Abstract
15B3 is a monoclonal IgM antibody that selectively detects pathological aggregates of the prion protein (PrP). We report the unexpected finding that 15B3 also recognizes oligomeric but not monomeric forms of amyloid-β (Aβ)42, an aggregating peptide implicated in the pathogenesis of Alzheimer’s disease (AD). The 15B3 antibody: i) inhibits the binding of synthetic Aβ42 oligomers to recombinant PrP and neuronal membranes; ii) prevents oligomer-induced membrane depolarization; iii) antagonizes the inhibitory effects of oligomers on the physiological pharyngeal contractions of the nematode Caenorhabditis elegans; and iv) counteracts the memory deficits induced by intracerebroventricular injection of Aβ42 oligomers in mice. Thus this antibody binds to pathologically relevant forms of Aβ, and offers a potential research, diagnostic, and therapeutic tool for AD.
Keywords: 15B3 antibody, Alzheimer’s disease, amyloid beta-protein (1– 42), oligomers, prion protein, prions, oligomers
INTRODUCTION
The aberrant aggregation of amyloid-β (Aβ), eventually leading to deposition of amyloid plaques in the brain, is a major hallmark of Alzheimer’s disease (AD). The main components of Aβ plaques are Aβ40 and Aβ42 peptides, in the form of ordered structures with fibrillar morphology and high β-sheet content. Aggregation, ultimately leading to insoluble Aβ fibrils, involves the formation of different intermediate structures, including soluble oligomers and protofibrils, which are thought to play key pathogenic roles in AD. Oligomers, in particular, disrupt brain synaptic plasticity at relatively low concentrations [1–5].
One of the main challenges in AD research is the development of tools to distinguish different aggregated forms of Aβ [6]. We recently developed a surface plasmon resonance (SPR)-based immunoassay that detects transient oligomeric species generated during the incubation of synthetic Aβ42 [7]. This assay is based on the ability of the anti-Aβ antibody 4G8, immobilized on the sensor chip, to bind oligomers in a pseudo-irreversible manner (i.e., with very slow dissociation), while monomers dissociate much faster. Using this assay, we serendipitously found that 15B3, a monoclonal antibody that recognizes misfolded/aggregated forms of the prion protein (PrP) implicated in prion diseases, could also bind oligomeric Aβ42.
15B3 is a mouse monoclonal IgM antibody obtained by immunizing PrP-null mice with full-length recombinant bovine PrP. The antibody was found to selectively recognize PrPSc, the infectious (prion) isoform of PrP [8], as well as non-infectious pathological aggregates of PrP [9–12]. Because of these properties, 15B3 is currently used to boost the sensitivity of a real-time quaking-induced conversion (RT-QuIC) assay for the detection of prions in biological samples [13]. Given the role of Aβ oligomers in AD pathogenesis and the need for reagents that target these species for research, diagnostic, and therapeutic purposes, we thoroughly investigated the Aβ binding properties of 15B3, and its ability to interfere with the toxic effects of Aβ42 oligomers in animal models.
MATERIALS AND METHODS
Materials
15B3 was obtained from Prionics (now Thermo Fisher Scientific, Rockford, IL USA). Three batches of 15B3 were used: most of the studies were carried out with batch # 071114A, with a nominal concentration of 5 mg/mL; when indicated, batches # 110531 (0.9 mg/mL) and # 061013 (0.8 mg/mL) were also used.
Control mouse IgM was from Thermo Fisher Scientific, Rockford, IL, USA; anti-Aβ monoclonal antibody 4G8 was from Covance, Princeton, NJ, USA; anti-Aβ oligomer polyclonal antibodies OC and A11 were from Merck Millipore, Darmstadt, Germany; mouse monoclonal anti-Aβ 6E10 was from Covance, Emerville, CA, USA; rabbit anti-β-tubulin was from Sigma, St. Louis, MO, USA and guinea pig anti-Bassoon was from Synaptic System, Gottingen, Germany.
Aβ42 preparation
Depsi-Aβ42 was synthesized in-house, as previously described [14]. In comparison with the highly aggregating Aβ42, the more soluble depsi form has a much lower propensity for spontaneous aggregation [14, 15] preventing the formation of seeds in solution. Aβ42 was then obtained from the depsi-peptide by a “switching” procedure involving a change in pH [16]. The solution was diluted in 10 mM PBS, pH 7.4, to a final Aβ42 concentration of 100 μM. This procedure allows the preparation of reproducible, seed-free solutions of monomeric Aβ42, as shown by circular dichroism, size exclusion chromatography, and SPR [7, 14]. To obtain aggregated Aβ42, the solution was incubated at 25°C in quiescent conditions for different times. We used freshly prepared solutions (t = 0) to have Aβ42 monomers only, or Aβ42 solutions incubated for 5 h (t = 5 h) for maximal oligomer enrichment [7].
SPR studies
The SPR apparatus we employed (ProteOn XPR36 Protein Interaction Array System; Bio-Rad) has six parallel flow-channels that can be used to uniformly immobilize six different ligands on the sensor surface. The fluidic system can automatically rotate 90° so up to six different analytes can be injected, allowing simultaneous monitoring of up to 36 individual molecular interactions in a single run on a single chip [17]. 15B3 was immobilized in a flow channel of GLC sensor chips (Bio-Rad) using amine coupling chemistry, as described [7]. Briefly, after surface activation, the antibody (30 μg/mL in 10 mM acetate buffer, pH 5.0) was injected for 5 min at a flow rate of 30 μL/min, and the remaining activated groups were blocked with ethanolamine, pH 8.0. The final immobilization level was about 7000 resonance units (1 RU = 1 pg of protein/mm2). A “reference” surface was prepared in parallel using the same immobilization procedure without adding the antibody. Recombinant mouse PrP, prepared as previously described [18, 19], was also immobilized by amine coupling chemistry, as previously described [20] (immobilization 3000 RU). After rotation of the microfluidic system, Aβ42 was injected for 2 min at a flow rate of 30 μL/min. Dissociation was measured in the next 11 min. The running buffer, also used to dilute the samples, was 10 mM PBS containing 0.005% Tween 20 (PBST). Assays were run at 25°C. The sensorgrams (time course of the SPR signal in RU) were normalized to a baseline of 0. The signal in the surfaces immobilizing the antibody was corrected by subtracting the nonspecific response observed in the reference surface.
Enzyme-linked immunosorbent assay (ELISA)
Synthetic Aβ42 was dissolved in PBS to a final concentration of 100 μM and incubated at 25°C in quiescent conditions. Aliquots were taken at different incubation times (from 0 to 72 h), diluted to 1 μM in PBS, loaded in triplicate on a 15B3-precoated 96-well microplate (Prionics, now Thermo Fisher Scientific, Rockford, IL, USA) and incubated 1 h at room temperature under shaking. The plate was washed four times with PBS containing 0.005% Tween-20 (PBST) and Aβ42 oligomers were detected with HRP-labeled 4G8 (Covance), 1 : 50 000 in PBST, incubated for 1 h at room temperature, protected from light. After four washes with PBST, Luminata Forte Western HRP Substrate (Millipore) was added and luminescence was measured with a F500 Infinite plate reader (Tecan Italia Srl, Italy).
Kinetics of fibril formation
The kinetics of Aβ42 aggregation was monitored using an in situ Thioflavin-T (ThT) fluorescence assay based on the increase of the fluorescence signal of ThT when bound to β-sheet-rich structures. Aβ42, 4 μM, was incubated, with and without 15B3 or control IgM, under quiescent conditions at 37°C in microplate wells (Microplate Corning 3881, 96-well, low-binding, Corning Incorporated Life Sciences, Acton, MA) in the presence of 20 μM ThT (100 μL solution per well). ThT fluorescence was measured every 2.5 min using an F500 Infinity plate reader (Tecan Italia Srl, Italy). The dye was excited at 448 nm (bandwidth 7 nm) and the emission measured at 485 nm (bandwidth 20 nm).
