisation factor Cronbach’s coefficient alpha was 0.31 (raw) and 0.26 (standardised), respectively, suggesting a low level of consistency. When deleting some of the items from the factor, the raw coefficient alpha increased without item 13 (general somatic symptoms) or 17 (insight) to 0.33. This increment indicates that items 13 and 17 contribute to the poor homogeneity of the anxiety/somatisation factor in the present population. These items were also negatively correlated with the items 10 (anxiety psychic) and 12 (gastrointestinal symptoms) from the anxiety/somatisation factor.
Concurrent Validity
Spearman’s correlation coefficients between the HAMD17 anxiety/somatisation subscale and the HAMA total score were low at baseline (0.46), but increased progressively to reach 0.81 at endpoint (Table 4). This increment suggests that the correlation is higher as anxiety symptoms improve over time. These findings were consistent with the correlation coefficients observed between the HAMD17 anxiety/somatisation factor and the HAMA somatic and psychic anxiety factors. The correlation coefficient between the mean change from baseline to endpoint of the anxiety/somatisation subscale and the HAMA total score was 0.69, with a value of 0.63 for the HAMA somatic anxiety factor and 0.59 for the psychic anxiety factor.
Table 4. Correlation Coefficients* between the HAMD17 Anxiety/Somatisation Factor and the HAMA at Each Visit; and between the HAMD17 Total Score Excluding the Anxiety/Somatisation Items and the HAMA at Each Visit.
| BASELINE | WEEK 1 | W 2 | W 3 | W 5 | W 7 | W 9 | ENDPOINT | |
|---|---|---|---|---|---|---|---|---|
| HAMD17 anxiety/ somatisation | ||||||||
| With: | ||||||||
| HAMA total | 0.46 | 0.56 | 0.62 | 0.7 | 0.75 | 0.76 | 0.76 | 0.81 |
| HAMA somatic anxiety | 0.32 | 0.45 | 0.51 | 0.61 | 0.69 | 0.7 | 0.7 | 0.75 |
| HAMA psychic anxiety | 0.46 | 0.52 | 0.57 | 0.63 | 0.63 | 0.65 | 0.64 | 0.7 |
| HAMD17 without anxiety/ somatisation | ||||||||
| With: | ||||||||
| HAMA total | 0.37 | 0.54 | 0.63 | 0.71 | 0.76 | 0.77 | 0.81 | 0.83 |
| HAMA somatic anxiety | 0.43 | 0.6 | 0.7 | 0.77 | 0.82 | 0.83 | 0.85 | 0.88 |
| HAMA psychic anxiety | 0.24 | 0.36 | 0.41 | 0.47 | 0.5 | 0.52 | 0.54 | 0.56 |
Spearman-rank correlation coefficients.
HAMA, Hamilton Anxiety scale; HAMD17, 17-item Hamilton Depression Rating scale.
The correlation coefficients between the HAMD17 total score, excluding the anxiety/somatisation items, and the HAMA total score are also presented in Table 4. They followed the same pattern as the anxiety/somatisation factor with higher correlation coefficients at endpoint than at baseline. The correlation coefficients were in the same range as those observed with the anxiety/somatisation subscale. This suggests that the HAMD17 without the anxiety items is correlated at almost the same degree with the HAMA, especially the HAMA somatic factor, as is the anxiety/somatisation subscale. The correlation coefficients between the anxiety/somatisation subscale and the HAMD17 total score excluding these anxiety items went from 0.16 at baseline to 0.81 at endpoint.
Clinical Validity
The HAMD17 anxiety/somatisation baseline scores were significantly higher in patients who reported an anxiety-related treatment-emergent adverse event during the course of the studies than in patients without such adverse events (Table 5). The mean (SD) baseline HAMD17 anxiety/somatisation subscore in patients with an anxious depression, as defined by a baseline HAMA total score of at least 15, was 7.6 (1.9) and was significantly higher than in the non-anxious group (6.0 [1.8], p < 0.0001).
