ABSTRACT
Necrotic enteritis toxin B (NetB) is a pore-forming toxin produced by Clostridium perfringens and has been shown to play a key role in avian necrotic enteritis, a disease causing significant costs to the poultry production industry worldwide. The aim of this work was to determine whether immunization with a non-toxic variant of NetB (NetB W262A) and the C-terminal fragment of C. perfringens alpha-toxin (CPA247–370) would provide protection against experimental necrotic enteritis. Immunized birds with either antigen or a combination of antigens developed serum antibody levels against NetB and CPA. When CPA247–370 and NetB W262A were used in combination as immunogens, an increased protection was observed after oral challenge by individual dosing, but not after in-feed-challenge.
KEYWORDS: Clostridium perfringens, necrotic enteritis, NetB, alpha-toxin, immunization
Introduction
Necrotic enteritis (NE) is a severe gastro-intestinal disease causing significant costs to the poultry industry worldwide (Parish, 1961; Keyburn et al., 2008; Cooper & Songer, 2009). Disease can occur either as an acute clinical or as a mild subclinical form. Acute NE typically leads to high mortality rates during the last weeks of the broiler rearing period. Disease can arise without any previous signs and cause mortality in a couple of hours (Helmboldt & Bryant, 1971; Wijewanta & Senevirtna, 1971). However, most of the NE cases are associated with relatively mild clinical signs (Kaldhusdal & Hofshagen, 1992; Brennan et al., 2001a, 2001b). This subclinical form of NE is chronic and induces intestinal damage. Diseased birds show reduced performance parameters such as less feed intake, decreased digestion and absorption of feedstuffs and consequently reduced weight gain over time (Elwinger et al., 1992; Kaldhusdal et al., 2001). The mild subclinical form is believed to be the most prevalent form of NE and mostly responsible for the associated economic losses as it may go undetected and remain untreated (Dahiya et al., 2006).
Clostridium perfringens, a commensal of the intestinal microbiota, has been shown to be the causative agent of NE. A number of predisposing factors have been identified which influence the gut environment of the host organism and favour the growth of NE-inducing C. perfringens strains. The nature of the feedstuff is the key predisposing factor for NE. Poorly digestible diets, such as non-starch polysaccharides and protein-rich feed, lead to ideal growth conditions for C. perfringens in the gut (Branton et al., 1987; Riddell and Kong, 1992; Palliyeguru et al., 2010). Sudden diet changes, high-density bird housing conditions or extreme environmental temperatures are other important factors that predispose to NE (McDevitt et al., 2006; Burkholder et al., 2008). Mucosal damage of the gut, caused by organisms such as Eimeria species, has often been reported before or during outbreaks of NE in the field (Helmboldt & Bryant, 1971; Broussard et al., 1986; Williams, 2005). Co-infection of C. perfringens with Eimeria species has been shown to synergistically induce NE (Alsheikhly & Alsaieg, 1980; Williams et al., 2003; Gholamiandehkordi et al., 2007; Park et al., 2008). The molecular makeup of C. perfringens strains present in the gut is another essential factor (Shojadoost et al., 2012). Most of C. perfringens isolates from cases of NE possess the netB gene (Chalmers et al., 2008; Cooper & Songer, 2009; Martin & Smyth, 2009), encoding the necrotic enteritis toxin B (NetB), a member of the Staphylococcus aureus α-hemolysin-like β-pore-forming toxin family (Keyburn et al., 2008; Savva et al., 2013). This toxin has a proven role in NE development (Keyburn et al., 2008).
Vaccine trials against NE initially focused on the use of C. perfringens alpha-toxin (CPA) as an antigen. Immunization studies with CPA have been shown to partially protect chickens from developing NE (Kulkarni et al., 2007; Cooper et al., 2009; Kulkarni et al., 2010). It has been shown that birds with high CPA titres showed lower mortality rates during the production period than those with low titres (Heier et al., 2001). Immunization with either C. perfringens crude toxoids or culture supernatants can also provide significant protection against experimental NE (Lanckriet et al., 2010; Saleh et al., 2011). In addition, a number of immunogenic proteins from C. perfringens have been evaluated as sub-unit vaccines providing partial protection against experimental NE. Although a variety of antigens have been tested as vaccine candidates against NE so far, complete protection against disease has not been reported yet. In a previous study, we showed that a non-toxic variant of NetB (NetB W262A) was able to induce partial protection against experimental NE in poultry (Fernandes da Costa et al., 2013). In this study we investigated whether a combination of NetB W262A and a fragment of the C-terminal domain of CPA (CPA247–370) (Williamson & Titball, 1993) could provide improved protection against disease as compared to vaccination with the individual antigens. Protection was evaluated using an in-feed and oral administration infection model.