Aβ42 binding to neurons
Primary hippocampal neurons were established from Sprague Dawley E18 fetal rats (Charles River Italia). The experimental procedures followed the guidelines established by European (Directive 2010/63/EU) and Italian legislation (L.D. no. 26/2014). They were reviewed and approved by the Animal Welfare Committee of the University of Milan and by the Italian Ministry of Health. Briefly, dissociated cells were plated onto poly-L-lysine-treated coverslips at 520 cells/mm2 density and maintained in Neurobasal medium with 2% B27 supplement and 2 mM glutamine (neuronal medium). 12–15 DIV hippocampal neurons were exposed to 1 μM Aβ42 monomers or Aβ42 oligomers for 1 h in neuronal medium. In one set of experiments, Aβ42 oligomers were preincubated with 10 nM 15B3 or control IgM for 30 min before being administered to neurons. Neurons were then washed, fixed with 4% paraformaldehyde, and immunostained using the following antibodies: mouse monoclonal anti-Aβ 6E10, rabbit anti-β-tubulin and guinea pig anti-Bassoon. Aβ binding to neurons was quantified using the Image J 1.46r software by a modification of the method previously described [21]. Briefly, Aβ42 and β-tubulin double-positive puncta were revealed by generating an Aβ42/β-tubulin double-positive image, using the ‘and’ option of ‘image calculator’. A fixed threshold was set in the double-positive image, and the total co-localizing area was quantified using the ‘analyze particle’ function and normalized to total β-tubulin area in each field.
Electrophysiology
5000 HEK293T cells were seeded on 35-mm Petri dishes and cultured for two days in DMEM with 4.5 g/L glucose, without L-glutamine (VWR International PBI S.r.l., Milan, Italy), 10% fetal bovine serum, 1% penicillin-streptomycin 100X (Life Technologies, Milan, Italy), 1% UltraGlutamine1 (Lonza Group Ltd, Basel, Switzerland). HEK293T cells were perfused with solutions containing 10 μM Aβ42 monomers, Aβ42 oligomers or Aβ42 oligomers pre-incubated for 30 min with 10 nM 15B3. Control cells were treated with the vehicle.
Membrane potential was monitored in single cells using the patch-clamp technique in configuration perforated-patch, current-clamp mode. In brief, patch-clamp pipettes (Garner Glass 7052) were made using a P97 Sutter Instruments puller (Novato, CA) and fire-polished to a tip diameter of 1–1.5 μm and 5–7 MΩ resistance. The Axopatch 200 B amplifier and pClamp 9 acquisition software and Clampfit 9 (both from Molecular Device, Novato, CA) were used to record and analyze cell membrane voltages. Experimental traces were digitized at 5 kHz and filtered at 1000 Hz. The bath solution contained (in mM) 136.5 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 5.5 glucose, 5.5 HEPES, pH 7.4. The perforated-patch clamp configuration was achieved by adding to the pipette solution (in mM) 135 KCl, 10 NaCl, 1 MgCl2, 10 HEPES pH 7.2, and the antibiotic gramicidin (Sigma Aldrich) diluted to a final concentration of 7.5 μg/mL. This solution was used to fill the patch pipette allowing the pores in the membrane to open, to obtain electrical access to the cell after about 5–10 min. With this technique we can monitor the cell membrane potential for more than 30 min since cytoplasm dialysis is hampered by the sieve formed by the antibiotic apertures through the membrane which is permeable only to monovalent cations.
C. elegans experiments
Bristol N2 strain, from the Caenorhabditis elegans Genetic Center (CGC; University of Minnesota), was propagated at 20°C on solid nematode growth medium (NGM) seeded with OP50 Escherichia coli (from CGC) for food. To prepare age-synchronized animals, nematodes were transferred to fresh NGM plates on reaching maturity at three days of age and allowed to lay eggs overnight. Isolated hatchlings from the synchronized eggs (day 1) were cultured on fresh NGM plates at 20°C. For pumping-rate assays, nematodes (L3-L4 larval stage) were collected with M9 buffer, centrifuged, and washed twice with 5 mM PBS, pH 7.4 to eliminate bacteria. The worms were incubated with Aβ42 without E. coli to avoid interference and bacteria-mediated peptide degradation. Worms (100 worms/100 μL) were incubated with 10 μM oligomeric Aβ42 in 10 mM PBS (pH 7.4) alone or with the 15B3 antibody or control IgM. After 2 h, worms were transferred onto NGM plates seeded with OP50 E. coli. The pharyngeal pumping rate was scored 2 h later by counting the number of times the terminal bulb of the pharynx contracted in a 1-min interval (pumps/min).
Mouse studies
C57BL/6 mice were obtained from Charles River Laboratories (Calco, Italy). Procedures involving animals and their care were conducted in conformity with the institutional guidelines at the IRCCS – Mario Negri Institute for Pharmacological Research in compliance with national (Decreto Legislativo 4 marzo 2014, n.26) and international laws and policies (EEC Council Directive 2010/63/UE; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council (Eighth Edition) 2011). They were reviewed and approved by the Mario Negri Institute Animal Care and Use Committee which includes ad hoc members for ethical issues, and by the Italian Ministry of Health. Animal facilities meet international standards and are regularly checked by a certified veterinarian who is responsible for health monitoring, animal welfare supervision, experimental protocols, and review of procedures.
Intracerebroventricular (i.c.v.) cannulation was done as described [5]. Briefly, 10-week-old mice were anesthetized with Forane (Abbott Laboratories) and mounted on a stereotaxic apparatus (model 900, David Kopf Instruments, Tujunga, CA). A 7-mm-long guide cannula was implanted into the cerebral lateral ventricle (lateral ± 1.0 and dorsal-ventral –3.0 from the dura with an incisor bar at 0°) and secured to the skull with two stainless steel screws and dental cement. Mice were allowed 10–15 days to recover from surgery before the experiment.
Aβ42 oligomers were prepared as described above, and diluted to a final concentration of 1 μM before i.c.v. microinfusion (7.5 μL/mouse). To test the effect of 15B3, mice were treated 5 min before the injection of Aβ42 oligomers with 0.25 μg of antibody in 2 μL of PBS. The novel object recognition test (NORT) was run as described [5]. Briefly, mice are trained in an arena containing two objects that they can explore freely (familiarization phase). Twenty-four hours later, the mice are exposed to one familiar and one new object (test phase). Memory was expressed as a discrimination index, i.e., the time spent exploring the novel object minus the time spent exploring the familiar object, divided by the total time spent exploring both objects; the higher the discrimination index, the better the performance.
