Abstract
Background
Fine-needle aspiration (FNA) biopsy of lung lesions is a highly accurate method for diagnosing and staging of lung cancers, particularly in patients with advanced cancer. Although, the majority of FNA cases of non-small cell lung carcinoma (NSCLC) can be subclassified by hematoxylin and eosin (H&E) sections, immunohistochemical (IHC) markers are usually necessary for difficult cases. Our previous study has shown that both P40 and P63 demonstrate differential sensitivity and specificity in the subclassification of squamous cell carcinoma (SqCC) using tumor tissue microarrays (TMA). In the present study, we further evaluated the utility of P40 and P63 and the potential pitfalls and limitations associated with the usefulness of these stains in FNA cases.
Methods
By a computer search of pathology archives, 144 FNA biopsies with diagnoses of lung cancers and P40/P63 stains were identified, including 50 adenocarcinomas (ADCs), 56 SqCCs, 8 small cell lung carcinomas (SCLCs), and 12 cases of poorly differentiated carcinoma (PD CA). Ten benign FNA lung lesions and 8 other malignant neoplasms were also included as controls. Nuclear staining patterns of P40 and P63 were scored semi-quantitatively as 0 (negative), 1 (<10%, weak and focal), or 2 (>10%, strong and diffuse).
Results
In lung SqCCs, P40 and P63 were positive in 77.3% and 89.5% cases, respectively. In ADCs, P40 was weakly and focally positive in 6.1% cases, and P63 was variably positive in 62.8% cases. In SCLCs, P40 and P63 were focally positive in 12.5% and 50% cases. In PD CAs, no P40 or P63 immunoreactivity was detected. In the group of other neoplasms (n=8) both P40 and P63 were positive in the case of metastatic non-seminomatous germ cell tumor (NSGCT) (n=1), and P63 was positive in the case of metastatic Merkel cell carcinoma (n=1). The sensitivity and specificity of P40 and P63 were 76.9%/93.3%, and 90.2%/50.7% in the lung SqCC.
Conclusions
P63 has a better sensitivity, and P40 has a better specificity for SqCC. A positive staining pattern with both markers was also found in certain non-SqCC cases. Recognizing limitations of these markers are particularly important in FNA cases.
Keywords: non-small cell lung carcinoma (NSCLC), immunohistochemical markers, P40 and P63, fine needle aspiration of lung cancers
Introduction
Currently, lung cancer is the leading cause of cancer-related mortality in the world and in the United States in both sexes [1]. Morphologically, lung cancer can be divided in two major histological types, non-small cell (NSCLC) and small cell carcinoma (SCLC). NSCLC is the most common type and represents approximately 80% of all lung cancers. Historically, all subtypes of NSCLC received the same treatment. However, recent studies have shown that lung cancer is a heterogeneous group of tumors at morphologic, immunophenotypic and molecular genetic levels [2-5]. Current data has demonstrated that different histological subtypes of tumors are frequently associated with different genetic alterations [5-8]. These findings have advanced lung cancer treatment into a new era of personalized therapy. For example, the discovery of alterations of epidermal growth factor receptor (EGFR) gene and the rearrangement of the echinoderm microtubule-associated protein-like 4-the anaplastic lymphoma kinase (EML4-ALK) gene in a subset of lung adenocarcinomas (ADC) lead to successful targeted tumor therapy with tyrosine kinase inhibitors (TKIs) and crizotinib [9-13]. In contrast to EGFR mutations, NSCLC tumors with the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations show little or no response to TKI therapy [14-18]. In other words, clinical application of targeted therapies depends on accurate histological sub-classification of NSCLC. This information is particularly important in patients with advanced NSCLC (stage III and IV NSCLC) and in patients with metastatic lung cancers, since the majority of these patients are not candidates for surgical resection of the tumor and in those patients fine needle aspiration (FNA) biopsy of the tumor/lesion is frequently performed to obtain tumor tissue for the diagnosis, histologic and molecular testing of the tumor [2,3,19,20].
Although, the majority of NSCLC, including FNA cases, can be sub-classified based on morphologic examination using hematoxylin and eosin (H&E) stained slides, in day to day practice, an accurate diagnosis might be challenging in some of the small biopsy specimens due to: paucity of tumor cells in a given specimen, loss of characteristic architecture in FNA and small core biopsies, preparation-related artifacts and factors related to differentiation and heterogeneity of tumor cells. In these situations, immunohistochemical (IHC) markers come to play a crucial role in the sub-classification of NSCLC.
Our previous study and those of others have shown that the most commonly used markers for identification of lung ADC are thyroid transcription factor 1 (TTF1), Napsin A and mucin; whereas, cytokeratin 5/6 (CK5/6), P63 and P40 are commonly employed for SqCC [20]. These markers have shown different sensitivity and specificity in the sub-classification of NSCLC. In ADCs, our previous study using lung tumor tissue microarrays (TMAs) has shown that TTF1 and Napsin A have a sensitivity and specificity of 85.7% and 75.0%; and 89.6% and 90.0% respectively, whereas using cytological material TTF1 has a sensitivity and specificity of 81% each; and Napsin A has a sensitivity of 65% and a specificity of 96% [20-21].