Materials and methods
Expression and purification of NetB W262A and CPA247–370
Expression and purification of NetB W262A or CPA247–370 was carried out as described previously (Titball et al., 1993; Williamson and Titball, 1993; Fernandes da Costa et al., 2013; Savva et al., 2013). In short, recombinant Escherichia coli expressing the toxin variants were grown in terrific broth supplemented with ampicillin (100 μg/ml) at 37°C and shaken at 300 rpm. For NetB W262A expression, cultures were induced at an optical density (OD595nm) of 0.5 for 6 h by adding arabinose at a final concentration of 0.02% (w/v). Expression of CPA247–370 was induced at an OD595nm of 0.5 for 6 h by the addition of IPTG (1 mM final concentration). In both cases, bacterial cells were harvested by centrifugation, lysed enzymatically using BugBuster (Invitrogen, Paisley, UK) and NetB or CPA247–370 were purified with Ni-NTA or GST GraviTrap chromatography columns (GE Healthcare Life Sciences, Little Chalfont, UK), respectively, according to the manufacturer's instructions. Buffer was exchanged by size-exclusion chromatography using PD-10 desalting columns (GE Healthcare) equilibrated with Tris-buffered saline (TBS; 20 mM Tris pH 7.5, 150 mM NaCl) and protein concentrations were measured with a UV–VIS spectrophotometer (Thermo Scientific, Cramlington, UK).
Birds and housing conditions
Ross 308 broiler chickens were obtained as one-day-old chickens (Vervaeke-Belavi Hatchery, Tielt, Belgium) and the parent flock had not been vaccinated with the commercial Netvax™ or any other C. perfringens vaccine. All birds were housed in the same room. The birds were reared in pens at a density of 26–30 birds per 1.5 m2 on wood shavings. All pens were separated by solid walls to prevent contact between birds from different treatment groups. Before the trials, housing rooms were decontaminated with Metatectyl HQ (Clim'oMedic®, Metatecta, Belgium) and a commercial anticoccidial disinfectant (OOCIDE, DuPont Animal Health Solutions, Wilmington, DE, USA). The chickens received ad libitum drinking water and feed. Bird experiments were carried out according to the recommendations and following approval of the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium.
Strains and culture conditions
C. perfringens strain 56 was grown during 18 h at 37°C in Brain Heart Infusion broth (Oxoid, Basingstoke, UK) with 0.375% glucose in an anaerobic (84% N2, 8% CO2 and 8% H2) cabinet (Ruskinn Technology, Bridgend, UK) and used as such.
Vaccine preparation and delivery
On days 3, 9 and 15, chickens were immunized with 30 μg of NetB W262A, CPA247–370 or a combination of both. In each case, Quil-A (50 μg; Brenntag Biosector, Frederikssund, Denmark) was used as an adjuvant. The mixture was diluted in PBS to a total volume of 200 µl, mixed by vortexing and filter-sterilised (0.2 µm pore size). Birds were vaccinated subcutaneously in the neck with a 200 µl dose. Controls consisted of an untreated group and a group receiving only the Quil-A adjuvant.
In vivo NE model
For each trial, five groups of 26–30 (indicated in Table 1) one-day-old Ross 308 broiler chickens were fed a wheat/rye-based (43%/7.5%) diet, with soybean meal as protein source. The feed composition was as described elsewhere (Gholamiandehkordi et al., 2007). Briefly, the diet contained high levels of (bird) proteins and non-starch polysaccharides which predispose to the development of NE. Nobilis Gumboro D 78 vaccine (Schering-Plough Animal Health, Brussels, Belgium) was given in the drinking water on day 16. From day 17 onwards, soy bean meal was replaced by fishmeal (30%) as a protein source.