RESULTS
15B3 recognizes oligomeric but not monomeric forms of synthetic Aβ42
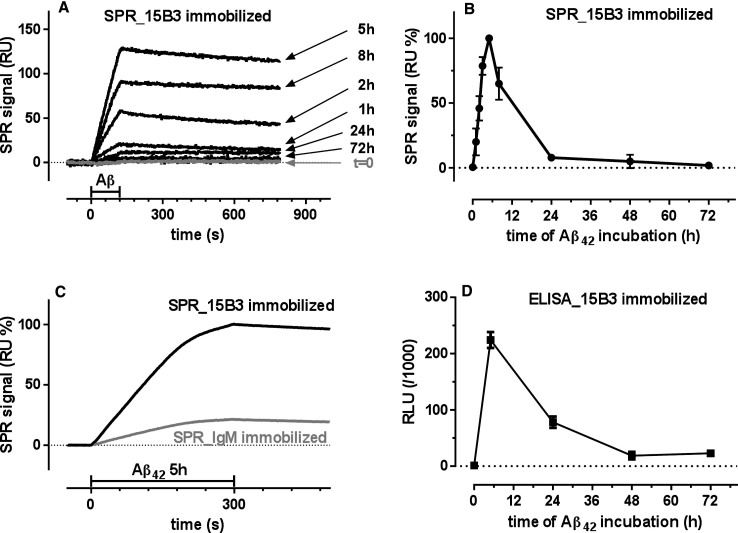
Aβ42 was dissolved in PBS to a final concentration of 100 μM and incubated at 25°C in quiescent conditions. Aliquots were then taken at different times (from 0 to 72 h), diluted to 1 μM in PBS and flowed over SPR sensor chips on which the 15B3 antibody had been immobilized. Figure 1A shows that the binding signal on 15B3 was markedly affected by the length of incubation of Aβ42. No binding signal was observed on injecting the freshly prepared solution (t = 0), whereas a marked increase was seen with solutions analyzed after 1, 2, and 5 h; with longer incubation (8, 24, and 72 h), the signal progressively declined (Fig. 1A,B). Injection of the 5-h Aβ42 solution over immobilized control IgM resulted in a binding signal about 80% lower than that on immobilized 15B3 (Fig. 1C), supporting the specificity of the interaction. Results were confirmed by sandwich ELISA: 96-well microplates pre-coated with 15B3 were incubated with aliquots of Aβ42 solution taken at 0–72 h. After washing, 15B3-captured Aβ42 was detected using HRP-conjugated 4G8 antibody and chemiluminescence. The time-course of binding was similar to that obtained by SPR, with the highest signal at 5 h (Fig 1D).
Fig.1.
Surface plasmon resonance (SPR) and ELISA studies. Synthetic Aβ42 (100 μM) was incubated at 25°C, and samples were taken at different times (from 0 to 72 h), diluted to 1 μM in 10 mM PBS, pH 7.4, and flowed (SPR) or incubated (ELISA) on immobilized 15B3. A) Sensorgrams (time course of the SPR signal expressed in resonance units, RU) obtained from a representative experiment in which Aβ42 solutions were flowed for 2 min (bar), followed by 11 min of dissociation. B) Effect of the length of incubation of Aβ42 on its binding to 15B3 immobilized on the SPR sensor chip. Each value is the mean ± SD of three different experiments. C) Sensorgrams obtained flowing the 5-h Aβ42 solution over parallel sensor surfaces on which 15B3 or control IgM had been previously immobilized (immobilization levels respectively 7040 and 7050 RU); this experiment was replicated twice with identical results. D) Effect of the length of incubation of Aβ42 on its binding to 15B3 immobilized on ELISA plates. Mean ± SD of three replicates.
We previously demonstrated [7] that freshly prepared Aβ42 solution (t = 0) contained only monomers, whereas the solution incubated for 5 h contained a heterogeneous population of SDS-labile aggregates, including globular species and short protofibrils, with a main hydrodynamic diameter of 10–30 nm. The solution incubated for 24 h mainly contained larger, SDS-stable species [7]. Thus, 15B3 selectively recognized a specific population of soluble Aβ42 oligomers, but not Aβ42 monomers or higher-order aggregates.
The pseudo-irreversible binding observed around t = 5 h suggests multivalent interactions between different epitopes on a single oligomeric assembly and several immobilized 15B3 molecules. The affinity of the oligomers for 15B3 could not be determined because of the lack of information about their actual concentration, due to uncertainties about their precise molecular mass. However, based on previous analysis by size exclusion chromatography we can assume that after 5 h of incubation 40% of the monomers have assembled into oligomers with a mass range from 90 to 400 kDa [7, 22]; it follows that the concentration of oligomers is in the low nanomolar range (4–20 nM) and their affinity for 15B3 is exceptionally high (KD < 1 nM).
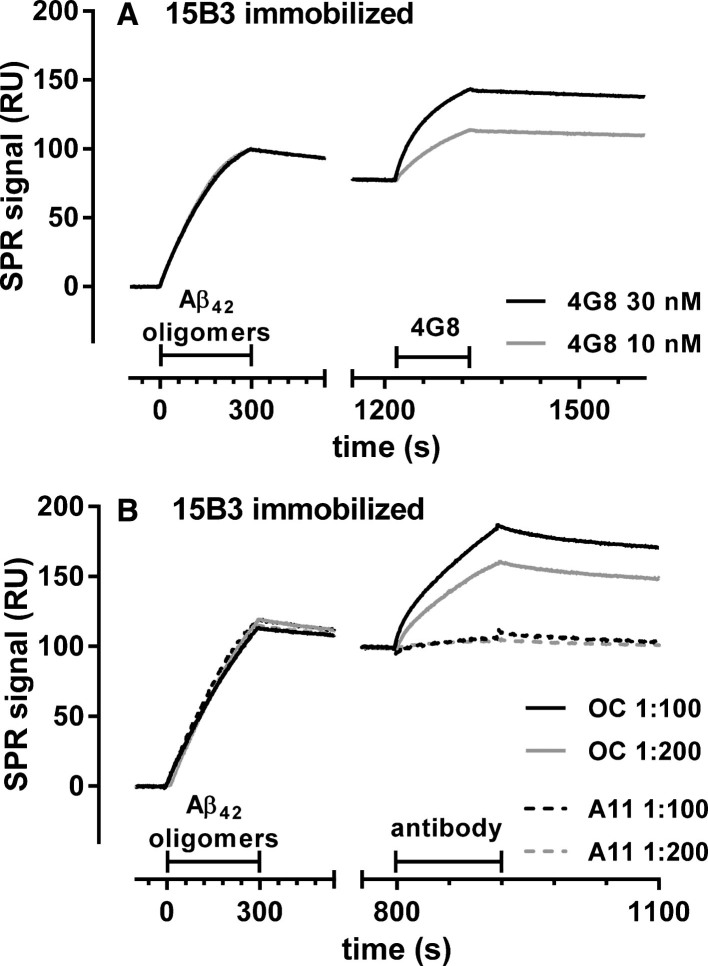
The oligomeric population selectively recognized by 15B3 was also recognized by the anti-Aβ antibody 4G8, and by OC, a conformation-specific antibody believed to selectively target fibrillar oligomers [23], as shown by an SPR assay in which 4G8 or OC were flowed onto 15B3-captured oligomers (Fig. 2). This oligomeric population was not recognized by the other widely-used anti-oligomer antibody A11, neither in this SPR format (Fig. 2), nor when the Aβ42 solution was flowed onto A11 immobilized on the sensor chip (data not shown). Dot-blot analysis confirmed the presence of species recognized by OC but not A11 in the 5-h Aβ42 solution (data not shown).
Fig.2.
SPR studies showing binding of 4G8 and OC, but not A11, to 15B3-captured Aβ42 oligomers—Synthetic Aβ42 (100 μM) was incubated at 25°C, sampled after 5 h, diluted to 1 μM in 10 mM PBS, pH 7.4, and injected over immobilized 15B3 (batch # 071114A) for 5 min, followed by injection of two different concentrations of 4G8 (A), OC or A11 (B) antibodies for 2 min (bars). The binding of 4G8 to captured oligomers (A) confirms the data shown in Fig. 1D. The experiment shown in B was replicated three times with very similar results.