Table 5. Clinical Validity of the HAMD17 Anxiety/Somatisation Factor.
| N | HAMD17 ANXIETY/SOMATISATION BASELINE SCORE MEAN ± SD | p-VALUE | |
|---|---|---|---|
| Adverse event (AE) related to anxiety | <0.0001 | ||
| Yes | 92 | 6.4 ± 2.9 | |
| No | 1348 | 4.2 ± 2.6 | |
| Baseline HAMA score | <0.0001 | ||
| 0‒14 | 383 | 6.0 ± 1.8 | |
| ≥15 | 1083 | 7.6 ± 1.9 |
HAMA, Hamilton Anxiety scale; HAMD17, 17-item Hamilton Depression Rating scale;
SD, standard deviation.
Psychometric Properties Assessment of the Factors from the Factor Analysis
Our factor analysis yielded the 6 factors and did not confirm the presence of the Cleary and Guy’s anxiety/somatisation factor in our sample, but yielded another anxiety factor that we labelled the anxiety/hypochondriasis factor.
The main factors identified by the factor analysis in our sample were assessed by calculating their correlations with the CGI-S at each visit, as well as with the HAMA total and factor scores and the anxiety/somatisation factor for the anxiety/hypochondriasis (items 10, 11, and 15) factor (Table 6).
Table 6. Concurrent Validity of the Newly Identified HAMD17 Factors 1 to 4 (Spearman’s Coefficient).
| CORRELATION | BASELINE | WEEK 1 | W 2 | W 3 | W 5 | W 7 | W 9 | ENDPOINT |
|---|---|---|---|---|---|---|---|---|
| HAMD17 factor 1 (items 4, 5, 6) Sleep | ||||||||
| With: | ||||||||
| CGI-S (mean score) | 0.21 | 0.28 | 0.31 | 0.4 | 0.42 | 0.44 | 0.41 | 0.48 |
| HAMD17 factor 2 (items 2, 3, 17) Severity | ||||||||
| With: | ||||||||
| CGI-S (mean score) | 0.16 | 0.27 | 0.38 | 0.47 | 0.49 | 0.47 | 0.46 | 0.6 |
| HAMD17 factor 3 (items 1, 7, 8) Core symptoms | ||||||||
| With: | ||||||||
| CGI-S (mean score) | 0.26 | 0.46 | 0.61 | 0.71 | 0.74 | 0.77 | 0.79 | 0.84 |
| HAMD17 factor 4 (items 10, 11, 15) Anxiety/hypochondriasis | ||||||||
| With: | ||||||||
| HAMD anxiety/somatisation (6 items) | 0.9 | 0.89 | 0.9 | 0.92 | 0.92 | 0.91 | 0.91 | 0.93 |
| HAMA total | 0.46 | 0.54 | 0.6 | 0.66 | 0.71 | 0.71 | 0.7 | 0.77 |
| HAMA somatic anxiety | 0.34 | 0.45 | 0.51 | 0.58 | 0.65 | 0.66 | 0.65 | 0.71 |
| HAMA psychological anxiety | 0.44 | 0.49 | 0.53 | 0.6 | 0.6 | 0.61 | 0.59 | 0.66 |
| CGI-S (mean score) | 0.25 | 0.35 | 0.44 | 0.5 | 0.56 | 0.56 | 0.58 | 0.63 |
CGI-S, Clinical Global Impression-Severity scale; HAMA, Hamilton Anxiety scale; HAMD17, 17-item Hamilton Depression Rating scale.
As observed with the anxiety/somatisation subscale, the correlation of the anxiety/hypochondriasis factor with the HAMA total score was low at baseline (0.46) and increased progressively over time up to 0.77 at endpoint.
The third factor (core symptoms of depression [items 1, 7, and 8]) correlated weakly with the CGI-S score at baseline (0.26), but progressively increased up to 0.79. The second (“severity”) and first factors (“sleep”) had low correlation coefficients with the CGI-S scores. The mean score of these factors at each visit is shown on Figure 1.
