Abstract
The RNA exosome is a 3′-5′ ribonuclease complex that is composed of nine core subunits and an essential catalytic subunit, Rrp44. Two distinct conformations of Rrp44 were revealed in previous structural studies, suggesting that Rrp44 may change its conformation to exert its function. In the channeling conformation (Rrp44ch) RNA accesses the active site after traversing the central channel of the RNA exosome, while in the other conformation (Rrp44da) RNA gains direct access to the active site. Here, we show that the Rrp44da-exosome is important for nuclear function of the RNA exosome. Defects caused by disrupting the direct access conformation are distinct from those caused by channel-occluding mutations, indicating specific functions for each conformation. Our genetic analyses provide in vivo evidence that the RNA exosome employs a direct-access route to recruit specific substrates, indicating that the RNA exosome uses alternative conformations to act on different RNA substrates.
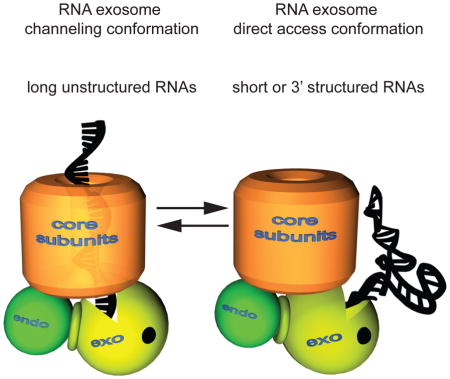
Graphical Abstract
INTRODUCTION
The RNA exosome is an essential 3′-5′ exoribonuclease complex with a wide variety of molecular functions (Chlebowski et al., 2013; Houseley and Tollervey, 2009; Januszyk and Lima, 2014; Makino et al., 2013b). In the nucleus, it processes 3′-ends of various RNA species and degrades aberrant RNAs (Porrua and Libri, 2013). In the cytoplasm, it is involved in regular mRNA turnover and mRNA surveillance (Klauer and van Hoof, 2012; Schaeffer et al., 2010). These molecular functions are best defined in yeast, but many are conserved in human cells (Staals and Pruijn, 2011). In human patients, different mutations that affect RNA exosome activity are associated with distinct human diseases: multiple myeloma (Weissbach et al., 2015), pontocerebellar hypoplasia (Boczonadi et al., 2014; Wan et al., 2012), trichohepatoenteric syndrome (Fabre et al., 2012; Hartley et al., 2010), and most recently, a distinct disorder that has not yet been named (Di Donato et al., 2016). A thorough investigation of the structure and function of the RNA exosome is essential to understand how the RNA exosome carries out all of these functions, and how RNA exosome defects cause these very diverse diseases.
The exo-9 core of the eukaryotic RNA exosome is composed of nine essential subunits and the overall architecture of this core is conserved among bacteria, archaea, and eukaryotes (Januszyk and Lima, 2010, 2014; Liu et al., 2006). The exo-9 core is formed by six RNase PH-like subunits that form a ring structure and are capped on one side by three RNA binding proteins. In the bacterial and archaeal enzymes, the catalytic active sites are located inside of the PH-ring and a single-stranded RNA is threaded into the central channel of this ring for degradation (Evguenieva-Hackenberg, 2010; Evguenieva-Hackenberg et al., 2014; Lin-Chao et al., 2007; Lorentzen et al., 2005). Unlike its bacterial and archaeal counter parts, the exo-9 core of the eukaryotic RNA exosome is catalytically inert. Instead, it interacts with two nucleases, Rrp44/Dis3 and Rrp6 (Bonneau et al., 2009; Butler and Mitchell, 2011; Dziembowski et al., 2007; Makino et al., 2013a; Malet et al., 2010; Wasmuth et al., 2014). Rrp44 is responsible for most of the 3′ to 5′ exoribonuclease activity of the RNA exosome (Dziembowski et al., 2007), while Rrp6 is restricted to the nucleus and its 3′ to 5′ exoribonuclease activity appears to be important for a subset of RNA exosome functions (Briggs et al., 1998; Butler and Mitchell, 2011). In addition to 3′-5′ exoribonuclease activity, Rrp44 has endoribonuclease activity that is mediated by a separate active site (Lebreton et al., 2008; Schaeffer et al., 2009; Schneider et al., 2009). While yeast has one RRP44/DIS3 gene, the human genome encodes three homologs Dis3, Dis3L1 and Dis3L2. Dis3 and Dis3L1 associate with the Exo-9 core in the nucleus and cytoplasm, respectively (Januszyk and Lima, 2014), and appear functionally very similar to the single yeast Rrp44 (Shiomi et al., 1998; Staals et al., 2010; Tomecki et al., 2010). In contrast, Dis3L2 is not known to be associated with the Exo-9 core, indicating that Rrp44 family members can have RNA exosome-independent functions (Chang et al., 2013; Lubas et al., 2013; Malecki et al., 2013).
The exo-9 core RNA exosome in association with Rrp44 has been studied by X-ray crystallography and electron microscopy (EM) (Bonneau et al., 2009; Liu et al., 2014; Liu et al., 2016; Makino et al., 2013a; Makino et al., 2015; Malet et al., 2010; Wang et al., 2007). These studies revealed that the ability of RNA to bind inside the exo-9 ring is conserved between eukaryotic, bacterial and archaeal enzymes. However, since there is no active site in the ring, an important difference is that in the eukaryotic enzyme the RNA substrate is thought to pass all the way through the exo-9 ring to access the Rrp44 active site. This channeling of RNA substrates in vitro requires a long (30nt) unstructured 3′ end (Bonneau et al., 2009; Liu et al., 2014; Makino et al., 2013a; Malet et al., 2010). A different conformation of Rrp44 has also been described by both X-ray crystallography and EM (Bonneau et al., 2009; Liu et al., 2014; Liu et al., 2016; Makino et al., 2013a; Makino et al., 2015). This second conformation is seen in vitro in either the absence of RNA, or with very short (<12 nts) RNAs of unclear physiological relevance, and has been suggested to be important in vivo for acting on RNAs with highly structured 3′ ends (Liu et al., 2014). In this alternative conformation, RNA is thought to access the exonuclease active site of Rrp44 without going through the central channel. For convenience, we will refer to these two conformations as Rrp44ch (channel) RNA exosome conformation and Rrp44da (direct access) RNA exosome conformation, indicating the substrate recruitment site (supplemental movie). The exo-9 core of the RNA exosome contains the same proteins for both conformations and undergoes only minor conformational change (Liu et al., 2014). Thus the only significant difference between the direct access and channel conformations of the RNA exosome is a large rotation of the exonuclease domain of Rrp44, while the endonuclease domain and the exo-9 core are essentially identical in both conformations. The importance of the Rrp44ch conformation is supported by the observation that mutations that sterically and electrostatically interfere with RNA channeling through exo-9 cause specific RNA processing and growth defects (Drazkowska et al., 2013; Malet et al., 2010; Wasmuth and Lima, 2012), but whether the Rrp44da-exosome indeed allows direct access of substrates to the active site in vivo, whether the direct access conformation is only adopted when the RNA exosome is not RNA bound, or whether it is formed at all in vivo is not clear.
The large rotation of the exoribonuclease domain results in a different side of Rrp44 facing exo-9 in the two conformations. In this study we show that the amino acid residues at the interface between Rrp44da and exo-9 are important for association between the Rrp44 and exo-9, and thus that the direct access conformation is relevant in vivo. Furthermore, we show that specific RNA processing and degradation effects result from disrupting the rrp44da-exosome and thus provide evidence that this conformation is used for specific RNA exosome functions. Shortly after the RNA exosome was discovered, two models for its overall function were proposed (Mitchell and Tollervey, 2000; van Hoof and Parker, 1999). The proteasome-like model proposed that to be degraded a substrate had to access a central channel of an overall ring-shape structure, and many aspect of this model have since proven correct (Makino et al., 2013b). The now disfavored allosteric activation model was suggested as an alternative and one important aspect of it was that the RNA exosome adopts different conformations for different functions. Here, we provide evidence for the importance of alternative conformation. Our results unite key tenets of both models, with a channel conformation that closely fits the proteasome-like model and an alternative conformation that is required for specific functions.