15B3 inhibits Aβ42 fibrillogenesis
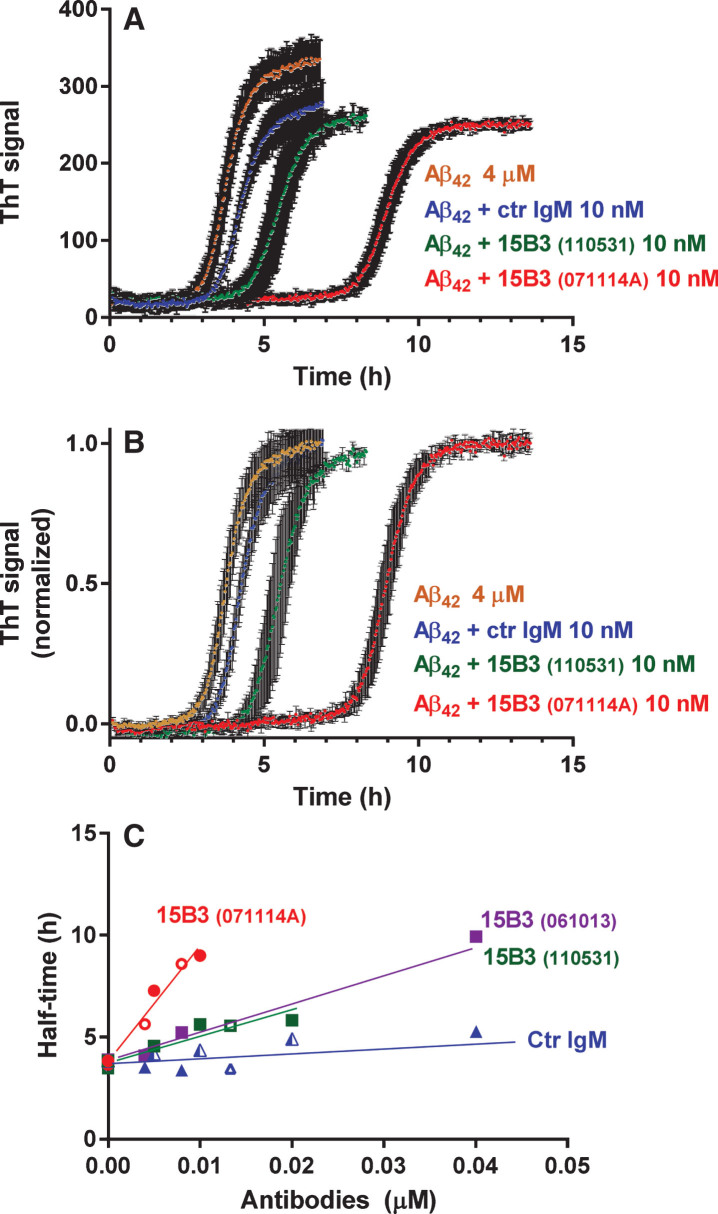
Next we tested whether 15B3 binding to the soluble Aβ42 assemblies formed early in the aggregation process affected subsequent fibril formation. A freshly prepared 4- μM solution of Aβ42 was incubated with 20 μM thioflavinT (ThT) with or without 15B3 or control IgM, and the ThT fluorescence was monitored for 24 h [14]. Aβ42 fibrillogenesis had a lag phase of 2 h, followed by very rapid growth, reaching a plateau after 5 h (Fig. 3A and B). Control IgM, up to 40 nM, did not significantly affect this process, whereas 15B3 shifted the curves in a dose-dependent manner, increasing the half-time of transition (Fig. 3C). There was some batch-to batch variability in the effects of 15B3, with batch 071114A about four times more potent than batches 061013 and 110531 (Fig. 3B). This might reflect subtle structural differences between antibodies of different batches, perhaps due to variable expression conditions, which might affect glycosylation and/or the intrinsic tendency of IgM to aggregate.
Fig.3.
Effect of 15B3 on Aβ42 fibrillogenesis, evaluated by ThT fluorescence—Synthetic Aβ42 (4 μM) was incubated with ThT (20 μM) with or without 15B3 or control IgM, and ThT fluorescence was monitored every 2.5 min. Three batches of 15B3 were used for these studies, as indicated. A) Representative raw fluorescent values. B) Normalization of the data in A on the corresponding maximal values to illustrate better the shift in the half-time of transition, i.e., the time corresponding to half the maximum ThT signal. C) Half-time of transition of Aβ42 in the presence of different concentrations of the antibodies. Antibodies are identified by the colors; open or solid symbols indicate results of independent experiments.
These data suggest that 15B3 binds to oligomeric intermediates on the pathway of Aβ42 fibrillation. A similar effect on Aβ42 fibrillogenesis was observed with N1, a physiological N-terminal cleavagefragment of PrP, which also selectively interacts with Aβ42 oligomers [24].
15B3 inhibits the binding of Aβ42 oligomers to recombinant PrP and to rat hippocampal neurons, and prevents oligomer-induced membrane depolarization
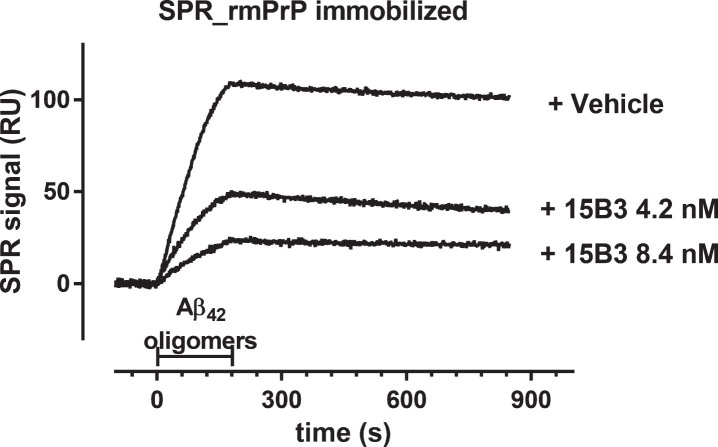
As a first step to assess the biological importance of the 15B3-Aβ42 interaction, we investigated the antibody’s ability to counteract the binding of Aβ42 oligomers to recombinant mouse PrP (rmPrP), since cellular PrP (PrPC) may mediate oligomer-induced neurotoxic signaling [25]. Aβ42 oligomers bound rmPrP immobilized on the sensor chip with high affinity, confirming previous results [5, 20] (Fig. 4). Preincubation of oligomers with 15B3 for 30 min resulted in dose-dependent reduction of binding (Fig. 4). 15B3 did not bind to immobilized rmPrP (data not shown).
Fig.4.
Effect of 15B3 on the binding of Aβ42 oligomers to recombinant mouse PrP (rmPrP) immobilized on the sensor chip—Synthetic Aβ42 (100 μM) was incubated at 25°C, sampled after 5 h, diluted to 1 μM in 10 mM PBS, pH 7.4, and incubated for another 30 min with or without 15B3 (4.2 and 8.4 nM, lot# 061013). Aliquots were then injected for 3 min (bar) over immobilized rmPrP. The figure shows the sensorgrams (time course of the SPR signal expressed in resonance units, RU) from a representative experiment. This study was replicated twice with very similar results.