Figure 1.
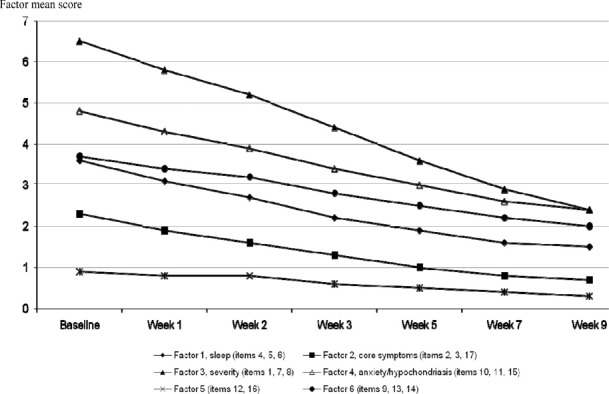
Hamilton Depression Scale 17 Items (HAMD17) Factors from the Factor Analysis Mean Score at Each Visit.
For these factors indentified at baseline, the mean scores at each visit were compared between treatment groups using a MMRM method. The mean baseline HAMD factor scores were not statistically different in the duloxetine and placebo groups, except for factor 5 (p = 0.01). Following therapy, the anxiety/hypochondriasis factor 4 score was significantly decreased at weeks 5, 7, and 9 in the duloxetine group as compared to the placebo group (p = 0.02; p = 0.005; p = 0.001, respectively). A significant difference – i.e., decrease in HAMD factor score—favouring duloxetine over placebo was also observed for factor 2 (severity of depression) at weeks 3, 5, 7, and 9 (p = 0.045; p = 0.0008; p = 0.0011; p = 0.007, respectively), factor 3 (core symptoms of depression) at weeks 5, 7, and 9 (p = 0.001; p < 0.0001; and p < 0.0001, respectively), and factor 6 at weeks 7 and 9 (p = 0.002 and p = 0.0002, respectively). In contrast, factors 1 (sleep) and 5 were not affected by the different therapies (NS), except at week 2 (p = 0.02 and 0.003, respectively).
Discussion
We performed a factor analysis of the HAMD17 in a total of 1466 depressed patients. To our knowledge, this is the largest factor analysis of the HAMD17 ever performed on a patient population. Other studies (published from 1980 to 2003) reporting factor analysis of the HAMD17 included smaller samples, i.e., between 89 and 1204 patients.13 Our sample was very homogenous. It included patients from four identical trials that were selected using the same structured diagnostic interview, the MINI, providing DSM IV diagnostic consistency for MDD across all studies. In contrast, the factor analyses done by Hamilton and Cleary and Guy were in more heterogeneous samples, not selected based on common criteria. Hamilton used a sample of 272 patients divided in 4 groups in his original factor analysis, i.e., original cases, other cases collected by the same physicians, patients admitted in a mental hospital, and finally, outpatient cases.2
Our analysis yielded 6 factors that were different from those of Cleary and Guy but are congruent with the main dimensions of depression. Sleep disturbance emerged as the first factor; the severity of depression was reflected in the second factor (guilt, suicide, loss of insight). The third factor included the core symptoms of depression (namely, depressed mood, psychomotor retardation, and work and interests). The fourth factor captured anxiety and included 3 items (somatic anxiety, psychic anxiety, and hypochondriasis). This factor was only partially similar to the anxiety/somatisation factor identified by Clearly and Guy3 and defined a priori as anxiety measure in these trials. This factor was previously identified by Onega and Abraham in 206 geriatric psychiatric outpatients along with 3 other factors.21 All the other factorial analyses reviewed by Bagby et al. in their extensive literature review described other item associations, confirming the unstable nature of the HAMD17 structure and the inherent variability in any factor analysis.13 We reconfirmed this point by performing the factor analysis in each of the 4 study samples separately. We found different factors in each analysis. In their review, Bagby et al. reported that the 15 studies reviewed identified from 2 to 8 factors and that the items and number of items included in each factor was highly variable. The most consistent factor seems to be the “sleep factor” that regroups the insomnia items in 13 of these studies. In 6 studies, the anxiety items (psychic and somatic anxiety) were associated with the agitation item; 4 studies include our 3 anxiety/hypochondriasis items in their anxiety factor.21-24
Our pooled analysis showed that the Cleary and Guy’s anxiety/somatisation factor was only weakly correlated to the HAMA, especially at the beginning of the treatment period. This correlation increased during treatment to a maximum of 0.81. These increasing correlation coefficients over time in treatment were also found in the analysis of the anxiety/hypochondriasis factor.