Similarly to Napsin A, P40 is a relatively new marker and has been considered to be more specific for SqCC than P63. Both P40 and P63 are products of the P63 gene, which is located on chromosome 3q27-29 [22]. The full-length protein TAp63 (containing the N-terminal transactivation domain), can be identified using antibody 4A4 (P63), and the truncated protein TAp63, ie ΔNp63 (N-terminal-truncated protein isoform of TA63), can be identified by an antibody designated as P40 [23]. Recent studies have shown that the P40 has sensitivity and specificity of 100% and 98-100% in identifying SqCCs in surgically resected specimens [24,25,26,34,35]. By using tumor tissue microarrays (TMAs), we have recently demonstrated that P40 and P63 have the sensitivity and specificity of 80.95% and 90.0% (P40) and 93.5% and 80.0% (P63) in SqCC of the lung [20]. Our previous study was also consistent with these findings in that P40 had a higher specificity and lower sensitivity than that of P63.
Although P40 and P63 were tested in larger series as highly sensitive and specific markers for SqCC [24-26], the sensitivity and specificity of these markers in FNA biopsies are not well represented in literature. As with many newly introduced IHC markers, understanding limitations and pitfalls is essential to avoid misdiagnosis and misguided patient management, particularly in small biopsy specimens. Here, we evaluate P40 and P63 expression in NSCLC by using FNA biopsy specimens, and provide evidence-based knowledge regarding the utility of P40 and P63 in FNA cases.
Material and methods
Case collection
A computer search of the department of pathology archive was performed over a period of 48 months. A total of 144 FNA cases with diagnoses of lung cancers and P40/P63 stains were identified and retrieved. Among them, 56 SqCCs (45 pairs of P63 and P40), 50 ADCs (34 pairs of P63 and P40), 8 small cell lung carcinomas (SCLCs), 12 poorly differentiated carcinoma (PDCA), and 8 non-pulmonary neoplasms. 10 benign lung lesions were also included. In FNA cases, 61.8% (89 of 144 FNA cases) had follow up resections of tumors. The World Health Organization (WHO) and International Association for the Study of Lung Cancer/American Thoracic Society classification criteria were used for determination of histological subtypes of lung NSCLC; and the American Joint Committee on Cancer (AJCC) 7th edition was used to determine the pathological stage (pT) of the primary tumor at the time of initial diagnosis [27]. The histological diagnoses were correlated with cytological findings. The study was approved by the institutional review board of the Johns Hopkins Medical Institutions.
Preparation of cytological smears
FNA smears were prepared using both air-dried and wet-fixed methods. The air-dried smears were stained with Diff-Quik method (DQ stain) and used for the immediate on-site evaluation. Additional smears were wet-fixed with 95% alcohol and stained by Papanicolaou method (Pap stain) in the cytopathology laboratory.
Cell block and core biopsy material preparations
For cell block preparation, the aspiration needle was rinsed with 10 to 20 cc of Hanks balanced salt solution (Sigma, St Louis, MO) into a 50 cc centrifuge tube. The samples were transferred to the cytological laboratory within half hour. The cellular material was then harvested using a micro centrifuge at 1870 rpm for 10 min (Hettich, Beverly, MA). One to 2 cc of 10% neutral buffered formalin was added to the cell pellet and fixed overnight. After fixation in formalin, the pellet was embedded with paraffin and processed in the histology laboratory. The section was cut at 5-micron thickness and stained with hematoxylin and eosin (H&E). The core biopsy materials were fixed in 10% neutral buffered formalin and processed in the histology laboratory, with subsequent H&E staining.
Immunohistochemical (IHC) stain of individual markers
IHC was performed on cell block and/or core biopsy material. The sections were cut at 4 microns and deparaffinized prior to incubation with primary antibodies. Mouse anti-human P40 monoclonal antibody (clone BC28, BioCare, Concord, CA) was used at 1:100 dilutions. Monoclonal anti-human p63 antibody (clone 4a4, BioCare, Concord, CA) was prediluted and used as the manufacturer's suggestions. The cell conditioning solution (CC1 Mild, Ventana, Tucson, AZ) was used as a pretreatment condition according to the standard protocol and/or the manufacturer's suggestions. Heat antigen retrieval at 70°C for 40 minutes was used to enhance signal detection.
P40 and P63 were stained using a Ventana XT autostainer (Ventana Medical System, Tucson, AZ). Both p63 and P40 have well-known nuclear staining patterns. The staining pattern and intensity of these markers were scored semi-quantitatively using a four tier system: 0, undetectable (0% positive cells); 1+ (<10% positive cells); 2+ (more than 10% positive cells). Care was taken not to interpret entrapped normal bronchial epithelium or alveolar macrophages as positive for tumor cell staining. Appropriate positive and negative controls were also included in the assay.
Evaluation of data and statistical analysis
The Student t, Fisher exact and/or Chi-square tests were used for statistical analyses to characterize the expressions of markers in different types of tumors, and correlated with clinical characteristics. Differences were considered statistically significant when the P-value was less than or equal to 0.05 (P≤0.05). All P value statistical tests were two-sided.
Results
Clinical information
A total of 144 cytological specimens were included. Among them, 10 cases of benign lung lesions were used as a control group. Of 56 SqCC cases, 44 were primary lung SqCCs, and 12 were metastatic lung SqCCs to other sites, including mediastinal lymph nodes (n=7), anterior mediastinal soft tissue (n=3), pleural cavity (n=1) and soft tissue of the chest wall (n=1). Of 50 ADC cases, 31 were primary lung ADCs, and 19 cases were metastatic lung ADCs to other sites, including pleural cavity (n=7), bone (n=3), soft tissue of the chest wall (n=4), mediastinal lymph nodes (n=4) and brain (n=1). Of 8 SCLCs, 3 cases were primary lung lesions and 5 cases were metastatic SCLCs to the other sites. Of 12 PD CAs, 5 cases were primary lung lesions and 7 cases were metastatic lung PD CAs to other sites.