RESULTS
Identification of residues in the exonuclease domain of Rrp44 that contribute to interaction with the RNA exosome core
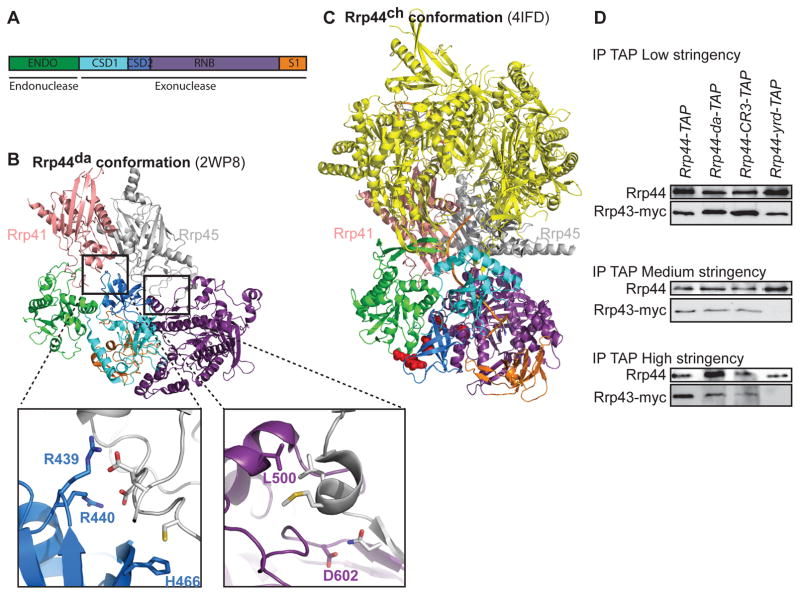
Comparison of multiple X-ray crystallography and EM studies of the RNA exosome suggests that the endonuclease domain does not undergo major conformational changes between the direct access conformation and the channel conformation of the RNA exosome (Figure 1A, B, and C and Supplemental movie; Bonneau et al., 2009; Liu et al., 2014; Liu et al., 2016; Makino et al., 2013a; Makino et al., 2015). In contrast, the exonuclease domain forms two distinct Rrp44-exosome interfaces with the core subunits Rrp41 and Rrp45 (Figures 1B and C and supplemental movie). Initial experiments suggested that the exonuclease domain has non-catalytic functions, possibly including contributing to exo-9 interaction (supplemental figure S1 and supplemental text). Structural studies indicate that such exonuclease domain interaction with exo-9 would be specific to one or the other conformation. Five conserved residues of Rrp44 (R439, R440, H466, L500, D602) appear to be important for exonuclease domain interaction with exo-9 in the direct access conformation (Figure 1B and S2A; highlighted in green in supplemental movie). Mutation of these residues would specifically disrupt the interaction of Rrp44da with the RNA exosome core because they do not seem to participate in the Rrp44ch-exosome core interaction and are largely solvent exposed in Rrp44ch (Figure 1C, red spheres; supplemental movie). Therefore, we constructed a mutant allele of RRP44, rrp44-da, in which these five residues are changed to alanine and used co-immunoprecipitation to test for effects on exo-9 interaction.
Figure 1. Identification of residues important for the direct access conformation of the RNA exosome.
(A) Domain organization of Rrp44. ENDO: endonuclease domain; CSD1/2: Cold Shock Domain 1/2; RNB: RNase II family catalytic domain; S1: S1 RNA Binding Domain. The domains are color coded as in panels B and C (B) Five conserved residues (R439, R440, H466, L500, and D602) that are important for the formation of the Rrp44da-conformation. (C) The five residues (shown as red spheres) are located on the bottom and exposed to solvent in the Rrp44ch-conformation. Cartoon versions of the X-ray crystal structures were generated by MacPyMol (Schrödinger, LLC). (D) Mutations in the five resides of panel B disrupt the Rrp44-exosome interaction. TAP-tagged variants of Rrp44 were immunoprecipitated at different wash conditions, and western blot was conducted by using α-Protein A and α-Myc antibodies.
Rrp44-TAP variants were immunoprecipitated from a yeast strain, which expresses Myc-tagged Rrp43 (one of the exo-9 subunits), and western blot was performed to detect co-precipitation of Rrp43-Myc (Figure 1D). Furthermore, we performed these experiments under low, medium and high stringency conditions (no NaCl, 50mM NaCl and 1M NaCl). Under the high stringency condition, similar to what we used previously (Schaeffer et al., 2012), the amount of Rrp43-Myc that co-purified with Rrp44-da-TAP or the previously analyzed Rrp44-CR3-TAP was reproducibly reduced compared to wild-type Rrp44-TAP, suggesting that residues in both the endonuclease and exonuclease domains are important for the interaction of Rrp44 with the RNA exosome core (Figure 1D). The CR3 motif within the endonuclease domain forms a zinc coordination site that is important for the proper positioning of the YRD motif that directly interacts with the exo-9 core (Makino et al., 2013a; Schaeffer et al., 2012). Rrp44-yrd-TAP, in which the YRD motif is changed to alanines, showed no detectable co-purified Rrp43-Myc at high stringency, consistent with the idea that the YRD motif directly interacts, while the CR3 motif has a less important role by positioning the YRD residues. Under medium stringency conditions, wild-type Rrp44, Rrp44-CR3 and Rrp44-da reproducibly co-immunoprecipitated approximately equal amounts of Rrp43-myc, while Rrp44-yrd copurified strongly reduced amounts. Finally under low stringency conditions, all three mutant forms of Rrp44-TAP co-purified Rrp43-myc. These data indicate that residues in both the endonuclease and exonuclease domains contribute to interaction with the core RNA exosome, although the contribution of the R439, R440, H466, L500, and D602 residues in the exonuclease domain is not as important as the contribution of the YRD motif in the endonuclease domain. In addition, the contribution of the R439, R440, H466, L500, and D602 residues for exo-9 interaction suggests the presence of the direct access conformation in vivo.
The RNA exosome direct access conformation is required for its normal function
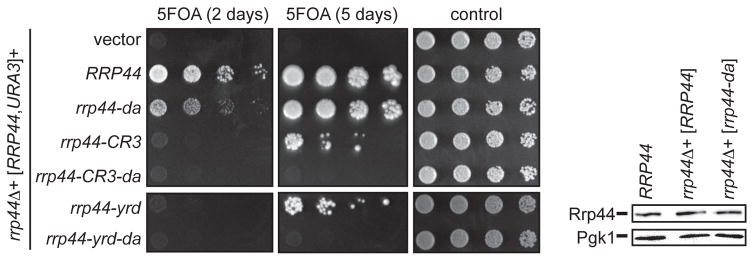
To determine whether the Rrp44da conformation is required for the function of the RNA exosome in vivo, we tested the growth of the rrp44-da mutant using a plasmid shuffle assay. Briefly, an RRP44 deletion strain that carries a wild-type RRP44 allele on a plasmid with a URA3 marker was transformed with a second plasmid carrying a wild-type RRP44 or rrp44 mutant alleles and a LEU2 marker. Resulting transformants were plated on 5FOA containing media that selects for cells that have lost the RRP44, URA3 plasmid as well as on control media. The strain transformed with rrp44-CR3 or rrp44-yrd grew slowly after losing the wild-type RRP44 gene, which is consistent with previous studies (Figure 2; Schaeffer et al., 2012; Schaeffer and van Hoof, 2011). Importantly, rrp44-da also caused a growth defect compared to wild type, although this growth defect was less severe than that of rrp44-CR3 or rrp44-yrd. The slow growth of the rrp44-da strain is not due to reduced expression of the mutant allele because western blot analysis shows that the mutant and wild-type allele expressed from a plasmid are expressed at similar levels to each other and to the endogenous Rrp44 (Figure 2). The slow growth of the rrp44-da strain suggests that the direct access conformation is required for RNA exosome function. Interestingly, combining the rrp44-da mutation with either rrp44-CR3 or rrp44-yrd resulted in lethality (Figure 2), suggesting that the two contact sites with exo-9 are partially redundant (see discussion).