Despite this weak correlation with the HAMA, the anxiety/somatisation subscale was able to discriminate the anxious vs. non-anxious depressed patients as defined by a HAMA threshold demonstrating a clinically meaningful score difference of 1.6 between these two subgroups of patients. However, this sole discriminant validity is not enough to support the use of this subscale as an anxiety measure in depressed patients.
The anxiety/hypochondriasis factor identified in our sample was highly correlated to the anxiety/somatisation factor (correlation coefficients from 0.89 to 0.93), but was not better correlated to the HAMA (correlation coefficients from 0.46 to 0.77). This raises the question of the validity of any HAMD subscale to assess the anxiety symptoms in a depressed patient population, even if this subscale is identified in the sample where it is used. Alternatively, it may reflect the lack of validity of the HAMA in assessing anxious symptoms in an MDD population.
However, our analysis suggests that a scale factor should not be used as an outcome measure valid for any sample if it cannot be found in that study sample. One solution is to perform a factor analysis (identified a priori in the protocol as a “post enrolment” analysis to yield a valid subscale/factor) on the baseline sample where the subscale is to be used as an outcome measure. Such an analysis would need to be done on the locked baseline dataset and before the blind is broken, and could not be based on any post hoc analysis of the sample. The factor analysis should be performed on the baseline dataset before any treatment to ensure that the factors are identified independently from any treatment effect. This option has regulatory implications since an outcome measure needs usually to be defined a priori in the protocol before randomising the first patient to be acceptable to regulatory agencies.
Conclusion
In conclusion, our findings indicate that use of the Cleary and Guy’s anxiety/somatisation factor from the HAMD17 is unstable across studies and is not an appropriate routine measure of anxiety in depressed patients. We suggest that in clinical trials, other scales specifically designed and validated for anxiety assessment should be used. An alternative strategy is to assess the factorial structure of the HAMD17 scale for each studied sample, and use for outcome assessment the factors identified by this analysis. Such “tailored” factor analysis would retrospectively allow the identification of the set of items appropriate for each specific study sample.
Acknowledgments
This work was sponsored by Eli Lilly and Company. The authors thank Claire Cropet for her assistance with statistical analyses and Catherine Soubrouillard for her medical writing.
Footnotes
Declaration of Interest
Funding for this analysis was provided by Eli Lilly and Company (Indianapolis, IN, USA). The sponsor was involved in the interpretation of the data, the writing of the report and in the decision to submit the paper for publication. The clinical trial summaries can be found on www.lillytrials.com (Clinical Trial Registry number 4298 and 4091). The statistical analysis was performed by MAPI-NAXIS on behalf of Eli Lilly and Company. Celine Goldberger is a full-time employee and minor share holder of Eli Lilly and Company. David V Sheehan has received honoraria and travel expenses related to lectures from Eli Lilly.