In addition, 8 cases of other neoplasms were also included. Four of them were metastatic malignant lesions to the lung, including one case each of sarcomatoid carcinoma from laryngeal SqCC, synovial sarcoma, non-seminoma germ cell tumor (NSGCT) and Merkel cell carcinoma (MCC). The remaining four included one case each of a metastatic non-pulmonary small cell carcinoma to the brain, a metastatic medullary thyroid carcinoma to cervical lymph node, a metastatic pancreatic adenocarcinoma to the liver, and a metastatic primitive neuroendocrine tumor of the brain.
The clinical information is summarized in Table 1. In our study, the overall patients' median age was 66.4 years and ranged from 37 to 88 years. The male to female ratio was: 1:1.1.
Table 1.
Clinical characteristics of the study groups.
| Characteristics | ADC(%) (n=50) | SqCC(%) (n=56) | SCLC(%) (n=8) | PD Ca (%) (n=12) | Other (%) (n=8) |
|---|---|---|---|---|---|
|
| |||||
| Gender | |||||
| Male | 18(36.0%) | 32(57.1%) | 5(62.5%) | 7(58.3%) | 4(50.0%) |
| Female | 32(64.0%) | 24(42.9%) | 3(37.5%) | 5(41.7%) | 4(50.0%) |
|
| |||||
| Age (years) | |||||
| Mean | 64.0 | 68.9 | 68.3 | 68.4 | 64.8 |
| Range | 37-86 | 46-88 | 58-79 | 53-86 | 45-83 |
|
| |||||
| Tumor size (cm) | |||||
| Mean | 3.07 | 4.32 | 4.09 | 4.22 | 3.87 |
| Range | 0.5- 9.0 | 0.2-13.5 | 0.7- 8.0 | 0.5- 9.0 | 0.3- 8.0 |
ADC: adenocarcinoma. SqCC: squamous cell carcinoms. SCLC: small cell lung carcinoma. PD CA: poorly differentiated carcinoma.
Immunostaining pattern of P40 and P63 in squamous cell carcinomas
The nuclear staining pattern of intensity and distribution of P40 and P63 were scored semi-quantitatively using a three-tier system: 0, undetectable (0% positive cells); 1+ (<10% positive cells, weak and focal); 2+ (more than 10% positive cells, strong and diffuse); and shown in Figure 1.
Figure 1.
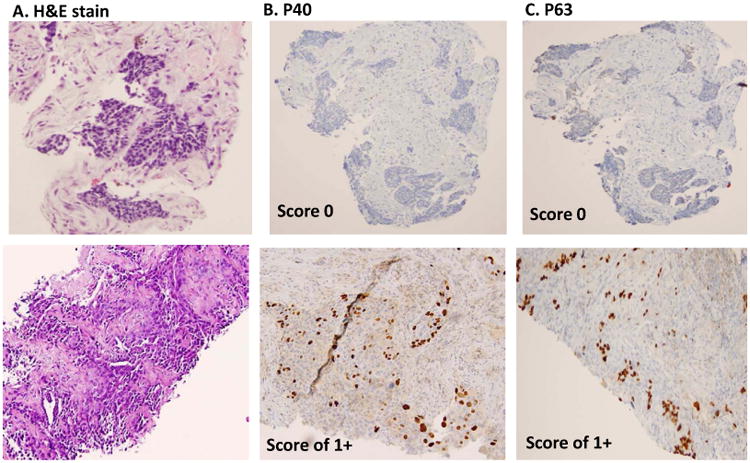
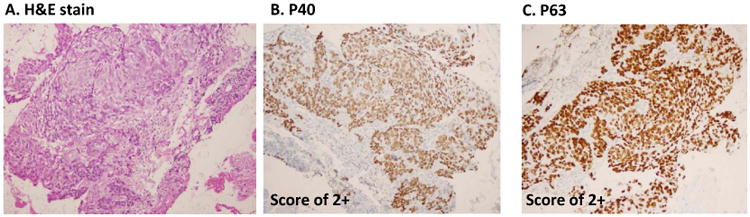
Semi-quantitative scoring of P40 and P63 in lung SqCC. A, histomorphology of SqCC on H&E slide. B, immunostain of P40, and C, immunostain of P63. The upper panel shows score 0 (negative staining patterns), the mid panel shows score 1+ (focally staining patterns), and the lower panel shows score 2+ pattern (diffusely staining patterns). All photos are taken at 20× magnification.
In primary lung SqCCs, the typical immunopattern of P40 and P63 are shown in Figure 2. Of P40, the strong nuclear staining pattern (score 2+) was found in 66.66% (28/42 cases), focal staining pattern (score 1+) were found in 9.52% (4/42 cases), negative cases (score 0) were found in 23.8% (10/42 cases), and 2 cases lacked sufficient tumor cells for scoring. Of P63, a strong nuclear staining pattern (score 2+) was found in 84.21% (32/38 cases), focal staining pattern (score 1+) was found in 5.26% (2/38 cases), negative staining (score 0) was found in 10.52% (4/38 cases), and 6 cases lacked sufficient tumor cells for scoring. In metastatic SqCC, 11 cases (11 stained with P40 and 10 stained with P63) had sufficient tumor cells for comparison. P40 was positive (1+ and 2+ cases) in 81.81% (9/11 cases), and P63 was positive (1+ and 2+ cases) in 90% (9/10 cases). The data was summarized in Figure 2B and Table 2.