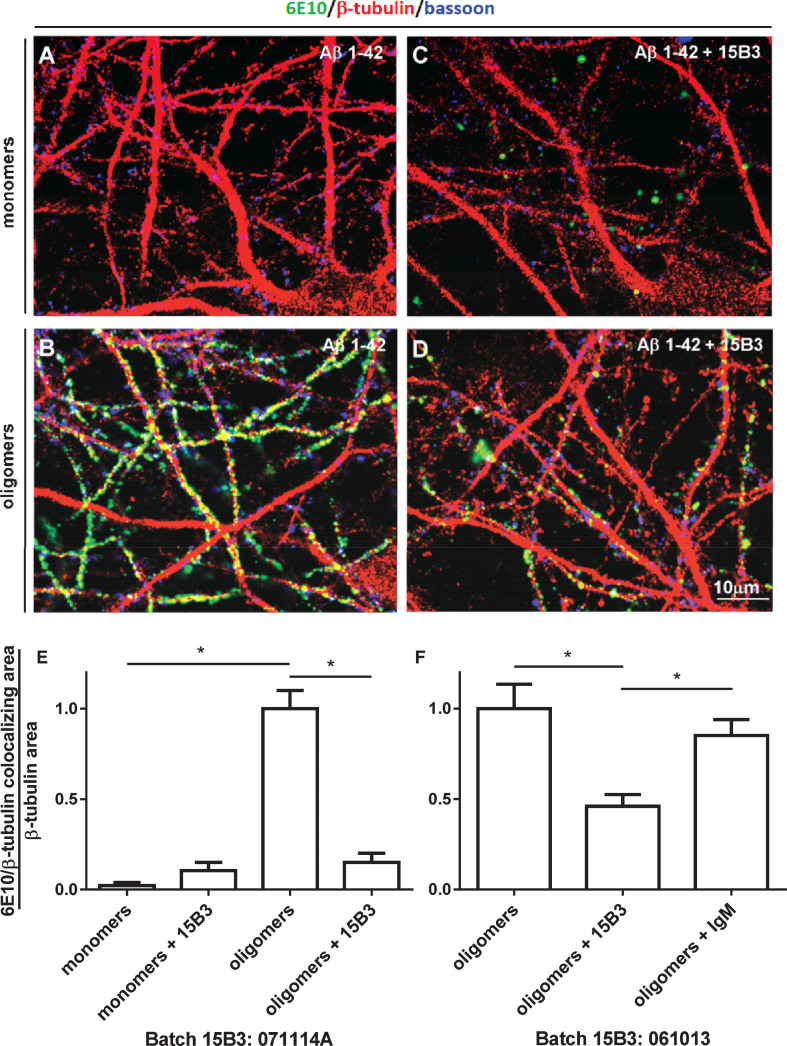
Next, we analyzed the antibody’s ability to counteract the binding of Aβ42 oligomers to rat hippocampal neurons [21]. Incubating neurons with monomeric Aβ42 did not result in Aβ binding to the neuronal surface, as shown by the lack of staining with the anti-Aβ antibody 6E10 (Fig. 5A). In contrast, a strong immunopositive signal was seen on the surface of neurons incubated with the Aβ oligomer-containing solution (Fig. 5B). Preincubation of oligomers with 15B3 significantly reduced binding. This was clearly seen with batch 071114A (Fig. 5E); less so with batch 061013 (Fig. 5F), consistent with their different ability to inhibit Aβ42 fibrillogenesis (Fig. 3). Control IgM (10 nM) had no effect on binding (Fig. 5F), and there was no 6E10-positive signal when neurons were incubated with 15B3 alone (data not shown). Experiments in which 15B3 was used to immunostain neurons that had been previously incubated with synthetic Aβ42 oligomers were unrewarding. In fact, 15B3 could not even immunodetect pathological PrP in brain sections from prion diseased patients and mice ([10] and unpublished results), indicating that this antibody is not suitable for immunostaining.
Fig.5.
Effect of 15B3 on the binding of Aβ42 oligomers to rat hippocampal neurons—A–D) Representative images obtained exposing 12–15 DIV hippocampal neurons for 1 h to solutions containing (A) Aβ42 monomers or (B) Aβ42 oligomers. The final concentration of Aβ42 was 1 μM in both cases. C, D) Neurons exposed to 1 μM Aβ42 monomers or oligomers pre-incubated for 30 min with 15B3 (batch # 071114A, 10 nM). Neurons were washed, fixed with 4% paraformaldehyde and stained using the following antibodies: mouse anti-Aβ, 6E10 (green), rabbit anti-β tubulin (red) and guinea pig anti-Bassoon (blue). E) Corresponding quantification of 6E10 binding to cultured neurons expressed as colocalizing area between 6E10 and β-tubulin, relative to total β-tubulin. Mean ± SEM of 20 fields from two independent experiments, *p < 0.05 Dunn’s test after Kruskal-Wallis One Way Analysis of Variance on Ranks (this statistical analysis was used because the normality test failed). F) Quantification of a third experiment with 15B3 batch # 061013 and control IgM, both 10 nM. Mean ± SEM of 10 fields, *p < 0.05 Holm-Sidak test after One Way Analysis of Variance (used because the normality test passed).
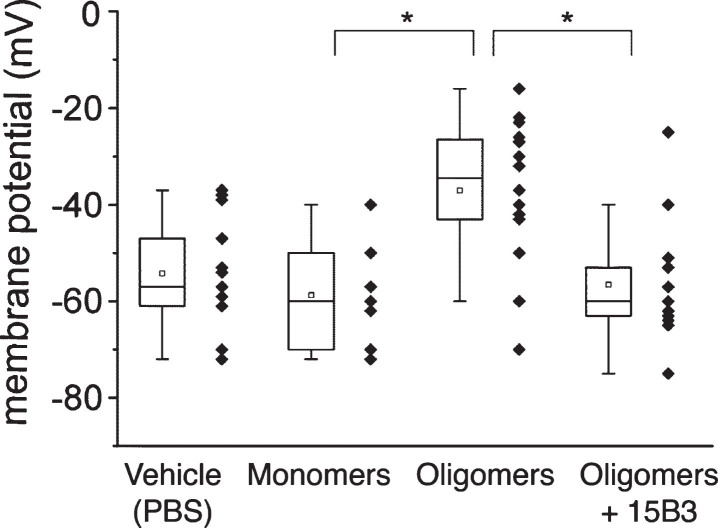
Finally, we tested whether 15B3 protected cells from the effects of Aβ42 oligomers on cell membrane potential. We used HEK293T cells, monitoring the cell membrane resting potential by a perforated-patch current-clamp technique [26]. HEK293T cells have a reduced set and a lower number of ion channels, and compensate small changes in membrane potential less efficiently than primary neurons, and are therefore more sensitive to the effects of Aβ42. Acute exposure of cells to Aβ42 oligomers, but not monomers, resulted in significant membrane depolarization, and this effect was completely abolished by 15B3 (Fig. 6).
Fig.6.
Effect of 15B3 on membrane potential depolarization caused by Aβ42 oligomers—HEK293T cells were perfused with solutions containing 10 μM Aβ42 monomers, 10 μM Aβ42 oligomers, or 10 μM Aβ42 oligomers pre-incubated for 30 min with 10 nM 15B3, batch # 071114A. Control cells were treated with the vehicle. Data are expressed as median and interquartile range, as well as single values (12, 16, 14, and 11 cells for the four groups). *p < 0.05; one-way ANOVA, Tukey’s post hoc test.