Table 1. Description of experimental groups used in this study.
| Trial | Group | Birds/group | Vaccine | Vaccination dose | Vaccination day(s) | Serum collecting day | Challenge | Number of birds with lesions/total number | Percentage of birds with necrotic enteritis (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 27 | – | – | – | 16 | Orally on days 17, 18, 19 and 20 | 10/27 | 37 |
| 2 | 28 | Quil A + PBS | – | 3, 9, 15 | 16 | 9/28 | 32 | ||
| 3 | 28 | NetB W262A | 30µg | 3, 9, 15 | 16 | 5/28 | 18 | ||
| 4 | 26 | CPA247–370 | 30µg | 3, 9, 15 | 16 | 3/26 | 12A | ||
| 5 | 28 | NetB W262A + CPA (247–370) | 30µg + 30µg | 3, 9, 15 | 16 | 0/28 | 0B | ||
| 2 | 1 | 26 | – | – | – | 17 | Culture/feed mixture (3:4) twice a day on days 19, 20, 21, 22 | 11/26 | 42 |
| 2 | 27 | Quil A + PBS | – | 3, 9, 15 | 17 | 14/27 | 52 | ||
| 3 | 29 | NetB W262A | 30µg | 3, 9, 15 | 17 | 8/29 | 28 | ||
| 4 | 30 | CPA247–370 | 30µg | 3, 9, 15 | 17 | 6/30 | 20B | ||
| 5 | 30 | NetB W262A + CPA247–370 | 30µg + 30µg | 3, 9, 15 | 17 | 9/30 | 30 |
Values with uppercase(A) superscripts differ significantly (P < 0.01 for trial 1); values with uppercase (B) superscripts differ significantly (P < 0.05 for trial 2).
Trial 1 was carried out to compare the efficacy of vaccination of individual antigens with a combination of antigens using an infection model causing mild disease. The NE model of the first trial was based on a subclinical in vivo model described previously (Mot et al., 2012). The birds were challenged orally, using a plastic tube inserted in the crop, on days 17, 18, 19 and 20 with a single dose of approximately 4 × 108 colony-forming units of C. perfringens strain 56. On day 18, all birds were orally inoculated with a 10-fold dose of Paracox-5 (Schering-Plough Animal Health). On days 21, 22 and 23, one-third of the birds in each group were euthanized and necropsied.
Trial 2 was carried out to clarify whether vaccination yielded the same protection when a more severe challenge model was used. In the second trial an in-feed-challenge was performed based on the model by Keyburn et al. (2006). High level protein feed (30% fishmeal) and Brain Heart Infusion broth culture were manually mixed in a ratio of 3:4 (v/w). The mixture was then placed into feed trays. Birds were fed the culture/feed mixture twice a day, on days 19, 20, 21 and 22. The feed trays were cleaned and the remaining feed discarded prior to each subsequent feeding. On day 20, all birds were orally inoculated with a 10× dose of Paracox-5 (Schering-Plough Animal Health). The birds were euthanized and necropsied on day 23.
Measurement of antibodies to NetB and CPA using ELISA
Antibody responses to NetB W262A and CPA247–370 immunization were determined using an enzyme-linked immunosorbent assay (ELISA). Serum from 10 chickens was collected individually in all groups, on day 16 (first trial) or day 17 (second trial), and pooled. For NetB, ELISA was performed as described previously (Fernandes da Costa et al., 2013). First, 96-well microtitre plates (Nunc-Immuno Plates, MaxiSorp, Thermo Scientific, Cramlington, UK) were coated with 0.5 µg/well of recombinant wild-type NetB with N-terminal His tag and incubated overnight at 4°C. Plates were then washed three times with TBS-T (TBS, Tween 0.5% v/v) and blocked with TBS-3% skimmed milk for 1 h at 37°C. Following incubation, plates were rinsed three times with TBS-T and incubated with 100 µl/well of pooled sera (1:20) in TBS-1% skimmed milk for 1 h at 37°C. Wells were then rinsed three times with TBS-T and incubated with a horseradish peroxidase (horseradish peroxidase)-conjugated goat anti-chicken IgY (H + L) secondary antibody (Abcam, Cambridge, UK) at a dilution of 1:10,000 in TBS-1% skimmed milk. For detection, 100 μl of tetramethylbenzidine substrate solution was added to each well and plates were incubated for 30 min at room temperature. The reaction was stopped by the addition of 100 μl of 3 M H2SO4 and absorbance was measured at 450 nm using a Model 680 Microplate Reader (Bio-Rad Laboratories Ltd., Hemel Hempstead, UK). For CPA detection, the Bio-X CPA ELISA kit (Bio-X Diagnostics, Jemelle, Belgium) was used according to the manufacturer's instructions. In brief, pooled sera samples (1:2) were added to a recombinant CPA sensitized 96-well microtitre plate and incubated for 2 h at 37°C. Wells were then rinsed three times with washing buffer, HRP-conjugated anti-CPA antibodies added and plates incubated for 30 min at 37°C. Antibody detection was performed using tetramethylbenzidine as described above. Each ELISA was performed in triplicate.