Figure 2.
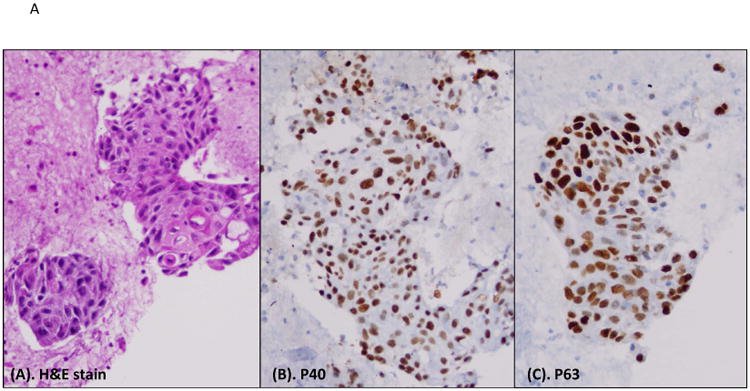
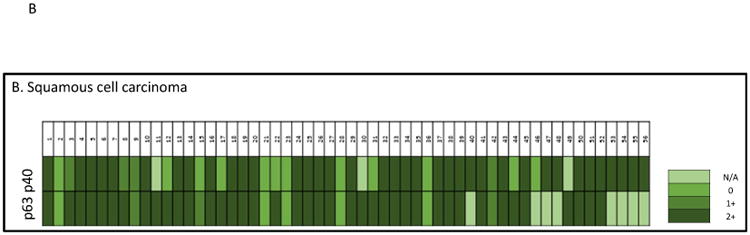
Heat map of P40 and P63 expression in individual SqCC cases. A, histomorphology of SqCC on the H&E slide, and immunostains of P40 and P63 (all photos are taken at 20× magnification). B, heat map of P40 and P63 expression in individual SqCC cases. N/A indicates lack of tumor cells on the IHC stains.
Table 2.
P40 and P63 expression in primary and metastatic pulmonary squamous cell carcinomas.
| Tumors | Score 0 | Score +1 | Score +2 | |||
|---|---|---|---|---|---|---|
| P40 | P63 | P40 | P63 | P40 | P63 | |
| Primary tumors (n=44) | 10/42 | 4/38 | 4/42 | 2/38 | 28/42 | 32/38 |
| 23.8% | 10.52% | 9.52% | 5.26% | 66.66% | 84.21% | |
| Metastatic tumors (n=12) | 2/11 | 1/10 | 1/11 | 1/10 | 8/11 | 8/10 |
| 18.18% | 10% | 9.09% | 10% | 72.72% | 80% | |
Interestingly, we also found that 8.88% (4/45 pairs) of P40 negative cases were P63 positive, but no P63 negative cases were P40 positive. The results are shown in Figure 2B. Taken together, P40 was positive in 77.3% (41/53) of cases, and P63 was positive in 89.5% (43/48) of cases.
Immunostaining pattern of P40 and P63 in adenocarcinomas and other carcinomas
In primary lung ADCs, the typical immunopattern of P40 and P63 are shown in the Figure 3. Of P40, the strong nuclear staining pattern (score 2+) was found in 0% (0/31 cases), focal staining pattern (score 1+) was found in 9.67% (3/31 cases), and negative staining (score 0) was found in 90.32% (28/31 cases). For P63, the strong nuclear staining pattern (score 2+) was found in 25% (6/24 cases), focal staining pattern (score 1+) was found in 45.83% (11/24 cases), negative staining (score 0) was found in 29.16% (7/24 cases), and 7 cases lacked sufficient tumor cells for scoring. In metastatic ADCs with sufficient tumor cells for scoring, P40 was negative in all cases (0/11 cases), and P63 was positive (1+ and 2+ cases) in 45.45% (5/10 cases). Taken together P40 was positive in 6.12% (3/49) of cases, and P63 was positive in 62.8% (22/35) of cases (Figure 3B and Table 3).
Figure 3.
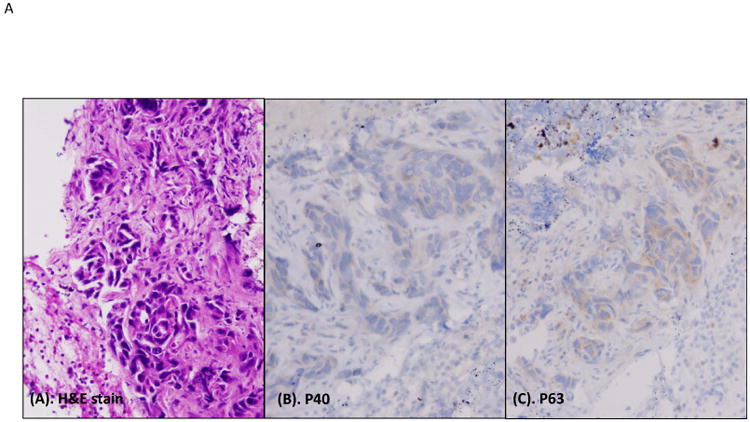
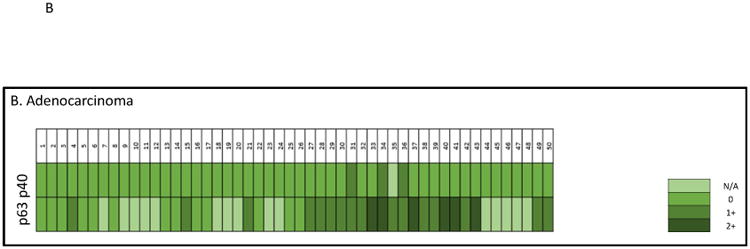
Heat map of P40 and P63 expression in individual ADC cases. A, histomorphology of ADC on the H&E slide, and immunostains of P40 and P63 (all photos are taken at 20× magnification). B, heat map of P40 and P63 expression in individual ADC cases. N/A indicates lack of tumor cells on the IHC stains.