15B3 prevents the toxic effects of Aβ42 oligomers on C. elegans pharynx
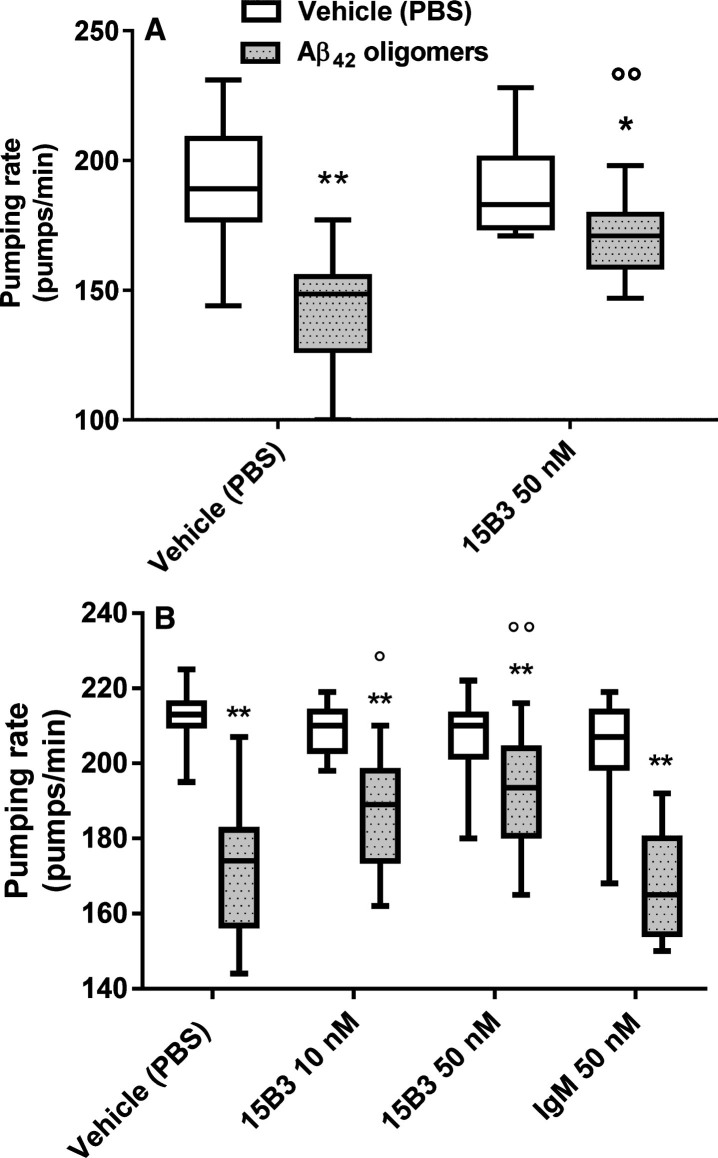
We investigated the ability of 15B3 to counteract Aβ oligomer toxicity in vivo. First we used the invertebrate nematode C. elegans, whose pharyngeal behavior is sensitive to sublethal doses of chemical stressors. We previously reported that both rhythmic contraction and relaxation of the pharyngeal muscle in C. elegans, scored as “pumping rate”, were significantly impaired by feeding the nematodes synthetic Aβ42 oligomers, but not monomers or fibrils [7]. Here we tested whether pre-incubation of 15B3 with Aβ42 oligomers prevented this effect (Fig. 7). Consistent with previous observations, Aβ42 oligomers significantly reduced the worm pumping rate, and this effect was dose-dependently antagonized by 15B3 but not by control IgM. 15B3 alone had no effect on the pumping rate (Fig. 7).
Fig.7.
Effect of 15B3 on the ability of Aβ42 oligomers to reduce the pharyngeal motility of C. elegans—Synthetic Aβ42 (100 μM) was incubated at 25°C for 5 h, diluted to 10 μM and incubated with 10 nM or 50 nM 15B3 antibody or 50 nM control IgM, or the vehicle (PBS). The solutions were incubated for another 30 min before being given to the worms. Nematodes were fed for 2 h with these solutions, then plated on Nematode Growth Medium plates seeded with OP50 E. coli. The pharyngeal pumping rate was scored 2 h after plating. Data are expressed as minimum to maximum box and whisker plots (10–20 worms/group from one or two independent experiments). Panels A and B show the results with 15B3 batches # 071114A and # 110531, respectively. **p < 0.01 effect of Aβ42 oligomers versus corresponding vehicle; °p < 0.05, °°p < 0.01 effect of 15B3 versus corresponding vehicle, Bonferroni’s test after two-way ANOVA.
15B3 prevents the memory deficits induced by Aβ42 oligomers in mice
We previously reported that synthetic Aβ42 oligomers injected i.c.v. in C57BL/6 mice caused memory impairment in the novel-object recognition task [5]. This was not seen using either Aβ monomers or fibrils, indicating that Aβ oligomers are the molecular species responsible for the amnesic effect [5, 24].
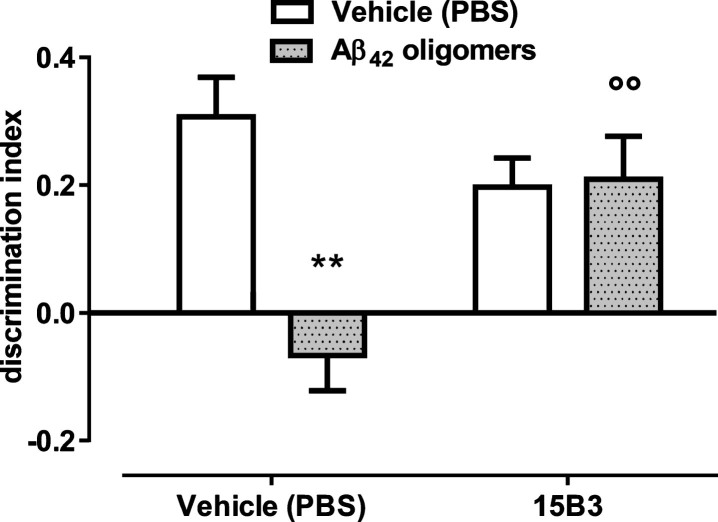
We used this acute in vivo mouse model to evaluate whether 15B3 prevented the deleterious effects of Aβ42 oligomers on memory. As expected, mice receiving 1 μM Aβ42 oligomers, i.c.v., had a significantly lower discrimination index compared to mice injected with the vehicle solution (Fig. 8). No effect of Aβ42 oligomers was seen in mice pre-treated i.c.v. with 15B3 (0.25 μg/2 μL, 5 min before oligomers) (Fig. 8).
Fig.8.
Aβ42 oligomer-induced memory impairment in mice is prevented by pretreatment with 15B3—Bars show the discrimination index, calculated as follows: (time on novel object – time on familiar object)/(total time on objects). Data are expressed as the mean ± standard error (SE) (n = 5–7). Two-way ANOVA showed a significant interaction between Aβ42 and 15B3 (p < 0.01). **p = 0.01 effect of Aβ42 oligomers versus corresponding vehicle; °°p < 0.01 effect of 15B3 versus corresponding vehicle, post hoc Bonferroni’s test. Batch # 071114A of 15B3 was used for these experiments.
DISCUSSION
In the present study we show that 15B3, an anti-prion antibody known for its ability to specifically recognize PrPSc and other pathogenic PrP aggregates [8–11], also recognizes aggregated, but not monomeric, Aβ42 . In particular, our data show that 15B3 interacts with a transient population of oligomers of synthetic Aβ42 that have substantial biological and pathogenic effects.
The soluble oligomers recognized by 15B3 form progressively during incubation of synthetic Aβ42, reaching a peak concentration after 5 h, then disappearing. Previous analysis showed that the 5-h solution contains a heterogeneous population of SDS-labile structures, including globular oligomers and short protofibrils, with a main hydrodynamic diameter of 10–30 nm [7]. These species have important biophysical and biological properties: i) they bind recombinant PrP and may therefore be responsible for the previously described PrPC-mediated neurotoxic effects [5, 20, 24, 25, 27–32]; ii) they bind to neurons and raise the cell membrane potential, a mechanism that may contribute to their toxicity [33, 34]; iii) they slow the pumping rate of the C. elegans pharynx [7, 35, 36]; and iv) they impair recognition memory when injected i.c.v. in mice [5]. All these effects are prevented by mixing the 5-h Aβ42 solution with nanomolar concentrations of 15B3. This is probably due to the antibody’s ability to directly bind a subpopulation of bioactive Aβ42 oligomers. 15B3 might promote oligomer disassembly. However, if this were the case the SPR signal indicative of their interaction should rapidly decrease, whereas we observed a very stable interaction (Fig. 1A). Alternatively, 15B3 might shield certain patches on the surface of Aβ oligomers, responsible for their interaction with PrPC and/or other components of cell membranes, and for their in vitro and in vivo toxicity. Structurally similar patches are probably also exposed by toxic PrP assemblies which interact with 15B3 [8–10, 13].