Figure 2. The Rrp44da-exosome interface and the CR3/YRD RNA exosome interface are partially redundant.
rrp44-da causes a slow growth phenotype and is synthetic lethal with rrp44-CR3 and rrp44-YRD. An rrp44Δ strain carrying a wild-type RRP44 allele in a URA3 plasmid was transformed with LEU2 plasmids carrying RRP44 variants. Transformants were serially diluted and spotted on 5FOA and SC-LEU-URA (control) media. The western blot indicates that the plasmid-encoded rrp44-da allele is expressed at the same level as endogenous Rrp44 (first lane), or plasmid-encoded wild-type Rrp44 (second lane).
Because the rrp44-CR3-da and rrp44-yrd-da alleles were lethal, we could not assess whether these proteins are expressed at the normal level. We thus repeated the analysis with TAP-tagged plasmids. The plasmid shuffle assay with RRP44-TAP variants confirmed that rrp44-CR3-da and rrp44-yrd-da alleles were lethal. Importantly, when introduced into a wild-type strain (that contains the endogenous RRP44 gene) the Rrp44-CR3-da-TAP and Rrp44-YRD-da-TAP proteins were detected by western blot, ruling out the possibility that the lethal phenotype is due to the lack of expression (supplemental Fig. S3). Taken together, these data suggest that both the five residues mutated in rrp44-da and the CR3/YRD motif are important for the function of the RNA exosome, and that they are partially redundant. In addition, the importance of the five residues indicates that the Rrp44da-exosome contributes to the essential function of the RNA exosome.
The RNA exosome direct access conformation utilizes both the exo- and endoribonuclease activities
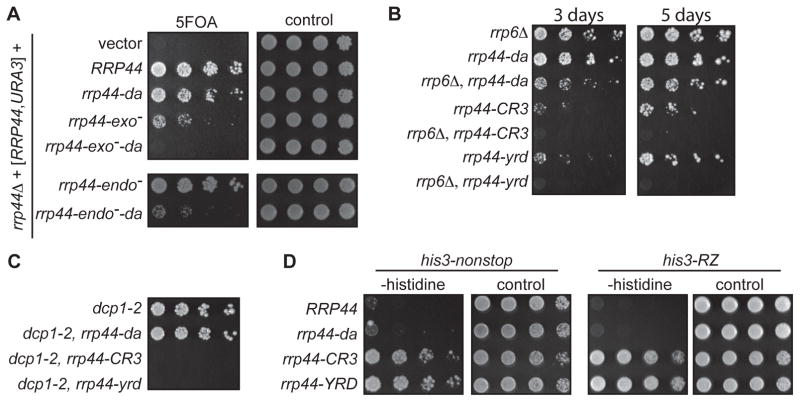
Since the RNA exosome processes both exo- and endonuclease activities, we tested what activities require the Rrp44da-exosome conformation. For this experiment, we used well-characterized mutations that generate an RNA exosome with only endonuclease activity (rrp44-exo−) or an RNA exosome with only exonuclease activity (rrp44-endo−) (Dziembowski et al., 2007; Lebreton et al., 2008; Schaeffer et al., 2009; Schneider et al., 2009). Introduction of the rrp44-da mutation into the RNA exosome that has only endonuclease activity resulted in lethality (Figure 3A). This suggests that the Rrp44da exo-9 interface is required for endonuclease activity. Similarly, introducing the rrp44-da mutation into the RNA exosome that has only exonuclease activity resulted in severe growth defect (Figure 3A). Therefore, the Rrp44da conformation is also required for exonuclease activity. A similar experiment using TAP-tagged variants showed the same lethal phenotype, and the expression level of the TAP-tagged proteins was comparable to TAP-tagged wild-type Rrp44, suggesting that the lethality is not due to failure to express the variant (supplemental Fig. S3). This indicates that disrupting the direct access RNA exosome conformation affects both the exo- and endonuclease activities of the RNA exosome.
Figure 3. The Rrp44da-exosome utilizes both the exo- and endonuclease activities and functions in the nucleus.
(A) rrp44-da is synthetic lethal with rrp44-exo− and rrp44-endo−. An rrp44Δ strain carrying a wild-type RRP44 allele in a URA3 plasmid was transformed with LEU2 plasmids carrying RRP44 variants. Transformants were serially diluted and spotted on 5FOA and SC-LEU-URA (Control) media. (B) (C) Synthetic growth defect of rrp44-da with Δrrp6 and dcp1-2. rrp44Δ rrp6Δ or rrp44Δ dcp1-2 strains carrying a wild-type RRP44 allele in a URA3 plasmid were transformed with LEU2 plasmids carrying a wild-type RRP44, rrp44-da, rrp44-CR3, or rrp44-yrd. Transformants were serially diluted and spotted on 5FOA. (D) Strains carrying RRP44 variants were transformed with reporter constructs encoding aberrant HIS3 mRNAs. Transformants were serially diluted and spotted on media lacking histidine or control plates containing histidine. The his3-nonstop reporter is the HIS3 gene with its stop codon removed. The his3-RZ reporter has a hammerhead ribozyme cleavage site immediately upstream of stop codon of the HIS3 gene.
The RNA exosome direct access conformation is important for nuclear functions but may be dispensable in the cytoplasm
The RNA exosome is present in both the nucleus and cytoplasm (Januszyk and Lima, 2014). The nuclear form of the RNA exosome is essential, while the cytoplasmic form is not (Jacobs Anderson and Parker, 1998; Mitchell et al., 1997). Thus, the analysis of growth and viability described above assesses the essential nuclear function of the RNA exosome and suggests that the Rrp44da conformation is important for nuclear function of the RNA exosome. This predicts that the rrp44-da mutation may show genetic interactions with mutations of nuclear RNA exosome cofactors, such as Rrp6. Rrp6 is an additional exonuclease that associates with the RNA exosome in the nucleus, but also has non-catalytic roles including mediating interactions with additional cofactors such as Rrp47 and Mtr4 (Butler and Mitchell, 2011; Feigenbutz et al., 2013; Schuch et al., 2014). As expected, the rrp44-da mutation shows a synthetic growth defect with rrp6Δ (Figure 3B) confirming that the Rrp44da conformation is important for the nuclear functions of the RNA exosome.
To investigate whether the Rrp44da conformation is also essential for the function of the cytoplasmic RNA exosome, we carried out two experiments. First, we tested for genetic interactions with the dcp1-2 mutation. The cytoplasmic RNA exosome functions in one of two general mRNA decay pathways, and the cytoplasmic RNA exosome is not essential because of redundancy between these pathways (Jacobs Anderson and Parker, 1998) . Therefore, the cytoplasmic RNA exosome becomes essential if the alternative pathway is inactivated. dcp1-2 is a temperature sensitive mutation in the alternative pathway, such that the cytoplasmic RNA exosome is essential in a dcp1-2 strain incubated at the restrictive temperature (Schaeffer and van Hoof, 2011). rrp44-da did not show a significant growth defect when combined with dcp1-2 (Figure 3C). This is in contrast with rrp44-CR3, which is synthetic lethal with dcp1-2 at the restrictive temperature as previously shown (Schaeffer et al., 2012). The rrp44-yrd mutation is also synthetic lethal with dcp1-2 as expected. This suggests that the Rrp44da conformation is not essential for mRNA degradation by the cytoplasmic RNA exosome.
In addition to its function in general mRNA decay, the cytoplasmic RNA exosome is required for the rapid degradation of specific aberrant mRNAs (Frischmeyer et al., 2002; Klauer and van Hoof, 2012; Meaux and van Hoof, 2006; van Hoof et al., 2002). Thus, in the second experiment we tested the effect of the rrp44-da mutation on this mRNA quality control function. The his3-nonstop reporter mRNA lacks a stop codon and therefore is rapidly degraded by the cytoplasmic RNA exosome (van Hoof et al., 2002). Mutations that inactivate the cytoplasmic RNA exosome stabilize the his3-nonstop mRNA, which allows the cell to synthesize sufficient histidine to grow in the absence of added histidine. As previously reported (Schaeffer et al., 2012; Schaeffer and van Hoof, 2011), the rrp44-CR3 mutation allows a his3-nonstop strain to grow in the absence of added histidine, indicating a defect in cytoplasmic RNA exosome function (Figure 3D, left two panels). As expected, the rrp44-yrd mutation has the same effect. In contrast, the rrp44-da mutation does not affect the his3-nonstop reporter mRNA, suggesting that the Rrp44da conformation is not required for nonstop mRNA degradation by the cytoplasmic RNA exosome.