Assessment of protection
NE severity was assessed by scoring lesions within the small intestine of each bird (duodenum to ileum) as described by Keyburn et al. (2006) as follows: 0 = no gross lesions; 1 = congested intestinal mucosa; 2 = focal necrosis or ulceration (1–5 foci); 3 = focal necrosis or ulceration (6–15 foci); 4 = focal necrosis or ulceration (≥ 16 foci); 5 = patches of necrosis 2–3 cm long; 6 = diffuse necrosis typical of field cases. Birds showing lesion scores of two or higher were classified as NE positive.
Statistical analyses
To compare the mean values of antibody levels for the enzyme linked immunoassay, 1-way analysis of variance analysis was carried out followed by Dunnett's multiple comparisons test using GraphPad Prism 5.01 software (GraphPad Software, La Jolla, CA, USA). For the in vivo NE model, differences between groups in the occurrence of NE-positive birds were evaluated by binary logistic regression analysis with the SPSS Statistics software 22.0 (SPSS Inc., Chicago, IL, USA). In both analyses, a P value of less than 0.05 was considered as significant.
Results
Immune response to NetB W262A and CPA247–370
An ELISA was used to measure serum antibody responses to NetB or CPA in the immunized birds. Blood samples were taken on day 16 or 17, one day before the first C. perfringens challenge. In the first trial chickens immunized with NetB W262A, CPA247–370 or a combination of both increased antibody responses to the immunizing antigen relative to the Quil-A immunized control group (Figure 1(a)). In particular, a statistically significant increase (P < 0.001) was detected for NetB antibody levels in the NetB W262A immunized group and when a combination of NetB W262A and CPA247–370 was used. The second trial confirmed these results (Figure 1(b)). A significant increase (P < 0.05) was detected for NetB antibody levels in the NetB W262A immunized group and when the combination NetB W262A and CPA247–370 was used. A significant increase was detected for CPA antibody levels in the CPA247–370 immunized group (P < 0.001) and when the combination NetB W262A and CPA247–370 was used (P < 0.05).
Figure 1.

Antibody responses to NetB and CPA using ELISA. Chickens were immunized with NetB W262A, CPA247–370 or a combination of both, on days 3, 9 and 15. Sera were taken on day 16 prior to C. perfringens challenge. Each bar represents mean ± SEM. Asterisks indicate a statistically significant difference relative to the Quil-A immunized control (*P < 0.05, **P < 0.01 and ***P < 0.001). (a) First trial and (b) second trial.
Protection against experimental NE after immunization with genetic toxoids
Trial 1
The chickens were challenged orally once a day at days 17, 18, 19 and 20. Immunization with NetB W262A, CPA247–370 or a combination of both reduced lesion scores and the occurrence of NE-positive birds relative to the control groups. While mean lesion scores were 0.74 and 0.75 in the control groups of untreated chickens and those dosed with adjuvant only, respectively, birds immunized with either NetB W262A or CPA247–370 showed reduced mean lesion scores to 0.39 and 0.23, respectively (Figure 2(a)). No lesions were observed after immunization with a combination of NetB W262A and CPA247–370. In the untreated chickens or chickens dosed with adjuvant only, 37% and 32% were NE-positive, respectively, whereas only 18% of the birds immunized with NetB W262A and 12% of the birds immunized with CPA247–370 were NE-positive (Figure 2(b)). Birds immunized with a combination of NetB W262A and CPA247–370 showed no signs of NE.
Figure 2.