It was suggested that one of the main determinants of oligomer toxicity is surface hydrophobicity [37]. Supporting this, it was found that Aβ oligomers interact with and disrupt cellular membranes depending on the degree of solvent exposure of their central and C-terminal hydrophobic segments [38]. Our SPR data indicate that the 15B3-captured Aβ42 oligomers are recognized by 4G8, a monoclonal antibody that binds to the central hydrophobic region of Aβ, indicating that they expose hydrophobic toxic domains. However, in sharp contrast to 4G8 [7], 15B3 does not bind monomeric Aβ42.
Different antibodies have been described which target different Aβ oligomeric species [39], consistent with the highly heterogeneous nature of these assemblies [40]. These include A11 and OC, polyclonal IgGs targeting “prefibrillar” and “fibrillar” oligomers, respectively [41, 42]; NU-1, A-887755a and 11A1, monoclonal IgGs targeting Aβ-derived diffusible ligands (ADDLs) [43], globulomers [44], and “toxic Aβ42 conformers” [45], respectively; and the “oligomer-specific” IgM OMAB [46]. All these antibodies were generated using an Aβ antigen whereas15B3 was raised against a heterologous protein (PrP). Other monoclonal antibodies have been raised against PrP that can bind soluble oligomers of both PrPSc and Aβ [47]; however, the Aβ-binding properties of these antibodies were not characterized. Our SPR data indicate that the oligomeric population detected by 15B3 is also recognized by the OC, but not the A11 antibody. OC is a conformational antibody raised by immunizing rabbits with Aβ42 fibrils, but detecting different types of amyloid, including α-synuclein, islet amyloid polypeptide and polyQ fibrils, in addition to soluble oligomeric fragments of Aβ42 fibril protofilament [42, 48]. The fibrillar oligomers recognized by OC are immunologically distinct from the prefibrillar oligomers recognized by A11, even though their sizes broadly overlap [48]. Analysis in brain extracts from AD patients [49] and transgenic mice [50] showed that an increase in OC-positive oligomers correlated with cognitive decline and neuropathological changes, whereas increased levels of A11-positive oligomers did not. These data therefore suggest that the Aβ oligomers recognized by 15B3 are pathologically relevant species that expose the OC but not the A11 epitope.
The 15B3-based SPR immunoassay may represent a convenient technique for checking the formation of bioactive oligomers of synthetic Aβ42, e.g., for investigating the effect of mutations on the kinetics of Aβ oligomerization [51] or for characterizing the mechanism of action of compounds that inhibit Aβ aggregation, such as EGCG and N1 [7, 24]. The 15B3 antibody is particularly suitable for SPR-based immunoassay, since only highly purified antibodies can be reliably immobilized on sensor chips, and other commercially available anti-oligomer antibodies, such as OC antiserum, lack this requisite. The present study was carried out using synthetic preparations of Aβ42 oligomers, and further studies are underway to evaluate if 15B3 also recognizes Aβ oligomers in AD patients.
If this were the case, 15B3-based immunoassays could be used for implementing diagnostic AD tests based on detection of Aβ oligomers in biological fluids, for example to boost the sensitivity of protein misfolding cyclic amplification or QuIC assays of Aβ [52]. 15B3 may also be used for immunopurifying biologically active Aβ species, as has been done for pathogenic forms of PrP [11]. Finally, the evidence that 15B3 neutralizes Aβ42 oligomer toxicity in C. elegans and mice calls for further studies to assess its potential for the immunotherapy of AD.
ACKNOWLEDGMENTS
Bristol N2 C. elegans strain and OP50 E. Coli bacteria were provided by CGC, which is funded by NIH Office of Research Infrastructure Program (P40 OD010440).
We thank Alessandro Negro (University of Padua, Italy) for production of recombinant PrP and Pietro Lavitola for his help with treatment of the mice and behavioral studies.
This work was supported by grants from Telethon Italy (TDRC00508TU) to RC. EB was supported by a Young Investigator Award (GR-2010-2312769) from the Italian Ministry of Health. EB is an Assistant Telethon Scientist at the Dulbecco Telethon Institute (TCP14009, Fondazione Telethon, Italy).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0882r2).
REFERENCES
- [1]. Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8, 79–84. [DOI] [PubMed] [Google Scholar]
- [2]. Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95, 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Walsh DM (2002) Naturally secreted oligomers of amyloid [beta] protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539. [DOI] [PubMed] [Google Scholar]
- [4]. Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27, 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G (2010) Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A 107, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Holtzman DM, Morris JC, Goate AM (2011) Alzheimer’s disease: The challenge of the second century. Sci Transl Med 3, 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Stravalaci M, Bastone A, Beeg M, Cagnotto A, Colombo L, Di Fede G, Tagliavini F, Cantu L, Del Favero E, Mazzanti M, Chiesa R, Salmona M, Diomede L, Gobbi M (2012) Specific recognition of biologically active amyloid-beta oligomers by a new surface plasmon resonance-based immunoassay and an in vivo assay in Caenorhabditis elegans. J Biol Chem 287, 27796–27805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B (1997) Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390, 74–77. [DOI] [PubMed] [Google Scholar]
- [9]. Nazor KE, Kuhn F, Seward T, Green M, Zwald D, Purro M, Schmid J, Biffiger K, Power AM, Oesch B, Raeber AJ, Telling GC (2005) Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J 24, 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Biasini E, Seegulam ME, Patti BN, Solforosi L, Medrano AZ, Christensen HM, Senatore A, Chiesa R, Williamson RA, Harris DA (2008) Non-infectious aggregates of the prion protein react with several PrPSc-directed antibodies. J Neurochem 105, 2190–2204. [DOI] [PubMed] [Google Scholar]
- [11]. Biasini E, Tapella L, Mantovani S, Stravalaci M, Gobbi M, Harris DA, Chiesa R (2009) Immunopurification of pathological prion protein aggregates. PLoS One 4, e7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Chiesa R, Piccardo P, Biasini E, Ghetti B, Harris DA (2008) Aggregated, wild-type prion protein causes neurological dysfunction and synaptic abnormalities. J Neurosci 28, 13258–13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Orru CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, Caughey B (2011) Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio 2, e00078–00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Beeg M, Stravalaci M, Bastone A, Salmona M, Gobbi M (2011) A modified protocol to prepare seed-free starting solutions of amyloid-beta (Abeta)(1-40) and Abeta(1-42) from the corresponding depsipeptides. Anal Biochem 411, 297–299. [DOI] [PubMed] [Google Scholar]
- [15]. Sohma Y, Taniguchi A, Skwarczynski M, Yoshiya T, Fukao F, Kimura T, Hayashi Y, Kiso Y (2006) ‘O-Acyl isopeptide method’ for the efficient synthesis of difficult sequence-containing peptides: Use of ‘O-acyl isodipeptide unit’. Tetrahedron Lett 47, 3013–3017. [Google Scholar]
- [16]. Taniguchi A, Sohma Y, Hirayama Y, Mukai H, Kimura T, Hayashi Y, Matsuzaki K, Kiso Y (2009) “Click peptide”: pH-triggered production and aggregation of monomer Abeta1-42. Chembiochem 10, 710–715. [DOI] [PubMed] [Google Scholar]
- [17]. Bravman T, Bronner V, Lavie K, Notcovich A, Papalia GA, Myszka DG (2006) Exploring “one-shot” kinetics and small molecule analysis using the ProteOn XPR36 array biosensor. Anal Biochem 358, 281–288. [DOI] [PubMed] [Google Scholar]