We repeated the assay for mRNA quality control defects with a different reporter mRNA (Figure 3D, right two panels). The his3-RZ reporter contains a hammerhead ribozyme and therefore generates a truncated mRNA that lacks a poly(A) tail (Meaux and van Hoof, 2006). Such mRNA cleavage fragments are also degraded by the cytoplasmic RNA exosome, regardless of whether they contain a stop codon or not. As with his3-nonstop, mutations that inactivate the cytoplasmic RNA exosome stabilize the his3-RZ mRNA, which allows the cell to synthesize sufficient histidine to grow in the absence of added histidine. As previously reported, the rrp44-CR3 mutation allows a his3-RZ strain to grow in the absence of added histidine, and as expected, the rrp44-yrd mutation has the same effect. However, the rrp44-da mutation does not have this effect. Together, genetic analyses suggest that the Rrp44da-exosome conformation functions in the nucleus but is dispensable for cytoplasmic RNA exosome functions.
The RNA exosome direct access conformation is required for specific RNA degradation events, but makes minor contributions to others
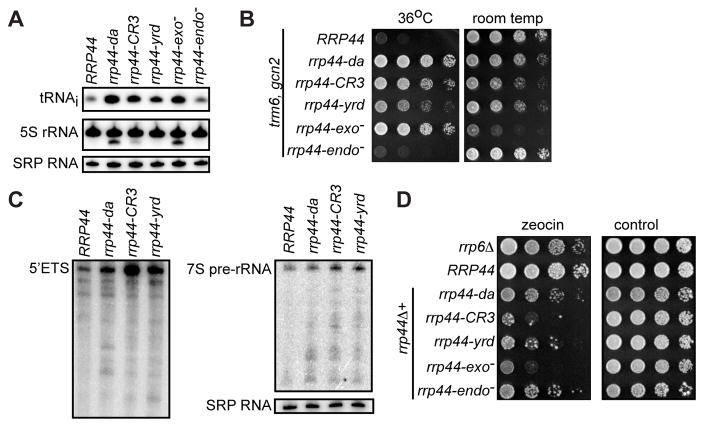
It has previously been shown that unmodified initiator tRNA (tRNAiMet) binds to the direct access conformation of the RNA exosome in vitro (Liu et al., 2014) and that the RNA exosome degrades hypomodified tRNAiMet in vivo (Kadaba et al., 2004). We therefore hypothesized that the direct access conformation of the RNA exosome may be especially important for the degradation of hypomodified tRNAiMet in vivo. Thus, we tested the hypomodified tRNAiMet level by northern blot analysis as previously described. This analysis used a trm6-504 rrp44Δ strain transformed with plasmids encoding either wild-type or mutant Rrp44. The trm6-504 mutation causes a defect in m1A58 methylation and thus causes the hypomodification that triggers exosome-mediated degradation of tRNAiMet in the strain containing the wild-type RRP44 gene. In contrast, tRNAiMet accumulates to approximately two-fold higher levels in the rrp44-da strain (Figure 4A). Although the rrp44-da mutation only caused a 2-fold increase, this increase was highly reproducible and similar to previous reports (Kadaba et al., 2004; Wang et al., 2008). The rrp44-exo− mutation caused similarly high tRNAiMet levels, while the rrp44-endo− mutation had no effect, suggesting that the exonuclease is the major activity responsible for hypomodified tRNAiMet degradation. The rrp44-CR3 and rrp44-yrd mutations also increased hypomodified tRNAiMet levels, but this effect was reproducibly smaller than the effect of the rrp44-da mutation (see discussion).
Figure 4. The Rrp44da-exosome is required for specific functions of the RNA exosome.
(A) Total RNA isolated from trm6, gcn2, rrp44Δ strains carrying wild-type or mutant alleles of RRP44 in a LEU2 plasmid were subjected to northern blot. Shown is a representative result of two independent biological replicates. (B) Strains used in (A) were serially diluted and spotted on SC-LEU. (C) Total RNAs were isolated from rrp44Δ strains carrying a wild-type or mutant RRP44 allele followed by northern blot probing 5′ETS and 7S pre-rRNA. SRP RNA serves a loading control. (D) rrp6Δ, and rrp44Δ strains carrying wild-type or mutant RRP44 alleles were serially diluted and spotted on media containing 10 μg/ml zeocin and YPD media as a control.
We confirmed the role of the direct access conformation in pre-tRNAiMet degradation using a growth assay. The temperature sensitive growth of a trm6-504 strain is caused by reduced tRNAimet level and therefore this temperature sensitivity is suppressed by rrp44 mutations that affect tRNAiMet degradation. The trm6-504 strain with a wild-type RRP44 allele failed to grow at 36 degrees (Figure 4B). This growth phenotype was strongly suppressed by rrp44-exo− and rrp44-da, while rrp44-CR3 and rrp44-yrd were slightly less effective at restoring growth. This growth phenotype mirrors the effects seen by northern blot confirming that the exonuclease activity and direct access conformation of Rrp44 are required for the rapid degradation of hypomodified tRNAiMet.
The effect of Rrp44 on hypomodified tRNAiMet was initially found in a strain that carries the rrp44-20 point mutation. This same rrp44-20 mutation also causes the accumulation of a truncated 5S rRNA (Kadaba et al., 2004). We therefore next analyzed the effect of the same RRP44 mutations on 5S rRNA and the results mirrored what we observed for hypomodified tRNAiMet. Specifically, the rrp44-da and rrp44-exo− strains reproducibly accumulated relatively high amounts of the truncated 5S rRNA, while the rrp44-CR3 and rrp44-yrd mutations had a much smaller effect and the rrp44-endo−mutation had no effect (Figure 4A).
To investigate the role of the direct access conformation on other specific nuclear RNA exosome functions, we next tested the effect of rrp44-da on the 5′ETS and 5.8S rRNA, two prototypical RNA exosome substrates. The RNA exosome degrades the 5′ external transcribed spacer (5′ETS) that is generated from 35S pre-rRNA processing events (de la Cruz et al., 1998). The RNA exosome is also involved in the maturation of 5.8S rRNA by processing the 3′-end of 7S pre-rRNA (Allmang et al., 1999). Using northern blot analysis, we reproducibly observed a 2-fold increase of the full-length 5′ETS and an accumulation of its degradation intermediates in rrp44-da compared to wild type (Figure 4C, left panel). In addition, rrp44-da showed a minor accumulation of the processing intermediates of 7S pre-rRNA (Figure 4C, right panel). Importantly, these defects are not as severe as the defects in rrp44-CR3 or rrp44-yrd (e.g. 6-fold increase in 5′ETS), indicating that the Rrp44da-exosome has a minor contribution to the degradation 5′ETS and processing of 7S rRNA to 5.8S rRNA (see below).
Recent studies have implicated the nuclear RNA exosome as important for the DNA damage response both in the budding yeast and HeLa cells (Hieronymus et al., 2004; Manfrini et al., 2015; Marin-Vicente et al., 2015). Specifically, the RNA exosome cofactors, Rrp6, Trf4, and the NEXT (Nuclear EXosome Targeting) complex were implicated in the DNA damage response (Gavalda et al., 2013; Hieronymus et al., 2004; Manfrini et al., 2015). We therefore tested whether mutations in the catalytic subunit of the RNA exosome itself cause sensitivity to zeocin, an agent that induces double-strand breaks (Chankova et al., 2007). As reported previously rrp6Δ strain was sensitive to zeocin (Figure 5D; Manfrini et al., 2015). We found that rrp44-CR3 and rrp44-exo− strains are extremely sensitive to zeocin, while the rrp44-da strain showed sensitivity similar to rrp6Δ. This shows that the exonuclease activity of Rrp44 is required for the DNA damage response, but the direct access conformation is less critical.
Figure 5. The balance between two RNA exosome conformations is required for growth.