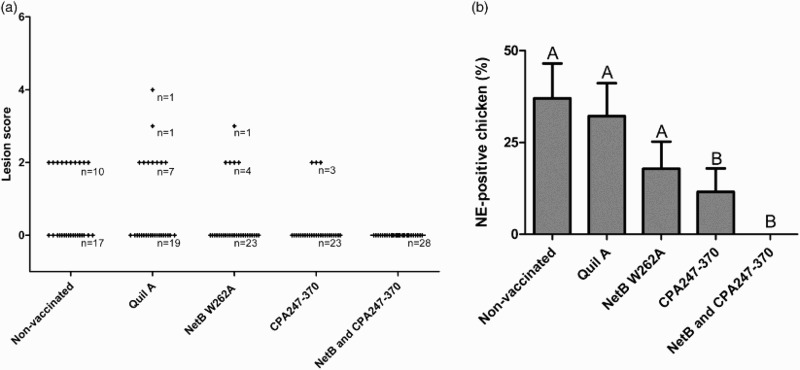
Data from the in vivo NE model of the first trial. Chickens were challenged orally once a day at days 17, 18, 19 and 20. (a) Lesion scores of individual chickens. According to severity, lesions in the small intestine were scored from 0 (no gross lesions) to 6 (diffuse necrosis). Individual chickens are marked as (+). n = number of birds. (b) NE-positive chickens. Birds with lesion scores of 2 or higher were classified as NE-positive. Black bars represent SEM. Groups not sharing the indicated letters are significantly different (P < 0.01).
Trial 2
Chickens were challenged in-feed at days 19, 20, 21 and 22. Again, immunization with NetB W262A, CPA247–370 or a combination of both reduced lesion scores and the occurrence of NE-positive birds relative to the control groups. Mean lesion scores were 1.23 and 1.70 in the control groups of untreated chickens and those dosed with adjuvant only, respectively. Chickens immunized with NetB W262A showed a mean lesion score of 0.79. The group immunized with CPA247–370 showed a mean lesion score of 0.56. After immunization with the combination of NetB W262A and CPA247–370 a mean lesion score of 0.93 was observed (Figure 3(a)). In the untreated chickens or chickens dosed with adjuvant only, 42% and 52% were NE-positive, respectively, whereas only 27% of the birds immunized with NetB W262A and 20% of the birds immunized with CPA247–370 were NE-positive (Figure 3(b)). In the group immunized with the combination of NetB W262A and CPA247–370 30% of the chickens were NE-positive.
Figure 3.
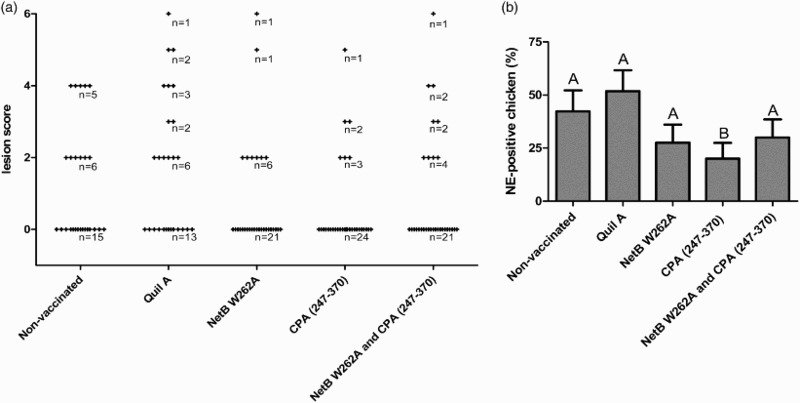
In vivo NE model of the second trial. Chickens were challenged in-feed at days 19, 20, 21 and 22. (a) Lesion scores of individual chickens. According to severity, lesions in the small intestine were scored from 0 (no gross lesions) to 6 (diffuse necrosis). Individual chickens are marked as (+). n = number of birds. (b) NE-positive chickens. Birds with lesion scores of 2 or higher were classified as NE-positive. Black bars represent SEM. Groups not sharing the indicated letters are significantly different (P < 0.05).