- [18]. Zahn R, von Schroetter C, Wuthrich K (1997) Human prion proteins expressed in Escherichia coli and purified by high-affinity column refolding. FEBS Lett 417, 400–404. [DOI] [PubMed] [Google Scholar]
- [19]. Botto L, Cunati D, Coco S, Sesana S, Bulbarelli A, Biasini E, Colombo L, Negro A, Chiesa R, Masserini M, Palestini P (2014) Role of lipid rafts and GM1 in the segregation and processing of prion protein. PLoS One 9, e98344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Chen S, Yadav SP, Surewicz WK (2010) Interaction between human prion protein and amyloid-beta (Abeta) oligomers: Role OF N-terminal residues. J Biol Chem 285, 26377–26383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, Giussani P, Magnani G, Comi G, Legname G, Ghidoni R, Furlan R, Matteoli M, Verderio C (2014) Microglia convert aggregated amyloid-beta into neurotoxic forms through the shedding of microvesicles. Cell Death Differ 21, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Freir DB, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, Asante EA, Farrow MA, Sessions RB, Saibil HR, Clarke AR, Rowan MJ, Walsh DM, Collinge J (2011) Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nat Commun 2, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Glabe CG (2008) Structural classification of toxic amyloid oligomers. J Biol Chem 283, 29639–29643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Fluharty BR, Biasini E, Stravalaci M, Sclip A, Diomede L, Balducci C, La Vitola P, Messa M, Colombo L, Forloni G, Borsello T, Gobbi M, Harris DA (2013) An N-terminal fragment of the prion protein binds to amyloid-beta oligomers and inhibits their neurotoxicity in vivo. J Biol Chem 288, 7857–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM (2012) Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci 15, 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Averaimo S, Abeti R, Savalli N, Brown LJ, Curmi PM, Breit SN, Mazzanti M (2013) Point mutations in the transmembrane region of the clic1 ion channel selectively modify its biophysical properties. PLoS One 8, e74523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Beland M, Roucou X (2014) Taking advantage of physiological proteolytic processing of the prion protein for a therapeutic perspective in prion and Alzheimer diseases. Prion 8, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Dohler F, Sepulveda-Falla D, Krasemann S, Altmeppen H, Schluter H, Hildebrand D, Zerr I, Matschke J, Glatzel M (2014) High molecular mass assemblies of amyloid-beta oligomers bind prion protein in patients with Alzheimer’s disease. Brain 137, 873–886 . [DOI] [PubMed] [Google Scholar]
- [29]. Nicoll AJ, Panico S, Freir DB, Wright D, Terry C, Risse E, Herron CE, O’Malley T, Wadsworth JD, Farrow MA, Walsh DM, Saibil HR, Collinge J (2013) Amyloid-beta nanotubes are associated with prion protein-dependent synaptotoxicity. Nat Commun 4, 2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Nieznanski K, Choi JK, Chen S, Surewicz K, Surewicz WK (2012) Soluble prion protein inhibits amyloid-beta (Abeta) fibrillization and toxicity. J Biol Chem 287, 33104–33108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Muller V, Krishnan R, Vabulas RM, Kretzschmar HA, Lindquist S, Hartl FU, Multhaup G, Winklhofer KF, Tatzelt J (2011) The cellular prion protein mediates neurotoxic signalling of beta-sheet-rich conformers independent of prion replication. EMBO J 30, 2057–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457, 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Shankar GM, Walsh DM (2009) Alzheimer’s disease: Synaptic dysfunction and Abeta. Mol Neurodegener 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Blanchard BJ, Thomas VL, Ingram VM (2002) Mechanism of membrane depolarization caused by the Alzheimer Abeta1-42 peptide. Biochem Biophys Res Commun 293, 1197–1203. [DOI] [PubMed] [Google Scholar]
- [35]. Beeg M, Diomede L, Stravalaci M, Salmona M, Gobbi M (2013) Novel approaches for studying amyloidogenic peptides/proteins. Curr Opin Pharmacol 13, 797–801. [DOI] [PubMed] [Google Scholar]
- [36]. Jones D, Candido EP (1999) Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: Relationship to the cellular stress response. J Exp Zool 284, 147–157. [DOI] [PubMed] [Google Scholar]
- [37]. Mannini B, Mulvihill E, Sgromo C, Cascella R, Khodarahmi R, Ramazzotti M, Dobson CM, Cecchi C, Chiti F (2014) Toxicity of protein oligomers is rationalized by a function combining size and surface hydrophobicity. ACS Chem Biol 9, 2309–2317. [DOI] [PubMed] [Google Scholar]
- [38]. Ladiwala AR, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, Davis J, Van Nostrand WE, Tessier PM (2012) Conformational differences between two amyloid beta oligomers of similar size and dissimilar toxicity. J Biol Chem 287, 24765–24773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Murakami K (2014) Conformation-specific antibodies to target amyloid beta oligomers and their application to immunotherapy for Alzheimer’s disease. Biosci Biotechnol Biochem 78, 1293–1305. [DOI] [PubMed] [Google Scholar]
- [40]. Benilova I, Karran E, De Strooper B (2012) The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci 15, 349–357. [DOI] [PubMed] [Google Scholar]
- [41]. Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. [DOI] [PubMed] [Google Scholar]
- [42]. Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG (2007) Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL (2007) Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem 100, 23–35. [DOI] [PubMed] [Google Scholar]
- [44]. Hillen H, Barghorn S, Striebinger A, Labkovsky B, Muller R, Nimmrich V, Nolte MW, Perez-Cruz C, van der Auwera I, van Leuven F, van Gaalen M, Bespalov AY, Schoemaker H, Sullivan JP, Ebert U (2010) Generation and therapeutic efficacy of highly oligomer-specific beta-amyloid antibodies. J Neurosci 30, 10369–10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Murakami K, Horikoshi-Sakuraba Y, Murata N, Noda Y, Masuda Y, Kinoshita N, Hatsuta H, Murayama S, Shirasawa T, Shimizu T, Irie K (2010) Monoclonal antibody against the turn of the 42-residue amyloid beta-protein at positions 22 and 23. ACS Chem Neurosci 1, 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Lindhagen-Persson M, Brannstrom K, Vestling M, Steinitz M, Olofsson A (2010) Amyloid-beta oligomer specificity mediated by the IgM isotype–implications for a specific protective mechanism exerted by endogenous auto-antibodies. PLoS One 5, e13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Tayebi M, Jones DR, Taylor WA, Stileman BF, Chapman C, Zhao D, David M (2011) PrP(Sc)-specific antibodies with the ability to immunodetect prion oligomers. PLoS One 6, e19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Hatami A, Albay R 3rd, Monjazeb S, Milton S, Glabe C (2014) Monoclonal antibodies against Abeta42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain. J Biol Chem 289, 32131–32143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Tomic JL, Pensalfini A, Head E, Glabe CG (2009) Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis 35, 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Sarsoza F, Saing T, Kayed R, Dahlin R, Dick M, Broadwater-Hollifield C, Mobley S, Lott I, Doran E, Gillen D, Anderson-Bergman C, Cribbs DH, Glabe C, Head E (2009) A fibril-specific, conformation-dependent antibody recognizes a subset of Abeta plaques in Alzheimer disease, Down syndrome and Tg2576 transgenic mouse brain. Acta Neuropathol 118, 505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Diomede L, Di Fede G, Romeo M, Bagnati R, Ghidoni R, Fiordaliso F, Salio M, Rossi A, Catania M, Paterlini A, Benussi L, Bastone A, Stravalaci M, Gobbi M, Tagliavini F, Salmona M (2014) Expression of A2V-mutated Abeta in Caenorhabditis elegans results in oligomer formation and toxicity. Neurobiol Dis 62, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C (2014) Detection of misfolded Abeta oligomers for sensitive biochemical diagnosis of Alzheimer’s disease. Cell Rep 7, 261–268. [DOI] [PubMed] [Google Scholar]