(A) (B) rrp44-da suppresses the growth defect of rrp41-L and rrp45-L. rrp44Δ rrp41Δ or rrp44Δ rrp45Δ strains carrying RRP44 variants in a LEU2 plasmid and a wild-type RRP41 or RRP45 in a URA3 plasmid were transformed with a TRP1 plasmid carrying the rrp41-L or rrp45-L allele. Transformants were serially diluted and spotted on 5FOA media. (C) rrp44Δ rrp41Δ strains carrying RRP44 and RRP41 variants were subjected to total RNA isolation followed by northern blot analysis probing 7S pre-rRNA, 5′ETS, 5S rRNA, snR128 snoRNA and SRP RNA. Asterisk (*) indicates 5′ETS, 5S and snR128 RNA species specifically and reproducibly detected in the rrp44-da strain.
Taken together, the observations that rrp44-da has stronger effects on tRNAiMet and 5S rRNA than the rrp44-CR3 and rrp44-yrd mutations, while rrp44-CR3 and rrp44-yrd has stronger effects of growth, RNA exosome interaction, other RNA degradation reactions, and zeocin sensitivity suggest that the effects on tRNAiMet and 5S rRNA reflect a specific requirement of the direct access conformation for these two RNA exosome functions (see discussion).
The balance between the two RNA exosome conformations is required for growth
We next sought to identify the relationship between the two conformations, Rrp44da- and Rrp44ch-exosome, since the EM studies suggest dynamic conformational change between them (Liu et al., 2014; Liu et al., 2016). Instead of being maintained by protein contacts, the Rrp44ch conformation is thought to be stabilized by simultaneous interactions of long RNAs with the channel and exonuclease domain (Liu et al., 2016). Specifically, the interaction surface between the exonuclease domain and the RNA exosome core is much larger and electrostatically more favorable in the direct access conformation than in the channel conformation (Liu et al., 2016). Because of this reliance on RNA to stabilize the channel conformation, we could not identify specific Rrp44 residues required for the channel conformation. As an alternative way to disrupt channeling through the exosome core, we took advantage of the previously reported and characterized channel occluding mutations of Rrp41 and Rrp45 (rrp41-L and rrp45-L), in which an 11 amino acid residue insertion physically and electrostatically blocks the central channel of the RNA exosome (Wasmuth and Lima, 2012). rrp41-L and rrp45-L have slow growing and lethal phenotypes, respectively, which suggests that the central channel is essential. To test the relationship between the two RNA exosome conformations, we tested the genetic interaction between the rrp44-da and rrp41-L or rrp45-L mutations (Figure 5A). Strikingly, rrp44-da suppressed the slow growing phenotype of rrp41-L. This suppression is specific for the rrp44-da allele as rrp44-exo− and rrp44-endo− do not have a significant effect, and rrp44-CR3 is synthetic lethal with rrp41-L (and thus has the opposite effect of the rrp44-da allele). Similarly, the rrp44-da mutation suppressed the lethality of the rrp45-L channel occluding mutation (Figure 5B). We conclude that a proper balance between two conformations is important for the essential function of the RNA exosome (see discussion).
Having generated strains with either the direct access conformation or the channel disrupted, we further compared the role of the two conformations in specific RNA exosome functions by northern blotting for known RNA exosome substrates. In addition, we tested whether the suppression of rrp41-L growth phenotype by rrp44-da was accompanied by restoration of RNA processing and degradation defects. For 7S pre-rRNA to 5.8S rRNA processing we detected intermediates in the rrp41-L strain and lower levels of the same intermediates in the rrp44-da strain (Figure 5C). This confirms the conclusion that the direct access route makes a much smaller contribution to 5.8S processing than the channel route. Furthermore, the processing defect seen in the rrp41-L rrp44-da double mutant closely resembled that seen in rrp41-L, indicating that suppression of the rrp41-L growth phenotype is not accompanied by suppression of this rRNA processing defect.
As described above, the rrp44-da strain accumulated full-length 5′ETS as well as some degradation intermediates. The rrp41-L strain also accumulated 5′ETS degradation intermediates but not the full length 5′ETS. The rrp41-L strain accumulated much higher levels of degradation intermediates than the rrp44-da strain, again confirming that the channel route is the major degradation route for 5′ETS. Several intermediates were specific for rrp41-L, while one specific intermediate was reproducibly only detected in rrp44-da, although at low levels (Figure 5C, asterisk). Rather than suppressing the rrp41-L phenotype, the effect of combining rrp41-L with rrp44-da appeared additive, such that both sets of intermediates from the single mutants and the accumulation of full length 5′ETS were seen in the double mutant.
Although, as pointed out above, for some RNA exosome substrates we saw no suppression of the rrp41-L phenotype by rrp44-da, for other substrates we did see a suppression that correlates with the suppression of the growth phenotype. Specifically, the RNA subunit of the signal recognition particle (SRP) is commonly used as a loading control, but we noted that it was reproducibly 3-fold more abundant in the rrp41-L mutant than in the RRP41 control strain, consistent with a recent report that this RNA is also a substrate for the RNA exosome (Leung et al., 2014). The rrp41-L rrp44-da double mutant strain accumulated only 2-fold more SRP than the RRP41, RRP44 control strain (Figure 5C). Thus, the increased growth rate of this double mutant correlates with a smaller defect in the processing of this particular RNA. We noticed a similar trend with the snR128 snoRNA. 3′ extended species of this snoRNA accumulate in RNA exosome mutants, and we observed this phenotype for the rrp41-L strain as well. Strikingly however, the mature snR128 also over accumulated in the rrp41-L strain, and this over accumulation was slightly, but reproducibly less severe in the rrp41-L rrp44-da double mutant (Figure 5C). Overall, these data indicate that although the rrp44-da mutation suppresses the growth phenotype of the blocked RNA exosome channel in rrp41-L, most of the RNA processing defects in rrp41-L are not suppressed. We did see some minor suppression of SRP and snR128 defects, but whether this suppression is cause or effect of the suppression of the growth defect is not yet clear. In addition, the comparison of the rrp41-L and rrp44-da strains confirmed the above conclusion that the direct access conformation of the RNA exosome is required for a few specific functions while the channel conformation is required for many other functions.
DISCUSSION
Structural studies have captured the RNA exosome in two conformations in vitro (Liu et al., 2014; Makino et al., 2015). One conformation is consistent with RNA threading through the central channel of the exo-9 core to access the exonuclease active site, while in the other conformation RNA substrates directly access the active site, bypassing the channel. Here, we provide evidence that the Rrp44da-exosome is present in vivo, and that it has specific functions. We identified and mutated five residues in the exonuclease domain that interact with exo-9 in the direct access conformation, but are facing the solvent in the channel conformation. We show that mutation of these five residues reduces the co-immunoprecipitation of Rrp44 with Rrp43 and causes a slow growth phenotype. We conclude that the direct access conformation of the RNA exosome exists in vivo and contributes to RNA exosome function.
Several observations suggest that the direct access conformation of the exosome has specific but limited functions. Most importantly, the severity of RNA exosome defects seen in different RRP44 alleles cannot be explained by quantitative differences in Rrp44 activity with some alleles more severely affecting overall exosome activity and others having a smaller effect. For example, the rrp44-da mutation has a smaller effect than rrp44-yrd on growth, cytoplasmic RNA exosome functions, and most nuclear RNA exosome functions. In contrast, the rrp44-da mutation has a larger effect than rrp44-yrd on degradation of hypomodified tRNAiMet and truncated 5S rRNA. In fact, for these latter two functions, the severity of the defect in rrp44-da is similar to that seen in the catalytically inactive rrp44-exo− mutant. Second, while the rrp44-da, rrp44-CR3 and rrp44-yrd alleles all affect Rrp43 co-immunoprecipitation, the severity of these defects does not correlate well with growth and RNA degradation defects. Specifically, the rrp44-da and rrp44-CR3 mutations have similar effects on RNA exosome core interactions and rrp44-yrd has a larger effect. This is in contrast to the growth defects that are milder for rrp44-da and more severe for rrp44-CR3 and rrp44-yrd. Because of this disconnect between the effect on RNA-exosome binding and growth, we conclude that the effects seen for rrp44-da are not simply due to reduced interaction with the RNA exosome core. Third, there is an allele specific suppression of the rrp41-L growth phenotype. Specifically, the slow growth phenotype of the rrp41-L mutant is suppressed by the rrp44-da mutation, but enhanced by the rrp44-CR3 mutation. Such an allele-specific interaction is difficult to explain by both rrp44 alleles reducing overall RNA exosome function, but is readily explained by one of the alleles disrupting a specific function. While we have not directly measured the effect of the rrp44-da mutation on in vitro catalytic activity, such an effect is unlikely given the location of the mutations. More importantly, even if the mutations reduced the overall catalytic activity, such an effect could not explain the specific in vivo phenotypes. Based on all of these data, we conclude that the rrp44-da allele disrupts a specific aspect of RNA exosome function. Based on the structural studies and the effect on RNA exosome core co-immunoprecipitation, the most likely explanation is that the rrp44-da allele specifically disrupts the direct access conformation of the RNA exosome.