Discussion
In recent years, a number of studies have been carried out on the development of a potential vaccine against NE. Significant protection has been shown by immunization with crude or inactivated C. perfringens supernatant (Lanckriet et al., 2010). However, the antigens responsible for the induction of protective immunity have not been identified. A range of recombinant proteins from C. perfringens has been evaluated as vaccines, including glyceraldehyde-3-phosphate dehydrogenase, pyruvate-ferredoxin oxidoreductase, fructose 1,6-biphosphate-aldolase and a hypothetical protein (Kulkarni et al., 2007). Immunization with any of these proteins provided partial protection against experimental NE. Oral immunization with an attenuated Salmonella enterica serovar Typhimurium vaccine vector expressing fructose 1,6-biphosphate-aldolase, the carboxy-terminal domain of CPA or a hypothetical protein induced protective responses against NE in chickens (Kulkarni et al., 2008; Zekarias et al., 2008). Partial protection against NE has also been reported after immunization with C. perfringens large cytotoxin TpeL, endo- β-N-acetylglucosaminidase or phosphoglyceromutase (Jiang et al., 2009). A more recent study in which CPA, NetB, pyruvate-ferredoxin oxidoreductase and elongation factor-Tu were compared as protective antigens concluded that NetB and pyruvate-ferredoxin oxidoreductase given with ISA71 adjuvant provided the best protective immunity (Jang et al., 2012). In an attempt to improve the level of protection afforded by these vaccine antigens, some of these have been expressed in attenuated mutants of S. enterica (Zekarias et al., 2008; Kulkarni et al., 2008, 2010). Whilst these recombinant Salmonella are well suited for oral delivery, these vaccines also failed to confer complete protection against disease.
In a previous study, we have shown that immunization of poultry with a formaldehyde NetB toxoid or NetB W262A resulted in the induction of antibody responses against NetB and provided partial protection against experimental NE (Fernandes da Costa et al., 2013). The current study was conducted to test if a combination of NetB W262A with CPA247–370, which individually have been shown to provide partial protection against disease, provided enhanced protection relative to single protein immunization. Immunization led in both trials to increased antibody responses to NetB and CPA and to protection against experimental NE. However, the enhanced protection by immunization with a combination of NetB W262A and CPA247–370 depended on the severity of challenge in the in vivo trials. In the first trial, in which an oral challenge was performed resulting in mild subclinical disease, the protection in the group vaccinated with the combination of NetB W262A and CPA247–370 was complete. In the second trial, in which a more severe in-feed model was used, the protection was partial.
The importance of the challenge method used in an in vivo NE-model was already mentioned by Shojadoost et al. (2012). The severity of the disease, and also the protection against the disease, depends strongly on the challenge method used.
Our data show that vaccination with the combination of both antigens enhances the protection against a mild challenge but is not sufficient enough against a severe challenge. Also, the vaccination scheme used would not be practical in the field since the vaccine was administered parenterally three times. An alternative route may lie in breeder hen vaccination, but the antibody decline in the progeny may decrease efficacy (Keyburn et al., 2013). Expression of NetB W262A and CPA247–370 in a bacterial vector could allow this vaccine to be given by an oral route, such as in drinking water or feed. Suitable vectors would include attenuated mutants of bacteria, such as attenuated strains of S. enterica. Alternatively, it may be possible to express NetB W262A and CPA247–370 in a bacterium that is normally a member of the poultry gut microbiota, such as Bacillus species. The use of a live bacterial vector for expressing antigens, however, would mean the vaccines would be classified as genetically modified organisms. In contrast to other vaccines they require special attention concerning their impact on the environment. The regulatory restrictions of genetically modified organisms products are significantly larger in many countries than those for the release of conventional live vaccines (Frey, 2007).
In conclusion, the present study shows the potential of CPA247–370 and NetB W262A to be used as a combination vaccine to provide protection against mild NE. Further studies are required to determine a suitable delivery route for practical immunization in the poultry industry, and to enhance protection.
Acknowledgements
SFdC, DM and SG carried out the experiments and drafted the manuscript. MB-B carried out ELISA on NetB and interpreted the data. RT and FVI designed the trials and drafted the manuscript. All authors read and approved the final manuscript.
Funding Statement
This research was supported in part by Wellcome Trust award [WT089618MA] and by the European Union Marie Curie Network [grant number 237942] to RT.
Disclosure statement
The use of this combination of antigens to provide protection against necrotic enteritis is detailed in patent application WO2015092406 (A1) filed by the University of Ghent and the University of Exeter.