Table 3.
P40 andP63 expression in primary and metastatic pulmonary adenocarcinomas.
| Tumors | Score 0 | Score +1 | Score +2 | |||
|---|---|---|---|---|---|---|
| P40 | P63 | P40 | P63 | P40 | P63 | |
| Primary tumors (n=31) | 28/31 | 7/11 | 3/31 | 11/24 | 0/31 | 6/24 |
| 90.32% | 29.16% | 9.67% | 45.83% | 0% | 25% | |
| Metastatic tumors (n=19) | 18/18 | 6/11 | 0/18 | 5/11 | 0/18 | 0/11 |
| 100% | 54.54% | 0% | 45.45% | 0% | 0% | |
In SCLCs with sufficient tumor cells, P40 was focally and weakly positive in 12.5% (1/8) of cases, and P63 was focally and weakly positive in 50% (2/4) of cases. In our study, all 12 cases of PD CA were negative for both P40 and P63. In the group of other neoplasms (n=8), both P40 and P63 were positive in the case of metastatic NSGCT (n=1). Additionally, P63 was positive in the case of metastatic Merkel cell carcinoma (n=1), and the rest of the cases were negative for both P40 and P63 (n=6).
In 10 cases of benign lung lesions, we found that the basal layer of the bronchial epithelium was positive for both P40 and P63 (Figure 4). Similarly, we also found in tumor cells that P40 and P63 were positive in entrapped basal cells rather than actual tumor cells (Figure 5).
Figure 4.
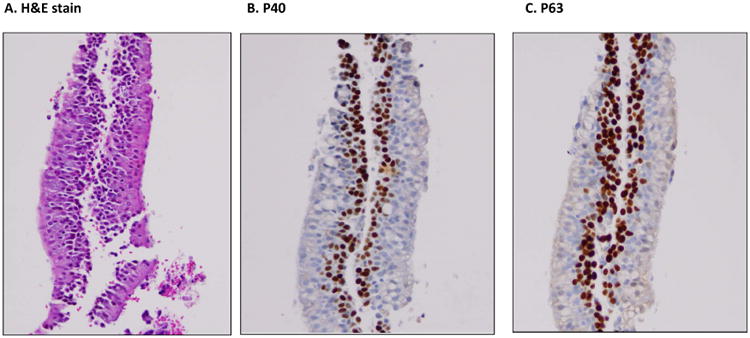
A cell block preparation of a FNA case with benign bronchial epithelium. A, H&E slide, B and C showing nuclear stating of basal cells for p40 and p63, respectively. All photos are taken at 20× magnification.
Figure 5.
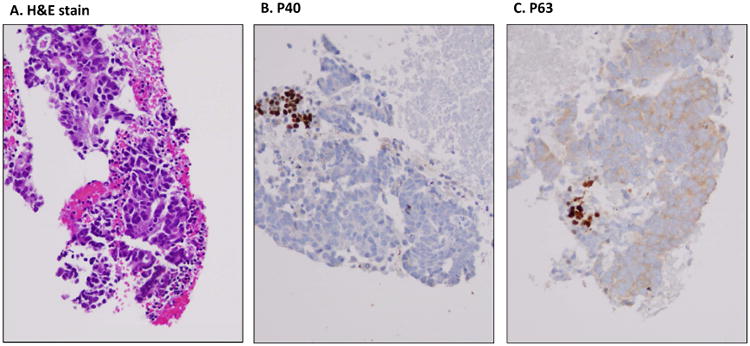
A cell block preparation of a FNA case with lung ADC. A, H & E slide, B, immunostaining of P40, and C, immuonstainig of P63. The P40 and P63 immunoreactive cells are entrapped bronchial basal cells, and the tumor cells are actually negative for both P40 and P63.
Finally, the overall sensitivity and specificity of P40 and P63 in SqCC FNA cases were 76.6% and 93.3%, 90.02% and 50.7%, respectively (P <0.0001) (Table 4).
Table 4.