By analyzing a variety of previously characterized RNA exosome functions either by Northern blot or growth phenotypes, we show that the channeling and direct access conformations of the RNA exosome have distinct functions. Using channel-occluding mutations and qRT-PCR, it has previously been shown that the channeling conformation is required for the degradation of CUTs and 5′ ETS, and processing of 5.8S rRNA and U4 snRNA by the nuclear exosome and mRNAs by the cytoplasmic exosome (Drazkowska et al., 2013; Wasmuth and Lima, 2012). We confirm the 5.8S rRNA processing and 5′ ETS degradation defects and show that channel-occluding mutations also lead to defects in snoRNA processing. We also noted that the RNA subunit of SRP was more abundant in the channel-occluding mutant, consistent with a recently described role of the RNA exosome in SRP quality control (Leung et al., 2014). Most of the substrates affected by the channel occluding mutations were affected much less strongly by the direct access mutation. Conversely, the direct access mutant accumulated a truncated 5S rRNA form, while the channel occluding mutation had no effect on 5S rRNA. We also found that the direct access mutant of Rrp44 was completely inactive in degrading hypomodified tRNAiMet.
Although we identified distinct functions for the direct access and channeling conformations, both appear to be important for degradation of some substrates, such as 5′ETS, although the pattern of intermediates that accumulate in the two mutants is distinct. This may be because the 5′ETS can be degraded by either pathway, or because 5′ETS degradation is initiated by the direct access conformation and then finished by channeling through exo-9. This switch between access routes would require that the 3′ end of 5′ETS dissociates from the Rrp44 catalytic site, a possibility consistent with oligo-adenylation by TRAMP at internal sites (Schneider et al., 2012).
Surprisingly, the growth defect of channel occluding mutations, rrp41-L and rrp45-L, is suppressed by disruption of the direct access route (Figure 5A and B). While many of the defects seen in the single mutants are not reversed in the double mutant, accumulation of full-length snR128 snoRNA, and SRP RNA in rrp41-L is decreased in the rrp41-L rrp44-da double mutant. A possible explanation is that when one conformation of the RNA exosome is inhibited, the other conformation inappropriately acts on these RNAs. Partially disrupting both conformations could suppress phenotypes by interfering with the inappropriate action of the alternative conformation.
Strikingly, the defects (in initiator tRNA and 5S rRNA) we describe for rrp44-da closely resemble those described previously for the rrp44-20 allele (Kadaba et al., 2004). The single amino acid substitution in rrp44-20 (Gly833 - Asp) is positioned within the RNA binding channel of the exonuclease domain near the −5 nucleotide (numbering from the active site). Although this part of the RNA binding channel is shared between the direct access route and the channel route, the mutation appears to have a larger effect on direct access-dependent substrates. We suggest that introduction of a bulky, negative charged residue at this position has a more disruptive effect on the short (12 nt) RNA path of the direct-access route than the much longer (30 nt) path through the channel. Our results raise the possibility that defects in the two different conformations cause different human diseases. Specifically, multiple myeloma genomes often contain mutations in the Rrp44 exonuclease domain, but not in other RNA exosome subunits (Weissbach et al., 2015). In contrast, pontocerebellar hypoplasia is caused by point mutations in the exo-9 core (EXOSC3 and EXOSC8; Boczonadi et al., 2014; Wan et al., 2012). The residues mutated in rrp44-da are highly conserved in the human ortholog (supplemental Fig. S2), suggesting that the direct access conformation is also important in humans. Thus, we speculate that defects in the direct access function of DIS3 might contribute to the development of multiple myeloma, while defects in the channel-dependent functions may lead to pontocerebellar hypoplasia. The specific mutations in multiple myeloma may either directly affect the ability to adopt the direct access conformation, analogous to rrp44-da, or affect RNA interactions more severely in the short direct access route than in the longer channel route, as we propose for rrp44-20.
Genetic analyses suggest that the direct access conformation of the RNA exosome is important for both exo- and endoribonuclease activities. How RNA substrates access the endonuclease active site in general is unknown. Multiple crystal structures indicate the endonuclease domain is static with the active site readily accessible from the solvent (Bonneau et al., 2009; Makino et al., 2013a; Makino et al., 2015). However, biochemical analyses indicate that channel occluding mutations also affect the endonuclease activity of the RNA exosome (Drazkowska et al., 2013; Wasmuth and Lima, 2012). This suggests that there may be an additional uncharacterized conformation of Rrp44 that orients the endonuclease site towards the channel. One explanation why the rrp44-da mutation inhibits endonuclease activity is that this hypothetical conformational change of the endonuclease domain likely breaks the contact of exo-9 with the YRD motif of Rrp44 and therefore integrity of the RNA exosome in this hypothetical conformation would depend more heavily on interactions between exo-9 and the exonuclease domain of Rrp44.
Regardless of whether substrates utilize the direct access route or the channel route, the RNA exosome function requires additional proteins that are thought to mediate substrate specificity. Mutations in the TRAMP subunit Trf4 also affect both the degradation of hypomethylated tRNAiMet and the accumulation of truncated 5S rRNA (Kadaba et al., 2004; Kadaba et al., 2006), suggesting that TRAMP is capable of delivering RNA substrates to the direct access route, in addition to its better characterized ability to deliver channel-dependent substrates. Thus, the route the RNA takes in the RNA exosome may not be dictated by the cofactor that delivers it to the substrate.
In summary, we show that the direct access conformation of the RNA exosome is present in vivo and functions on specific substrates in the nucleus. A major difference between the two conformations is the length of the paths, which is ~30nt and 12nt, respectively (Bonneau et al., 2009; Liu et al., 2014; Makino et al., 2015; Malet et al., 2010). The longer RNA binding path through a channel is likely to increase continuous binding to long single stranded RNAs and thus processivity of the RNA exosome, while direct access may be more suitable for structured RNAs or RNAs that are part of large RNPs that don’t fit through the central channel (such as tRNAiMet and 5S rRNA). The access point utilized by a particular substrate could therefore result in distinct outcomes in the processing or degradation reactions. Taken together, we propose that the RNA exosome adopts different conformations to accommodate RNA substrates with vastly different characteristics. This resembles the allosteric activation model that was proposed soon after the discovery of the RNA exosome, but has since lost favor (Mitchell and Tollervey, 2000).
MATERIALS AND METHODS
Plasmids and strains
Yeast strains were generated using standard genetic techniques and are listed in table S1. The leu2-Δ0 and trp1Δ::hisG alleles were introduced as described (Alani et al., 1987; Brachmann et al., 1998). Plasmids were generated using standard molecular biology techniques and are described in table S2. Rrp44 was expressed from low copy plasmids and driven by the RRP44 promoter. The rrp44-da allele was generated by gene synthesis (Genewiz).
Co-immunoprecipitation (Co-IP) assay and western blotting
Co-IP was performed as previously described with some modifications (Schaeffer et al., 2012). Briefly, a yeast strain (yAV1117) that carries Myc tagged-Rrp43 was transformed with TAP-tagged RRP44 alleles. Exponentially growing transformants in 50 ml media were resuspended in IP0 for low stringency (50 mM Tris–HCl pH 7.5, 2 mM MgCl2, 0.1% Triton X100, 0.5 mM β-mercaptoethanol, 0.1 mM PMSF, with complete EDTA-free protease inhibitors [Roche]) or IP50 (IP0 with 50mM NaCl) for medium and high stringency. Cells were lysed by vortexing with acid washed glass beads. 200 μl lysate was incubated with 12 μl IgG Sepharose 6 Fast Flow beads (Amersham Biosciences) for 2 hours at 4°C. The beads were washed four time s with 1 ml IP0 (low stringency), IP50 (medium stringency), or IP1000 buffer (IP0 with 1M NaCl; high stringency). The high stringency IP was washed once more with 1 ml IP50 buffer to remove residual salts. Washed beads were mixed with SDS-PAGE sample buffer and heated to 95°C for 5 min to elute bound proteins. Eluted proteins were analyzed by western blot with anti-protein A antibodies (Sigma), anti-Myc antibodies (generous donation from Dr. Eric Wagner), anti-Rrp44 (NeoBioLab) and anti-Pgk1 antibodies (Invitrogen).