References
- Alsheikhly F., Alsaieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Diseases. 1980;24:324–333. doi: 10.2307/1589700. [DOI] [PubMed] [Google Scholar]
- Branton S.L., Reece F.N., Hagler W.M., Jr. Influence of a wheat diet on mortality of broiler chickens associated with necrotic enteritis. Poultry Science. 1987;66:1326–1330. doi: 10.3382/ps.0661326. [DOI] [PubMed] [Google Scholar]
- Brennan J., Bagg R., Barnum D., Wilson J., Dick P. Efficacy of narasin in the prevention of necrotic enteritis in broiler chickens. Avian Diseases. 2001a;45:210–214. doi: 10.2307/1593030. [DOI] [PubMed] [Google Scholar]
- Brennan J., Moore G., Poe S.E., Zimmermann A., Vessie G., Barnum D.A., Wilson J. Efficacy of in-feed tylosin phosphate for the treatment of necrotic enteritis in broiler chickens. Poultry Science. 2001b;80:1451–1454. doi: 10.1093/ps/80.10.1451. [DOI] [PubMed] [Google Scholar]
- Broussard C.T., Hofacre C.L., Page R.K., Fletcher O.J. Necrotic enteritis in cage-reared commercial layer pullets. Avian Diseases. 1986;30:617–619. doi: 10.2307/1590433. [DOI] [PubMed] [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poultry Science. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Chalmers G., Bruce H.L., Hunter D.B., Parreira V.R., Kulkarni R.R., Jiang Y.F., Prescott J.F., Boerlin P. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. Journal of Clinical Microbiology. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Veterinary Microbiology. 2009;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Cooper K.K., Trinh H.T., Songer J.G. Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens . Veterinary Microbiology. 2009;133:92–97. doi: 10.1016/j.vetmic.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Animal Feed Science and Technology. 2006;129:60–88. doi: 10.1016/j.anifeedsci.2005.12.003. [DOI] [Google Scholar]
- Elwinger K., Schneitz C., Berndtson E., Fossum O., Teglof B., Engstom B. Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks. Acta Veterinaria Scandinavica. 1992;33:369–378. doi: 10.1186/BF03547304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes da Costa S.P., Mot D., Bokori-Brown M., Savva C.G., Basak A.K., Van Immerseel F., Titball R.W. Protection against avian necrotic enteritis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine. 2013;31:4003–4008. doi: 10.1016/j.vaccine.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J. Biological safety concepts of genetically modified live bacterial vaccines. Vaccine. 2007;25:5598–5605. doi: 10.1016/j.vaccine.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi A.R., Timbermont L., Lanckriet A., Van den Broeck W., Pedersen K., Dewulf J., Pasmans F., Haesebrouck F., Ducatelle R., Van Immerseel F. Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathology. 2007;36:375–382. doi: 10.1080/03079450701589118. [DOI] [PubMed] [Google Scholar]
- Heier B.T., Lovland A., Soleim K.B., Kaldhusdal M., Jarp J. A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks. Avian Diseases. 2001;45:724–732. doi: 10.2307/1592919. [DOI] [PubMed] [Google Scholar]
- Helmboldt C.F., Bryant E.S. The pathology of necrotic enteritis in domestic fowl. Avian Diseases, 1971;15:775–780. doi: 10.2307/1588866. [DOI] [PubMed] [Google Scholar]
- Jang S.I., Lillehoj H.S., Lee S.H., Lee K.W., Lillehoj E.P., Hong Y.H., An D.J., Jeong W., Chun J.E., Bertrand F., Dupuis L., Deville S., Arous J.B. Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine. 2012;30:5401–5406. doi: 10.1016/j.vaccine.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Kulkarni R.R., Parreira V.R., Prescott J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins. Avian Diseases. 2009;53:409–415. doi: 10.1637/8656-021109-Reg.1. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poultry Science. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Schneitz C., Hofshagen M., Skjerv E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Diseases. 2001;45:149–156. doi: 10.2307/1593022. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens . PLoS Pathogens. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Portela R.W., Sproat K., Ford M.E., Bannam T.L., Yan X., Rood J.I., Moore R.J. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Veterinary Research. 2013;44:54. doi: 10.1186/1297-9716-44-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Moore R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infection and Immunity. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R.R., Parreira V.R., Jiang Y.F., Prescott J.F. A live oral recombinant Salmonella enterica serovar Typhimurium vaccine expressing Clostridium perfringens antigens confers protection against necrotic enteritis in broiler chickens. Clinical and Vaccine Immunology. 2010;17:205–214. doi: 10.1128/CVI.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R.R., Parreira V.R., Sharif S., Prescott J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clinical and Vaccine Immunology. 2007;14:1070–1077. doi: 10.1128/CVI.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R.R., Parreira V.R., Sharif S., Prescott J.F. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine. 2008;26:4194–4203. doi: 10.1016/j.vaccine.2008.05.079. [DOI] [PubMed] [Google Scholar]
- Lanckriet A., Timbermont L., Eeckhaut V., Haesebrouck F., Ducatelle R., Van Immerseel F. Variable protection after vaccination of broiler chickens against necrotic enteritis using supernatants of different Clostridium perfringens strains. Vaccine. 2010;28:5920–5923. doi: 10.1016/j.vaccine.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Martin T.G., Smyth J.A. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Veterinary Microbiology. 2009;136:202–205. doi: 10.1016/j.vetmic.2008.10.026. [DOI] [PubMed] [Google Scholar]
- McDevitt R.M., Brooker J.D., Acamovic T., Sparks N.H.C. Necrotic enteritis; a continuing challenge for the poultry industry. World's Poultry Science Journal. 2006;62:221–247. doi: 10.1079/WPS200593. [DOI] [Google Scholar]
- Mot D., Timbermont L., Delezie E., Haesebrouck F., Ducatelle R., Van Immerseel F. Day-of-hatch vaccination is not protective against necrotic enteritis in broiler chickens. Avian Pathology. 2012;42:179–184. doi: 10.1080/03079457.2013.778955. [DOI] [PubMed] [Google Scholar]
- Palliyeguru M.W., Rose S.P., Mackenzie A.M. Effect of dietary protein concentrates on the incidence of subclinical necrotic enteritis and growth performance of broiler chickens. Poultry Science. 2010;89:34–43. doi: 10.3382/ps.2009-00105. [DOI] [PubMed] [Google Scholar]
- Parish W.E. Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii . Journal of Comparative Pathology. 1961;71:377–393. doi: 10.1016/S0368-1742(61)80043-X. [DOI] [PubMed] [Google Scholar]
- Park S.S., Lillehoj H.S., Allen P.C., Park D.W., FitzCoy S., Bautista D.A., Lillehoje E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Diseases. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- Riddell C., Kong X.M. The influence of diet on necrotic enteritis in broiler chickens. Avian Diseases. 1992;36:499–503. doi: 10.2307/1591740. [DOI] [PubMed] [Google Scholar]
- Saleh N., Fathalla S.I., Nabil R., Mosaad A.A. Clinicopathological and immunological studies on toxoids vaccine as a successful alternative in controlling clostridial infection in broilers. Anaerobe. 2011;17:426–430. doi: 10.1016/j.anaerobe.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Savva C.G., Fernandes da Costa S.P., Bokori-Brown M., Naylor C.E., Cole A.R., Moss D.S., Titball R.W., Basak A.K. Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens . The Journal of Biological Chemistry. 2013;288:3512–3522. doi: 10.1074/jbc.M112.430223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Veterinary Research. 2012;43:74–86. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball R.W., Fearn A.M., Williamson E.D. Biochemical and immunological properties of the C-terminal domain of the alpha-toxin of Clostridium perfringens . FEMS Microbiology Letters. 1993;110:45–50. doi: 10.1111/j.1574-6968.1993.tb06293.x. [DOI] [PubMed] [Google Scholar]
- Wijewanta E.A., Senevirtna P. Bacteriological studies of fatal Clostridium perfringens type-A infection in chickens. Avian Diseases. 1971;15:654–561. doi: 10.2307/1588852. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathology. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Williams R.B., Marshall R.N., La Ragione R.M., Catchpole J. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitology Research. 2003;90:19–26. doi: 10.1007/s00436-002-0803-4. [DOI] [PubMed] [Google Scholar]
- Williamson E.D., Titball R.W. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas-gangrene. Vaccine. 1993;11:1253–1258. doi: 10.1016/0264-410X(93)90051-X. [DOI] [PubMed] [Google Scholar]
- Zekarias B., Mo H., Curtiss R. Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clinical and Vaccine Immunology. 2008;15:805–816. doi: 10.1128/CVI.00457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