The sensitivity and specificity of P40 and P63 in squamous cell carcinomas.
| Markers | Sensitivity* | Specificity* | ||
|---|---|---|---|---|
| Average ± SD | Range | Average ± SD | Range | |
| p40 | 0.769 ± 0.0544 | 0.642 – 0.925 | 0.933 ± .0345 | 0.838 – 1.000 |
| p63 | 0.902 ± 0.0388 | 0.771 – 1.000 | 0.507 ± 0.0735 | 0.321 – 0.698 |
| P value | P < 0.0001 | P < 0.0001 | ||
SqCC vs all other groups
Discussion
The World Health Organization (WHO) lung classification system lists the following major subcategories for malignant epithelial tumors: squamous cell carcinoma, adenocarcinoma, large cell carcinoma, adenosquamous carcinoma, sarcomatoid carcinoma, carcinoid tumor, salivary gland tumors and unclassified carcinoma. NSCLC (which is mainly comprised of SqCC and ADC) accounts for approximately 80% of all lung cancers [1,2]. From a management standpoint, NSCLC has been subdivided into two major treatment protocols for either SqCC or non-SqCC—including ADC, large cell carcinoma, and poorly differentiated carcinoma (PD CA), which is also known as NSCLC not otherwise specified (NOS). EGFR and ALK testing is recommended for the NSCLC, particularly in the last three categories where, if positive, tyrosine kinase inhibitors or ALK-inhibitors are included in therapeutic regimens [12,13,16].
In advanced lung cancer patients (stage III and IV NSCLC), FNA is frequently performed to obtain tumor tissue for the diagnosis as well as histological subclassification and molecular testing of the tumor. Understanding pitfalls and limitations of these markers in FNA cases, particularly by using paired FNA cases is essential to reach an appropriate interpretation of the IHC result and accurate subclassifiction of NSCLC. It is important to point out here that both P63 and P40 also stain basal bronchial cells [24-26]. This finding may be a challenge in FNA specimens where the characteristic architecture is not preserved, and not infrequently entrapped native non neoplastic basal bronchial cells may be seen admixed with the negative-stained tumor cells
Although p40 and P63 sensitivity and specificity for SqCC were found to approach 100% in some recent studies [24-26, 34], many of these studies were performed on surgically resected specimens. Collins et al, studied p40 and p63 expression in FNA specimens in patients with primary pulmonary NSCLC, P40 sensitivity and specificity were: 89.4% and 100% while P63: 86.8% and 96.7%. [35]. P40 specificity and sensitivity for SqCC in FNA cell blocks were both 100% in FNA specimens in Vogt et al series, in the same study P63 sensitivity and specificity were 97% and 80% respectively.
In the current study, we found that the sensitivity and specificity of P40 and P63 in SqCC FNA cases were 76.9% and 93.3%; 90.2% and 50.7% respectively. Furthermore, comparing findings of SqCC FNA specimens to surgical resection specimens of our previous study, the sensitivity and specificity of the p40 and p63 in 77 SqCC TMA were 80.5% and 90.0%; 93.5% and 80.0% respectively [20]. Our findings have confirmed the previous reports using surgical specimens that P40 has a higher specificity and lower sensitivity than that of P63, but both sensitivity and specificity did not approach the 100% in FNA specimens. In many of the prior studies P40 and P63 expression were studied on NSCLC (SqCC and ADC), in our cohort we also included other lung neoplasms, benign lung lesions and non-pulmonary neoplasm that metastasized to the lung to avoid sampling bias. Interestingly, we have found that the specificity of P63 was 80.0% by using TMAs and only 50.7% by using FNA cases in our current study. Several factors may have played roles and potentially caused this variable specificity by using different type of specimens. First, tumor heterogeneity is a well-known problem in biomarker study. In general, TMAs are constructed using well-select tumor tissues, however, the FNA cases are represent randomly sampled specimens, thus, the sampling error may have potential effect on the specificity. Second, the period of tumor sample in normal saline and the time of tumor fixation may also have potential effect on the detection of cellular proteins [37]. Taken together, the variable sensitivity and specificity might be related to type of the specimen, tumor heterogeneity, tissue sampling and cross-reaction.
The full-length protein TAp63 (containing the N-terminal transactivation domain), can be identified using antibody 4A4 (P63), and the truncated protein TAp63, i.e. ΔNp63 (N-terminal-truncated protein isoform of TA63), can be identified by an antibody designated as P40 [22,23]. ΔNP63 (N-terminal-truncated protein isoform of TA63), the truncated form of P63 without the transactivation domain, can be identified by the antibody designated as P40. Many investigators have addressed the cross-reactivity of P63 with bronchpulmonary ADC; in fact this cross-reactivity had led to the emergence of the more specific P40 [24-26]. Bishop JA et al, have also reported a cross reactivity of P63 in large B cell lymphoma, and NUT midline carcinoma (NMC) [26,28]. Masai K et al, analyzed SqCC and ADC markers on neuroendocrine carcinoma (NEC) and reported that P63 was focally positive in 14% of the studied cases [29]. In our results, 1/8 SCLC cases was positive for P40 and 2/4 were positive for P63. Additionally, one case consisting of a pulmonary neoplasm with small round blue morphology and negative staining for P40 and ADC markers but with focal immunostaining for P63 was identified. On follow up, however, this patient was diagnosed with MCC. P63 was also expressed in a metastatic NSGCT to the lung. The expression of P40 and P63 in non-SqCC was one of the factors that lowers the specificity of these markers in our studies.
P40 has been reported to have better sensitivity and specificity than P63 in the identification of SqCCs. Interestingly, recent studies have shown P40 immunostaining in benign and malignant neoplasms other than SqCC. A recent study has demonstrated the role of P40 as a marker for sebaceous lineage, noting that this antibody can be utilized for diagnosing sebaceous carcinoma in the setting of poorly differentiated carcinoma [30]. P40 is also expressed in the cuboidal tumor cells of sclerosing hemangioma of the lung, but not in the polygonal tumor cells [31,34,35].