Growth assays
For growth assays, strains were serially diluted and spotted on the indicated media. Zeocin sensitivity was tested on YPD plates containing 10 μg/ml zeocin.
Northern blot analysis
RNA was isolated from cells growing exponentially at 30°C, with the exception of the experiment described in Figure 5A where cells were grown at 30°C and incubated at 37°C for 4 hours to reveal the trm6 effect before harvesting as previously described (Kadaba et al., 2004). 10 μg RNA was subjected to 6% polyacrylamide (19:1) 8 M urea gel electrophoresis and transferred to Zeta-Probe GT Blotting membranes (Bio-Rad). Blots were probed using 32P-radiorabeled oligonucleotides listed in table S3.
Supplementary Material
Acknowledgments
We thank members of the van Hoof laboratory for critical discussions, Jim Anderson and Chris Lima for providing strains, Eric Wagner for providing antibodies, and Nayun Kim for help with the DNA damage response assays. This work was supported by NIH R01 grant GM099790 to AvH.
Footnotes
AUTHOR CONTRIBUTIONS
JH conducted all experiments. JH and AvH designed and analyzed experiments and wrote the paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE CITED
- Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999;18:5399–5410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczonadi V, Muller JS, Pyle A, Munkley J, Dor T, Quartararo J, Ferrero I, Karcagi V, Giunta M, Polvikoski T, et al. EXOSC8 mutations alter mRNA metabolism and cause hypomyelination with spinal muscular atrophy and cerebellar hypoplasia. Nature communications. 2014;5:4287. doi: 10.1038/ncomms5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau F, Basquin J, Ebert J, Lorentzen E, Conti E. The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell. 2009;139:547–559. doi: 10.1016/j.cell.2009.08.042. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Briggs MW, Burkard KT, Butler JS. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J Biol Chem. 1998;273:13255–13263. doi: 10.1074/jbc.273.21.13255. [DOI] [PubMed] [Google Scholar]
- Butler JS, Mitchell P. Rrp6, rrp47 and cofactors of the nuclear exosome. Adv Exp Med Biol. 2011;702:91–104. doi: 10.1007/978-1-4419-7841-7_8. [DOI] [PubMed] [Google Scholar]
- Chang HM, Triboulet R, Thornton JE, Gregory RI. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature. 2013;497:244–248. doi: 10.1038/nature12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chankova SG, Dimova E, Dimitrova M, Bryant PE. Induction of DNA double-strand breaks by zeocin in Chlamydomonas reinhardtii and the role of increased DNA double-strand breaks rejoining in the formation of an adaptive response. Radiat Environ Biophys. 2007;46:409–416. doi: 10.1007/s00411-007-0123-2. [DOI] [PubMed] [Google Scholar]
- Chlebowski A, Lubas M, Jensen TH, Dziembowski A. RNA decay machines: the exosome. Biochim Biophys Acta. 2013;1829:552–560. doi: 10.1016/j.bbagrm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Tollervey D, Linder P. Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J. 1998;17:1128–1140. doi: 10.1093/emboj/17.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato N, Neuhann T, Kahlert AK, Klink B, Hackmann K, Neuhann I, Novotna B, Schallner J, Krause C, Glass IA, et al. Mutations in EXOSC2 are associated with a novel syndrome characterised by retinitis pigmentosa, progressive hearing loss, premature ageing, short stature, mild intellectual disability and distinctive gestalt. Journal of medical genetics. 2016;53:419–425. doi: 10.1136/jmedgenet-2015-103511. [DOI] [PubMed] [Google Scholar]
- Drazkowska K, Tomecki R, Stodus K, Kowalska K, Czarnocki-Cieciura M, Dziembowski A. The RNA exosome complex central channel controls both exonuclease and endonuclease Dis3 activities in vivo and in vitro. Nucleic Acids Res. 2013;41:3845–3858. doi: 10.1093/nar/gkt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowski A, Lorentzen E, Conti E, Seraphin B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat Struct Mol Biol. 2007;14:15–22. doi: 10.1038/nsmb1184. [DOI] [PubMed] [Google Scholar]
- Evguenieva-Hackenberg E. The archaeal exosome. Adv Exp Med Biol. 2010;702:29–38. [PubMed] [Google Scholar]
- Evguenieva-Hackenberg E, Hou L, Glaeser S, Klug G. Structure and function of the archaeal exosome. Wiley Interdiscip Rev RNA. 2014;5:623–635. doi: 10.1002/wrna.1234. [DOI] [PubMed] [Google Scholar]
- Fabre A, Charroux B, Martinez-Vinson C, Roquelaure B, Odul E, Sayar E, Smith H, Colomb V, Andre N, Hugot JP, et al. SKIV2L mutations cause syndromic diarrhea, or trichohepatoenteric syndrome. Am J Hum Genet. 2012;90:689–692. doi: 10.1016/j.ajhg.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbutz M, Garland W, Turner M, Mitchell P. The exosome cofactor Rrp47 is critical for the stability and normal expression of its associated exoribonuclease Rrp6 in Saccharomyces cerevisiae. PLoS One. 2013;8:e80752. doi: 10.1371/journal.pone.0080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer PA, van Hoof A, O’Donnell K, Guerrerio AL, Parker R, Dietz HC. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- Gavalda S, Gallardo M, Luna R, Aguilera A. R-loop mediated transcription-associated recombination in trf4Delta mutants reveals new links between RNA surveillance and genome integrity. PLoS One. 2013;8:e65541. doi: 10.1371/journal.pone.0065541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley JL, Zachos NC, Dawood B, Donowitz M, Forman J, Pollitt RJ, Morgan NV, Tee L, Gissen P, Kahr WH, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy) Gastroenterology. 2010;138:2388–2398. 2398 e2381–2382. doi: 10.1053/j.gastro.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus H, Yu MC, Silver PA. Genome-wide mRNA surveillance is coupled to mRNA export. Genes Dev. 2004;18:2652–2662. doi: 10.1101/gad.1241204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Jacobs Anderson JS, Parker R. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 1998;17:1497–1506. doi: 10.1093/emboj/17.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januszyk K, Lima CD. Structural components and architectures of RNA exosomes. Adv Exp Med Biol. 2010;702:9–28. [PMC free article] [PubMed] [Google Scholar]
- Januszyk K, Lima CD. The eukaryotic RNA exosome. Current opinion in structural biology. 2014;24:132–140. doi: 10.1016/j.sbi.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18:1227–1240. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA. 2006;12:508–521. doi: 10.1261/rna.2305406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauer AA, van Hoof A. Degradation of mRNAs that lack a stop codon: a decade of nonstop progress. Wiley Interdiscip Rev RNA. 2012;3:649–660. doi: 10.1002/wrna.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton A, Tomecki R, Dziembowski A, Seraphin B. Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature. 2008;456:993–996. doi: 10.1038/nature07480. [DOI] [PubMed] [Google Scholar]
- Leung E, Schneider C, Yan F, Mohi-El-Din H, Kudla G, Tuck A, Wlotzka W, Doronina VA, Bartley R, Watkins NJ, et al. Integrity of SRP RNA is ensured by La and the nuclear RNA quality control machinery. Nucleic Acids Res. 2014;42:10698–10710. doi: 10.1093/nar/gku761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Chao S, Chiou NT, Schuster G. The PNPase, exosome and RNA helicases as the building components of evolutionarily-conserved RNA degradation machines. J Biomed Sci. 2007;14:523–532. doi: 10.1007/s11373-007-9178-y. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Bratkowski MA, Liu X, Niu CY, Ke A, Wang HW. Visualization of distinct substrate-recruitment pathways in the yeast exosome by EM. Nat Struct Mol Biol. 2014;21:95–102. doi: 10.1038/nsmb.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Niu CY, Wu Y, Tan D, Wang Y, Ye MD, Liu Y, Zhao W, Zhou K, Liu QS, et al. CryoEM structure of yeast cytoplasmic exosome complex. Cell research. 2016 doi: 10.1038/cr.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Greimann JC, Lima CD. Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell. 2006;127:1223–1237. doi: 10.1016/j.cell.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Lorentzen E, Walter P, Fribourg S, Evguenieva-Hackenberg E, Klug G, Conti E. The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat Struct Mol Biol. 2005;12:575–581. doi: 10.1038/nsmb952. [DOI] [PubMed] [Google Scholar]