References
- 1.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 3.Cleary P, Guy M. Factor analyses of the Hamilton Depression Scale presented at the International Symposium on the Evaluation of New Drugs in Clinical Psychopharmacology, Pisa, September 1975. Guy W, editor. Assessment manual for psychopharmacology. ECDEU. [Google Scholar]
- 4.Fava M, Rush AJ, Alpert JE et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 5.Papakostas GI, Trivedi MH, Alpert JE et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J Psychiatr Res. 2008;42:134–140. doi: 10.1016/j.jpsychires.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Farabaugh A, Mischoulon D, Fava M et al. The relationship between early changes in the HAMD-17 anxiety/somatization factor items and treatment outcome among depressed outpatients. Int Clin Psychopharmacol. 2005;20:87–91. doi: 10.1097/00004850-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Alpert JE, Franznick DA, Hollander SB, Fava M. Gepirone extended-release treatment of anxious depression: evidence from a retrospective subgroup analysis in patients with major depressive disorder. J Clin Psychiatry. 2004;65:1069–1075. [PubMed] [Google Scholar]
- 8.Beasley CM, Jr, Nilsson ME, Koke SC, Gonzales JS. Efficacy, adverse events, and treatment discontinuations in fluoxetine clinical studies of major depression: a meta-analysis of the 20-mg/day dose. J Clin Psychiatry. 2000;61:722–728. doi: 10.4088/jcp.v61n1003. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph RL, Entsuah R, Chitra R. A meta-analysis of the effects of venlafaxine on anxiety associated with depression. J Clin Psychopharmacol. 1998;18:136–144. doi: 10.1097/00004714-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett J, Barkin RL. A meta-analysis of eight randomized, double-blind, controlled clinical trials of mirtazapine for the treatment of patients with major depression and symptoms of anxiety. J Clin Psychiatry. 1998;59:123–127. [PubMed] [Google Scholar]
- 11.Guelfi JD, Dreyfus JF, Ruschel S et al. Structure factorielle de l’échelle de dépression de Hamilton, I. Ann Med Psychol (Paris) 1981;139:199–214. [PubMed] [Google Scholar]
- 12.Dreyfus JF, Guelfi JD, Ruschel S et al. Structure factorielle de l’échelle de dépression de Hamilton, II. Ann Med Psychol (Paris) 1981;139:446–453. [PubMed] [Google Scholar]
- 13.Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 15.Perahia DG, Wang F, Mallinckrodt CH et al. Duloxetine in the treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Psychiatry. 2006;21:367–378. doi: 10.1016/j.eurpsy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DJ, Lu Y, Detke MJ et al. Duloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetine. J Clin Psychopharmacol. 2004;24:389–399. doi: 10.1097/01.jcp.0000132448.65972.d9. [DOI] [PubMed] [Google Scholar]
- 17.Detke MJ, Wiltse CG, Mallinckrodt CH et al. Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Neuropsychopharmacol. 2004;14:457–470. doi: 10.1016/j.euroneuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan DV, Lecrubier Y, Sheehan KH et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 19.Guy W. National Institute of Mental Health; Rockville, MD: 1976. ECDEU assessment manual for psychopharmacology, revised. [Google Scholar]
- 20.Kaiser HF. The varimax criterion for analytic rotation in factor analysis. Psychometrika. 1958;23:187–200. [Google Scholar]
- 21.Onega LL, Abraham IL. Factor structure of the Hamilton Rating Scale for Depression in a cohort of community-dwelling elderly. Int J Geriatr Psychiatry. 1997;12:760–764. [PubMed] [Google Scholar]
- 22.Berrios G, Bulbena-Villarasa A. In The Hamilton Scales. Vol. 12. Berlin: Springer-Verlag; 1997. The Hamilton Depression Scale and the numerical description of the symptoms of depression; pp. 760–764. [DOI] [PubMed] [Google Scholar]
- 23.Daradkeh T, Abou-Saleh M, Karim L. The factorial structure of the 17-item Hamilton Depression Rating Scale. Arab J Psychiatry. 1997;8:6–12. [Google Scholar]
- 24.Zheng YP, Zhao JP, Phillips M et al. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry. 1988;152:660–664. doi: 10.1192/bjp.152.5.660. [DOI] [PubMed] [Google Scholar]