An additional challenge is created by large cell carcinoma (LCC), which accounts for 3-9% of all primary pulmonary malignancies. According to the 2004 World Health Organization (WHO) classification of lung tumors, large cell carcinoma (LCC) is considered an “undifferentiated non-small cell carcinoma that lacks the cytologic and architectural features of small cell carcinoma and glandular or squamous differentiation” [32,33]. This definition does not significantly differ from that of NSCLC-NOS in FNA material, when presented as a poorly differentiated carcinoma lacking overt glandular, squamous, or small cell carcinoma morphology. Practically speaking some authors recommend using the term NSCLC-NOS for cytology specimens and small biopsies, while the term LCC for the same neoplasms but on surgical resection specimens [32,33]. The definition of LCC is based on morphology not immunohistochemical profile, and it is not infrequent to have a poorly differentiated morphology with focal immunostaing for either ADC or SqCC markers. In fact, in our results one of the two cases of LCC in which the diagnosis was made on surgical resection material, was immunreactive for P40 and P63. On FNA biopsy, this particular case was interpreted as poorly differentiated SqCC. We and others believe that despite the large cell morphology, in the presence of immunohistochemical evidence, these cases should be considered poorly differentiated SqCC or poorly differentiated adenocarcinoma as appropriate. [32,33]
In the current pathology practice P40 has become a valuable marker for SqCC, especially when encountered with poorly differentiated NSCLC [34]
In summary, our study demonstrates that P40 has a better specificity, but a lower sensitivity, than that of P63 for SqCC. The sensitivity and specificity of P40 and P63 on FNA material are similar to that on the TMA's; however, a positive staining pattern with both markers was also found in certain non-SqCC cases. Recognizing limitations of these stains are particularly important in the interpretation of P40/P63 IHC patterns in FNA cases secondary to the scant biopsy material and limited tumor cellularity. The interpretation of the immunopattern of these two markers should be correlated with the cytomorphological features of tumor cells.
Highlights.
P40 has a better specificity, but a lower sensitivity, than that of P63 for Squamous cell carcinoma (SqCC)
The sensitivity and specificity of P40 and P63 on FNA material are similar to that on the tissue microarray (TMA) sections.
A positive staining pattern with both markers was also found in certain non-SqCC cases.
Recognizing limitations of these markers are particularly important in FNA cases.
Acknowledgments
This work is partially supported by Drs. Ji and Li Family Cancer Research Foundation (QKL) and U01CA152813 (HZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Travis W, Brambilla E, Noguchi M, Nicholson A, Geisinger K, Yatabe Y, Ishikawa Y, Wistuba I, Flieder D, Franklin W, Gazdar A, Hasleton P, Henderson D, Kerr K, Nakatani Y, Petersen I, Roggli V, Thunnissen E, Tsao M. Diagnosis of Lung Adenocarcinoma in Resected Specimens: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Archives of Pathology & Laboratory Medicine. 2013;137(5):685–705. doi: 10.5858/arpa.2012-0264-RA. [DOI] [PubMed] [Google Scholar]
- 3.Li QK, Singh A, Biswal S, Askin F, Gabrielson E. KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma. J Hum Genet. 2011;56:230–234. doi: 10.1038/jhg.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Science. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto N. High Expression of ErbB Family Members and Their Ligands in Lung Adenocarcinomas That Are Sensitive to Inhibition of Epidermal Growth Factor Receptor. Cancer Research. 2005;65(24):11478–11485. doi: 10.1158/0008-5472.CAN-05-1977. [DOI] [PubMed] [Google Scholar]
- 10.Tsao M, Sakurada A, Cutz J, Zhu C, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd F. Erlotinib in Lung Cancer - Molecular and Clinical Predictors of Outcome. New England Journal of Medicine. 2005;353(2):133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 11.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 12.Ka-Fai To, MBChB, Tong Joanna HM, Mok SK, MBBS, et al. Detection of ALK rearrangement by immunohistochemistry in lung adenocarcinoma and the identification of a novel EML4-ALK variant. J Thorac Oncol. 2013;8:883–891. doi: 10.1097/JTO.0b013e3182904e22. [DOI] [PubMed] [Google Scholar]
- 13.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson DH, Klein P, Miller VA, Ostland MA, Ramies DA, Sebisanovic D, Stinson JA, Zhang YR, Seshagiri S, Hillan KJ. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Steinberg SM, Oie HK, Oie HK, Mulshine JL, Phelps R, Viallet J, Pass H, Minna JD, Gazdar AF. ras gene mutations in non-small cell lung cancers are associated with shortened survival irrespective of treatment intent. Cancer Res. 1991;51:4999–5002. [PubMed] [Google Scholar]
- 15.Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, Papadimitriou CA, Murray S. Assessment of somatic KRAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–972. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 16.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG, Varmus HE. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoSMed. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janku F, Garrido-Laguna I, Petruzelka LB, Stewart DJ, Kurzrock R. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol. 2011;6:1601–1612. doi: 10.1097/JTO.0b013e31822944b3. Review. [DOI] [PubMed] [Google Scholar]