- Lubas M, Damgaard CK, Tomecki R, Cysewski D, Jensen TH, Dziembowski A. Exonuclease hDIS3L2 specifies an exosome-independent 3′-5′ degradation pathway of human cytoplasmic mRNA. The EMBO journal. 2013;32:1855–1868. doi: 10.1038/emboj.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino DL, Baumgartner M, Conti E. Crystal structure of an RNA-bound 11-subunit eukaryotic exosome complex. Nature. 2013a;495:70–75. doi: 10.1038/nature11870. [DOI] [PubMed] [Google Scholar]
- Makino DL, Halbach F, Conti E. The RNA exosome and proteasome: common principles of degradation control. Nat Rev Mol Cell Biol. 2013b;14:654–660. doi: 10.1038/nrm3657. [DOI] [PubMed] [Google Scholar]
- Makino DL, Schuch B, Stegmann E, Baumgartner M, Basquin C, Conti E. RNA degradation paths in a 12-subunit nuclear exosome complex. Nature. 2015;524:54–58. doi: 10.1038/nature14865. [DOI] [PubMed] [Google Scholar]
- Malecki M, Viegas SC, Carneiro T, Golik P, Dressaire C, Ferreira MG, Arraiano CM. The exoribonuclease Dis3L2 defines a novel eukaryotic RNA degradation pathway. The EMBO journal. 2013;32:1842–1854. doi: 10.1038/emboj.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet H, Topf M, Clare DK, Ebert J, Bonneau F, Basquin J, Drazkowska K, Tomecki R, Dziembowski A, Conti E, et al. RNA channelling by the eukaryotic exosome. EMBO Rep. 2010;11:936–942. doi: 10.1038/embor.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfrini N, Trovesi C, Wery M, Martina M, Cesena D, Descrimes M, Morillon A, d’Adda di Fagagna F, Longhese MP. RNA-processing proteins regulate Mec1/ATR activation by promoting generation of RPA-coated ssDNA. EMBO Rep. 2015;16:221–231. doi: 10.15252/embr.201439458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Vicente C, Domingo-Prim J, Eberle AB, Visa N. RRP6/EXOSC10 is required for the repair of DNA double-strand breaks by homologous recombination. J Cell Sci. 2015;128:1097–1107. doi: 10.1242/jcs.158733. [DOI] [PubMed] [Google Scholar]
- Meaux S, van Hoof A. Yeast transcripts cleaved by an internal ribozyme provide new insight into the role of the cap and poly(A) tail in translation and mRNA decay. RNA. 2006;12:1323–1337. doi: 10.1261/rna.46306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′-->5′ exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Tollervey D. Musing on the structural organization of the exosome complex. Nat Struct Biol. 2000;7:843–846. doi: 10.1038/82817. [DOI] [PubMed] [Google Scholar]
- Porrua O, Libri D. RNA quality control in the nucleus: the Angels’ share of RNA. Biochim Biophys Acta. 2013;1829:604–611. doi: 10.1016/j.bbagrm.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Schaeffer D, Clark A, Klauer AA, Tsanova B, van Hoof A. Functions of the cytoplasmic exosome. Adv Exp Med Biol. 2010;702:79–90. [PubMed] [Google Scholar]
- Schaeffer D, Reis FP, Johnson SJ, Arraiano CM, van Hoof A. The CR3 motif of Rrp44p is important for interaction with the core exosome and exosome function. Nucleic acids research. 2012;40:9298–9307. doi: 10.1093/nar/gks693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D, Tsanova B, Barbas A, Reis FP, Dastidar EG, Sanchez-Rotunno M, Arraiano CM, van Hoof A. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat Struct Mol Biol. 2009;16:56–62. doi: 10.1038/nsmb.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D, van Hoof A. Different nuclease requirements for exosome-mediated degradation of normal and nonstop mRNAs. Proc Natl Acad Sci U S A. 2011;108:2366–2371. doi: 10.1073/pnas.1013180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Kudla G, Wlotzka W, Tuck A, Tollervey D. Transcriptome-wide analysis of exosome targets. Mol Cell. 2012;48:422–433. doi: 10.1016/j.molcel.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Leung E, Brown J, Tollervey D. The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res. 2009;37:1127–1140. doi: 10.1093/nar/gkn1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch B, Feigenbutz M, Makino DL, Falk S, Basquin C, Mitchell P, Conti E. The exosome-binding factors Rrp6 and Rrp47 form a composite surface for recruiting the Mtr4 helicase. EMBO J. 2014;33:2829–2846. doi: 10.15252/embj.201488757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi T, Fukushima K, Suzuki N, Nakashima N, Noguchi E, Nishimoto T. Human dis3p, which binds to either GTP- or GDP-Ran, complements Saccharomyces cerevisiae dis3. J Biochem. 1998;123:883–890. doi: 10.1093/oxfordjournals.jbchem.a022020. [DOI] [PubMed] [Google Scholar]
- Staals RH, Bronkhorst AW, Schilders G, Slomovic S, Schuster G, Heck AJ, Raijmakers R, Pruijn GJ. Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J. 2010;29:2358–2367. doi: 10.1038/emboj.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staals RH, Pruijn GJ. The human exosome and disease. Adv Exp Med Biol. 2011;702:132–142. doi: 10.1007/978-1-4419-7841-7_11. [DOI] [PubMed] [Google Scholar]
- Tomecki R, Kristiansen MS, Lykke-Andersen S, Chlebowski A, Larsen KM, Szczesny RJ, Drazkowska K, Pastula A, Andersen JS, Stepien PP, et al. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J. 2010;29:2342–2357. doi: 10.1038/emboj.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A, Frischmeyer PA, Dietz HC, Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science. 2002;295:2262–2264. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- van Hoof A, Parker R. The exosome: a proteasome for RNA? Cell. 1999;99:347–350. doi: 10.1016/s0092-8674(00)81520-2. [DOI] [PubMed] [Google Scholar]
- Wan J, Yourshaw M, Mamsa H, Rudnik-Schoneborn S, Menezes MP, Hong JE, Leong DW, Senderek J, Salman MS, Chitayat D, et al. Mutations in the RNA exosome component gene EXOSC3 cause pontocerebellar hypoplasia and spinal motor neuron degeneration. Nat Genet. 2012;44:704–708. doi: 10.1038/ng.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Wang J, Ding F, Callahan K, Bratkowski MA, Butler JS, Nogales E, Ke A. Architecture of the yeast Rrp44 exosome complex suggests routes of RNA recruitment for 3′ end processing. Proc Natl Acad Sci U S A. 2007;104:16844–16849. doi: 10.1073/pnas.0705526104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jia H, Jankowsky E, Anderson JT. Degradation of hypomodified tRNA(iMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA. 2008;14:107–116. doi: 10.1261/rna.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth EV, Januszyk K, Lima CD. Structure of an Rrp6-RNA exosome complex bound to poly(A) RNA. Nature. 2014;511:435–439. doi: 10.1038/nature13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth EV, Lima CD. Exo- and endoribonucleolytic activities of yeast cytoplasmic and nuclear RNA exosomes are dependent on the noncatalytic core and central channel. Mol Cell. 2012;48:133–144. doi: 10.1016/j.molcel.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach S, Langer C, Puppe B, Nedeva T, Bach E, Kull M, Bargou R, Einsele H, Rosenwald A, Knop S, et al. The molecular spectrum and clinical impact of DIS3 mutations in multiple myeloma. Br J Haematol. 2015;169:57–70. doi: 10.1111/bjh.13256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.