- 18.Yung RCW, Otell S, Illei P, Clark DP, Feller-Kopman D, Yarmus L, Askin F, Gabrilson E, Li QK. Improvement of cellularity on cellblock preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120:185–195. doi: 10.1002/cncy.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feller-Kopman D, Yung RCW, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). A study of 135 cases with histology correlation. Cancer Cytopathol. 2009;117:482–490. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- 20.Ao MH, Zhang H, Sakowski L, Sharma R, Illei PB, Gabrielson E, Askin F, Li QK. The utility of a novel triple marker (combination of TTF1, Napsin A and p40) in the subclassification of non-small cell lung carcinoma (NSCLC) Human Pathol. 2014 doi: 10.1016/j.humpath.2014.01.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoll LM, Johnson MW, Gabrielson E, Askin F, Clark DP, Li QK. The utility of Napsin A in the identification of primary and metastatic lung adenocarcinoma among cytologically “poorly differentiated carcinoma”. Cancer Cytopathol. 2010;118:441–449. doi: 10.1002/cncy.20108. [DOI] [PubMed] [Google Scholar]
- 22.Nobre A, Albergaria A, Schmitt F. p40: A p63 Isoform Useful for Lung Cancer Diagnosis – A Review of the Physiological and Pathological Role of p63. Acta Cytologica. 2013;57(1):1–8. doi: 10.1159/000345245. [DOI] [PubMed] [Google Scholar]
- 23.Pelosi G, Rossi G, Cavazza A, Righi L, Maisonneuve P, Barbareschi M, Graziano P, Pastorino U, Garassino M, de Braud F, Papotti M. Np63 (p40) Distribution Inside Lung Cancer: A Driver Biomarker Approach to Tumor Characterization. International Journal of Surgical Pathology. 2013;21(3):229–239. doi: 10.1177/1066896913476750. [DOI] [PubMed] [Google Scholar]
- 24.Sethi S, Geng L, Shidham VB, Archuletta P, Bandyophadhyay S, Feng J, Madan S, Shi D, Tranchida P, Giorgadze T. Dual color multiplex TTF1 + Napsin A and p63 + CK5 immunostaining for subcategorizing of poorly differentiated pulmonary non-small carcinomas into adenocarcinoma and squamous cell carcinoma in fine needle aspiration specimens. Cytojournal. 2012;9:10. doi: 10.4103/1742-6413.94570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown AF, Sirohi D, Fukuoka J, Cagle P, Policarpio-Nicolas M, Tacha D, Jagirdar J. Tissue-Preserving Antibody Cocktails to Differentiate Primary Squamous Cell Carcinoma, Adenocarcinoma, and Small Cell Carcinoma of Lung. Arch Pathol Lab Med. 2013;137:1274–1281. doi: 10.5858/arpa.2012-0635-OA. [DOI] [PubMed] [Google Scholar]
- 26.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 27.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th. Springer; 2010. [Google Scholar]
- 28.Bishop JA, 1, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012 Aug;36(8):1216–21. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masai K, Tsuta K, Kawago M, Tatsumori T, Kinno T, Taniyama T, Yoshida A, Asamura H, Tsuda H. Expression of Squamous Cell Carcinoma Markers and Adenocarcinoma Markers in Primary Pulmonary Neuroendocrine Carcinomas. Appl Immunohistochem Mol Morphol. 2013;21:292–297. doi: 10.1097/PAI.0b013e31826fd4f3. [DOI] [PubMed] [Google Scholar]
- 30.Jain D, Mathur Sandeep R, Sharma Mehar C, Iyer Venkateswaran K. Diagnostic Cytopathology. Cytomorphology of Sebaceous Carcinoma With Analysis of p40 Antibody Expression. Published online 00 Month 2015 in Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- 31.Jian Wu, Zhang Chang, Qiao Haiguo. The significance of p40 expression in sclerosing hemangioma of lung. Scientific Reports. 2014 Aug;4:6102. doi: 10.1038/srep06102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/ American Thoracic Society/ European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelosia G, Barbareschi M, Cavazza A, Graziano P, Rossi G, Papotti G. Large cell carcinoma of the lung: A tumor in search of an author. A clinically oriented critical reappraisal. Lung Cancer. 2015 Jan 17; doi: 10.1016/j.lungcan.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Vogt A, Cohen C, Siddiqui M. p40 (ΔNp63) is more specific than p63 and cytokeratin 5 in identifying squamous cell carcinoma of bronchopulmonary origin: A review and comparative analysis. Diagn Cytopathol. 2013;42(5):453–458. doi: 10.1002/dc.23045. [DOI] [PubMed] [Google Scholar]
- 35.Collins B, Wang J, Bernadt C. Utilization of p40 (ΔNp63) with p63 and Cytokeratin 5/6 Immunohistochemistry in Non-Small Cell Lung Carcinoma Fine-Needle Aspiration Biopsy. Acta Cytologica. 2013;57(6):619–624. doi: 10.1159/000354213. [DOI] [PubMed] [Google Scholar]
- 36.Jain D, Mathur SR, Guleria R, et al. Utility and pattern of positivity of P40 in the diagnosis of squamous cell carcinoma of the lung by cytology: the first study on fine needle aspiration smears. Cytopathology. 2014;25:330–335. doi: 10.1111/cyt.12105. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita-Kashima Y, Shu S, Yorozu K, et al. Imprtance of formalin fixing conditions for Her2 testing in gastric cancer: immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer. 2014 Oct;17(4):648. doi: 10.1007/s10120-013-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


